Abstract
Systemic lupus erythematosus is a prototypic model for B-cell epitope spread in autoimmunity. Autoantibodies to numerous molecularly distinct self-antigens emerge in a sequential manner over several years, leading to disease manifestations. Among the earliest autoantibodies to appear are those targeting phospholipid-binding proteins, particularly β2-glycoprotein I. Notably, mice immunized with β2-glycoprotein I and lipopolysaccharide develop a strong T cell response to β2-glycoprotein I that is associated with autoantibody production and renal disease, similar to that seen in human SLE. Here we hypothesized that mice with murine systemic lupus erythematosus, whether induced or spontaneous, should have T cells that recognize β2-glycoprotein I. We evaluated the response of splenic T cells from mice with induced (C57BL/6 and C3H/HeN) and spontaneous (MRL/lpr) systemic lupus erythematosus to peptides spanning the entire sequence of human β2GPI. We found that mice with induced and spontaneous systemic lupus erythematosus recognize a common T cell epitope (peptide 31; LYRDTAVFECLPQHAMFG) in domain III of β2-glycoprotein I. β2GPI-reactive CD4+ T cells from the two models differed primarily in cytokine production: T cells from mice with induced SLE expressed IFN-γ, while T cells from MRL/lpr mice expressed both IL-17 and IFN-γ, indicating that IL-17-expressing T cells are not necessary for generating a β2GPI-reactive T cell response. These data suggest that the generation of a β2-glycoprotein I-reactive T cell response is shared by both induced and spontaneous models of systemic lupus erythematosus and that this T cell response may mediate epitope spread to autoantibodies in both models.
Keywords: β2-glycoprotein I, T cells, systemic lupus erythematosus, autoantibodies, MHC class II haplotypes
Introduction
Systemic lupus erythematosus (SLE) is a heterogeneous autoimmune disease in which individuals develop multiple different autoantibodies, as well as a diversity of organ-related pathologies.1 Despite this heterogeneity, autoantibodies emerge in a sequential manner, with anti-β2-glycoprotein I (β2GPI) being among the first to appear.2 We have previously shown that immunization of nonautoimmune mice with β2GPI and lipopolysaccharide (LPS) induces a murine model of SLE that closely mimics human disease.3 These mice develop a strong and persistent T cell response to β2GPI, which is associated with epitope spread to other SLE-related autoantibodies and the development of SLE-like disease.4 The order of appearance of autoantibodies in our induced model mimics that seen in spontaneous human SLE.2,3 We have shown that multiple β2GPI T cell epitopes are associated with the production of SLE autoantibodies in this induced model.4 Here we hypothesized that mice with spontaneous SLE should also have T cells recognizing β2GPI and that a common β2GPI T cell epitope might exist between induced and spontaneous models of SLE. We evaluated the T cell response to peptides spanning the entire sequence of β2GPI in two major histocompatibility complex (MHC) class II-distinct strains of mice with induced SLE and in the MRL/lpr spontaneous model of SLE, and we characterized the differences between β2GPI-reactive T cells in the two models.
CD4+ T cells, particularly T helper type 1 and type 17 cells (Th1/Th17), have been shown to be necessary for disease pathogenesis in murine and human SLE.5–7 Interferon-γ (IFN-γ)- and interleukin (IL)-17-producing T cells are important for the development of nephritis in MRL/lpr mice, a spontaneous murine model of SLE.8 However, characterization of the epitopes recognized by these pathogenic autoreactive T cells has been less well-studied and is limited to a few autoantigens, primarily in models of spontaneous SLE.9–11 In the current study, we use an induced model of SLE with a known initiating antigen, β2GPI, in which the generation of a strong T cell response to that initiating antigen is involved in disease progression.4 We map the dominant β2GPI-reactive T cell epitopes in our induced model across two MHC haplotypes and evaluate whether T cells from a spontaneous model of SLE share the same epitopes. Furthermore, we characterize the phenotypes of the β2GPI-reactive T cells. We propose that a common β2GPI-reactive T cell epitope may be responsible for SLE autoantibody production in both induced and spontaneous SLE.
Experimental procedures
Mice and immunization
Specific pathogen-free (SPF) female C57BL/6 mice (8–12 weeks of age) were purchased from The Jackson Laboratory (Bar Harbor, Maine). SPF female C3H/HeN mice were generously provided by Dr. Salman Qureshi and bred in-house. SPF MRL/MpJ-Tnfrsf6lpr (MRL/lpr) mice were purchased from The Jackson Laboratory and bred in-house. The MRL/lpr strain was derived originally from crosses among mouse strains LG, AKR, C3H/Di, and C57BL/6.12 These mice develop systemic autoimmunity and immune complex glomerulonephritis that closely resembles human SLE. Mice were maintained and bred according to the Canadian Council on Animal Care guidelines, and provided food and water ad libitum. Animal experiments were approved by the McGill University Animal Care Committee.
C57BL/6 and C3H/HeN mice were immunized with 20 µg human β2GPI and 10 µg LPS, as described previously.13 Mice were injected intravenously every 2 weeks and bled for serum 10 days following the second and third immunizations. The number of immunizations required was determined by the observed levels of anti-β2GPI antibodies. C57BL/6 mice received four immunizations, and C3H/HeN mice received two immunizations.4 MRL/lpr mice (4–8 weeks of age) were injected with a single dose of 20 µg GP-F (full-length recombinant human β2GPI; see below) and 10 µg LPS. Untreated MRL/lpr mice were used at 9–14 weeks of age.
Reagents
Unless stated otherwise, all reagents were obtained commercially from the following sources and used without further purification: human β2GPI (≥95% pure; Crystal Chem, Downers Grove, IL); LPS (Escherichia coli-derived, serotype O111:B4; List Biological Laboratories, Campbell, CA); bovine heart cardiolipin (CL; Avanti Polar Lipids, Alabaster, AL); E. coli DNA (double-stranded (dsDNA); Worthington Biochemical Corporation, Lakewood, NJ); Anti-Mouse Ig κ/Negative Control Compensation Particles Set, Anti-Rat and Anti-Hamster Ig κ/Negative Control Compensation Particles Set, Fc block (FcgIII/II receptor-CD16/32), mouse IL-2 enzyme-linked immunosorbent assay (ELISA) set (BD OptEIA kit), mouse IFN-γ ELISA set (BD OptEIA kit), and 3,3′,5,5′-tetramethylbenzidine substrate reagent set (BD OptEIA kit) from BD Biosciences (Mississauga, ON); alkaline phosphatase (AP)-conjugated goat anti-rabbit IgG and AP-conjugated streptavidin from Southern Biotech (Birmingham, AL); p-nitrophenyl phosphate, phorbol 12-myristate 13-acetate (PMA), and ionomycin from Sigma-Aldrich (St. Louis, MO); Alexa Fluor 488 anti-mouse IFN-γ (XMG1.2), Alexa Fluor 647 anti-mouse forkhead box P3 (MF-14), Alexa Fluor 700 anti-mouse CD8α (53–6.7), brilliant violet 421 anti-mouse IL-4 (11B11), brilliant violet 605 anti-mouse CD185 (CXCR5; L138D7), brilliant violet 785 anti-mouse CD4 (RM4–5), PE anti-human/mouse B-cell lymphoma 6 protein (Bcl-6; 7D1), PE/Dazzle 594 anti-mouse IL-17α (TC11–18H10.1), monensin solution, True-Nuclear transcription factor buffer set, Zombie NIR fixable viability kit, and recombinant human IL-7 from BioLegend (San Diego, CA); and recombinant human IL-2 (generously provided by Dr. Piccirillo [gift of the Surgery Branch, National Cancer Institute, National Institutes of Health]).
