ABSTRACT
Background The prognostic value of Chemokine (C-X-C motif) ligand 1 (CXCL1) in various types of cancer remains controversial. Here we aimed to evaluate the prognostic role of CXCL1 for cancer.
Methods A comprehensively search of the PubMed, Embase, Web of Science, Wanfang and China National Knowledge Internet databases was conducted to retrieve eligible studies meeting the inclusion criteria. Overall survival (OS), progression-free survival (PFS) and various clinicopathological parameters were defined as endpoints. Stata SE12.0 software was used for quantitative meta-analysis.
Results A total of 17 studies encompassing 2265 cancer patients were included. Our meta-analysis showed that patients with higher CXCL1 expression had significantly shorter OS, according to both multivariate (HR 1.51, 95% CI 1.19–1.83, P < .01) and univariate analysis (HR 2.08, 95% CI 1.62–2.54, P < .01). Furthermore, higher CXCL1 expression was significantly correlated with advanced TNM stage and lymph node metastasis (both P < .05).
Conclusions High CXCL1 expression is a risk factor for cancer prognosis indicating a poor OS, and advanced TNM stage and lymph node metastasis, demonstrating that it may be a promising prognostic biomarker for different cancers.
KEYWORDS: CXCL1, GRO alpha, cancer, prognosis
Introduction
Cancer is a global disease with increasing morbidity and lethality, which significantly increases the economic burden to society and families of patients with cancer.1 The National Cancer Center released the latest issue of national cancer statistics in February 2018. The data showed that about 10,000 people were diagnosed with cancer every day in China and the cumulative risk for a person to develop cancer is 36% by the age of 85. Cancer has become the second-ranking cause of death in the USA, and is therefore a major public health problem.2 The chemokine (C-X-C motif) ligand 1 (CXCL1), also named GRO-1 oncogene as a small cytokine that belongs to the family of CXC chemokines originally characterized by Richmond et al.3 This cytokine, secreted by melanoma cells, is involved in the pathogenesis of melanoma partially due to its mitogenic properties. In humans, this protein, encoded by the CXCL1 gene, plays a major role in inflammation, angiogenesis, tumorigenesis, and wound healing.4–6 In recent years, many lines of evidence have indicated that aberrant expression of CXCL1 is closely correlated with tumorigenesis and metastasis in various malignancies, such as primary human cancers of the breast, gastric, bladder, colorectal, lung, liver, and pancreas.7–10 Moreover, several studies have demonstrated that the overexpression of CXCL1 was correlated with poor prognosis of patients with cancer.11,12 However, there has been no meta-analysis assessing the usefulness of CXCL1 expression for the prognosis of tumors. Hence, it was necessary to conduct a systematic review and meta-analysis to elucidate if CXCL1 has prognostic potential for cancer. Here we aimed to evaluate the prognostic and clinicopathological significance of CXCL1 expression from tissue and serum samples of patients with cancer.
Materials and methods
Literature retrieval strategy
Studies included in the meta-analysis were obtained by conducting a systematic computerized literature search of the PubMed, Embase, Web of Science, Wanfang and China National Knowledge Internet databases. The following combinations of keywords were used in the search: (“CXCL1, Chemokine” OR “CXCL1 Chemokine” OR “Gro-alpha Protein C” OR “GRO alpha Protein”) AND (“neoplasm” OR “cancer” OR “tumor”). Additional studies were obtained by manually screening the reference lists. The studies included were published from 1996 to June 2018.
Inclusion criteria and exclusion criteria
Eligible studies were included in the meta-analysis if they fulfilled the following criteria: 1) containing comparisons of different levels of CXCL1 expression; 2) focusing on the prognostic role of CXCL1 for cancer; 3) reporting OS, PFS, or other clinicopathological parameters; and 4) having sufficient data. Exclusion criteria were: 1) insufficient data or animal research data; 2) overlapping data; and 3) expert opinions, case reports, reviews or letters.
Data extraction and quality assessment
The information and data we obtained were extracted from the included studies by Zulei Zhang and Yaofei Jiang, and a third investigator (Yan Luo) was called upon to resolve any disagreements. For each study, we recorded the information and encompassing the first author’s family name, publication year, country, cancer type, total sample size, sample type, analysis method, cut-off value, follow-up times (years), outcome measures, prognostic parameters (e.g., OS, or PFS) and some other clinicopathological parameters such as gender, age, TNM stage, lymph node metastasis, tumor size, distant metastasis, depth of invasion, vascular invasion and neural invasion. Multivariate analysis results of OS were preferentially extracted from the included studies. For those studies that only provided an overall-survival curve, we extracted the corresponding survival data using Engauge Digitizer version 4.1.
Statistical analysis
The statistical analysis of HRs for OS and risk ratios (RRs) for clinicopathological parameters was conducted using Stata SE12.0. We used the random-effects model to pool data with statistical heterogeneity determined by the inconsistency index (I2 ≥ 50%) and the chi-squared test (P ≤ 0.10). The robustness of the results was investigated using sensitivity analysis. Begg’s rank correlation test was used to assess the publication bias, determined as positive if Pr > |z| ≤0.1.13
Results
Literature search
After searching for the indicated keywords in PubMed, Web of Science, Embase, Wanfang and China National Knowledge Internet databases, 386 relevant studies were identified. Three additional records were identified by manually searching the references of these studies. Among them, 267 articles were retained after excluding duplicates. However, 250 studies were excluded after screening the titles, abstracts and data. Finally, we included 17 studies in this meta-analysis7–10,12,14–25 (Figure 1), encompassing 12 prospective studies7–9,12,15,17–19,21–23,25 and 5 retrospective studies.10,14,16,20,24
Figure 1.
