Abstract
Purpose
In the phase III METEOR trial (ClinicalTrials.gov identifier: NCT01865747), 658 previously treated patients with advanced renal cell carcinoma were randomly assigned 1:1 to receive cabozantinib or everolimus. The cabozantinib arm had improved progression-free survival, overall survival, and objective response rate compared with everolimus. Changes in quality of life (QoL), an exploratory end point, are reported here.
Patients and Methods
Patients completed the 19-item Functional Assessment of Cancer Therapy–Kidney Symptom Index (FKSI-19) and the five-level EuroQol (EQ-5D-5L) questionnaires at baseline and throughout the study. The nine-item FKSI–Disease-Related Symptoms (FKSI-DRS), a subset of FKSI-19, was also investigated. Data were summarized descriptively and by repeated-measures analysis (for which a clinically relevant difference was an effect size ≥ 0.3). Time to deterioration (TTD) was defined as the earlier of date of death, radiographic progressive disease, or ≥ 4-point decrease from baseline in FKSI-DRS.
Results
The QoL questionnaire completion rates remained ≥ 75% through week 48 in each arm. There was no difference over time for FKSI-19 Total, FKSI-DRS, or EQ-5D data between the cabozantinib and everolimus arms. Among the individual FKSI-19 items, cabozantinib was associated with worse diarrhea and nausea; everolimus was associated with worse shortness of breath. These differences are consistent with the adverse event profile of each drug. Cabozantinib improved TTD overall, with a marked improvement in patients with bone metastases at baseline.
Conclusion
In patients with advanced renal cell carcinoma, relative to everolimus, cabozantinib generally maintained QoL to a similar extent. Compared with everolimus, cabozantinib extended TTD overall and markedly improved TTD in patients with bone metastases.
INTRODUCTION
Renal cell carcinoma (RCC) is diagnosed every year in approximately 330,000 individuals worldwide (North America: 64,000; Europe: 115,000),1 and the incidence has generally increased in recent years.2 RCC affects more men than women; peak incidence occurs between 60 and 70 years of age. Many patients present with advanced or unresectable disease at initial diagnosis. Quality of life (QoL) in patients with advanced RCC is adversely affected by disease-related symptoms (such as fatigue, weakness, bone pain, hematuria, weight loss, and shortness of breath) and treatment-related adverse effects.3,4
Major first-line systemic standard-of-care treatments for patients with advanced clear cell RCC include the vascular endothelial growth factor receptor-tyrosine kinase inhibitors (VEGFR-TKIs) sunitinib and pazopanib. Second-line standard-of-care therapies include the VEGFR-TKIs cabozantinib, axitinib, and sorafenib; the mammalian target of rapamycin inhibitor everolimus; and the programmed cell death-1 checkpoint inhibitor nivolumab.5-7
Cabozantinib is an oral inhibitor of TKs, including MET, VEGFR, and AXL.8 Upregulation of MET and AXL in clear cell RCC, a consequence of von Hippel-Lindau protein dysfunction, has been implicated in tumor progression and VEGFR-TKI resistance in preclinical studies and has been associated with a poor prognosis in patients with advanced RCC.9-14 Cabozantinib gained regulatory approval in the United States and in Europe as a second-line treatment for patients with advanced RCC after prior therapy with an antiangiogenic drug on the basis of results of the randomized phase III METEOR trial. In this trial, cabozantinib treatment resulted in improvements compared with everolimus treatment for progression-free survival (PFS; primary end point) and the two secondary end points of overall survival (OS) and objective response rate (ORR).15-17 According to an independent radiology review, the median PFS was 7.4 months in the cabozantinib arm versus 3.8 months in the everolimus arm (hazard ratio [HR], 0.58; 95% CI, 0.45 to 0.74; P < .0001), and the ORR was 17% versus 3% (P < .0001). The median OS was 21.4 months (95% CI, 18.7 months to not estimable) versus 16.5 months (95% CI, 14.7 to 18.8 months) (HR, 0.66; 95% CI, 0.53 to 0.83; P < .0003). Changes from baseline in QoL, an exploratory end point in this study,18,19 are reported herein.
PATIENTS AND METHODS
Eligibility and Study Treatments
METEOR was a randomized, open-label, phase III study with patients enrolled at hospitals and outpatient clinics in 26 countries. Patients 18 years or older with advanced or metastatic RCC and a clear cell histology were eligible for enrollment if they had measurable disease per Response Evaluation Criteria in Solid Tumors (RECIST version 1.1),20 had received at least one previous VEGFR-TKI (there was no limit to the number of previous treatments), and had disease progression during or within 6 months of the most recent VEGFR-TKI treatment and within 6 months before randomization. Patients were required to have a Karnofsky performance status score ≥ 70% and adequate organ function, on the basis of standard laboratory tests, and no clinically significant morbidities. Patients with previous mammalian target of rapamycin inhibitor therapy, including everolimus, were not eligible for the study. The study adhered to the Good Clinical Practice guidelines and the Declaration of Helsinki. The institutional review board or ethics committee of the participating centers approved the study protocol. All patients provided written informed consent.
Patients were randomly assigned (1:1) to receive either cabozantinib or everolimus. Randomization was stratified by the number of prior VEGFR-TKI treatments (one or two or more) and Memorial Sloan Kettering Cancer Center risk groups (favorable, intermediate, or poor) for previously treated RCC.21 Patients and investigators were not masked to study treatment to allow appropriate management of adverse events (AEs).
Cabozantinib was given orally 60 mg once daily, and everolimus was given orally 10 mg once daily. Treatment interruptions and reductions were allowed for managing AEs. Patients with clinical benefit could continue study treatment beyond radiographic progression at the discretion of the investigator. On-study crossover between treatment arms was not permitted.