Β2GPI recombinant fragments and synthetic peptides
Recombinant maltose-binding protein (MalBP) fusion proteins encoding different regions of human β2GPI were used as antigens for T cell stimulation, as previously described.4,14 These fusion proteins, expressed as recombinant proteins in E. coli and described in detail previously,15 included the following: GP-F, encoding the entire amino-acid sequence of β2GPI (amino-acid residues 1–326); GP-1, encoding domains I and II (amino-acid residues 1–133); GP-2, encoding domains III and IV (amino-acid residues 119–254); and GP-3, encoding domains IV and V (amino-acid residues 182–326). Recombinant MalBP was used as a control antigen. All recombinant fragments were used at a concentration of 20 µg/ml in 0.01 M phosphate-buffered saline (PBS), pH 7.3.
Twenty-six 15-mer peptides (10 residue overlap) spanning domains I and II and 32 18-mer peptides (12 residue overlap) spanning domains III–V of human β2GPI were synthesized, and their purity was determined by high-performance liquid chromatography (Sigma-Aldrich). The peptides were dissolved in 200 μl of dimethyl sulfoxide (DMSO) and further diluted in 500 μl of PBS. Peptide stock solutions in DMSO and PBS were stored at −70 °C. The peptides were added to T cells at a final concentration of 10 μg/ml in PBS, as described below (see “Evaluation of domain and epitope specificity of T cells”).
Cell culture
Unless stated otherwise, all cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM; 4.5 g/l glucose and 110 mg/ml sodium pyruvate) containing 10% heat-inactivated fetal bovine serum (FBS), 1% penicillin-streptomycin, 1% l-glutamate, 1% HEPES, 1% non-essential amino acids, and 0.1% 2-mercaptoethanol (medium and supplements were from Life Technologies Inc., Burlington, ON), hereafter referred to as complete DMEM. Splenic T cells from immunized mice were isolated using an EasySep T cell kit (StemCell Technologies, Vancouver, BC) and were cultured in complete DMEM containing β2GPI-depleted FBS. FBS was depleted of β2GPI using a HiTrap Heparin HP column (GE Healthcare, Piscataway, NJ) to eliminate the potential influence of bovine β2GPI. β2GPI depletion was verified by the inability of treated FBS to support the binding of a bovine β2GPI-dependent murine monoclonal antibody to CL by ELISA (data not shown).
Evaluation of domain and epitope specificity of T cells
Splenocytes from β2GPI/LPS-immunized C57BL/6 and C3H/HeN or naive or GP-F/LPS-injected MRL/lpr mice were plated at 1 × 106 cells/well in complete DMEM containing β2GPI-depleted FBS. Commercial human β2GPI, recombinant protein fragments of human β2GPI or MalBP, or domain I–II and domain III–V peptides were then added to the T cells and incubated for 48 h (37 °C, 5% CO2). Cell supernatants were collected, and IFN-γ and IL-2 levels were quantified by ELISA. The results were expressed as the mean IFN-γ or IL-2 concentration (pg/ml) of duplicate samples, as determined from a standard curve using recombinant IFN-γ or IL-2.
Expansion of β2GPI-reactive T cells
Splenocytes from β2GPI/LPS- or PBS/LPS-immunized C57BL/6 or C3H/HeN mice were plated at 1 × 107 cells/well (24-well plates) in complete DMEM containing β2GPI-depleted FBS. Splenocytes were cultured for 5 days in the presence of commercial human β2GPI (15 µg/ml), IL-2 (10 units/ml), and IL-7 (10 ng/ml). For MRL/lpr mice, splenocytes from GP-F/LPS- or PBS/LPS-injected mice (10 weeks of age) were cultured for 5 days with GP-F (15 µg/ml), IL-2, and IL-7. The cells were then washed, transferred into 6-well plates, and cultured for an additional 2 days in the presence of only IL-2 and IL-7. The cells were then stimulated with PMA (20 ng/ml) and ionomycin (750 ng/ml) for 3 h in the presence of monensin solution, and then harvested and stained for surface and intracellular markers. The cells were analyzed using a BD LSRFortessa flow cytometer and FACSDiva software (BD Bioscience), and plots, gated on viable cells, were generated using FlowJo X software.
Detection of autoantibodies
Serum antibodies to β2GPI, recombinant β2GPI domain fragments, CL, and dsDNA were determined by ELISA, as previously described.3,4 The anti-β2GPI (direct binding) assay detects antibodies that bind to human β2GPI, while the anti-CL assay detects antibodies that recognize CL-bound β2GPI (bovine β2GPI from FBS in the assay buffer and, potentially, murine β2GPI within the serum sample). Reactivity to bound β2GPI was confirmed by a similar anti-CL ELISA where plates were incubated with either bovine or human serum, or purified human β2GPI, for 16 h at 37 °C prior to the addition of murine serum samples, as previously described.3 Sera from β2GPI/LPS-immunized C3H/HeN were collected 10–14 days after the first, second, and third immunizations. Sera from untreated MRL/lpr female mice were collected every 2 weeks, starting at 4 weeks of age.
Statistical analysis
Statistical significance was determined by a two-tailed unpaired non-parametric T-test using Prism 7.0 (GraphPad Software Inc., San Diego, CA). The minimal threshold for significance was p < 0.05.
RESULTS
T cells from mice with induced SLE recognize common β2GPI epitopes
We have previously evaluated the T cell response to epitopes within domains I and II of β2GPI in mice immunized with β2GPI and LPS, and we found no common epitopes between mice of different haplotypes.4,13 Here we extend our analysis to determine whether T cells from β2GPI/LPS-immunized mice also recognize epitopes within domains III–V of β2GPI, and we ask whether these epitopes are shared between haplotypes. Furthermore, we evaluate whether mice with induced and spontaneous SLE recognize shared T cell epitopes on β2GPI.