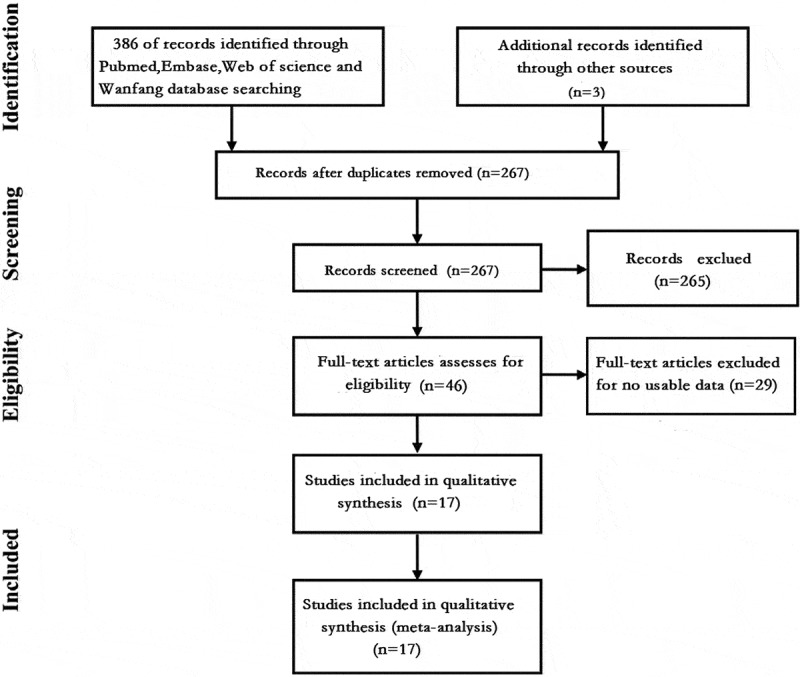
Flow chart showing the steps of document retrieval and selection.
Characteristics of the included studies
As shown in Table 1, 17 studies encompassing 2265 cancer patients were included for analysis, with sample sizes ranging from 48 to 292.7–10,12,14–25 Regarding the sample types, 14 studies7–10,12,14–18,20,22,24,25 were based on tissue samples, 2 used serum samples,21,23 and one investigated the patients’ urine.19 With respect to the level of CXCL1 expression and cut-off values, 12 studies used immunohistochemical methods (IHC) for detection, and immunoreactivity scores for the cut-off value determination,9,10,12,14–18,20,22,24,25 while several other studies used different detection methods and cut-off values,8,19,21 and 4 studies failed to report the details of how they arrived at the cut-off values.7,8,16,23 Among the 17 studies, 12 were from China,8,9,12,14–16,18,20–23,25 2 were from Japan,17,19 2 from the United States,10,24 and 1 from France.7 Among the predefined endpoints, 13 studies reported OS,9,12,14–22,24,25 3 studies reported PFS8,10,21 and 10 studies reported clinicopathological parameters.10,12,14–18,20,22,25 Moreover, 10 different types of carcinoma were analyzed, including esophageal squamous cell carcinoma (ESCC),15 hepatocellular carcinoma (HCC),22,23 bladder cancer,19,24 breast cancer,7 gastric cancer,9,12,16–18,20 cervical squamous cell carcinoma (CSCC),21 colorectal cancer (CRC),14 non-small cell lung cancer (NSCLC),8 pancreatic cancer and urothelial cancer of the bladder (UCB).10 Regarding the types of treatment, fourteen studies used surgical resection,7–10,12,14,16–20,22,23,25 one study used radio-chemotherapy,15 and two other studies did not report the treatment modalities.21,24 Multivariate analysis was employed in 11 studies,9,12,14,15,17–19,21,22,24,25 while univariate analysis was used in 8 studies12,14–16,19,20,24,25 as the analysis model for OS. In addition, the Newcastle-Ottawa scale (NOS) score was 2 (6–9 stars) in all of the included studies.
Table 1.
Basic information of the included studies.
