Abstract
PURPOSE
The overexpression of cyclooxygenase 2 (COX-2) gene, also known as prostaglandin-endoperoxide synthase 2 (PTGS2), occurs in breast cancer, but whether it affects response to anticox drugs remains unclear. We investigated the relationships between PTGS2 expression, celecoxib use during neoadjuvant chemotherapy (NAC), and both event-free survival (EFS) and overall survival (OS).
MATERIALS AND METHODS
We analyzed a cohort of 156 patients with human epidermal growth factor receptor 2–negative breast cancer from the REMAGUS02 (ISRCTN Registry No. 10059974) trial with pretreatment PTGS2 expression data. Patients were treated by sequential NAC (epirubicin plus cyclophosphamide followed by docetaxel with or without celecoxib). Experimental validation was performed on breast cancer cell lines. The Cancer and Leukemia Group B (CALGB) 30801 (ClinicalTrials.gov identifier: NCT01041781) trial that tested chemotherapy with or without celecoxib in patients with lung cancer served as an independent validation cohort.
RESULTS
After 94.5 months of follow-up, EFS was significantly lower in the celecoxib group (hazard ratio [HR], 1.7; 95% CI, 1 to 2.88; P = .046). A significant interaction between PTGS2 expression and celecoxib use was detected (Pinteraction = .01). In the PTGS2-low group (n = 100), EFS was lower in the celecoxib arm (HR, 3.01; 95% CI, 1.45 to 6.24; P = .002) than in the standard treatment arm. Celecoxib use was an independent predictor of poor EFS, distant relapse–free survival, and OS.
Celecoxib in addition to docetaxel enhanced cell viability in PTGS2-low cell lines but not in PTGS2-high cell lines. In CALGB 30801, a trend toward poorer progression-free survival was observed in the patients with low urinary metabolite of prostaglandin E2 who received celecoxib (HR = 1.57; 95% CI, 0.87 to 2.84; P = .13).
CONCLUSION
Celecoxib use during chemotherapy adversely affected survival in patients with breast cancer, and the effect was more marked in PTGS2-low and/or estrogen receptor–negative tumors. COX-2 inhibitors should preferably be avoided during docetaxel use in patients with breast cancer who are undergoing NAC.
INTRODUCTION
Cyclooxygenase-2 (COX-2; also known as PTGS2 [prostaglandin-endoperoxide synthase 2]) is an isoform of the key enzyme in eicosanoid biosynthesis PTGS, which catalyzes the rate-limiting step in prostaglandin synthesis. COX-2 overexpression has been observed in various malignant tumors, including lung,1 colon,2 and breast3,4 cancers. Preclinical studies have shown that COX-2 overexpression and the resulting production of prostaglandins stimulated angiogenesis and proliferation, which promoted cell invasion and metastasis development.5,6 High COX-2 levels are associated with poor outcome in many tumor models and clinical studies.7-9 However, there is no consensus about the prognostic or predictive value of COX-2 expression in invasive breast carcinoma.10-12
The selective COX-2 inhibitor celecoxib was released onto the market in 2000 for the symptomatic treatment of arthritis. Celecoxib binds reversibly to a hydrophilic pocket near the active site of COX-2 and thus inhibits the conversion of arachidonic acid to prostaglandin H2. This results in anti-inflammatory and pain-relieving effects. Selective COX-2 inhibitors have also been explored as therapeutic or preventive agents in various oncologic settings.13,14 Several studies have evaluated celecoxib in the neoadjuvant setting for breast cancer as a monotherapy15,16 or combined with endocrine therapy.17,18 In addition to toxicity and safety concerns, the benefits of such strategies to patients with breast cancer were not sufficiently high for these agents to be incorporated into standard care, and the development of COX-2 inhibitors in oncology thus fell short of initial expectations.19,20
The REMAGUS02 (ISRCTN Registry No. 10059974) study was a multicenter, randomized, phase II trial that included 340 patients with locally advanced breast cancer. Patients were randomly assigned to receive neoadjuvant sequential chemotherapy (NAC; either epirubicin plus cyclophosphamide, followed by docetaxel alone or docetaxel plus celecoxib [400 mg twice per day orally] for human epidermal growth factor receptor 2 (HER2)–negative tumors [n = 220]; or docetaxel alone or docetaxel plus trastuzumab for HER2-positive tumors [n = 120]). The trial found no benefit of celecoxib in terms of pathologic complete response21 (primary objective) or disease-free survival22 (DFS; secondary objective).