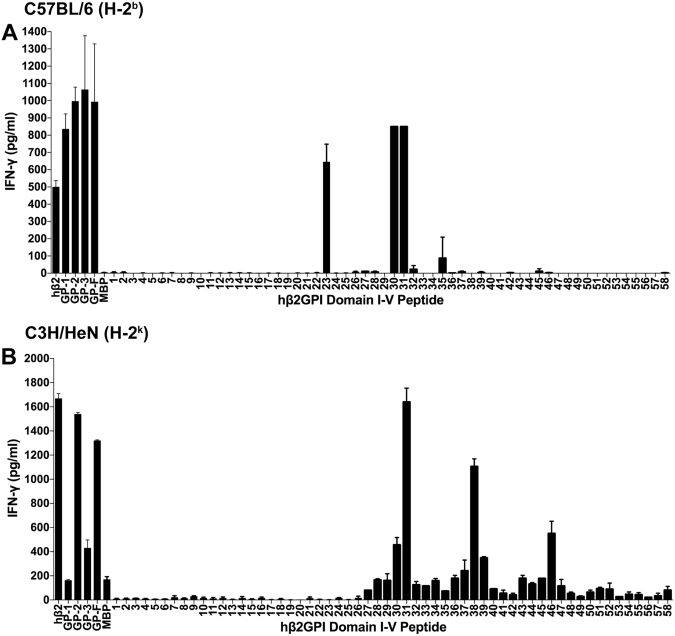
Splenic T cells from β2GPI/LPS-immunized mice of two different MHC class II haplotypes (C57BL/6 [H-2b] and C3H/HeN [H-2k]) were evaluated for their response to native human β2GPI, recombinant proteins encoding domains I–V of human β2GPI (or MalBP as control), and synthetic peptides covering the entire human β2GPI sequence. T cells from both C57BL/6 (Fig. 1A) and C3H/HeN (Fig. 1B) mice recognized native human β2GPI and full-length recombinant β2GPI (GP-F), but domain recognition differed between the two strains. While C57BL/6 T cells recognized all recombinant fragments (GP-1, 2, and 3) of human β2GPI, C3H/HeN T cells were most responsive to recombinant fragment GP-2 (domains III–IV). T cell recognition of peptides revealed both distinct and common epitopes. C57BL/6 T cells recognized peptides 23 (domain II) and 30/31 (domain III), while C3H/HeN T cells recognized peptides 30/31, 38, and 46 (domains III and IV). T cell responses were specific to human β2GPI, as shown by the lack of recognition of the control recombinant protein MalBP. These data demonstrate that despite different MHC class II haplotypes, T cells from mice with induced SLE respond to a common β2GPI epitope (peptides 30/31 [SAGNNSLYRDTAVFECLP/LYRDTAVFECLPQHAMFG]).
Fig. 1.
Mice with induced SLE recognize a common β2GPI T cell epitope. Splenocytes from β2GPI/LPS-immunized mice (C57BL/6 [H-2b] or C3H/HeN [H-2k]) were cultured with either human β2GPI (hβ2), GP-1 (domains I–II), GP-2 (domains III–IV), GP-3 (domains IV–V), GP-F (full-length β2GPI), or MalBP (MBP; control fusion protein) at a concentration of 20 µg/ml, or individual peptides from domains I–II (peptides 1–26) or domains III–V (peptides 27–58) at 10 µg/ml. Cells were incubated for 48 h, and IFN-γ production in the supernatant was measured by ELISA. Values represent the mean IFN-γ concentration (pg/ml) ± SE of duplicate samples, and the data shown are pooled from three independent experiments
T cells from mice with induced SLE are Th1-biased and produce IFN-γ
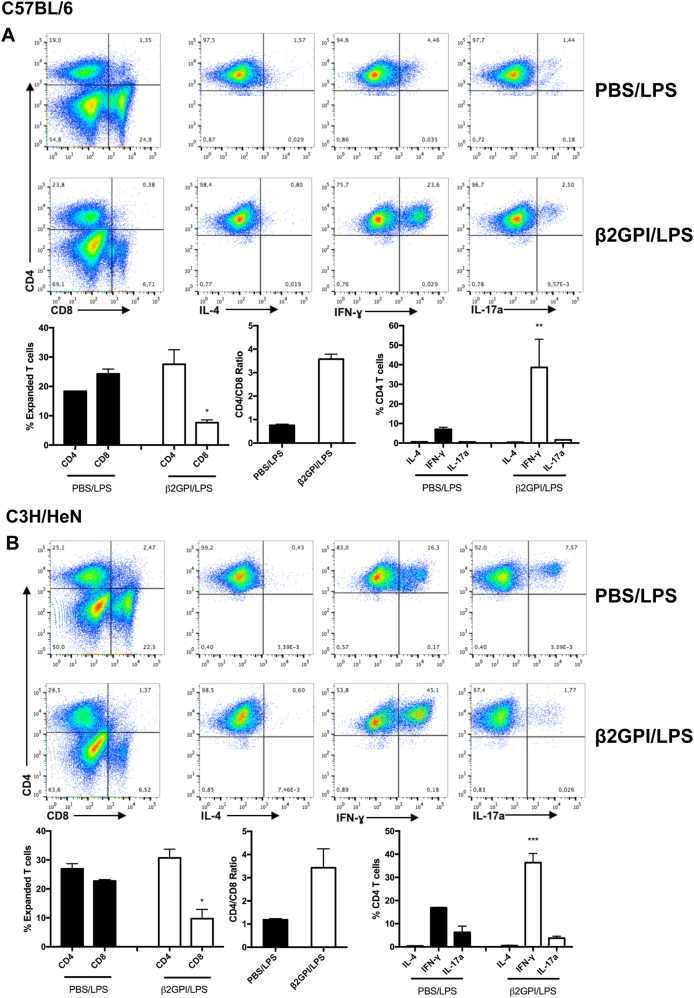
We next evaluated the characteristics of β2GPI-reactive T cells from C57BL/6 or C3H/HeN mice that had been expanded in vitro with β2GPI. β2GPI-expanded T cells from β2GPI/LPS-immunized C57BL/6 and C3H/HeN mice both had a much higher proportion of CD4+ (24–28%) than CD8+ (<10%) T cells, compared with approximately equal proportions (18–25% CD4+ and 22–23% CD8+) in similarly expanded T cells from PBS/LPS-immunized mice (Fig. 2). These findings suggest expansion of CD4+, but not CD8+, cells in response to β2GPI. This is also reflected by the ratio of CD4 to CD8 T cells, which was three- to four-fold higher in β2GPI/LPS-immunized mice than in control mice. Approximately 50% of the β2GPI-expanded T cells were IFN-γ+, with minimal IL-4+ T cells seen in this population, suggesting a clear Th1 orientation. The percentage of IFN-γ+ cells in the β2GPI-expanded population from β2GPI/LPS-immunized mice was two- to five-fold greater than that in expanded T cells from PBS/LPS-immunized mice. In contrast, IL-17+ cells were minimally changed in β2GPI-expanded T cells relative to the PBS control.