| Study | Year | Country | Diseases | Total (M/F) | Treatment types | Sample | Assay | Cut-off value | Follow-up (year) |
Outcome measures | Analysis type |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bieche7 | 2007 | France | Breast cancer | 48 | Surgery | Tissue | RT-PCR | NR | 10 | RFS | U |
| Cao8 | 2017 | China | NSCLC | 50 (14/36) | Surgery | Tissue | CBA kit | NR | ≥2 | PFS | U |
| Cheng9 | 2011 | China | GC | 116 (65/51) | Surgery | Tissue | IHC | Strong staining | 5 | OS | M |
| Miyake10 | 2016 | USA | UCB | 142 | Surgery | Tissue | IHC | IS | 10 | PFS | U |
| Xiang12 | 2015 | China | GC | 127 (92/35) | Surgery | Tissue | IHC | IS>97.2% | 7 | OS | M and U |
| Zhuo14 | 2018 | China | CRC | 276 (166/100) | Surgery | Tissue | IHC | IS4 | 5 | OS | M and U |
| Zhang15 | 2017 | China | ESCC | 141 (75/66) | Radio- chemotherapy |
Tissue | IHC | Strong staining | 3 | OS | M and U |
| Wang16 | 2017 | China | GC | 105 (71/34) | Surgery | Tissue | IHC | NR | ≥8 | OS | U |
| Kasashima17 | 2017 | Japan | GC | 264 (114/150) | Surgery | Tissue | IHC | IS>3 | 5 | OS | M |
| Wang18 | 2016 | China | GC | 100 (65/35) | Surgery | Tissue | IHC | IS3 | 5 | OS | M |
| Nakashima19 | 2015 | Japan | Bladder cancer | 175 (132/43) | Surgery | Urine | ELISA | 35 pg/mg | 4.5 | OS | M and U |
| Wei20 | 2015 | China | GC | 98 (62/36) | Surgery | Tissue | IHC | Strong staining | 5 | OS | U |
| Zhang21 | 2014 | China | CSCC | 292 (F) | NR | Serum | Luminex technology | <748.43 pg/ml | 3 | OS, DFS | M |
| Cao22 | 2014 | China | HCC | 48 (41/7) | Surgery | Tissue | IHC | Strong staining | >6 | OS | M |
| Chen23 | 2013 | China | HCC | 179 (160/19) | Surgery | Serum | Luminex technology | NR | 8 | DFS | U |
| Miyake24 | 2013 | USA | Bladder cancer | 142 | NR | Tissue | IHC | IS3 | 4 | OS, DFS | M and U |
| Lian25 | 2016 | China | Pancreatic cancer |
160 | Surgery | Tissue | IHC | IS3 | 5 | OS | M and U |
CRC: colorectal cancer; IHC: immunohistochemical method; IS: immunoreactivity score; OS: overall survival; M: multivariate analysis; U: univariate analysis; ESCC: esophageal squamous cell carcinoma; CRC: colorectal Cancer; ELISA: enzyme-linked immunoassay; NSCLC: non-small cell lung cancer; GC: gastric cancer; CBA kit: human proinflammatory chemokine panel (13-plex) (BioLegend, San Diego, CA); UCB: urothelial cancer of the bladder; CSCC: cervical squamous cell carcinoma; HCC: hepatocellular carcinoma; NR, not reported.
Meta-analysis of the association between CXCL1 expression levels and OS
Thirteen articles reporting OS were included in the meta-analysis.9,12,14–22,24,25 According to different methods, it was divided into multivariate analysis9,12,14,15,17–19,21,22,24,25 and univariate analysis.12,14–16,19,20,24,25 The fixed effects model was used because of no significant heterogeneity (I2 = 13.8%, P = .279). The results indicated that cancer patients with higher CXCL1 expression had significantly shorter OS, in both multivariate analysis (HR 1.51, 95% CI 1.19–1.83, P < .01) and univariate analysis (HR 2.08, 95% CI 1.62–2.54, P < .01), as shown in Figure 2. The robustness of both the results was confirmed by sensitivity analysis (Figure 3). However, the Begg’s rank correlation test indicated the presence of significant publication bias (Pr > |z| = 0.075) (Figure 4). Moreover, we performed subgroup analyses based on the types of treatment, and we found that higher levels of CXCL1 expression were significantly correlated with poorer OS in the patients that underwent surgical resection (HR 2.01, 95% CI 1.67–2.36, P < .01) or radio-chemotherapy (HR 3.78, 95% CI 1.89–5.67, P < .01), as shown in Fig. S1.
Figure 2.
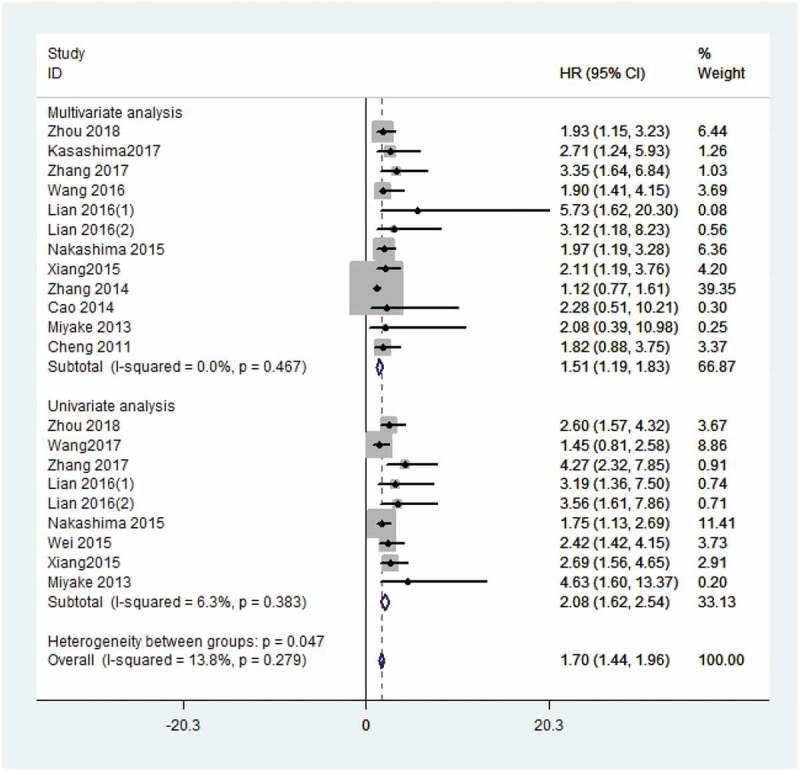
Forest plot for the meta-analysis of OS by multivariate analysis and univariate analysis.