Radiographic tumor imaging assessments occurred regularly every 8 to 12 weeks throughout the study. Assessment of AEs was performed throughout the study. Patients had a 30-day post-treatment visit with subsequent survival follow-up every 8 weeks. Efficacy and safety evaluations in this study have been previously described.15,16
QoL Assessments
QoL was measured using two validated patient self-reported instruments: the Functional Assessment of Cancer Therapy–Kidney Symptom Index-19 item (FKSI-19) and EuroQol Group’s five-level (EQ-5D-5L) questionnaires. Patients were required to complete the QoL questionnaires before any assessments at each clinic visit before the first dose, every 4 weeks through week 25, every 8 weeks thereafter for the first year, and then every 12 weeks thereafter. The QoL assessments were conducted per protocol before patients knew the results of their next tumor assessment. Regardless of whether study treatment was given, reduced, interrupted, or discontinued, these assessments were performed until the date of the last tumor imaging assessment. Questionnaires were provided on paper in the patient’s native language.
FKSI-19.
The FKSI-19 is a 19-item self-reported questionnaire with a total score and four subscales that assess disease-related symptoms (DRS), treatment side effects (TSE), and function/well-being associated with advanced kidney cancer. It captures symptom severity and interference in activity and general health perceptions.22,23 The FKSI-19 comprises four subscales (DRS-Physical, DRS-Emotional, TSE, and Function/Well-Being). Each of the items is scored on a five-point scale from zero (not at all) to four (very much). Higher FKSI-19 scores indicate improvement. The FKSI-19 ensures content validity congruent with US Food and Drug Administration Guidance24 and was developed from a previous nine-item DRS version (FKSI-DRS; lack of energy, pain, weight loss, fatigue, short of breath, fever, bone pain, cough, and blood in urine). The FKSI-19 includes selected TSE.
The earlier FKSI-DRS (nine-item) version was also investigated. The items from the FKSI-DRS (nine-item version) are included within the FKSI-19; general US population norms for each version have been calculated.25 The FKSI instrument has been used in other pivotal studies in RCC.26-30
EQ-5D-5L.
The standardized measure of health status EQ-5D-5L was also used in this study to measure generic health status.31 It comprises five functional and symptom dimensions: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. For each dimension, patients may indicate their health status from level 1 (no problem) to levels 2 through 5 (mild problem to extreme problem). The severity scores from the five dimensions were converted into a single population-based index value (EQ-Index) normalized across the nine countries in the study for which index value sets were available.32 A higher index score indicates better health. Patients also completed a 20-cm vertical visual analog scale (EQ-VAS) scored from zero (“worst health you can imagine”) to 100 (“best health you can imagine”). A prior (three-level) version of the EQ-5D instrument had been evaluated in a study of pazopanib versus placebo33 and the NCT00083889 study of sunitinib versus interferon.27
Statistical Analysis
The QoL analyses were performed on the intention-to-treat population with the same data cutoff date (May 22, 2015) as the primary PFS end point.15,16 OS analyses used a cutoff date of December 31, 2015. There was no prespecified statistical QoL hypothesis for this study. All available data were included in the analyses, and no data were imputed. No adjustment was made for multiplicity for any of the QoL analyses. The questionnaire completion rate was calculated as number completed/number expected at that time point.
Descriptive statistics were used to summarize QoL scores over time for each treatment arm. In addition, a prespecified repeated-measures model was used to compare changes from baseline between the treatment arms. The model included the outcome variable of QoL score change from baseline. The predictors were the baseline scores, treatment arms, and visit. Effect sizes in the range from 0.20 to 0.49 are generally considered to be small; for this study, an effect size ≥ 0.30 was prespecified as likely to be clinically relevant.34,35
To assess the effect of patient dropout, post hoc linear growth curve models were undertaken as sensitivity analyses using a mixed-effect regression technique. Fixed effects included baseline score, randomization stratification factors, treatment arm, time (a continuous variable), and the interaction between treatment arm and time. Random effects included intercept and slope. Models were fitted for three dropout tertiles, ie, early (before the week 25 visit), median (week 25 and 33 visits), and late (week 41 visit and later); the mean trajectory of each arm was estimated. Time of dropout was defined as the time of the last available analyzable score.
Time to deterioration (TTD; the earlier of date of death, radiographic disease progression (rPD), or at least a four-point decrease from baseline in the nine-item FKSI-DRS) was a post hoc analysis.
Finally, a post hoc analysis of the effect of FKSI-DRS scores on OS was performed in two groups of patients (at or above v below the median FKSI-DRS at baseline). The same Kaplan-Meier method was used as in a previous OS analysis.16
RESULTS
Baseline Characteristics and Study Disposition
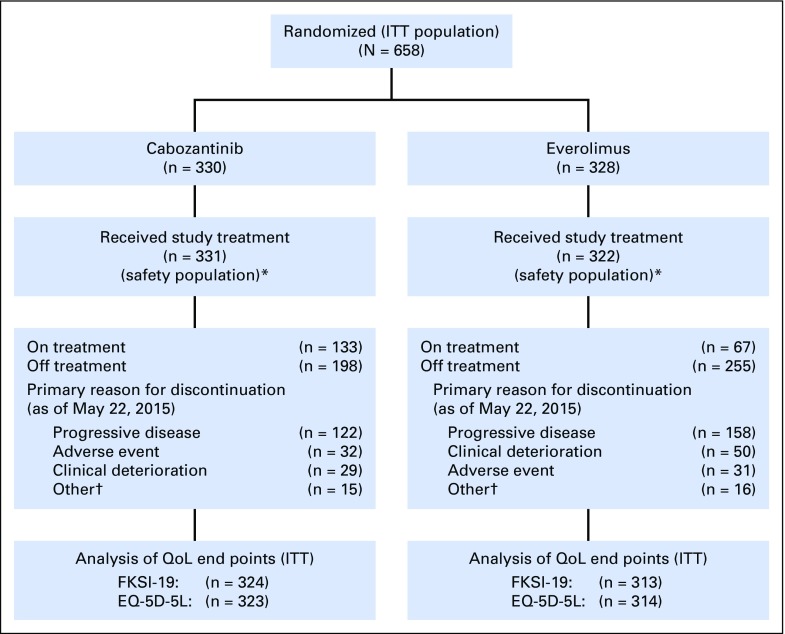
A total of 658 patients were randomly assigned and included in the intention-to-treat analysis (Fig 1). At the cutoff date for the QoL analysis, 40% of patients in the cabozantinib arm and 21% in the everolimus arm were still on study treatment. Dose reductions as a result of AEs occurred in 59.8% of cabozantinib-treated patients and 24.2% of everolimus-treated patients. The incidence of discontinuation due to a primary reason of AE (excluding disease progression) was similar (10%) in each arm.
