The incidence of germ cell cancer continues to increase,1 and clinical stage (CS) I seminoma is the most common presentation. Nonetheless, despite decades of prospective studies, the optimal treatment of patients with CS I seminoma remains controversial. Surveillance, radiation therapy (RT), and carboplatin are each viewed as acceptable by various guideline groups. In recent years, a risk-adapted approach has increasingly been used, with both RT and carboplatin administered to patients at higher risk of recurrence. However, the studies that justify reduced doses and fields of radiation and the use of carboplatin were performed in unselected patients. We believe that the role of RT and carboplatin needs to be re-evaluated in light of recent data, prompting us to conclude that surveillance is the treatment of choice for most, if not all, compliant patients with CS I seminoma. Some historical perspective is necessary.
Because seminoma is highly radiosensitive, adjuvant RT to the para-aortic and ipsilateral iliac lymph nodes had been the standard of care for unselected CS I seminoma for decades, with a risk of relapse of approximately 4%. In parallel, many surveillance studies revealed that metastatic disease would appear in only approximately 15% of unselected patients. When relapse occurred, salvage treatment was highly successful, with a disease-specific survival of almost 100%.2-4 As such, it was clear a substantial majority of patients with CS I seminoma were being overtreated with RT, leading to a search for interventions with less toxicity and predictive factors for relapse. Evidence also showed an increased long-term risk of RT-induced second malignant neoplasms (SMNs) and cardiovascular disease (CVD), evoking concern for its use in all patients with CS I disease.5 To address acute toxicity, the Medical Research Council (MRC) performed several randomized trials to reduce RT field and dose. In MRC TE10, a para-aortic port was compared with the classic dogleg port. It was designed to accept a higher pelvic failure rate. The results showed equivalent overall relapse rate (RR) and survival with lesser toxicity, but a significantly increased pelvic failure rate was observed as the cost for eliminating the pelvic RT port to reduce acute and late toxicity.6 Trial MRC TE18/European Organisation for Research and Treatment of Cancer (EORTC) 30942 compared 30 Gy in 15 fractions of RT with the lower dose of 20 Gy in 10 fractions; the lower dose was equally effective with fewer adverse events.7
Because of a toxicity profile preferable to that of cisplatin and greater convenience for patients, studies of adjuvant carboplatin were initiated. In 2005, the results of MRC TE19/EORTC 30982 were published. This noninferiority trial, initiated in 1995, prospectively compared RT with a single dose of carboplatin (area under the curve [AUC], 7) in 1,477 unselected patients with CS I seminoma. At 2 years of follow-up, the difference in the relapse-free rates between RT and chemotherapy was −1.0% (90% CI, −2.5 to 0.5), with fewer acute adverse events in the carboplatin group. With a predefined noninferiority margin of 3% (absolute difference), it was concluded that adjuvant carboplatin was noninferior to adjuvant RT.8 In 2011, the mature results of TE19 were published; 5-year relapse-free rates were −1.3% (90% CI, −3.5 to 0.7), demonstrating noninferiority only after the noninferiority margin was adjusted to a 5% absolute difference. In addition, a majority (74%) of relapses in the carboplatin group occurred in the retroperitoneum, forcing the need for abdominal computed tomography (CT) scanning after completion of therapy, testing that is not needed after dogleg RT. The 6.5-year median follow-up did not allow a meaningful assessment of late relapse or late chemotherapy toxicity, including CVD and SMNs.9 Powles et al10 reported statistically nonsignificant increases in the standardized mortality ratios at 9-year median follow-up after carboplatin treatment of CS I seminoma for ischemic heart disease and (especially) cerebrovascular disease. In addition, they were not able to confirm the finding for reduced incidence in second primary testicular cancers touted as an advantage of carboplatin over RT in the MRCTE19/EORTC 30982 study.8