Fig. 2.
T cells from mice with induced SLE are Th1-biased and produce IFN-γ. Splenocytes from β2GPI/LPS-immunized mice (C57BL/6 [A]) or C3H/HeN [B]) were cultured with human β2GPI (15 µg/ml), IL-2 (10 units/ml), and IL-7 (10 ng/ml) for 7 days. Cells were then stimulated with PMA/ionomycin for 3 h in the presence of monensin and stained for surface and intracellular markers. Flow cytometry plots depict CD4 vs. CD8 populations, followed by IL-4, IFN-γ, and IL-17a staining for live CD4+ cells. All cytokine data shown were gated on 3 × 104 live CD4+ T cells. Bar graphs summarizing the flow cytometry data show pooled data ± SE of two independent experiments (*P < 0.05; **P < 0.01; ***P < 0.001)
T cells from mice with spontaneous SLE share β2GPI epitopes with induced SLE
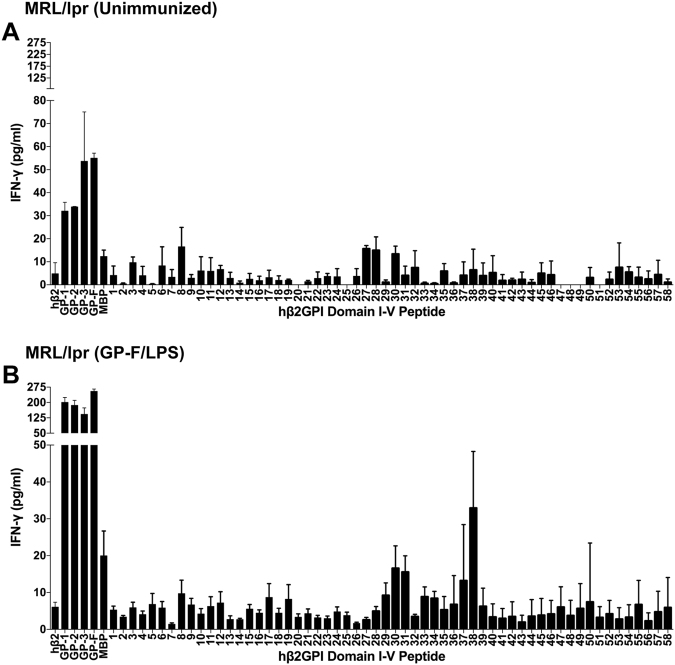
We were interested in determining whether T cells from a spontaneous model of SLE also respond to β2GPI. We found that T cells from MRL/lpr mice did not recognize native serum-derived human β2GPI but reacted very strongly to GP-F, a recombinant full-length β2GPI. The lack of recognition of native β2GPI by MRL/lpr T cells is similar to findings for human β2GPI-reactive T cells from patients with anti-phospholipid syndrome, which recognize recombinant but not native β2GPI.15,16 For this reason, recombinant GP-F (rather than native β2GPI) was used to increase the numbers of β2GPI-reactive T cells in MRL/lpr mice (see below). Splenic T cells isolated from naive 10-week-old MRL/lpr (H-2k) mice were evaluated for their response to native human β2GPI, recombinant proteins encoding domains I–V of human β2GPI (or MalBP as control), and synthetic peptides covering the human β2GPI sequence (Fig. 3A). T cells responded to all of the recombinant fragments of human β2GPI, but responded poorly to native β2GPI. T cells also responded to multiple peptides over the sequence of β2GPI, but IFN-γ levels were low and variable. These data from naive MRL/lpr mice suggest that β2GPI-reactive T cells are present but in very low numbers.
Fig. 3.
Mice with spontaneous SLE recognize the common β2GPI T cell epitope found in induced SLE. MRL/lpr mice (5 weeks of age) were either left unimmunized (A) or boosted once with GP-F and LPS (B), and splenocytes were harvested 10–14 days later. Splenocytes were cultured with either human β2GPI (hβ2), GP-1 (domains I–II), GP-2 (domains III–IV), GP-3 (domains IV–V), GP-F (full-length β2GPI), or MalBP (MBP; control fusion protein) at a concentration of 20 µg/ml, or individual peptides from domains I–II (peptides 1–26) or domains III–V (peptides 27–58) at 10 µg/ml. Cells were incubated for 48 h, and IFN-γ production in the supernatant was measured by ELISA. Values represent the mean IFN-γ concentration (pg/ml) ± SE of duplicate samples, and the data shown are pooled from three independent experiments
To increase the number of β2GPI-reactive T cells, MRL/lpr mice were injected once with recombinant full-length β2GPI (GP-F) and LPS. Splenic T cells from the GP-F-injected mice demonstrated a strong IFN-γ response to all recombinant fragments of β2GPI (Fig. 3B), as well as a clear pattern of peptide recognition, particularly for peptides 30/31 and 38. The IL-2 response of these T cells was similar to that of IFN-γ (data not shown). Of note, the pattern of peptide recognition for MRL/lpr T cells was virtually identical to that of T cells from β2GPI/LPS-immunized C3H/HeN mice, which share the same MHC class II haplotype (H-2k). Importantly, MRL/lpr mice responded to peptides 30/31, which both C3H/HeN and C57BL/6 mice recognize. These data demonstrate that T cells from both spontaneous (MRL/lpr) and induced (β2GPI/LPS-immunized C57BL/6 and C3H/HeN) mice recognize a shared epitope (peptides 30/31) on human β2GPI.
T cells from mice with spontaneous SLE are Th1/Th17-biased
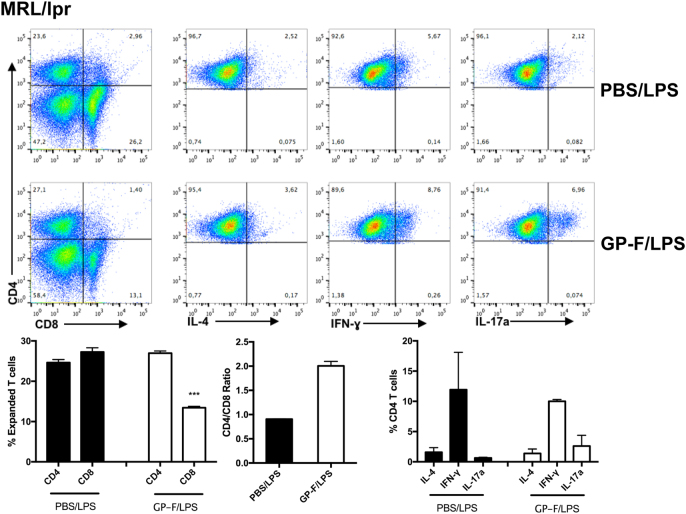
To determine the phenotype of β2GPI-reactive T cells from mice with spontaneous SLE, T cells from MRL/lpr mice (10 weeks) that had received a single injection of either GP-F/LPS or PBS/LPS were expanded in vitro with GP-F. Similar to T cells from mice with induced SLE (Fig. 2), GP-F-expanded T cells from GP-F/LPS-boosted MRL/lpr mice were predominantly CD4+. However, unlike mice with induced SLE, MRL/lpr-derived T cells expressed moderate levels of IFN-γ but two- to three-fold higher levels of IL-17a compared to PBS/LPS-boosted mice (Fig. 4). These data are consistent with a Th17 phenotype for β2GPI-reactive MRL/lpr T cells.17
Fig. 4.