Predictive biomarkers are biologic indicators of the likely response of a patient to a particular drug. Estrogen receptor (ER), progesterone receptor, and HER2 status, which are used to determine the potential benefits of endocrine and trastuzumab treatments, are currently the only predictive markers used in clinical settings in breast cancer. However, many patients still do not respond to these therapies, and the identification of additional biomarkers to provide personalized treatment to population subgroups remains an important task in breast oncology.
In this study, we investigated the dependence of the effects of celecoxib on COX-2 expression by performing a post hoc exploratory analysis of the REMAGUS02 trial to evaluate survival as a function of PTGS2 expression, as assessed by reverse transcription quantitative polymerase chain reaction (RT-qPCR). We validated our findings experimentally on breast cancer cell lines, and we performed analyses in an independent cohort of patients with non–small-cell lung cancer (NSCLC) from the Cancer and Leukemia Group B (CALGB) 30801 (ClinicalTrials.gov identifier: NCT01041781).
MATERIALS AND METHODS
Patients
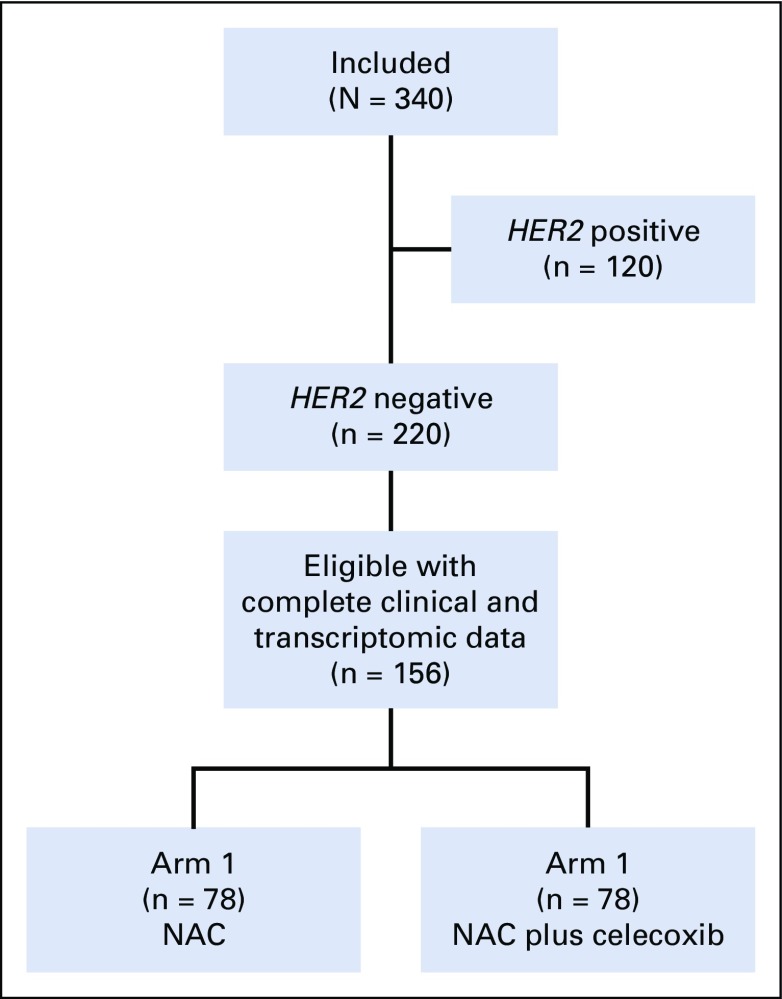
In total, 220 patients with locally advanced breast cancer were included in the HER2-negative stratum of the REMAGUS02 phase II randomized trial. The patients received sequential chemotherapy with, first, epirubicin plus cyclophosphamide alone followed by docetaxel with or without celecoxib 400 mg administered twice per day orally with random assignment to arm 1 (without celecoxib) or arm 2 (with celecoxib), as previously described.21,22 The full protocol (REMAGUS02 protocol; Appendix, online only), CONSORT diagram (Appendix Fig A1, online only), and results of the clinical trial (REMAGUS02 trial; Appendix) are provided. The use of celecoxib was suspended by the French Health Products Safety Agency from December 2004 to September 2005 because of safety concerns. Thereafter, the use of this agent was authorized but with a revision of the informed consent form. As a result, 13 patients randomly assigned to the celecoxib group did not receive this drug. Analyses of the results of this study were performed on an intention-to-treat basis and per-protocol analyses are provided in the Appendix. For the 220 patients who were randomly assigned, 156 (71%) had frozen pretreatment biopsy specimens that contained more than 30% invasive epithelial tumor cells and that were available for RT-qPCR analysis (raw data in Data Supplement). Among them, 139 patients had Affymetrix U133A chips (Thermo Fisher Scientific, Waltham, MA) with baseline gene expression data available (standard treatment, n = 72; celecoxib, n = 67).