Because restricting adjuvant therapy to patients at higher risk for recurrence was the next logical step, the search for high-risk features paralleled these studies of modified adjuvant RT and carboplatin. Tumor size (> 4 cm) and presence of rete testis invasion (RTI) were identified as independent risk factors for recurrence in an analysis of four pooled surveillance studies. Patients with neither, one, or both risk factors had a 12%, 16%, and 32% risk of relapse at 5 years and were considered at low, intermediate, and high risk, respectively.11 These two risk factors were used in recent risk-adapted studies to limit the use of carboplatin to a high-risk patient population. Two important studies were recently published addressing adjuvant carboplatin in patients with a high risk of disease recurrence.12,13
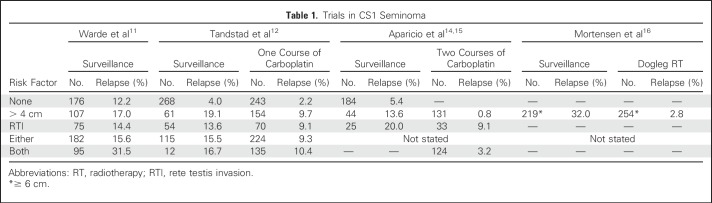
In one study, Tandstad et al,12 reporting for the Swedish and Norwegian Testicular Cancer Group (SWENOTECA), prospectively evaluated the efficacy of one cycle of carboplatin in a population-based cohort of 897 patients with CS I seminoma. Surveillance was recommended for patients whose tumors had zero or one risk factor, whereas patients whose tumors had both risk factors (size > 4cm and RTI) were offered one course of adjuvant carboplatin (AUC, 7). Regardless of the risk factor recommendation, patients were free to choose between adjuvant carboplatin and surveillance. With a median follow-up of 5.6 years, 69 relapses were reported. The RR in surveillance was 4.0% when tumors were without risk factors, 19.1% when the primary tumor was > 4 cm only, 13.6% with RTI only, and 16.7% when both risk factors were present; in the combined group of patients with either one or two risk factors, the RR was 15.5% (Table 1). In patients who received one cycle of carboplatin, the RR was 2.2% when tumors were without risk factors, 9.7% with a primary tumor > 4 cm only, 9.1% with RTI only, 9.3% with either risk factor, and 10.4% when both risk factors were present. Hence, in all risk groups, carboplatin reduced the RR by only approximately 50%. Moreover, the RR with surveillance in subgroups with one or two risk factors was similar (recognizing the small sample size of surveillance patients in the group with two risk factors). Notably, the RRs were similar to or lower than those reported in the original pooled estimate study11 (Table 1). Finally, the retroperitoneum was the only site of relapse in 90% of patients with recurrent disease after carboplatin. Although the study was not randomized, the sample size does allow meaningful conclusions. An absolute reduction in the risk of relapse by only 6.2%, resulting in approximately one in 10 patients relapsing despite carboplatin, as compared with one in seven patients relapsing during surveillance, is insufficient to justify the use of a single cycle of adjuvant carboplatin, even accounting for the low toxicity of carboplatin (administered unnecessarily to the considerable majority) and the cure of essentially all who relapse. Moreover, because the retroperitoneum was the predominant site of relapse, post-treatment CT surveillance remains mandatory.12
Table 1.
Trials in CS1 Seminoma
In a second analysis, Aparicio et al13 summarized the results of the three Spanish Germ Cell Cancer Group (SGCCG) studies.13 In two of these studies, the administration of two cycles of adjuvant carboplatin was used in patients whose primary tumors had both risk factors; relapse was observed in three (6.0%) of 50 patients in one study and one (1.4%) of 74 in their most recent trial, percentages that ought to be considered with some caution because median duration of follow-up was < 3 years in both studies, and 15% of relapses were recently noted to have occurred > 3 years after adjuvant carboplatin.17 Summing the second and third SGCCG studies, both of which used tumor size and RTI for risk stratification, only 23% of patients had two risk factors.14,15 RRs in patients with zero or one risk factor ranged from 5.4% to 20.0%, paralleling the observations of Tandstad et al.12 The RR in surveillance patients was not different from that in the SWENOTECA study (Table 1).