Figure 3.
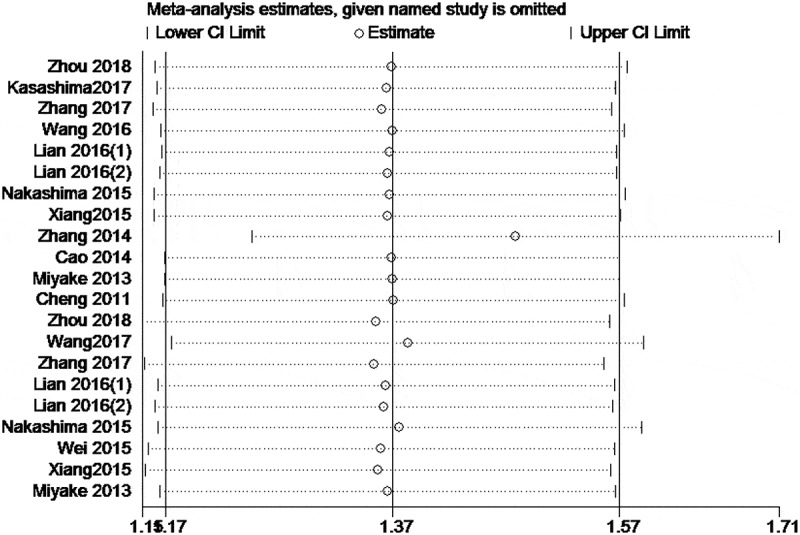
Sensitivity analyses for the meta-analysis of OS.
Figure 4.
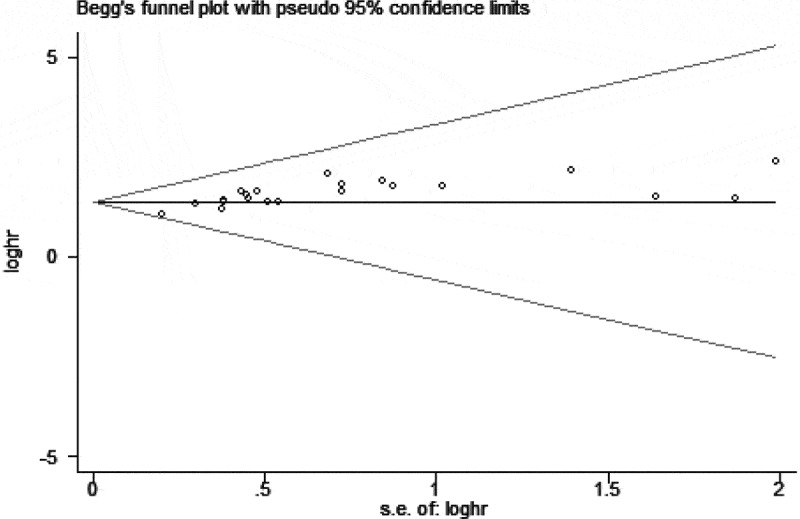
Assessment of publication bias for the meta-analysis of the association between CXCL1 level and OS using Begg’s rank correlation test.
Association between CXCL1 expression levels and PFS
Three studies reported PFS. Among them, two studies demonstrated significantly shorter PFS in cancer patients with higher CXCL1 expression compared to those with low CXCL1 expression by univariate analysis,8,10 while one demonstrated comparable outcomes by multivariate analysis.21
Meta-analyses of the association between CXCL1 levels and clinicopathological parameters
Ten articles were included for meta-analyses of clinicopathological parameters.10,12,14–18,20,22,25 As shown in Table 2, there was no significant correlation between CXCL1 expression and gender (RR 1.00, 95% CI 0.92–1.08; P = .967) (Figure 5a). Similar results were observed for age (RR 1.08, 95% CI 0.97–1.19; P = .145) (Figure 5b), tumor size (RR 1.40, 95% CI 0.92–2.14; P = .116) (Figure 5c), distant metastasis (RR 2.18, 95% CI 0.70–6.81; P = .181) (Figure 5d), degree of tumor differentiation (RR 0.98, 95% CI 0.67–1.43; P = .910) (Figure 6a), and neural invasion (RR 1.30, 95% CI 0.87–1.95; P = .199) (Figure 6b). However, higher CXCL1 expression was significantly correlated with a more advanced TNM stage (RR 1.73, 95% CI 1.47–2.04; P < .001) (Figure 6c) and lymph node metastasis (RR 1.77, 95% CI 1.07–2.92; P = .026) (Figure 6d).
Table 2.
The meta-analyses of clinical parameters.
| Variables | Included studies | Patients (n) | RR 95%CI | P | I2 | Mode |
|---|---|---|---|---|---|---|
| Gender (male versus female) | 10 | 1474 | 1.0 [0.92, 1.08] | 0.967 | 0.00% | Fixed |
| Age (old versus young) | 9 | 1319 | 1.08 [0.97, 1.19] | 0.145 | 16.60% | Fixed |
| TNM stage (III/IV versus I/II) | 5 | 478 | 1.73 [1.47, 2.04] | <0.001 | 38.20% | Fixed |
| LNM (yes versus no) | 3 | 681 | 1.77 [1.07, 2.92] | 0.026 | 89.20% | Random |
| Tumor size (large versus small) | 9 | 1210 | 1.40 [0.92, 2.14] | 0.116 | 84.40% | Random |
| Distant metastasis (yes versus no) | 3 | 451 | 2.18 [0.70,6.81] | 0.181 | 78.00% | Random |
| Differentiation (poor versus good) | 4 | 452 | 0.98 [0.67, 1.43] | 0.910 | 80.00% | Random |
| Neural invasion (yes versus no) | 2 | 346 | 1.30 [0.87, 1.95] | 0.199 | 0.00% | Fixed |
LNM: lymph node metastasis; OR: odds ratio; CI: confidence interval.