PTGS2 (COX-2) Expression
Total RNA extraction from frozen pretreatment biopsy specimens, reverse transcription, and qPCR analysis and quality control were performed as previously described.23,24 The RPLPO, TATA box-binding protein (TBP), transferrin receptor (TFR), beta-actin, beta-glucuronidase (GUS), and GAPDH genes were used as endogenous reference genes. Target quantities were normalized relative to the median value for the six reference genes. No consensus threshold has been defined for RT-qPCR analyses, so PTGS2 gene expression was classified on the basis of tertiles (low, intermediate, and high). The odds ratios (ORs) for pathologic complete response of tertiles 1 (OR, 1; four [7.7%] of 52); and 2 (OR, 0.77; three [6%] of 50) were essentially similar (v OR, 4.22; 13 [26%] of 50 for tertile 3), so we chose to merge those two tertiles (PTGS2-low) and compare them with the third tertile (PTGS2-high), as previously described.24
Statistical Analysis
To investigate if tumors were different between the celecoxib and noncelecoxib arms, we performed a differential expression analysis between the two groups of treatment (Appendix). Event-free survival (EFS) was defined as the time from random assignment to progression, locoregional recurrence, distant recurrence, or death, whichever occurred first. Distant relapse–free survival (DRFS) was defined as the time from random assignment to first distant metastasis or death; overall survival (OS) was defined as the time from random assignment to death. Patients for whom none of these events was recorded were censored at the date of last known contact. The cutoff date for the analysis was May 1, 2015. Predictive effects were evaluated with a test of interaction between treatment group and PTGS2 expression and ER status. EFS and OS were estimated using the Kaplan-Meier method, and survival curves were compared using a log-rank test. Univariable Cox proportional hazard models were performed to determine the variables associated with survival. Covariables selected for the multivariable analysis were those with P values no greater than .15 after univariable analysis. A multivariable model was then implemented using a forward stepwise selection procedure. Analyses were performed with R software, version 3.1.2.
Experimental Validation and Independent Human Validation Cohort
We performed an experimental validation on two PTGS2-low breast cancer cell lines (MDA-MB-231 and MDA-MB-157), and two PTGS2-high cell lines (BT549 and MDA-MB-436; Appendix). Cell lines were treated with increasing concentrations of docetaxel with or without celecoxib 25 µM. Cellular viability was assessed at 72 hours. Statistical analyses were performed using GraphPad Prism 5 software (GraphPad Software, San Diego, CA). The data were expressed as the mean and standard error of the mean (SEM). One-way analyses of variance followed by Bonferroni post hoc comparison tests were performed in all statistical analyses. The results were considered statistically significant at a P < .05, P < .01, or P < .001. To confirm our results, we also performed a post hoc reanalysis of the CALGB 30801 trial,25 in which 312 patients with advanced NSCLC were randomly assigned to receive celecoxib or placebo in addition to standard chemotherapy. We stratified the analyses by the expression levels of the urinary after they were stratified by the expression levels of the urinary metabolite of prostaglandin E2 (PGE-M; Appendix).
RESULTS
Analyses of the REMAGUS02 Trial
Patient population.