Because of the initial noninferior outcome in MRC TE19/EORTC 30982 and these risk-adapted results, many centers abandoned adjuvant RT, and adjuvant carboplatin emerged in international guidelines as the alternative approach to treat CS I disease, particularly in high-risk CS I seminoma. Surveillance was preferred for patients with CS I disease without risk factors.4,18
Although these studies support the notion that primary tumor size and RTI may be independent risk factors, multivariable analyses in other studies do not.12,19 A recent systematic review of prognostic factors in patients with CS I seminoma showed that the available evidence on the prognostic value of tumor size and RTI in those with CS I seminoma has significant limitations and that prudence is warranted on its routine use in clinical practice.20 It is important to keep in mind that, no matter whether these factors are used separately or combined, they fail to identify a high proportion of patients destined to relapse; > 90% of patients with no risk factors, > 80% of patients with one risk factor, and 70% of patients with both risk factors are cured after orchiectomy alone.
Regrettably, MRCTE19/EORTC 30982 did not collect data on tumor size or presence or absence of RTI. Because the trial was conducted in an era when adjuvant RT was still standard practice for all patients with CS I disease, it can safely be assumed that a majority of the patients in that study were at low risk for relapse. Likewise, MRC TE10 and TE18 did not collect data on tumor size and likely also comprised a substantial fraction of patients at low risk for recurrence.
One recent RT study focused exclusively on high-risk patients. Mortensen et al16 used the Danish Testicular Cancer Database to evaluate the outcome of 473 patients with a tumor size of > 6 cm who were treated with either active surveillance or adjuvant RT between 1985 and 2007. A dogleg port was used with a dose of 24 to 26 Gy. Surveillance was used in 219 of these patients, and 70 (32.0%) relapsed, essentially replicating their results from 199321; 2.8% of those treated with adjuvant RT relapsed. All cases of disease recurrence in patients treated with adjuvant RT were located outside the RT field. These results demonstrate that adjuvant dogleg RT effectively reduces the rate of disease recurrence (by 10-fold [90%]) in patients with CS I seminoma and a primary tumor ≥ 6 cm. Nonetheless, at least two thirds of patients with large (≥ 6 cm) primary tumors were cured after orchiectomy alone.
Taking all data together, we believe that one cycle of carboplatin (AUC, 7) should no longer be considered a treatment option in low-, intermediate-, or high-risk CS I seminoma. The data show that one cycle of carboplatin reduces the risk of relapse by no more than approximately 50% in all subgroups (Table 1), does not decrease the likelihood of a second primary germ cell tumor, and does not eliminate the need for regular CT scanning of the retroperitoneum. The use of two cycles of adjuvant carboplatin requires further study rather than incorporation into guidelines. Although the results of the SGCCG are encouraging, it must be remembered that single-agent carboplatin was inferior to combination cisplatin therapy in patients with metastatic seminoma,22 and a majority of patients (probably > 70%) will receive this therapy unnecessarily. Similar to one cycle, the long-term toxicities of two cycles of carboplatin also remain largely unknown and would be expected to be more frequent with the higher cumulative dose. Finally, the treatment of patients who relapse after adjuvant carboplatin has yet to be standardized, and recent data suggest that patients who relapse after adjuvant carboplatin with good-risk disease may have higher rates of subsequent relapse than historical controls.17,22 Adjuvant RT remains an option in selected patients who understand the long-term risks and the possibility of increased chemotherapy toxicity after RT relapse. In addition, although data are limited by small sample size, the proportion of patients treated by surveillance in the study by Mortensen et al16 who achieved a complete response to chemotherapy after relapse was greater in those proceeding directly to chemotherapy (24 [92%] of 26) than those who first received RT, relapsed, and then received chemotherapy (six [60%] of 10), and the only patients who died as a result of disease were those who received chemotherapy after RT.
Surveillance is clearly the treatment of choice for most, if not all, compliant patients with CS I seminoma, considering that even in high-risk CS I disease, 70% of patients will be overtreated, and relapse after surveillance is cured in nearly 100%. If a patient is unable or unwilling to comply with the surveillance protocol or prefers immediate definitive treatment, adjuvant RT is the only course of therapy that has been demonstrated to reduce the RR in high-risk patients. However, in that case, RT using the dogleg field with a total dose of 24 to 26 Gy is the only course of therapy that has been sufficiently investigated in this setting. Avoiding the dogleg or reducing the radiation dose has not been investigated in high-risk patients. The potentially increased risk of SMNs and CVD remains of concern, but in selected patients the risk-benefit ratio may support the choice for adjuvant RT, particularly in older patients or those with comorbidities. The short- and long-term advantages and disadvantages of RT should be discussed with the patient. In our experience, a majority of properly informed patients with tumors in all risk categories will choose surveillance.