Fig 1.
Patient disposition (intent-to-treat population). Baseline FKSI-19 (EQ-5D-5L) data were available for 324 (323) patients in the cabozantinib arm and 313 (314) patients in the everolimus arm, of whom 319 (317) and 303 (304), respectively, had at least one postbaseline assessment. (*) Five patients randomly assigned to the everolimus arm did not receive study treatment. In addition, one patient randomly assigned to the everolimus arm received cabozantinib as study treatment. (†) Includes withdrawals, protocol deviations, lack of efficacy, investigator decision. EQ-5D-5L, five-level EuroQol questionnaire; FKSI-19, 19-item Functional Assessment of Cancer Therapy–Kidney Symptom Index; ITT, intent to treat; QoL, quality of life.
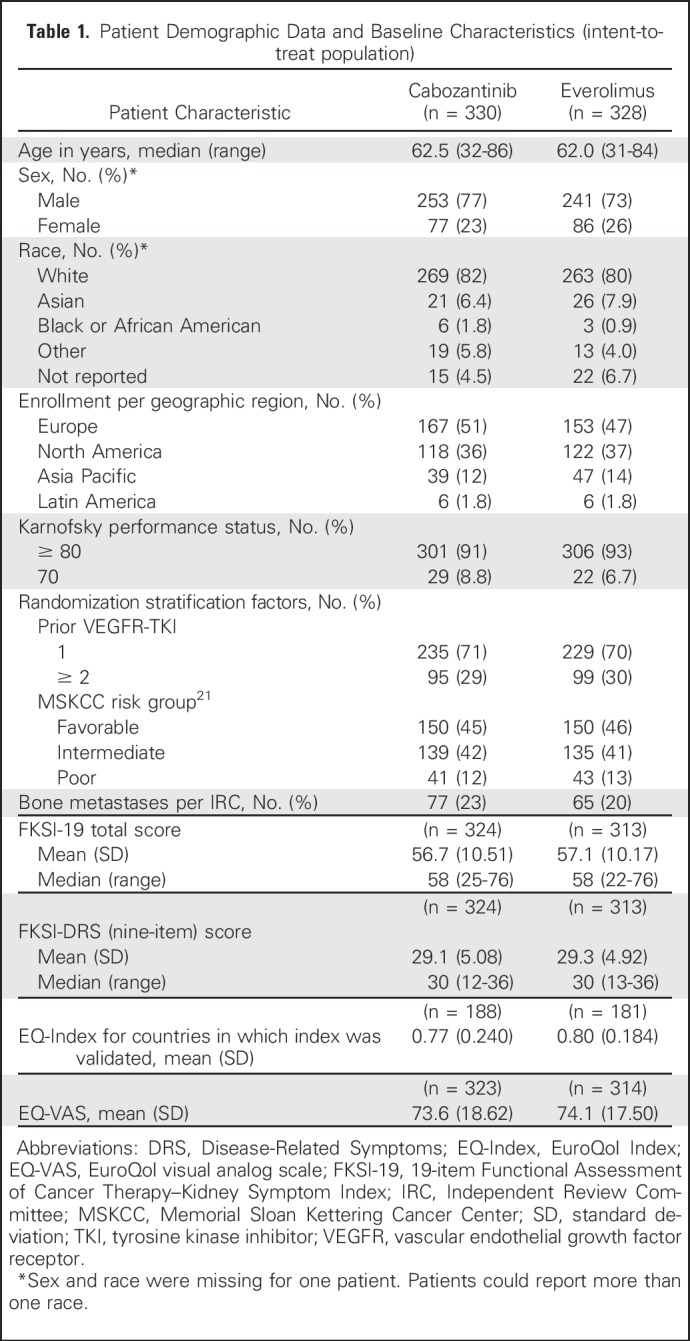
Baseline characteristics were also similar in each arm (Table 1). The mean age of patients was 62 years in the cabozantinib arm and 61 years in the everolimus arm; approximately 75% of patients were male. FKSI-19 and FKSI-DRS (nine-item) mean scores in each arm were similar to the US Adult Population values of 59.8 and 29.5, respectively.25
Table 1.
Patient Demographic Data and Baseline Characteristics (intent-to-treat population)
Questionnaire Completion Rate
For both instruments, questionnaire completion rates (number completed/expected at each time point) were high and similar in each treatment arm. At baseline, the rates for the FKSI-19 (EQ-5D) were 98% (96%) and 95% (95%) in the cabozantinib and everolimus arms, respectively, and remained ≥ 75% through week 48 in each arm for both instruments. The median duration of collection of QoL data was approximately 17 weeks in the cabozantinib arm and 13 weeks in the everolimus arm.