T cells from mice with spontaneous SLE are Th17-biased. Splenocytes from GP-F/LPS- or PBS/LPS-boosted MRL/lpr mice (10 weeks old) were cultured with GP-F (15 µg/ml), IL-2 (10 units/ml), and IL-7 (10 ng/ml) for 7 days. Cells were then stimulated with PMA/ionomycin for 3 h in the presence of monensin and stained for surface and intracellular markers. Flow cytometry plots depict CD4 vs. CD8 populations, followed by IL-4, IFN-γ, and IL-17a staining for live CD4+ cells. All cytokine data shown were gated on 3 × 104 live CD4+ T cells. Bar graphs summarizing the flow cytometry data show pooled data ± SE of two independent experiments (***P < 0.001)
β2GPI-reactive T cells precede the production of SLE autoantibodies
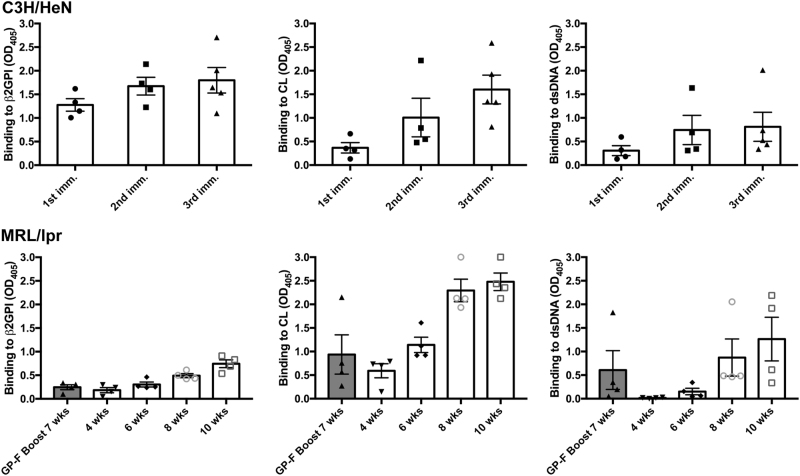
We wanted to ensure that the single injection of recombinant β2GPI (GP-F) and LPS used to expand the number of β2GPI-reactive T cells in the spontaneous SLE model (MRL/lpr mice) was not producing an “induced” autoantibody response, similar to that seen in our induced SLE model. In the induced model, we immunize mice repeatedly (three to five injections) with β2GPI and LPS, whereas MRL/lpr mice received a single injection of recombinant β2GPI (GP-F) and LPS. Moreover, we injected young (5-week-old) MRL/lpr mice, an age defined as being pre-diseased17 in this model. We evaluated SLE autoantibodies in both GP-F-immunized mice and unimmunized MRL/lpr mice of different ages. At 4–6 weeks, the levels of anti-β2GPI and anti-dsDNA were very low or undetectable in unimmunized MRL/lpr mice (Fig. 5). Anti-CL levels were low at 4 weeks, but were higher than the levels of anti-β2GPI and anti-dsDNA autoantibodies, and the levels increased quickly between 4 and 8 weeks. Importantly, in GP-F-injected mice that were used for T cell isolation (i.e., at 7 weeks of age), the levels of anti-β2GPI, anti-CL, and anti-dsDNA were comparable to those of unimmunized MRL/lpr mice of the same age.
Fig. 5.
Progression of SLE autoantibody production in induced and spontaneous SLE. Top panel: sera from β2GPI/LPS-immunized C3H/HeN mice were tested for IgG anti-β2GPI, anti-CL, and anti-dsDNA autoantibodies by ELISA 14 days after each immunization. As autoantibody titers increased over time, sera were evaluated at multiple dilutions. The dilution shown for each autoantibody and bleed is indicated in square brackets: anti-β2GPI (first immunization [1/100], second immunization [1/3000], and third immunization [1/5000]); anti-CL (first immunization [1/100], second and third immunizations [1/5000]); and anti-dsDNA (all immunizations [1/100]). Bottom panel: sera from MRL/lpr mice were obtained every 2 weeks, starting from 4 weeks until 10 weeks of age, and were tested for anti-β2GPI (1/100 dilution), anti-CL (1/1000 dilution), and anti-dsDNA (1/1000 dilution). Each dot represents the mean IgG antibody binding of duplicate samples for an individual mouse (n = 4–6 mice/group), and bars indicate the mean blanked OD405 ± SE for each group
Characterization of the anti-CL antibodies in immunized C3H/HeN and unimmunized MRL/lpr mice indicated that C3H/HeN antibodies recognized CL-bound bovine and human β2GPI, while those in MRL/lpr sera had the characteristics of β2GPI-independent antibodies and were inhibited by FBS and human serum (as described by others;18,19 Supplemental Fig. 1 [top]). In contrast, in direct binding assays on native or recombinant fragments of β2GPI, both C3H/HeN and MRL/lpr sera reacted with native human β2GPI, GP-2 (domains III and IV), and GP-3 (domains IV and V; Supplemental Fig. 1 [bottom]). These data indicate that the single injection of GP-F used to expand β2GPI-reactive T cells did not induce SLE autoantibodies in MRL/lpr mice, which had autoantibody levels similar to those of unimmunized mice. These findings suggest that β2GPI-reactive autoantibodies are among the earliest to develop in MRL/lpr mice, and that a β2GPI-reactive T cell response precedes the development of SLE autoantibodies in these mice.