Figure 5.
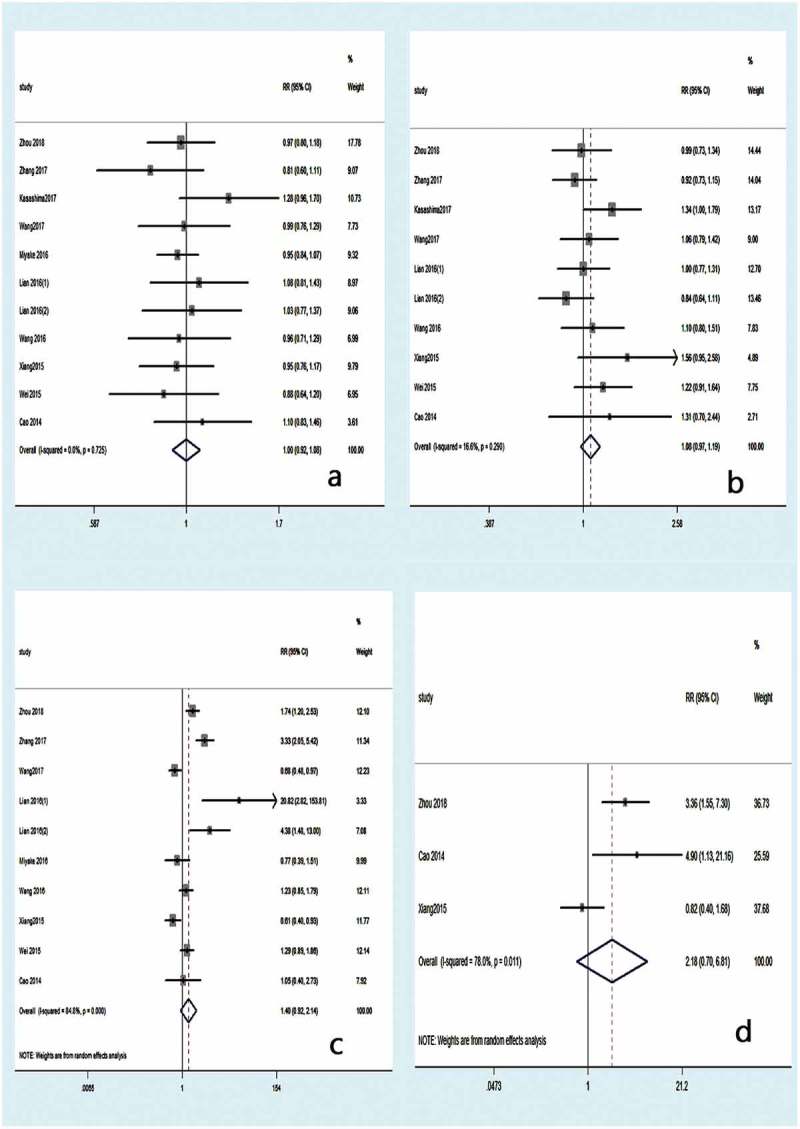
Forest plot for the meta-analysis according to gender (a), age (b), tumor size (c), and distant metastasis (d).
Figure 6.
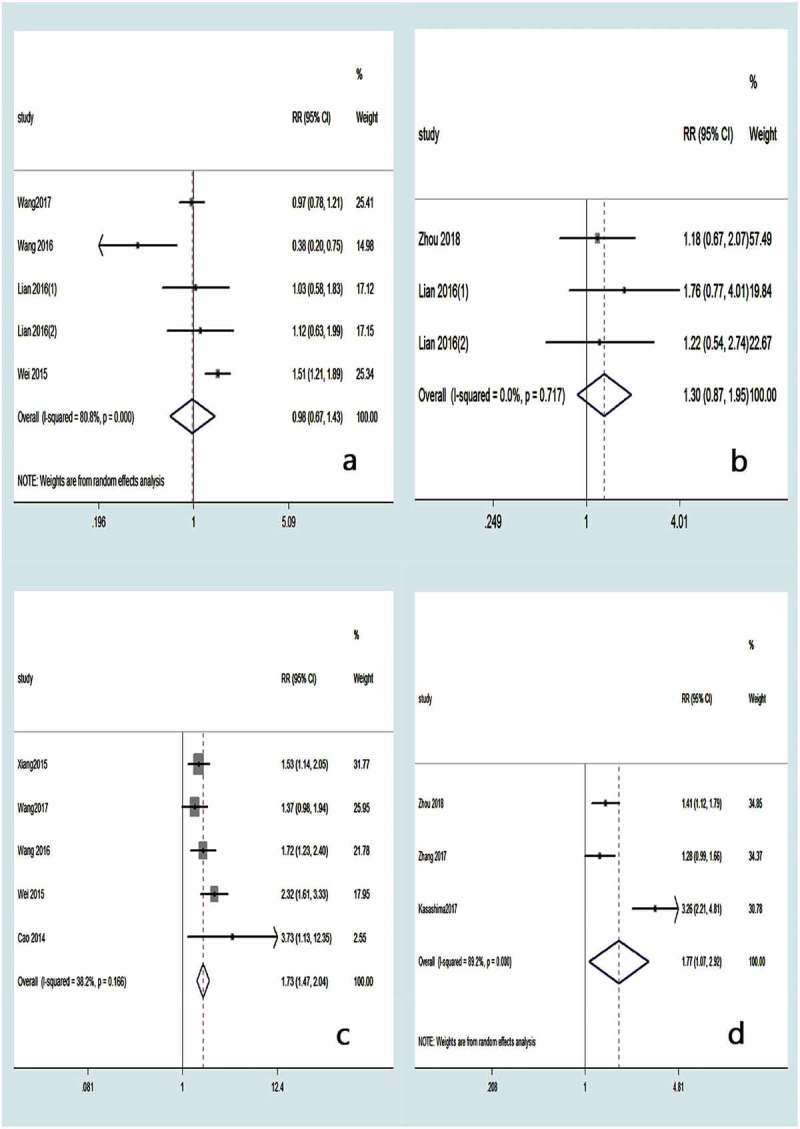
Forest plot for the meta-analysis according to degree of tumor differentiation (a), neural invasion (b), TNM stage (c), and lymph node metastasis (d).