FKSI Assessments
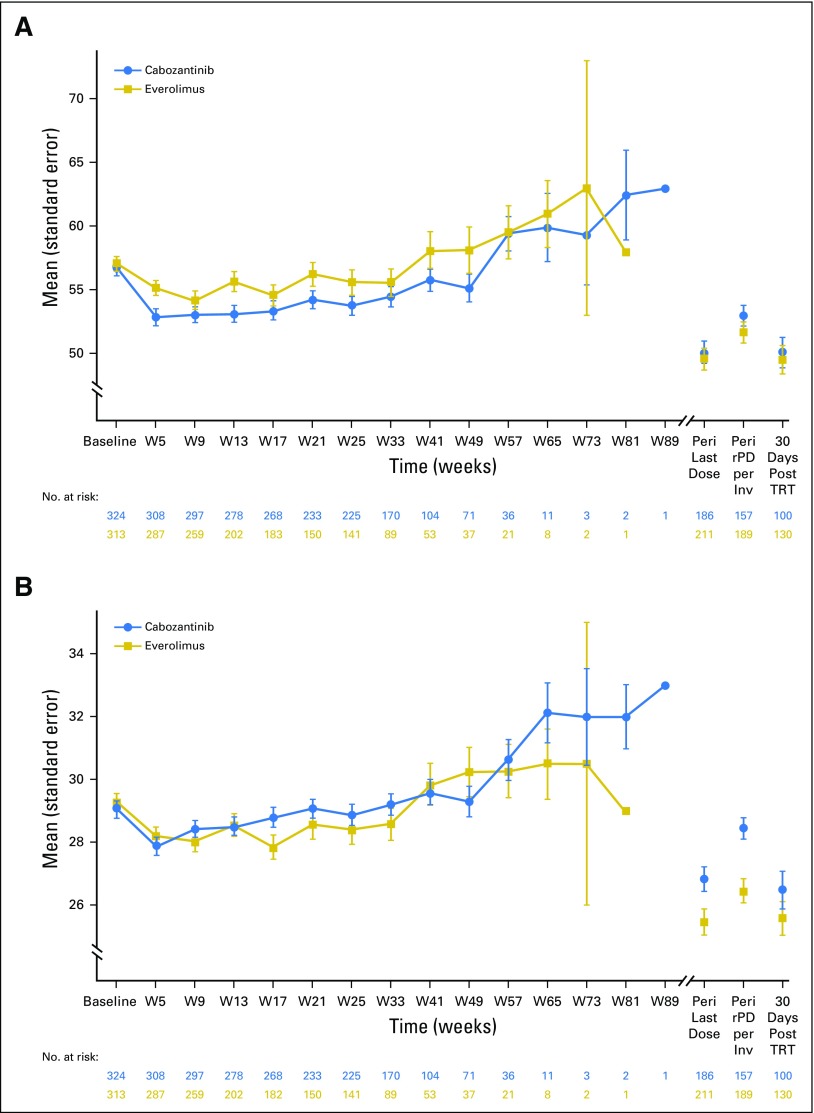
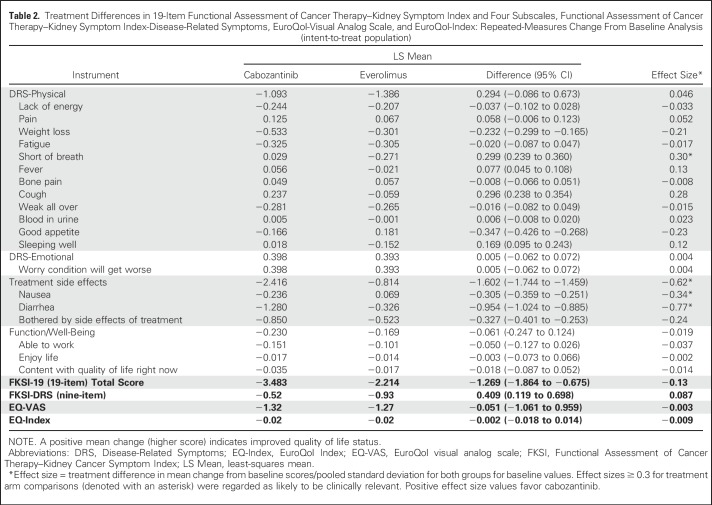
There were no differences over time between the treatment arms on the basis of descriptive summaries for the FKSI-19 Total or nine-item FKSI-DRS (Fig 2) or for the EQ instrument (data not shown). There was also no treatment difference (ie, the effect size was < 0.30) on the basis of a prespecified repeated-measures model change from baseline analysis for FKSI-19 Total or nine-item FKSI-DRS (Table 2). Among the 19 individual items, the only differences on the basis of effect size were lower scores in the cabozantinib arm for diarrhea and nausea and lower scores in the everolimus arm for shortness of breath. Among the four subscales, there was a treatment difference on the basis of effect size in favor of everolimus for the TSE subscale, with no difference observed for the other subscales; of note, the TSE subscale was not comprehensive and comprised three items: diarrhea, nausea, and bothered by side effects of treatment.
Fig 2.
Plot of absolute FKSI scores over time (intent-to-treat population). (A) FKSI-19 Total. (B) Nine-item FKSI-DRS. DRS, Disease-Related Symptoms; FKSI, Functional Assessment of Cancer Therapy–Kidney Symptom Index; PD, progressive disease; rPD, radiographic progressive disease; TRT, treatment; QoL, quality of life; W, week. Higher scores indicate improved QoL status. Peri Last Dose is the closest QoL assessment 2 weeks before to 4 weeks after last dose. Peri rPD per Inv is the closest QoL assessment 2 weeks before to 4 weeks after first date of PD per investigator.
Table 2.
Treatment Differences in 19-Item Functional Assessment of Cancer Therapy–Kidney Symptom Index and Four Subscales, Functional Assessment of Cancer Therapy–Kidney Symptom Index-Disease-Related Symptoms, EuroQol-Visual Analog Scale, and EuroQol-Index: Repeated-Measures Change From Baseline Analysis (intent-to-treat population)
EQ-5D-5L Assessments
There were no differences over time between the treatment arms (ie, the effect size was < 0.30) on the basis of a repeated-measures change from baseline analysis for EQ-Index or EQ-VAS (Table 2). There was no treatment difference on the basis of descriptive summaries over time for EQ-Index or EQ-VAS (data not shown).