Discussion
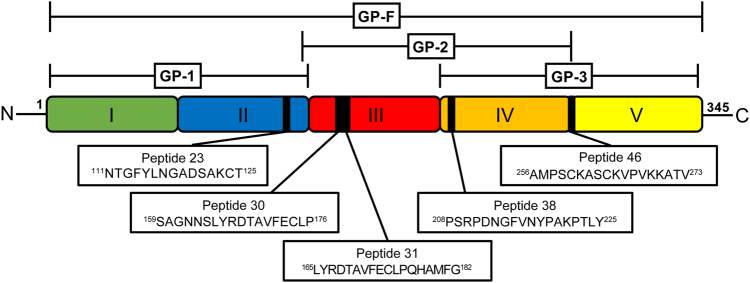
In this study, we show that mice with induced and spontaneous SLE share a common T cell epitope on human β2GPI. Both murine models develop the full spectrum of autoantibodies and renal disease seen in human SLE.2,3,20 T cells from β2GPI/LPS-immunized mice (induced SLE) and from MRL/lpr mice (spontaneous SLE) recognized peptide 31 (165LYRDTAVFECLPQHAMFG182) in domain III of β2GPI, as well as adjacent peptide 30 (159SAGNNSLYRDTAVFECLP176; Fig. 6). Interestingly, this T cell epitope was shared across two different MHC class II haplotypes. T cells from mice with induced and spontaneous SLE also shared MHC class II-restricted T cell epitopes; peptide 38 (208PSRPDNGFVNYPAKPTLY225) in domain IV was recognized by T cells from C3H/HeN and MRL/lpr mice, which both bear the H-2k haplotype. In contrast, there were other MHC class II-restricted peptides, such as peptide 23 (111NTGFYLNGADSAKCT125) and peptide 46 (256AMPSCKASCKVPVKKATV273), that were recognized solely by T cells from mice with induced SLE. The finding of shared T cell epitopes between mice with induced and spontaneous SLE suggests a potential common autoantigenic trigger and T cell potentiation of autoantibodies.
Fig. 6.
Human β2GPI-derived peptide sequences recognized by mice with induced and spontaneous SLE. This is a schematic representation of the full amino-acid sequence of human β2GPI (center) and its five domains (shown in color). Recombinant fusion proteins GP-1, GP-2, GP-3, and GP-F, and the domains that each fusion protein comprises are shown above the colored image of β2GPI. The epitopes (peptides) recognized by T cells from mice with induced (C57BL/6 [H-2b] and C3H/HeN [H-2k]) and spontaneous (MRL/lpr [H-2k]) SLE are shown by black bars within the colored image of β2GPI, with boxes below the image showing the amino-acid sequences of each peptide. Notably, peptide 31 in domain III is recognized by T cells from both induced and spontaneous SLE
Autoantibodies that are reactive toward β2GPI are among the earliest to occur2 in human SLE. Moreover, individuals positive for β2GPI-reactive antibodies develop other SLE-related autoantibodies earlier and appear to have a more severe clinical outcome than individuals negative for these antibodies,21 suggesting that β2GPI may be one of the first autoantigens to which immune tolerance is broken. In our induced model, we use a known initiating antigen (β2GPI) to induce a strong β2GPI-reactive T cell response that drives epitope spread to other SLE autoantibodies and ultimately leads to renal disease.3,4 Here our data suggest that a β2GPI-reactive T cell response may be important in driving autoantibody production in both induced and spontaneous murine SLE.
Our rationale for comparing the T cell response in this induced model to that of a spontaneous SLE model (MRL/lpr) that mimics human SLE is to provide evidence for common T cell mechanisms in murine and human SLE. Our induced murine model of SLE is generated by immunization of mice with human β2GPI with LPS,3 and we have recently shown that the T cell response associated with epitope spread to SLE autoantibodies is directed to T cell epitopes derived from human β2GPI.4 For this reason, human β2GPI was used throughout the study. The finding that T cells from MRL/lpr mice with spontaneous SLE recognize peptides derived from human β2GPI may at first seem surprising, but the finding becomes understandable when considering the high degree of homology between human and murine β2GPI (76.5% sequence identity). Notably, the human β2GPI epitopes recognized by T cells from MRL/lpr and human β2GPI/LPS-immunized mice are closely related to comparable amino-acid sequences within murine β2GPI (83.3% and 66.7% identity for peptides 30 and 31, and 77.8% and 72.2% identity for peptides 37 and 38, respectively). The recognition of human β2GPI-derived peptides by T cells from mice with spontaneous and induced SLE indicates that both models likely have T cells that recognize autologous murine β2GPI. Future studies will use murine β2GPI-derived peptides to define the precise autoreactive T cell epitopes within the autologous protein.
The finding that MRL/lpr T cells recognize recombinant human β2GPI (GP-F), but not native human β2GPI, is also consistent with published data for human T cells from healthy controls and from patients with anti-phospholipid syndrome (APS). Human β2GPI-reactive T cells respond to bacterially expressed recombinant β2GPI fragments and chemically reduced β2GPI, but they fail to respond to native β2GPI, suggesting that the generation of epitopes recognized by β2GPI-reactive T cells requires unfolding or structural modification of β2GPI. Kuwana et al.22 provided direct evidence that anionic phospholipid may be involved in the generation of these cryptic T cell epitopes. Dendritic cells or macrophages pulsed with vesicles containing anionic phospholipid and β2GPI, but not β2GPI or phospholipid alone, induced a response in domain V epitope (p276–290)-specific T cell lines generated from patients in an human leukocyte antigen – antigen D related (HLA-DR)-restricted manner. These data suggest that disease-relevant T cell epitopes in β2GPI may arise as a consequence of antigen processing of anionic phospholipid-bound β2GPI.
The sharing of β2GPI T cell epitopes in both spontaneous and induced SLE models also may speak to common mechanisms for generating these peptides. In particular, the induced model in C3H/HeN mice and spontaneous model in MRL/lpr mice can be compared, as they share the same MHC class II haplotype (H-2k). However, there are major differences between MRL/lpr and C3H/HeN mice. Both the autoimmune predisposition from the MRL/+ background and the lpr mutation in Fas impact immune cell composition and function in these mice.20 The finding that the T cell epitope response to β2GPI is so similar between nonautoimmune C3H/HeN mice and MRL/lpr mice suggests that abnormalities due to the MRL/+ background and the Fas mutation do not impact the antigenic processing and presentation of β2GPI-derived T cell epitopes. Divergence in T cell responses between these two strains therefore likely lies at a level distinct from antigenic processing and presentation.