Discussion
Chemokines are a class of single-subunit chemotactic cytokines ranging in size from 8 to 15 kDa. Their division into the four subfamilies CXC, CC, CX3L and C (where X stands for any amino acid) was determined by the position of conserved cysteines at the amino terminus.26 The CXC subfamily was further subdivided into ELR+ and ELR- chemokines according to whether the CXC domain has a tripeptide Glu-Leu-Arg (ELR motif) before, wherein the “ELR” motif is neutral. The interaction of granulocyte ligands/receptors is critical.27,28 As a member of the ELR+ subpopulation, CXCL1 (also known as GRO-α) was originally isolated from the culture supernatants of melanoma cells and is specific for its receptor CXCR2, a G protein-coupled receptor belonging to the seven-transmembrane family. Chemokine-ligand interactions play different roles in a variety of biological functions, such as angiogenesis, inflammation, wound healing and tumor development.29 In recent years, it has been found that CXCL1 plays an important role in tumor formation, since it is upregulated in melanoma, colorectal cancer, breast cancer, bladder cancer, ovarian epithelial cancer (epithelial ovarian cancer, EOC) and gastric cancer.30
To our best knowledge, this is the first comprehensive and systematical meta-analysis of the value of CXCL1 for cancer prognosis. In this study, we found that the cancer patients with higher CXCL1 expression had significantly worse OS compared to those with low CXCL1 expression. Thus, the findings of this meta-analysis indicate that high CXCL1 expression might serve as a risk factor for unfavorable prognosis of cancer. Furthermore, we also found that high CXCL1 expression was significantly correlated with a more advanced TNM stage and lymph node metastasis. All of these findings demonstrated that high CXCL1 expression might be used for predicting a worse prognosis in various kinds of carcinoma.
Some studies explained the underlying mechanisms of the relationship between CXCL1 dysregulation and cancer. Zhou et al. found that CXCL1 plays an important role in both cancer progression and metastasis in colorectal cancer patients by inducing glycolysis.14 Zhang et al. found that cancer-associated fibroblasts (CAF) secrete CXCL1, which inhibits the expression of the reactive oxygen species (ROS)-scavenging enzyme superoxide dismutase 1, leading to increased ROS accumulation following irradiation, which enhanced DNA damage repair and thereby mediated radio-resistance. CAF-secreted CXCL1 was found to mediate radio-resistance via the activation of the Mek/Erk pathway.15 Xu et al. found high expression of CXCR2 in gastric cancer tissues, which was positively correlated with disease stage and Ki67 proliferation index, as well as lymphatic vessel density (LVD), suggesting that CXCL1 and its receptor CXCR 2 play a crucial role in lymphatic metastasis of gastric cancer.31 In addition, Nakashima et al. found that patients with higher urine CXCL1 were statistically more likely to develop intravesical recurrence after transurethral resection (TUR), and multivariate analysis identified urine CXCL1 as an independent predictor of post-TUR intravesical recurrence.19 Li et al. established a homologous tumor cell clone library from an in situ mouse model of pancreatic cancer and found that tumor cell-derived CXCL1 directly inhibits T cell infiltration and reduces responsiveness to immunotherapy, while ablation of CXCL1 promoted T cell infiltration and sensitivity to a combination immunotherapy regimen.32 Understanding the role of CXCL1 in tumor progression may help to design strategies to inhibit tumor cell growth by selectively perturbing its function, which would make CXCL1 into an invaluable new biomarker for tumor therapy and potential therapeutic targets.14,16,20,24,25 However, only a few studies have investigated the mechanisms underlying the prognostic role of CXCL1 in cancer to date. Thus, more fundamental research is needed to decipher the prognostic value of CXCL1 for cancer.