Sensitivity Analyses
Post hoc growth curve sensitivity analyses of each of the QoL measures (FKSI-19, nine-item FKSI-DRS, EQ-Index, and EQ-VAS) showed that there was an inconsistent effect for the initial treatment difference at week 4 (first assessment after starting treatment) across the three tertiles (early, median, and late dropout). For the median and late dropout tertiles, initial QoL scores for each measure at week 4 were lower for cabozantinib relative to everolimus. There was no consistent initial treatment difference at week 4 across the QoL measures for the early dropout tertile. However, changes in QoL scores over time after week 4 consistently favored cabozantinib across each tertile for each QoL measure.19
TTD
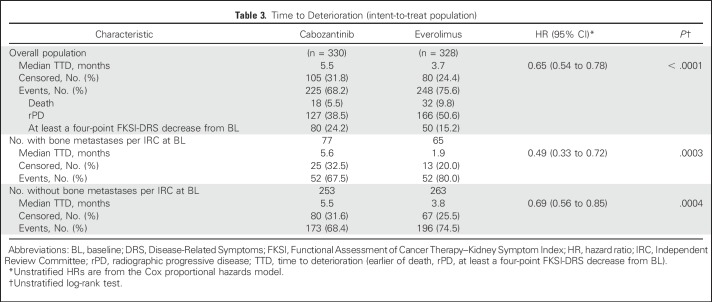
There was a notable decrease in QoL scores compared with baseline at the time of rPD per investigator. Progressive disease was the most frequent reason for study treatment discontinuation. Cabozantinib treatment improved TTD (earlier of date of death, rPD, or at least a four-point decrease from baseline in FKSI-DRS) compared with everolimus (median TTD, 5.5 v 3.7 months; P < .001; Table 3); this improvement seemed to be mainly driven by events of death or rPD rather than change in FKSI-DRS. In a subgroup analysis, there was a pronounced TTD improvement with cabozantinib treatment in patients with bone metastases (median TTD, 5.6 v 1.9 months; P < .001; Table 3).
Table 3.
Time to Deterioration (intent-to-treat population)
QoL as a Prognostic Factor for OS
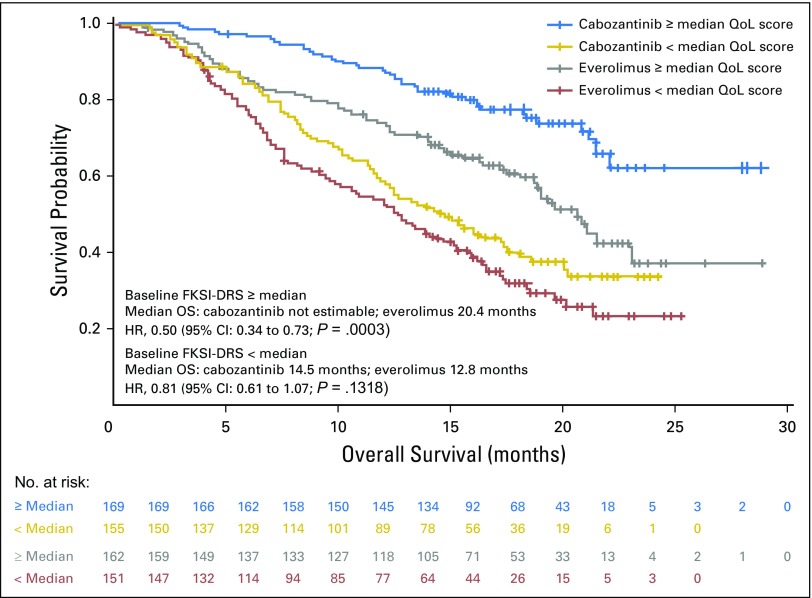
Baseline FKSI-DRS (nine-item) scores higher than the median population values were associated with improved OS in both treatment arms (Fig 3). HRs for OS favored the cabozantinib arm regardless of baseline QoL.
Fig 3.
Effect of baseline FKSI-DRS on OS (intent-to-treat population). DRS, Disease-Related Symptoms; FKSI, Functional Assessment of Cancer Therapy–Kidney Symptom Index; HR, hazard ratio; mo, month; OS, overall survival; QoL, quality of life.
DISCUSSION
As previously reported,15,16 the METEOR phase III trial demonstrated that cabozantinib (an oral inhibitor of TKs including MET, VEGFR, and AXL) improved key clinical end points of PFS, OS, and ORR compared with everolimus in patients with advanced RCC who had received a prior VEGFR-TKI. The improved efficacy for cabozantinib was demonstrated across all prespecified subgroups. The observed safety profiles of cabozantinib and everolimus in the METEOR study were reflective of their distinctive mechanisms of action. In the current study, we examined the QoL of patients, an exploratory end point in this trial, as assessed by standardized instruments (FKSI-19 and EQ-5D).
The FKSI-19 instrument was developed for patients with RCC to assess general QoL and the impact of specific treatment-related adverse effects. The results of the assessment showed that there was no clinically meaningful difference overall between treatment arms in the FKSI-19 Total scores. Of note, QoL data collection was more prolonged in the cabozantinib arm, which also had the greater PFS benefit. Of the 19 individual items, the observed treatment differences (worse diarrhea and nausea for cabozantinib, worse shortness of breath for everolimus) were consistent with the safety profiles of each agent.
Because this was an open-label trial, these treatment differences may also be observed in routine clinical practice. Therefore, management should be directed toward mitigating these adverse effects for either agent. It is noteworthy that in both treatment arms, a low percentage of patients discontinued study treatment because of AEs (approximately 10% of patients in each arm). In particular, diarrhea was not a major cause of treatment discontinuation (0.9% of patients in each arm), which indicates that proactive management of this symptom has been effective.
The (nine-item) FKSI-DRS instrument, which is part of the FKSI-19, examined the effects of study treatment on disease-related symptoms. Consistent with the results for the 19-item scale, no clinically meaningful treatment difference for the nine-item FKSI-DRS was observed for cabozantinib compared with everolimus. To put this finding into perspective, in the previous phase III RECORD-1 study using everolimus in a similar patient population, there was no evidence of a difference in FKSI-DRS over time compared with placebo.36 Finally, the results of the generic EQ-5D (health index and patient-based VAS) instrument in the METEOR trial were consistent with the findings from the overall FKSI-19 and FKSI-DRS scales of no clinically relevant treatment difference between the arms.
At the time of investigator-determined rPD, QoL of patients was markedly diminished in both treatment arms. This finding indicates the negative impact of disease progression on QoL and is suggestive of the benefit of extending PFS in this patient population. The observed prolongation of TTD in the cabozantinib arm further supports the overall clinical benefit of this agent in addition to improving PFS, ORR, and OS—albeit without a QoL advantage.