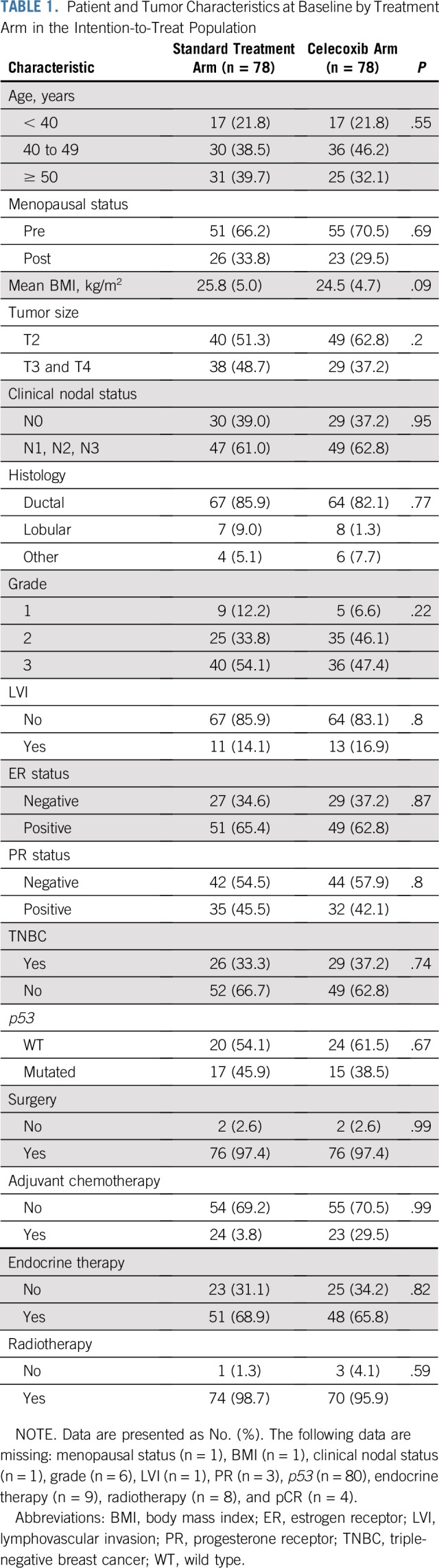
In total, 156 patients from the REMAGUS02 trial were included in this study; 78 were randomly assigned to the celecoxib arm, and 78 were randomly assigned to the arm with standard treatment only. Patient and tumor baseline characteristics were similar in the celecoxib and standard treatment arms (Table 1). In addition, no gene of 19,965 was differentially expressed between the celecoxib arm and the standard treatment arm, consistent with the random allocation of patients to the celecoxib arm.
TABLE 1.
Patient and Tumor Characteristics at Baseline by Treatment Arm in the Intention-to-Treat Population
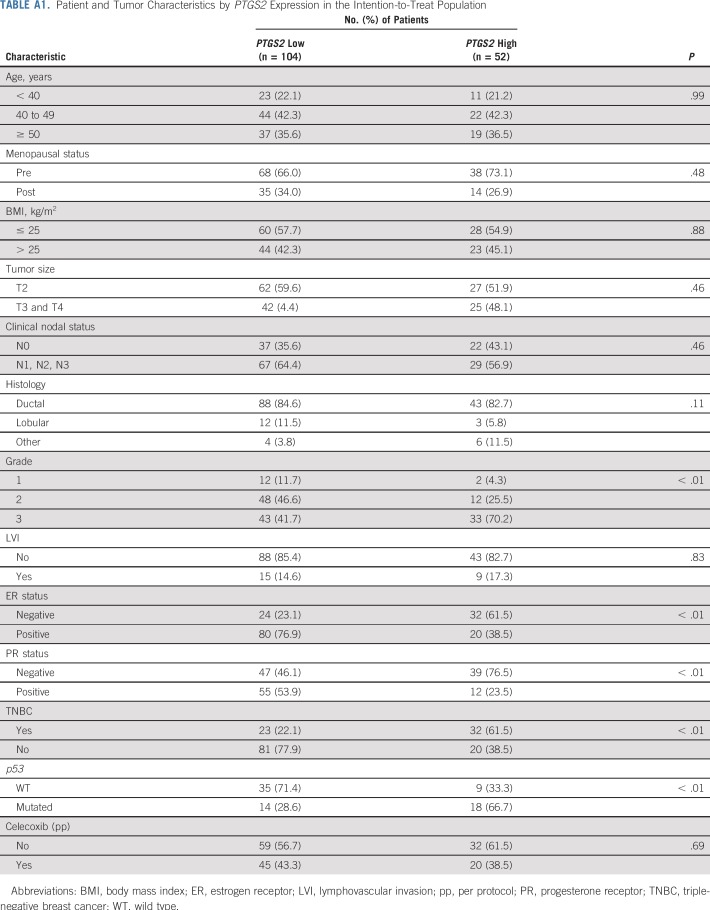
Notable differences in tumor characteristics according to PTGS2 status were observed. The frequencies of grade III, p53-mutated, ER-negative and progesterone receptor–negative tumors were higher in the PTGS2-high population than in the PTGS2-low population (Appendix Table A1, online only).