Finally, the constraints of the prognostic role of CXCL1 for human cancer found in this meta-analysis should be discussed. Firstly, potential risk bias might have been a factor. Because positive outcomes were more likely to be published than negative ones, we tried to identify all relevant information. However, some missing data was still unavoidable. Secondly, there was statistical heterogeneity in this analysis, which might be partially explained by different cut-off values for CXCL1 expression and different study protocols. Thirdly, in the selected studies, most of the patients were from Asia. Therefore, the results we obtained may be more representative of Asians. Fourthly, for those studies that only provided overall-survival curves, we extracted the survival data using Engauge Digitizer version 4.1, which compromises the precision of the data. Finally, only 17 studies with a total of 2265 patients were included in this meta-analysis, and even less studies were included in the subgroup analyses of OS and clinicopathological parameters, which led to relatively inadequate data. Since there are limitations in the recognized investigations and the current meta-analysis, our outcomes should be interpreted with caution, and the conclusions of this meta-analysis require detailed consideration.
In summary, high CXCL1 expression was found to be a risk factor for cancer prognosis indicating a poor OS, advanced TNM stage and lymph node metastasis, demonstrating that it may be a promising prognostic biomarker for cancer.
Disclosures
The authors have no conflicts of interest or financial ties to disclose.
References
- 1.Miller K, Siegel R, Lin C, Mariotto A, Kramer J, Rowland J, Stein KD, Alteri R, Jemal A.. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66(4):271–289. doi: 10.3322/caac.21349. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A.. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 3.Wang D, Yang W, Du J, Devalaraja M, Liang P, Matsumoto K, Tsubakimoto K, Endo T, Richmond A. MGSA/GRO-mediated melanocyte transformation involves induction of Ras expression. Oncogene. 2000;19(40):4647–4659. doi: 10.1038/sj.onc.1203820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haskill S, Peace A, Morris J, Sporn S, Anisowicz A, Lee S, Smith T, Martin G, Ralph P, Sager R. Identification of three related human GRO genes encoding cytokine functions. Proc Natl Acad Sci USA. 1990;87(19):7732–7736. doi: 10.1073/pnas.87.19.7732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dhawan P, Richmond A. Role of CXCL1 in tumorigenesis of melanoma. J Leukoc Biol. 2002;72(1):9–18. [PMC free article] [PubMed] [Google Scholar]
- 6.Anisowicz A, Bardwell L, Sager R. Constitutive overexpression of a growth-regulated gene in transformed Chinese hamster and human cells. Proc Natl Acad Sci USA. 1987;84(20):7188–7192. doi: 10.1073/pnas.84.20.7188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bieche I, Chavey C, Andrieu C, Busson M, Vacher S, Le Corre L, Guinebretière J-M, Burlinchon S, Lidereau R, Lazennec G. CXC chemokines located in the 4q21 region are up-regulated in breast cancer. Endocr Relat Cancer. 2007;14(4):1039–1052. doi: 10.1677/erc.1.01301. [DOI] [PubMed] [Google Scholar]
- 8.Cao Y, Huang H, Wang Z, Zhang G. The inflammatory CXC chemokines, GROalpha(high), IP-10(low), and MIG(low), in tumor microenvironment can be used as new indicators for non-small cell lung cancer progression. Immunol Invest. 2017;46(4):361–374. doi: 10.1080/08820139.2017.1280052. [DOI] [PubMed] [Google Scholar]
- 9.Cheng WL, Wang CS, Huang YH, Tsai MM, Liang Y, Lin KH. Overexpression of CXCL1 and its receptor CXCR2 promote tumor invasion in gastric cancer. Ann Oncol. 2011;22(10):2267–2276. doi: 10.1093/annonc/mdq739. [DOI] [PubMed] [Google Scholar]
- 10.Miyake M, Hori S, Morizawa Y, Tatsumi Y, Nakai Y, Anai S, Torimoto K, Aoki K, Tanaka N, Shimada K, et al. CXCL1-mediated interaction of cancer cells with tumor-associated macrophages and cancer-associated fibroblasts promotes tumor progression in human bladder cancer. Neoplasia. 2016;18(10):636–646. doi: 10.1016/j.neo.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zou A, Lambert D, Yeh H, Yasukawa K, Behbod F, Fan F, Cheng N. Elevated CXCL1 expression in breast cancer stroma predicts poor prognosis and is inversely associated with expression of TGF-β signaling proteins. BMC Cancer. 2014;14:781. doi: 10.1186/1471-2407-14-781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xiang Z, Jiang D, Xia G, Wei Z, Chen W, He Y, Zhang C-H. CXCL1 expression is correlated with Snail expression and affects the prognosis of patients with gastric cancer. Oncol Lett. 2015;10(4):2458–2464. doi: 10.3892/ol.2015.3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101. [PubMed] [Google Scholar]