In a recent phase III study (CheckMate 025) evaluating the checkpoint inhibitor nivolumab in a similar patient population, improvements were observed with the nine-item FKSI-DRS for nivolumab compared with everolimus.26,37 The CheckMate 025 study showed a treatment benefit for nivolumab for OS and ORR but no PFS benefits.37 In contrast with the QoL results from the METEOR study, QoL results in CheckMate 025 were limited to on-treatment data assessment, and the questionnaire in CheckMate 025 did not assess the effects of treatment-related adverse effects. The safety profiles of nivolumab and cabozantinib differ on the basis of their mechanisms of action; therefore, without a head-to-head comparison, it is speculative to identify the influence of treatment-related adverse effects on QoL differences between these agents. Severe immune-related adverse effects seem to be infrequent with nivolumab; however, these can occur even after treatment discontinuation and require aggressive management with immune suppression. In terms of potential prognostic indicators for clinical outcome, in both the METEOR and CheckMate 025 studies, higher baseline QoL was associated with improved OS.26
The METEOR study used an open-label design (patients and caregivers were aware of their study treatment allocation), which potentially could have led to an impact on the results of QoL evaluations. This was mitigated by the protocol requirement that QoL assessments be completed before patients knew their most recent treatment results. Although this was a multinational trial with no adjustment for by-country differences, it is unlikely that it affected QoL outcomes because of the randomized nature of the trial. In addition, key efficacy outcomes (PFS and OS) in the METEOR trial have been shown to be similar by region.15,16
Use of a repeated-measures model reduces the potential for bias resulting from dropouts in that all available data are used; if a score is missing, it has no effect on other scores from the same patient. In terms of validity of the METEOR QoL results, a lack of treatment difference has been demonstrated in both the unadjusted data and the prespecified repeated-measures modeled data. However, post hoc growth curve sensitivity analyses suggest heterogeneity in the initial treatment difference (at week 4), depending on when patients discontinued QoL assessments. Nevertheless, this circumstance did not meaningfully affect the overall interpretation from the study that QoL in each treatment arm is comparable.
In conclusion, the METEOR trial showed that QoL declined initially and was generally maintained over time to a similar extent in both the cabozantinib and everolimus arms. The totality of results (including PFS, OS, and ORR) shows that cabozantinib has a favorable clinical benefit compared with everolimus for previously treated patients with advanced RCC.
ACKNOWLEDGMENT
We thank the patients and their families, the investigators and site staff, and the study teams who participated in the METEOR trial. Editorial support was provided by Michael Raffin (Fishawack Communications, Conshohocken, PA).
Footnotes
Supported by Exelixis (South San Francisco, CA), which also supplied cabozantinib and funded editorial support. Patients treated at Memorial Sloan Kettering Cancer Center were supported in part by a Memorial Sloan Kettering Cancer Center Support Grant/Core Grant (P30 CA008748).
Presented in part at the European Society for Medical Oncology congress, Copenhagen, Denmark, October 7-11, 2016 and at the International Society for Quality of Life Research conference, Philadelphia, PA, October 18-21, 2017.
Clinical trial information: NCT01865747.
AUTHOR CONTRIBUTIONS
Conception and design: David Cella, Christian Scheffold, Robert J. Motzer, Toni K. Choueiri
Provision of study materials or patients: Bernard Escudier, Nizar M. Tannir, Thomas Powles, Frede Donskov, Katriina Peltola, Manuela Schmidinger, Daniel Y.C. Heng, Paul N. Mainwaring, Hans J. Hammers, Jae Lyun Lee, Thomas E. Hutson, Sumanta Pal, Robert J. Motzer, Toni K. Choueiri
Collection and assembly of data: David Cella, Bernard Escudier, Nizar M. Tannir, Thomas Powles, Frede Donskov, Katriina Peltola, Manuela Schmidinger, Daniel Y.C. Heng, Paul N. Mainwaring, Hans J. Hammers, Jae Lyun Lee, Bruce J. Roth, Milan Mangeshkar, Christian Scheffold, Thomas E. Hutson, Sumanta Pal, Robert J. Motzer, Toni K. Choueiri
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Quality of Life Outcomes for Cabozantinib Versus Everolimus in Patients With Metastatic Renal Cell Carcinoma: METEOR Phase III Randomized Trial
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
David Cella
Stock or Other Ownership: FACIT.org
Consulting or Advisory Role: AbbVie, Bayer, GlaxoSmithKline, Pfizer, Astellas Pharma, Novartis, PledPharma, Puma Biotechnology
Research Funding: Novartis (Inst), Genentech (Inst), Ipsen (Inst), Bayer (Inst), Pfizer (Inst)
Travel, Accommodations, Expenses: Ipsen
Bernard Escudier
Honoraria: Pfizer, Novartis, Bayer, Bristol-Myers Squibb, Ipsen, Exelixis, Genentech
Consulting or Advisory Role: Bayer, Novartis, Pfizer, Exelixis, Bristol-Myers Squibb, Ipsen
Travel, Accommodations, Expenses: Novartis, Bristol-Myers Squibb, Pfizer
Nizar M. Tannir
Honoraria: Pfizer, Novartis, Bristol-Myers Squibb, Exelixis, Nektar, Argos Therapeutics, Calithera Biosciences
Consulting or Advisory Role: Novartis, Exelixis, Bristol-Myers Squibb, Nektar, Pfizer, Argos Therapeutics, Calithera Biosciences
Research Funding: Bristol-Myers Squibb, Novartis, Exelixis, Epizyme, Miranti
Travel, Accommodations, Expenses: Pfizer, Novartis, Exelixis, Nektar, Bristol-Myers Squibb, Argos Therapeutics
Thomas Powles
Honoraria: Exelixis