AUTHOR CONTRIBUTIONS
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Controversies in the Management of Clinical Stage I Seminoma: Carboplatin a Decade in—Time to Start Backing Out
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Rune A.W. van de Wetering
No relationship to disclose
Stefan Sleijfer
Research Funding: AB Science (Inst), Sanofi (Inst), Merck KGaA (Inst), Eli Lilly (Inst)
Patents, Royalties, Other Intellectual Property: Test on circulating tumor cells in prostate cancer
Darren R. Feldman
Research Funding: Novartis, Seattle Genetics
Samuel A. Funt
Stock or Other Ownership: UroGen Pharma (I), Kite Pharma
Research Funding: AstraZeneca/MedImmune, Genentech
George J. Bosl
No relationship to disclose
Ronald de Wit
Honoraria: Sanofi, Eli Lilly, Roche/Genentech, Merck Sharp & Dohme
Consulting or Advisory Role: Sanofi, Merck Sharp & Dohme, Eli Lilly, Roche/Genentech
Research Funding: Sanofi (Inst)
REFERENCES
- 1.Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review. National Cancer Institute; 1975-2014. http://seer.cancer.gov/csr/1975_2014 [Google Scholar]
- 2.Groll RJ, Warde P, Jewett MAS. A comprehensive systematic review of testicular germ cell tumor surveillance. Crit Rev Oncol Hematol. 2007;64:182–197. doi: 10.1016/j.critrevonc.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 3.Nichols CR, Roth B, Albers P, et al. Active surveillance is the preferred approach to clinical stage I testicular cancer. J Clin Oncol. 2013;31:3490–3493. doi: 10.1200/JCO.2012.47.6010. [DOI] [PubMed] [Google Scholar]
- 4.de Wit R, Fizazi K. Controversies in the management of clinical stage I testis cancer. J Clin Oncol. 2006;24:5482–5492. doi: 10.1200/JCO.2006.07.9434. [DOI] [PubMed] [Google Scholar]
- 5.van den Belt-Dusebout AW, de Wit R, Gietema JA, et al. Treatment-specific risks of second malignancies and cardiovascular disease in 5-year survivors of testicular cancer. J Clin Oncol. 2007;25:4370–4378. doi: 10.1200/JCO.2006.10.5296. [DOI] [PubMed] [Google Scholar]
- 6.Fosså SD, Horwich A, Russell JM, et al. Optimal planning target volume for stage I testicular seminoma: A Medical Research Council randomized trial. J Clin Oncol. 1999;17:1146–1154. doi: 10.1200/JCO.1999.17.4.1146. [DOI] [PubMed] [Google Scholar]
- 7.Jones WG, Fossa SD, Mead GM, et al. Randomized trial of 30 versus 20 Gy in the adjuvant treatment of stage I testicular seminoma: A report on Medical Research Council Trial TE18, European Organisation for the Research and Treatment of Cancer Trial 30942 (ISRCTN18525328) J Clin Oncol. 2005;23:1200–1208. doi: 10.1200/JCO.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 8.Oliver RT, Mason MD, Mead GM, et al. Radiotherapy versus single-dose carboplatin in adjuvant treatment of stage I seminoma: A randomised trial. Lancet. 2005;366:293–300. doi: 10.1016/S0140-6736(05)66984-X. [DOI] [PubMed] [Google Scholar]
- 9.Oliver RT, Mead GM, Rustin GJM, et al. Randomized trial of carboplatin versus radiotherapy for stage I seminoma: Mature results on relapse and contralateral testis cancer rates in MRC TE19/EORTC 30982 study (ISRCTN27163214) J Clin Oncol. 2011;29:957–962. doi: 10.1200/JCO.2009.26.4655. [DOI] [PubMed] [Google Scholar]
- 10.Powles T, Robinson D, Shamash J, et al. The long-term risks of adjuvant carboplatin treatment for stage I seminoma of the testis. Ann Oncol. 2008;19:443–447. doi: 10.1093/annonc/mdm540. [DOI] [PubMed] [Google Scholar]