- 14.Zhuo C, Wu X, Li J, Hu D, Jian J, Chen C, Zheng X, Yang C. Chemokine (C-X-C motif) ligand 1 is associated with tumor progression and poor prognosis in patients with colorectal cancer. Biosci Rep. 2018;38:4. doi: 10.1042/BSR20180580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang H, Yue J, Jiang Z, Zhou R, Xie R, Xu Y, Wu S.. CAF-secreted CXCL1 conferred radioresistance by regulating DNA damage response in a ROS-dependent manner in esophageal squamous cell carcinoma. Cell Death Dis. 2017;8(5):e2790. doi: 10.1038/cddis.2017.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Z, Wang Z, Li G, Wu H, Sun K, Chen J, Feng Y, Chen C, Cai S, Xu J, et al. CXCL1 from tumor-associated lymphatic endothelial cells drives gastric cancer cell into lymphatic system via activating integrin β1/FAK/AKT signaling. Cancer Lett. 2017;385:28–38. doi: 10.1016/j.canlet.2016.10.043. [DOI] [PubMed] [Google Scholar]
- 17.Kasashima H, Yashiro M, Nakamae H, Masuda G, Kinoshita H, Morisaki T, Fukuoka T, Hasegawa T, Nakane T, Hino M, et al. Clinicopathologic significance of the CXCL1-CXCR2 axis in the tumor microenvironment of gastric carcinoma. PLoS One. 2017;12:6. doi: 10.1371/journal.pone.0178635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang L, Zhang C, Xu J, Wu H, Peng J, Cai S, He Y. CXCL1 gene silencing inhibits HGC803 cell migration and invasion and acts as an independent prognostic factor for poor survival in gastric cancer. Mol Med Rep. 2016;14(5):4673–4679. doi: 10.3892/mmr.2016.5843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakashima M, Matsui Y, Kobayashi T, Saito R, Hatahira S, Kawakami K, Nakamura E, Nishiyama H, Ogawa O. Urine CXCL1 as a biomarker for tumor detection and outcome prediction in bladder cancer. Cancer Biomark. 2015;15(4):357–364. doi: 10.3233/CBM-150472. [DOI] [PubMed] [Google Scholar]
- 20.Wei ZW, Xia GK, Wu Y, Chen W, Xiang Z, Schwarz RE, Brekken RA, Awasthi N, He Y-L, Zhang C-H. CXCL1 promotes tumor growth through VEGF pathway activation and is associated with inferior survival in gastric cancer. Cancer Lett. 2015;359(2):335–343. doi: 10.1016/j.canlet.2015.01.033. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y, Wu J-Z, Yang Y-Q, Ma R, Zhang J-Y, Feng J-F. Expression of growth‑regulated oncogene‑1, hepatocyte growth factor, platelet‑derived growth factor‑AA and soluble E‑selectin and their association with high‑risk human papillomavirus infection in squamous cell carcinoma of the uterine cervix. Mol Med Rep. 2014;10(2):1013–1024. doi: 10.3892/mmr.2014.2293. [DOI] [PubMed] [Google Scholar]
- 22.Cao Z, Fu B, Deng B, Zeng Y, Wan X, Qu L. Overexpression of Chemokine (C-X-C) ligand 1 (CXCL1) associated with tumor progression and poor prognosis in hepatocellular carcinoma. Cancer Cell Int. 2014;14:1. doi: 10.1186/1475-2867-14-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen ZY, Wei W, Guo ZX, Peng LX, Shi M, Li SH, Xiao C-Z, Zhong C, Qian C-N, Guo R-P. Using multiple cytokines to predict hepatocellular carcinoma recurrence in two patient cohorts. Br J Cancer. 2014;110(3):733–740. doi: 10.1038/bjc.2013.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miyake M, Lawton A, Goodison S, Urquidi V, Gomes-Giacoia E, Zhang G, Ross S, Kim J, Rosser CJ. Chemokine (C-X-C) ligand 1 (CXCL1) protein expression is increased in aggressive bladder cancers. BMC Cancer. 2013;13:322. doi: 10.1186/1471-2407-13-322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lian S, Zhai X, Wang X, Zhu H, Zhang S, Wang W, Yan J, Lin F. Elevated expression of growth-regulated oncogene-alpha in tumor and stromal cells predicts unfavorable prognosis in pancreatic cancer. Medicine (Baltimore). 2016;95(30):e4328. doi: 10.1097/MD.0000000000004864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000;12(2):121–127. [DOI] [PubMed] [Google Scholar]
- 27.Clark-Lewis I, Dewald B, Geiser T, Moser B, Baggiolini M. Platelet factor 4 binds to interleukin 8 receptors and activates neutrophils when its N terminus is modified with Glu-Leu-Arg. Proc Natl Acad Sci USA. 1993;90(8):3574–3577. doi: 10.1073/pnas.90.8.3574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hébert C, Vitangcol R, Baker J. Scanning mutagenesis of interleukin-8 identifies a cluster of residues required for receptor binding. J Biol Chem. 1991;266(28):18989–18994. [PubMed] [Google Scholar]
- 29.Balkwill F. The chemokine system and cancer. J Pathol. 2012;226(2):148–157. doi: 10.1002/path.3029. [DOI] [PubMed] [Google Scholar]
- 30.Ogata H, Sekikawa A, Yamagishi H, Ichikawa K, Tomita S, Imura J, Ito Y, Fujita M, Tsubaki M, Kato H, et al. GROα promotes invasion of colorectal cancer cells. Oncol Rep. 2010;24(6):1479–1486. [PubMed] [Google Scholar]
- 31.Xu J, Zhang C, He Y, Wu H, Wang Z, Song W, Li W, He W, Cai S, Zhan W. Lymphatic endothelial cell-secreted CXCL1 stimulates lymphangiogenesis and metastasis of gastric cancer. Int J Cancer. 2012;130(4):787–797. doi: 10.1002/ijc.26035. [DOI] [PubMed] [Google Scholar]
- 32.Li J, Byrne KT, Yan F, Yamazoe T, Chen Z, Baslan T, Richman LP, Lin JH, Sun YH, Rech AJ, et al. Tumor cell-intrinsic factors underlie heterogeneity of immune cell infiltration and response to immunotherapy. Immunity. 2018;49(1):178–93.e7. doi: 10.1016/j.immuni.2018.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]