One of the differences that we observed was the Th17 orientation of the β2GPI-reactive T cell response in MRL/lpr mice compared to the Th1 orientation of the response in C3H/HeN and C57BL/6 mice. β2GPI-reactive CD4+ T cells from MRL/lpr mice expressed IL-17 and IFN-γ, while T cells from mice with induced SLE expressed IFN-γ but not IL-17. Th17 cells play a central role in the pathogenesis of human and murine SLE.7,23 SLE patients have increased numbers of Th17 cells, as well as high serum levels of IL-17.23 Th17 cells, as well as TCRαβ double-negative (CD3+CD4−CD8) T cells and γδ T cells, produce increased amounts of IL-17 in the kidneys of SLE patients and SLE-prone mice.23 Of relevance to our findings, Yang et al.24 found an increased proportion of IL-17+ CD4+ T cells and higher expression of IL-17A mRNA in splenocytes from MRL/lpr mice compared to splenocytes from C57BL/6 mice. Dai et al.25 have shown that increased numbers of IL-17-expressing T cells are found in the spleens and lymph nodes of both MRL/lpr and C57BL/6-lpr mice relative to their control strains (MRL/MpJ and C57BL/6, respectively). In both lpr-bearing strains, the major population of IL-17-expressing cells was double-negative T cells. These data suggest that IL-17-expressing cells (particularly double-negative T cells) are increased in the presence of the lpr mutation. The genetic background, however, sets the baseline levels of these cells, which appear to be higher in MRL/MpJ compared with C57BL/6 mice.25 Dai et al.25 further showed that lymphocytes from SLE-prone animals expressed progressively higher levels of IL-23 receptor mRNA as their disease developed and that in vitro treatment of lymphocytes with IL-23 resulted in increased levels of IL-23 receptor mRNA and IL-17A mRNA, as well as increased numbers of IL-17A cells and double-negative cells. They suggest that IL-23 acts as a “trophic/inducing” cytokine in SLE-prone mice, particularly on double-negative T cells, and induces the production of IL-17 instead of IL-2 and IFN-γ. These findings fit well with our findings of IL-17-expressing cells in the spontaneous SLE of MRL/lpr, but not in an induced model of SLE, and the findings suggest that either the MRL/+ or the lpr mutation would be needed to observe IL-17-producing cells in our induced model. The data from our induced model further indicate that IL-17-expressing T cells are not necessary for generating a β2GPI-reactive T cell response or for epitope spread to multiple SLE autoantibodies.
The mechanism responsible for generating a β2GPI T cell epitope that is shared not only between spontaneous and induced SLE, but also across MHC class II haplotypes remains to be determined. In APS, de Moerloose et al.26 demonstrated an immunodominant T cell epitope in domain I of β2GPI and related it to anti-β2GPI antibody-binding motifs that could be shared among individuals with different MHC class II haplotypes. Currently, there is a paucity of knowledge about common T cell epitopes shared across MHC class II haplotypes in SLE. However, in the more general T cell literature, it is clear that the same peptide can be presented by different MHC class II haplotypes. Greenbaum et al.27 classified MHC class II molecules into groups (called “supertypes”) based on binding to shared peptide motifs or structures and found a surprising degree of sharing across MHC class II supertypes. They attribute this shared peptide binding to MHC class II backbone interactions rather than peptide anchor residues. There is also evidence for recognition of self-antigen-derived peptides across different MHC class II haplotypes. Individuals with different MHC class II haplotypes have identical endogenous peptides, suggesting that self-peptide expression can be quite promiscuous.28 Tsai and Santamaria29 propose that this MHC promiscuity may allow for both protective and pathogenic T cells to interact with the same peptide but with very different outcomes. They hypothesize that the same peptide may be recognized with higher affinity/avidity when bound to a protective MHC class II molecule, possibly resulting in negative selection or T regulatory cell development, whereas a lower affinity/avidity interaction with a disease-promoting MHC class II molecule would lead to positive selection of autoreactive T cells.29
The lack of consistent MHC class II associations in SLE, and the multitude of autoantigens targeted, make identification of critical T cell antigen(s) in this disease a major challenge. Multiple HLA alleles, including HLA-DR2 and HLA-DR3, are associated with SLE, but the strength of this association and the specific allele(s) depend on the ethnic group and clinical presentation studied.30 Identifying a dominant and shared T cell epitope across MHC class II haplotypes suggests a common initiating antigen and should help to identify the mechanisms involved in the initiation and progression of SLE. Previous T cell studies in the MRL/lpr model of SLE have focused mainly on CD4+ T cells reactive to an epitope on the 70K small nuclear ribonucleoprotein (snRNP).9–11 However, it is unclear how these snRNP-reactive T cell subsets fit into the temporal sequence of events leading to SLE. Autoantibodies to snRNP are produced relatively late in the sequential emergence of SLE autoantibodies, while autoantibodies to β2GPI are among the earliest autoantibodies detected in SLE patients.2 In our induced model, anti-β2GPI antibodies are produced first but are followed soon after by the emergence of multiple SLE autoantibodies in a progression mimicking human SLE.2–4 Of note, in MRL/lpr mice, anti-CL autoantibodies appeared early (4 weeks), while significant levels of anti-dsDNA autoantibodies appeared later (between 6 and 8 weeks; Fig. 5). This finding suggests that recognition of β2GPI epitopes occurs very early in the immune response in both spontaneous (murine and human) and induced SLE. We have previously proposed a model of epitope spread in SLE in which β2GPI-reactive T cells provide help to B cells of different specificities, as long as the B cell presents MHC class II-bound β2GPI epitopes on its surface.3
In summary, our current data suggest that a common β2GPI-reactive T cell epitope may promote SLE autoantibody production in both induced and spontaneous SLE. Identification of a shared T cell autoantigen in both murine models introduces the possibility that similar findings in human SLE could lead to T cell-based prognostic and therapeutic interventions.
Electronic supplementary material
Acknowledgements
This study has been funded in part by operating grants from the Canadian Institutes of Health Research (CIHR; MOP-67101 and MOP-97916; J.R.) and funding from the Department of Medicine of McGill University, the Research Institute of the McGill University Health Centre, the Division of Rheumatology of McGill University, and the Arthritis Society of Canada (Rheumatic Disease Unit grant). D.S. is the recipient of a studentship from the FRQS and a Merit Fellowship from the Department of Microbiology and Immunology. J.S.L. is supported by institutional funds from Dr. José A. Arruda and the Section of Nephrology, University of Illinois at Chicago. The authors are grateful to Dr. Salman Qureshi for providing the C3H/HeN mice used in this study, and to Annie Beauchamp for her expertise and technical assistance with the mice.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Jerrold S. Levine, Joyce Rauch.
Electronic supplementary material
Supplementary Information accompanies this paper at (10.1038/s41423-018-0013-3).