- 11.Warde P, Specht L, Horwich A, et al. Prognostic factors for relapse in stage I seminoma managed by surveillance: A pooled analysis. J Clin Oncol. 2002;20:4448–4452. doi: 10.1200/JCO.2002.01.038. [DOI] [PubMed] [Google Scholar]
- 12.Tandstad T, Ståhl O, Dahl O, et al. Treatment of stage I seminoma, with one course of adjuvant carboplatin or surveillance, risk-adapted recommendations implementing patient autonomy: A report from the Swedish and Norwegian Testicular Cancer Group (SWENOTECA) Ann Oncol. 2016;27:1299–1304. doi: 10.1093/annonc/mdw164. [DOI] [PubMed] [Google Scholar]
- 13.Aparicio J, Maroto P, García del Muro X, et al. Prognostic factors for relapse in stage I seminoma: A new nomogram derived from three consecutive, risk-adapted studies from the Spanish Germ Cell Cancer Group (SGCCG) Ann Oncol. 2014;25:2173–2178. doi: 10.1093/annonc/mdu437. [DOI] [PubMed] [Google Scholar]
- 14.Aparicio J, Germà JR, García del Muro X, et al. Risk-adapted management for patients with clinical stage I seminoma: The Second Spanish Germ Cell Cancer Cooperative Group study. J Clin Oncol. 2005;23:8717–8723. doi: 10.1200/JCO.2005.01.9810. [DOI] [PubMed] [Google Scholar]
- 15.Aparicio J, Maroto P, del Muro XG, et al. Risk-adapted treatment in clinical stage I testicular seminoma: The third Spanish Germ Cell Cancer Group study. J Clin Oncol. 2011;29:4677–4681. doi: 10.1200/JCO.2011.36.0503. [DOI] [PubMed] [Google Scholar]
- 16.Mortensen MS, Bandak M, Kier MGG, et al. Surveillance versus adjuvant radiotherapy for patients with high-risk stage I seminoma. Cancer. 2017;123:1212–1218. doi: 10.1002/cncr.30458. [DOI] [PubMed] [Google Scholar]
- 17.Fischer S, Tandstad T, Wheater M, et al. Outcome of men with relapse after adjuvant carboplatin for clinical stage I seminoma. J Clin Oncol. 2017;35:194–200. doi: 10.1200/JCO.2016.69.0958. [DOI] [PubMed] [Google Scholar]
- 18.Oldenburg J, Fosså SD, Nuver J, et al. Testicular seminoma and non-seminoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(Suppl 6):vi125–vi132. doi: 10.1093/annonc/mdt304. [DOI] [PubMed] [Google Scholar]
- 19.Chung P, Daugaard G, Tyldesley S, et al. Evaluation of a prognostic model for risk of relapse in stage I seminoma surveillance. Cancer Med. 2015;4:155–160. doi: 10.1002/cam4.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boormans JL, Mayor de Castro J, Marconi L, et al. Testicular tumour size and rete testis invasion as prognostic factors for the risk of relapse of clinical stage 1 seminoma testis patients under surveillance: A systematic review by the testicular cancer guidelines panel. Eur Urol [epub ahead of print on November 20, 2017] [DOI] [PubMed] [Google Scholar]
- 21.von der Maase H, Specht L, Jacobsen GK, et al. Surveillance following orchidectomy for stage I seminoma of the testis. Eur J Cancer. 1993;29:1931–1934. doi: 10.1016/0959-8049(93)90446-m. [DOI] [PubMed] [Google Scholar]
- 22.Bokemeyer C, Kollmannsberger C, Stenning S, et al. Metastatic seminoma treated with either single agent carboplatin or cisplatin-based combination chemotherapy: A pooled analysis of two randomised trials. Br J Cancer. 2004;91:683–687. doi: 10.1038/sj.bjc.6602020. [DOI] [PMC free article] [PubMed] [Google Scholar]