The Effect of Celecoxib on Survival is Modified by PTGS2 Expression and ER Status
EFS analysis.
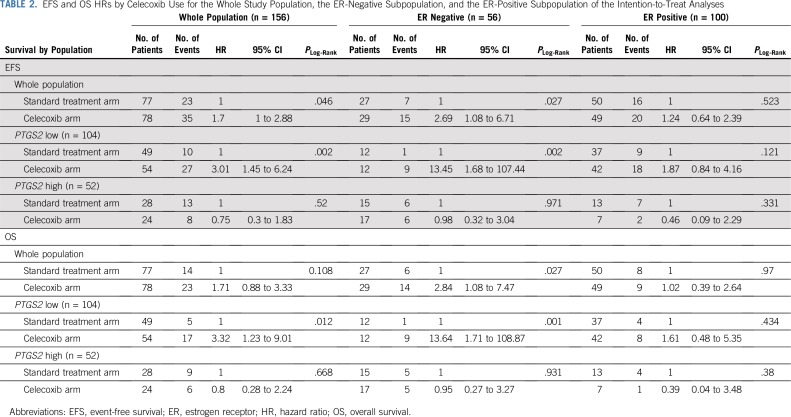
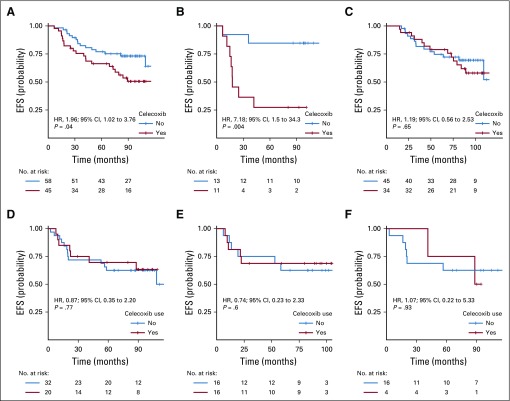
In the full study cohort of patients with HER2-negative disease (n = 156), celecoxib use was significantly associated with shorter EFS (hazard ratio [HR], 1.7; 95% CI, 1 to 2.88; P = .046; Table 2). There was a significant interaction between PTGS2 expression and celecoxib for EFS (Pinteraction = .01), which meant that the effect of celecoxib on EFS differed significantly between the PTGS2-low and PTGS2-high groups.
TABLE 2.
EFS and OS HRs by Celecoxib Use for the Whole Study Population, the ER-Negative Subpopulation, and the ER-Positive Subpopulation of the Intention-to-Treat Analyses
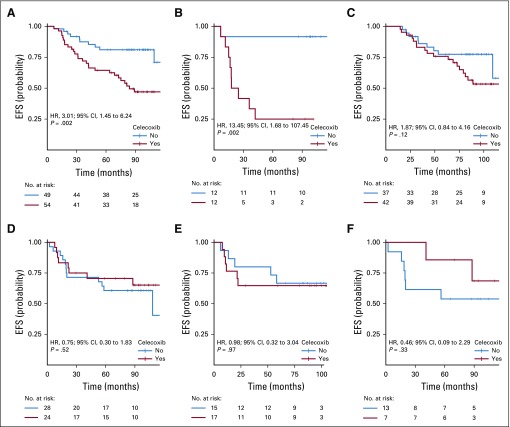
In the PTGS2-low group, celecoxib use was associated with shorter EFS (HR, 3.01; 95% CI, 1.45 to 6.24; P = .002; Fig 1A), and the obtained results differed by ER status. In ER-negative tumors, celecoxib use was strongly associated with shorter EFS (HR, 13.45; 95% CI, 1.68 to 107.44; P = .002; Fig 1B), whereas celecoxib had no effect on EFS in ER-positive tumors (HR, 1.87; 95% CI, 0.84 to 4.16; P = .121; Pinteraction = .02; Fig 1C). In the PTGS2-high group, celecoxib use did not affect EFS (Fig 1D) in either the ER-negative (Fig 1E) or ER-positive (Fig 1F) population.
FIG 1.
Kaplan-Meier curves for association between treatment arm and event-free survival (EFS), according to PTGS2 and estrogen receptor (ER) status: (A) PTGS2-low population; (B) PTGS2-low/ER-negative subpopulation; (C) PTGS2-low/ER-positive subpopulation; (D) PTGS2-high population; (E) PTGS2-high/ER-negative subpopulation; and (F) PTGS2-high/ER-positive subpopulation. HR, hazard ratio.