References
- 1.Tsokos GC, Lo MS, Costa Reis P, Sullivan KE. New insights into the immunopathogenesis of systemic lupus erythematosus. Nat. Rev. Rheumatol. 2016;12:716–730. doi: 10.1038/nrrheum.2016.186. [DOI] [PubMed] [Google Scholar]
- 2.Arbuckle MR, et al. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N. Engl. J. Med. 2003;349:1526–1533. doi: 10.1056/NEJMoa021933. [DOI] [PubMed] [Google Scholar]
- 3.Levine JS, et al. Immunization with an apoptotic cell-binding protein recapitulates the nephritis and sequential autoantibody emergence of systemic lupus erythematosus. J. Immunol. 2006;177:6504–6516. doi: 10.4049/jimmunol.177.9.6504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salem D, et al. beta2-Glycoprotein I-specific T cells are associated with epitope spread to lupus-related autoantibodies. J. Biol. Chem. 2015;290:5543–5555. doi: 10.1074/jbc.M114.619817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Masutani K, et al. Predominance of Th1 immune response in diffuse proliferative lupus nephritis. Arthritis Rheum. 2001;44:2097–2106. doi: 10.1002/1529-0131(200109)44:9<2097::AID-ART360>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 6.Shin MS, Lee N, Kang I. Effector T cell subsets in systemic lupus erythematosus: update focusing on Th17 cells. Curr. Opin. Rheumatol. 2011;23:444–448. doi: 10.1097/BOR.0b013e328349a255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suarez-Fueyo A, Bradley SJ, Klatzmann D, Tsokos GC. T cells and autoimmune kidney disease. Nat. Rev. Nephrol. 2017;13:329–343. doi: 10.1038/nrneph.2017.34. [DOI] [PubMed] [Google Scholar]
- 8.Hunemorder S, et al. TH1 and TH17 cells promote crescent formation in experimental autoimmune glomerulonephritis. J. Pathol. 2015;237:62–71. doi: 10.1002/path.4559. [DOI] [PubMed] [Google Scholar]
- 9.Monneaux F, Briand JP, Muller S. B and T cell immune response to small nuclear ribonucleoprotein particles in lupus mice: autoreactive CD4(+) T cells recognize a T cell epitope located within the RNP80 motif of the 70K protein. Eur. J. Immunol. 2000;30:2191–2200. doi: 10.1002/1521-4141(2000)30:8<2191::AID-IMMU2191>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 10.Monneaux F, Lozano JM, Patarroyo ME, Briand JP, Muller S. T cell recognition and therapeutic effect of a phosphorylated synthetic peptide of the 70K snRNP protein administered in MR/lpr mice. Eur. J. Immunol. 2003;33:287–296. doi: 10.1002/immu.200310002. [DOI] [PubMed] [Google Scholar]
- 11.Kattah NH, et al. Tetramers reveal IL-17-secreting CD4 + T cells that are specific for U1-70 in lupus and mixed connective tissue disease. Proc. Natl Acad. Sci. USA. 2015;112:3044–3049. doi: 10.1073/pnas.1424796112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andrews BS, et al. Spontaneous murine lupus-like syndromes. Clinical and immunopathological manifestations in several strains. J. Exp. Med. 1978;148:1198–1215. doi: 10.1084/jem.148.5.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tolomeo T, et al. T cells demonstrate a Th1-biased response to native beta2-glycoprotein I in a murine model of anti-phospholipid antibody induction. Autoimmunity. 2009;42:292–295. doi: 10.1080/08916930902828254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arai T, et al. Autoreactive CD4(+) T cell clones to beta2-glycoprotein I in patients with antiphospholipid syndrome: preferential recognition of the major phospholipid-binding site. Blood. 2001;98:1889–1896. doi: 10.1182/blood.V98.6.1889. [DOI] [PubMed] [Google Scholar]
- 15.Hattori N, et al. T cells that are autoreactive to beta2-glycoprotein I in patients with antiphospholipid syndrome and healthy individuals. Arthritis Rheum. 2000;43:65–75. doi: 10.1002/1529-0131(200001)43:1<65::AID-ANR9>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 16.Kuwana M. Beta2-glycoprotein I: antiphospholipid syndrome and T cell reactivity. Thromb. Res. 2004;114:347–355. doi: 10.1016/j.thromres.2004.06.029. [DOI] [PubMed] [Google Scholar]
- 17.Perry D, Sang A, Yin Y, Zheng YY, Morel L. Murine models of systemic lupus erythematosus. J. Biomed. Biotechnol. 2011;2011:271694. doi: 10.1155/2011/271694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hashimoto Y, et al. Anticardiolipin antibodies in NZW x BXSB F1 mice. A model of antiphospholipid syndrome. J. Immunol. 1992;149:1063–1068. [PubMed] [Google Scholar]
- 19.Verthelyi D, Ansar Ahmed S. Characterization of estrogen-induced autoantibodies to cardiolipin in non-autoimmune mice. J. Autoimmun. 1997;10:115–125. doi: 10.1006/jaut.1996.0121. [DOI] [PubMed] [Google Scholar]
- 20.Peng SL. Experimental use of mouse models of systemic lupus erythematosus. Methods Mol. Biol. 2012;900:135–168. doi: 10.1007/978-1-60761-720-4_7. [DOI] [PubMed] [Google Scholar]
- 21.McClain MT, et al. The prevalence, onset, and clinical significance of antiphospholipid antibodies prior to diagnosis of systemic lupus erythematosus. Arthritis Rheum. 2004;50:1226–1232. doi: 10.1002/art.20120. [DOI] [PubMed] [Google Scholar]
- 22.Kuwana M, et al. Binding of beta 2-glycoprotein I to anionic phospholipids facilitates processing and presentation of a cryptic epitope that activates pathogenic autoreactive T cells. Blood. 2005;105:1552–1557. doi: 10.1182/blood-2004-08-3145. [DOI] [PubMed] [Google Scholar]
- 23.Koga T, Ichinose K, Tsokos GC. T cells and IL-17 in lupus nephritis. Clin. Immunol. 2017;185:95–99. doi: 10.1016/j.clim.2016.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang J, et al. Th17 and natural Treg cell population dynamics in systemic lupus erythematosus. Arthritis Rheum. 2009;60:1472–1483. doi: 10.1002/art.24499. [DOI] [PubMed] [Google Scholar]
- 25.Dai H, He F, Tsokos GC, Kyttaris VC. IL-23 limits the production of IL-2 and promotes autoimmunity in lupus. J. Immunol. 2017;199:903–910. doi: 10.4049/jimmunol.1700418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Moerloose P, et al. Patient-derived anti-beta2GP1 antibodies recognize a peptide motif pattern and not a specific sequence of residues. Haematologica. 2017;102:1324–1332. doi: 10.3324/haematol.2017.170381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greenbaum J, et al. Functional classification of class II human leukocyte antigen (HLA) molecules reveals seven different supertypes and a surprising degree of repertoire sharing across supertypes. Immunogenetics. 2011;63:325–335. doi: 10.1007/s00251-011-0513-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Costantino CM, Spooner E, Ploegh HL, Hafler DA. Class II MHC self-antigen presentation in human B and T lymphocytes. PLoS ONE. 2012;7:e29805. doi: 10.1371/journal.pone.0029805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsai S, Santamaria P. MHC class II polymorphisms, autoreactive T cells, and autoimmunity. Front. Immunol. 2013;4:321. doi: 10.3389/fimmu.2013.00321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mohan C, Putterman C. Genetics and pathogenesis of systemic lupus erythematosus and lupus nephritis. Nat. Rev. Nephrol. 2015;11:329–341. doi: 10.1038/nrneph.2015.33. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.