Consulting or Advisory Role: Genentech, Bristol-Myers Squibb, Merck, Novartis, AstraZeneca
Research Funding: AstraZeneca/MedImmune, Genentech
Frede Donskov
Research Funding: Pfizer (Inst), Novartis (Inst)
Katriina Peltola
Employment: Orion Pharma, Docrates Cancer Clinic
Stock or Other Ownership: Faron Pharmaceuticals
Consulting or Advisory Role: MSD Oncology, Eli Lilly, Orion Pharma, Pfizer, Bristol-Myers Squibb, Novartis
Travel, Accommodations, Expenses: Bristol-Myers Squibb
Manuela Schmidinger
Honoraria: Pfizer, Novartis, Roche, Bristol-Myers Squibb, Ipsen, Exelixis, Eisai, Astellas
Consulting or Advisory Role: Pfizer, Novartis, Roche, Bristol-Myers Squibb, Ipsen, Exelixis, Eisai, Astellas
Research Funding: Pfizer, Roche, Novartis
Daniel Y.C. Heng
Consulting or Advisory Role: Pfizer, Novartis, Bristol-Myers Squibb, Janssen, Astellas Pharma
Research Funding: Pfizer (Inst), Novartis (Inst), Exelixis (Inst), Bristol-Myers Squibb (Inst)
Paul N. Mainwaring
Leadership: Xing Technologies
Stock or Other Ownership: Xing Technologies
Honoraria: Janssen, Genentech, Celgene, Pfizer, Ipsen
Consulting or Advisory Role: Janssen, Astellas Pharma, Pfizer, Pfizer (I), Ipsen
Speakers’ Bureau: Janssen Oncology, Genentech, Celgene, Pfizer
Research Funding: Merck
Patents, Royalties, Other Intellectual Property: Xing Technologies
Hans J. Hammers
Consulting or Advisory Role: Bristol-Myers Squibb, Pfizer, Exelixis, Bayer, Eisai
Research Funding: Bristol-Myers Squibb (Inst), Merck (Inst)
Travel, Accommodations, Expenses: Bristol-Myers Squibb
Jae Lyun Lee
Honoraria: Pfizer, Astellas Pharma, Novartis
Consulting or Advisory Role: Astellas Pharma, AstraZeneca, Eisai
Research Funding: Pfizer, Janssen, Novartis, Exelixis
Bruce J. Roth
Research Funding: Medivation (Inst)
Florence Marteau
Employment: Ipsen
Honoraria: Ipsen
Paul Williams
Employment: Mapi Group
Consulting or Advisory Role: Ipsen (Inst)
Research Funding: Ipsen (Inst)
John Baer
Employment: Exelixis
Stock or Other Ownership: Exelixis, GlaxoSmithKline
Milan Mangeshkar
Employment: Exelixis
Stock or Other Ownership: Exelixis
Christian Scheffold
Employment: Exelixis
Stock or Other Ownership: Exelixis
Thomas E. Hutson
Employment: Texas Oncology
Honoraria: Bayer/Onyx, Pfizer, Medivation, Novartis, Johnson & Johnson, GlaxoSmithKline, Dendreon
Consulting or Advisory Role: Bayer/Onyx, Pfizer, Novartis, Astellas Pharma, GlaxoSmithKline
Speakers’ Bureau: Dendreon, Pfizer, GlaxoSmithKline, Bayer/Onyx, Johnson & Johnson, Medivation
Research Funding: Pfizer (Inst), GlaxoSmithKline (Inst), Johnson & Johnson (Inst), Medivation (Inst)
Sumanta Pal
Honoraria: Novartis, Medivation, Astellas Pharma
Consulting or Advisory Role: Pfizer, Novartis, AVEO, Myriad Pharmaceuticals, Genentech, Exelixis, Bristol-Myers Squibb, Astellas Pharma, Ipsen, Eisai
Research Funding: Medivation
Robert J. Motzer
Consulting or Advisory Role: Pfizer, Novartis, Eisai, Exelixis, Merck
Research Funding: Pfizer (Inst), GlaxoSmithKline (Inst), Bristol-Myers Squibb (Inst), Eisai (Inst), Novartis (Inst), Genentech (Inst)
Toni K. Choueiri
Honoraria: NCCN, UpToDate
Consulting or Advisory Role: Pfizer, Bayer, Novartis, GlaxoSmithKline, Merck, Bristol-Myers Squibb, Genentech, Eisai, Foundation Medicine, Cerulean Pharma, AstraZeneca, Peloton Therapeutics, Exelixis, Prometheus, Alligent
Research Funding: Pfizer (Inst), Novartis (Inst), Merck (Inst), Exelixis (Inst), TRACON Pharma (Inst), GlaxoSmithKline (Inst), Bristol-Myers Squibb (Inst), AstraZeneca (Inst), Peloton Therapeutics (Inst), Genentech (Inst), Celldex (Inst), Agensys (Inst)
Travel, Accommodations, Expenses: Advisory boards/consultancy
REFERENCES
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al. : Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136:E359-E386, 2015 [DOI] [PubMed] [Google Scholar]
- 2.Znaor A, Lortet-Tieulent J, Laversanne M, et al. : International variations and trends in renal cell carcinoma incidence and mortality. Eur Urol 67:519-530, 2015 [DOI] [PubMed] [Google Scholar]
- 3.Cella D: Quality of life in patients with metastatic renal cell carcinoma: The importance of patient-reported outcomes. Cancer Treat Rev 35:733-737, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Palapattu GS, Kristo B, Rajfer J: Paraneoplastic syndromes in urologic malignancy: The many faces of renal cell carcinoma. Rev Urol 4:163-170, 2002 [PMC free article] [PubMed] [Google Scholar]
- 5. Motzer RJ, Jonasch E, Agarwal N, et al: National Comprehensive Cancer Network Guidelines - Kidney cancer, version 2.2017, 2016. [DOI] [PubMed]
- 6.Ljungberg B, Albiges L, Bensalah K, et al. : EAU Guidelines - Renal Cell Carcinoma. http://uroweb.org/guideline/renal-cell-carcinoma
- 7.Choueiri TK, Motzer RJ: Systemic therapy for metastatic renal-cell carcinoma. N Engl J Med 376:354-366, 2017 [DOI] [PubMed] [Google Scholar]
- 8.Yakes FM, Chen J, Tan J, et al. : Cabozantinib (XL184), a novel MET and VEGFR2 inhibitor, simultaneously suppresses metastasis, angiogenesis, and tumor growth. Mol Cancer Ther 10:2298-2308, 2011 [DOI] [PubMed] [Google Scholar]
- 9.Gibney GT, Aziz SA, Camp RL, et al. : c-Met is a prognostic marker and potential therapeutic target in clear cell renal cell carcinoma. Ann Oncol 24:343-349, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gustafsson A, Martuszewska D, Johansson M, et al. : Differential expression of Axl and Gas6 in renal cell carcinoma reflecting tumor advancement and survival. Clin Cancer Res 15:4742-4749, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Harshman LC, Choueiri TK: Targeting the hepatocyte growth factor/c-Met signaling pathway in renal cell carcinoma. Cancer J 19:316-323, 2013 [DOI] [PubMed] [Google Scholar]