The association between celecoxib use and impaired EFS (P < .001), the interactions between celecoxib use and PTGS2 expression (P = .008), and the interactions between celecoxib use and ER status (P = .005) were highly significant after multivariable analysis (Appendix Table A2, online only). Similar results were also found for DRFS (data not shown).
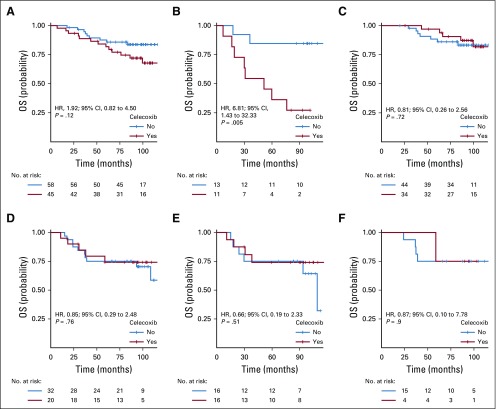
OS analyses.
Similar results were obtained for OS (Table 2). In the PTGS2-low group, celecoxib use was associated with a shorter OS (HR, 3.32; 95% CI, 1.23 to 9.01; P = .012; Appendix Fig A2A, online only), and its effects differed according to ER status (Pinteraction = .05). Celecoxib use was associated with a shorter OS in ER-negative tumors (HR, 13.64; 95% CI, 1.71 to 108.87; P = .001; Appendix Fig A2B) but had no significant effect on OS in ER-positive tumors (HR, 1.61; 95% CI, 0.48 to 5.35; P = .434; Appendix Fig A2C).
In the PTGS2-high group, celecoxib use had no effect on OS (Appendix Fig A2D) in the ER-negative population (Appendix Fig A2E) or in the ER-positive population (Appendix Fig A2F).
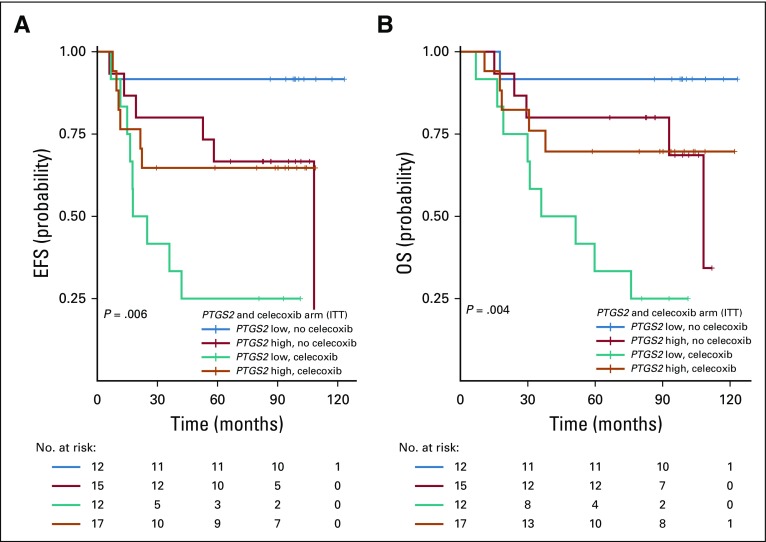
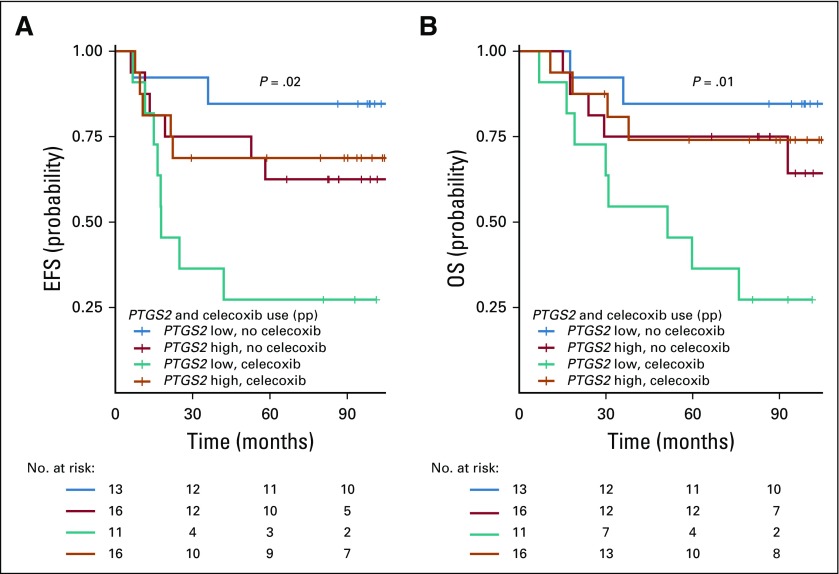
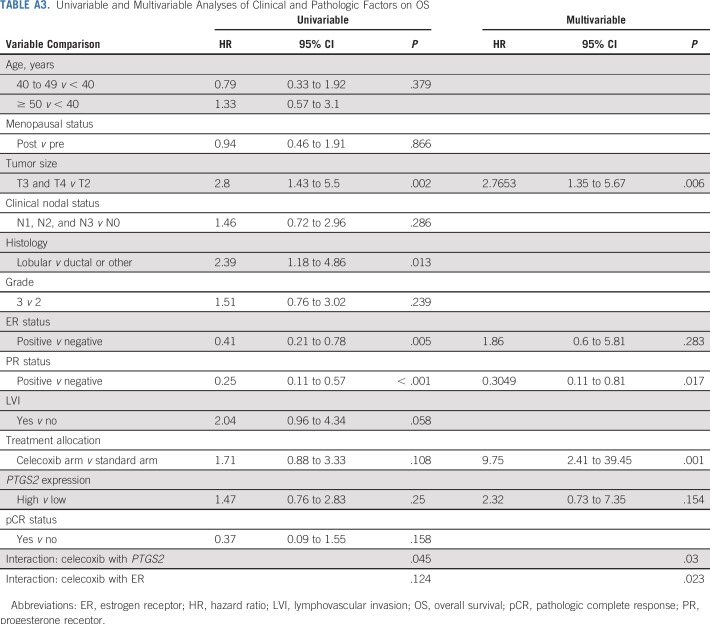
The association between celecoxib use and impaired OS (P = .001), the interactions between celecoxib use and PTGS2 expression (P = .03), and the interactions between celecoxib use and ER status (P = .02) were again significant after multivariable analysis (Appendix Table A3, online only). The combined Kaplan-Meier curves for EFS and OS as a function of PTGS2 expression and celecoxib use are shown for ER-negative tumors in Figure 2.
FIG 2.
Kaplan-Meier combined survival curves for the association between PTGS2 expression and treatment arm in the estrogen receptor (ER)–negative population. (A) Event-free survival (EFS) by PTGS2 expression and celecoxib use; (B) overall survival (OS) by PTGS2 expression and celecoxib use. ITT, intention to treat.
Per-protocol analyses.
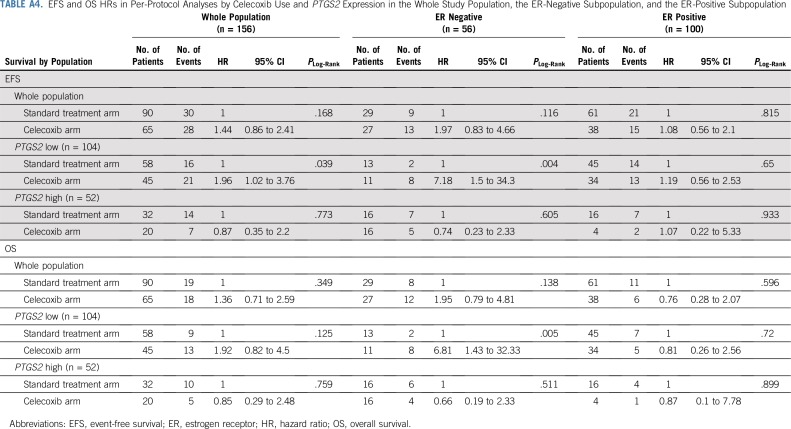
Analyses of this study on a per-protocol basis showed comparable results that are provided in the Appendix (Appendix Table A4, online only; Appendix Figs A3, A4, and A5, online only).
Experimental validation.
The addition of celecoxib to docetaxel enhances cell viability in PTGS2-low but not in PTGS2-high breast cancer cell lines.