- 12.Pinato DJ, Chowdhury S, Stebbing J: TAMing resistance to multi-targeted kinase inhibitors through Axl and Met inhibition. Oncogene 35:2684-2686, 2016 [DOI] [PubMed] [Google Scholar]
- 13.Rankin EB, Fuh KC, Castellini L, et al. : Direct regulation of GAS6/AXL signaling by HIF promotes renal metastasis through SRC and MET. Proc Natl Acad Sci USA 111:13373-13378, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou L, Liu XD, Sun M, et al. : Targeting MET and AXL overcomes resistance to sunitinib therapy in renal cell carcinoma. Oncogene 35:2687-2697, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choueiri TK, Escudier B, Powles T, et al. : Cabozantinib versus everolimus in advanced renal-cell carcinoma. N Engl J Med 373:1814-1823, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choueiri TK, Escudier B, Powles T, et al. : Cabozantinib versus everolimus in advanced renal cell carcinoma (METEOR): Final results from a randomised, open-label, phase 3 trial. Lancet Oncol 17:917-927, 2016 [DOI] [PubMed] [Google Scholar]
- 17.Cabometyx (cabozantinib). Prescribing information. Exelixis, Inc., South San Francisco, CA: 2016. [Google Scholar]
- 18.Cella D, Escudier B, Tannir N, et al. : Quality of life in the Phase 3 METEOR trial of cabozantinib vs everolimus for advanced renal cell carcinoma. Presented at the European Society for Medical Oncology Annual Congress, Copenhagen, Denmark, October 7-11, 2016 (abstract 816P). [Google Scholar]
- 19.Williams P, Marteau F, Gabriel S, et al. : Long-term trends in health-related quality of life in patients with metastatic renal cell carcinoma treated with cabozantinib or everolimus. Presented at the International Society for Quality of Life Research 24th Annual Conference, Philadelphia, PA, USA, October 18-21, 2017 [Google Scholar]
- 20.Eisenhauer EA, Therasse P, Bogaerts J, et al. : New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 45:228-247, 2009 [DOI] [PubMed] [Google Scholar]
- 21.Motzer RJ, Bacik J, Schwartz LH, et al. : Prognostic factors for survival in previously treated patients with metastatic renal cell carcinoma. J Clin Oncol 22:454-463, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Rao D, Butt Z, Rosenbloom S, et al. : A comparison of the Renal Cell Carcinoma-Symptom Index (RCC-SI) and the Functional Assessment of Cancer Therapy-Kidney Symptom Index (FKSI). J Pain Symptom Manage 38:291-298, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rothrock NE, Jensen SE, Beaumont JL, et al. : Development and initial validation of the NCCN/FACT symptom index for advanced kidney cancer. Value Health 16:789-796, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. US Food and Drug Administration. Guidance for Industry: Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims, 2009. [DOI] [PMC free article] [PubMed]
- 25.Butt Z, Peipert J, Webster K, et al. : General population norms for the Functional Assessment of Cancer Therapy-Kidney Symptom Index (FKSI). Cancer 119:429-437, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cella D, Grünwald V, Nathan P, et al. : Quality of life in patients with advanced renal cell carcinoma given nivolumab versus everolimus in CheckMate 025: A randomised, open-label, phase 3 trial. Lancet Oncol 17:994-1003, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cella D, Michaelson MD, Bushmakin AG, et al. : Health-related quality of life in patients with metastatic renal cell carcinoma treated with sunitinib vs interferon-alpha in a phase III trial: Final results and geographical analysis. Br J Cancer 102:658-664, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Motzer RJ, Escudier B, Oudard S, et al. : Efficacy of everolimus in advanced renal cell carcinoma: A double-blind, randomised, placebo-controlled phase III trial. Lancet 372:449-456, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Motzer RJ, Escudier B, Tomczak P, et al. : Axitinib versus sorafenib as second-line treatment for advanced renal cell carcinoma: Overall survival analysis and updated results from a randomised phase 3 trial. Lancet Oncol 14:552-562, 2013 [DOI] [PubMed] [Google Scholar]
- 30.Motzer RJ, Hutson TE, Cella D, et al. : Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med 369:722-731, 2013 [DOI] [PubMed] [Google Scholar]
- 31.Herdman M, Gudex C, Lloyd A, et al. : Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 20:1727-1736, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Hout B, Janssen MF, Feng YS, et al. : Interim scoring for the EQ-5D-5L: Mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health 15:708-715, 2012 [DOI] [PubMed] [Google Scholar]
- 33.Cella D, Pickard AS, Duh MS, et al. : Health-related quality of life in patients with advanced renal cell carcinoma receiving pazopanib or placebo in a randomised phase III trial. Eur J Cancer 48:311-323, 2012 [DOI] [PubMed] [Google Scholar]
- 34.Sloan JA, Cella D, Hays RD: Clinical significance of patient-reported questionnaire data: Another step toward consensus. J Clin Epidemiol 58:1217-1219, 2005 [DOI] [PubMed] [Google Scholar]
- 35.Yost KJ, Eton DT: Combining distribution- and anchor-based approaches to determine minimally important differences: The FACIT experience. Eval Health Prof 28:172-191, 2005 [DOI] [PubMed] [Google Scholar]
- 36.Beaumont JL, Butt Z, Baladi J, et al. : Patient-reported outcomes in a phase III study of everolimus versus placebo in patients with metastatic carcinoma of the kidney that has progressed on vascular endothelial growth factor receptor tyrosine kinase inhibitor therapy. Oncologist 16:632-640, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Motzer RJ, Escudier B, McDermott DF, et al. : Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med 373:1803-1813, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]