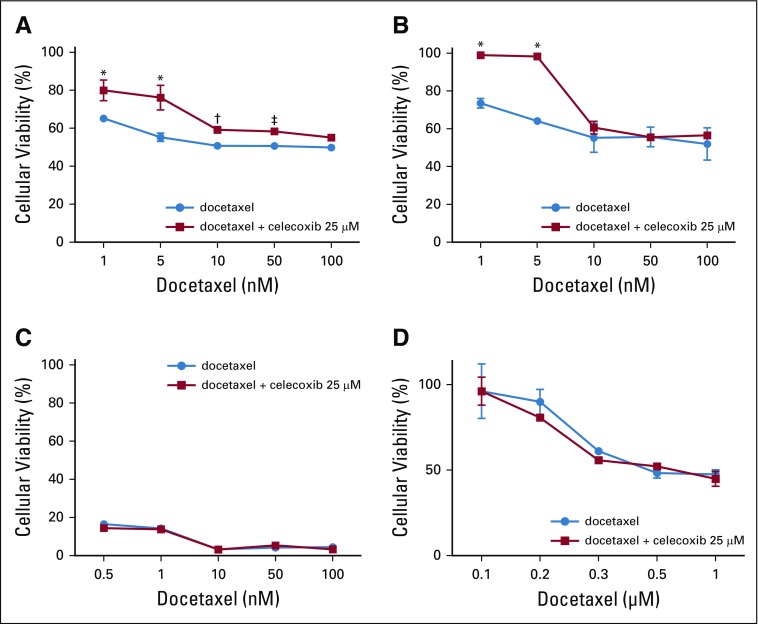
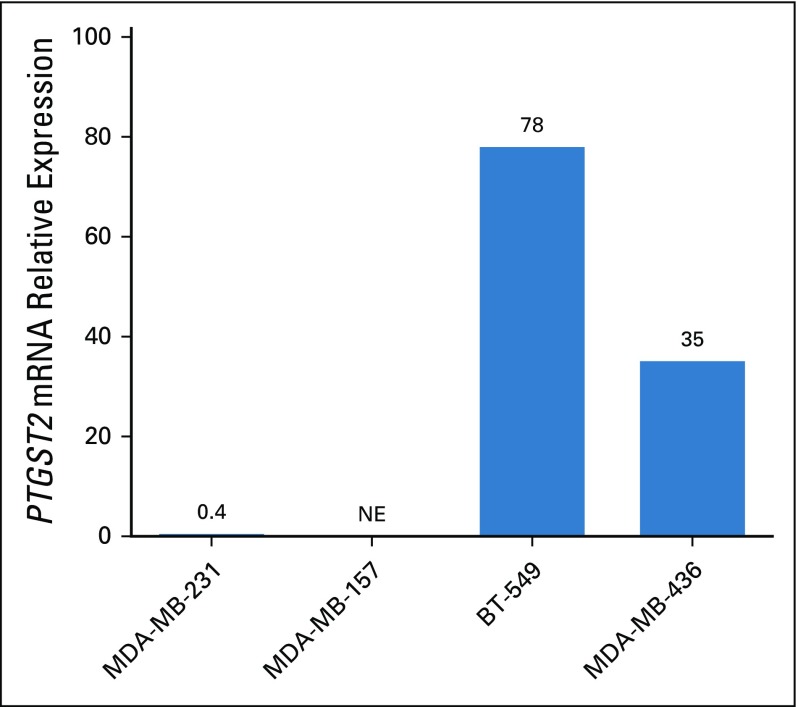
To assess whether preclinical models would mimic the clinical observations, we performed translational research by studying a panel of four ER-negative and HER2-negative breast cancer cell lines. PTGS2 expression was very low in MDA-MB-231 and MDA-MB-157, whereas it was high in BT549 and MDA-MB-436 (Appendix Fig A6, online only). In all four triple-negative breast cancer cell lines, celecoxib alone (5 to 200 µM) had no effect on cellular viability (data not shown).
In the PTGS2-low cell lines (MDA-MB-231 and MDA-MB-157), addition of celecoxib enhanced cellular viability compared with docetaxel treatment alone (Figs 3A and 3B). In PTGS2-high cell lines (BT549 and MDA-MB-436), celecoxib in association with docetaxel had no effect on cellular viability (Figs 3C and 3D). These cell culture results therefore match the clinical observations and suggest the following: (1) The effect of celecoxib in addition to chemotherapy varies with the expression levels of PTGS2, and this effect is restricted to PTGS2-low cell lines. (2) In PTGS2-low cell lines, the addition of celecoxib to taxanes enhances cellular viability compared with taxanes alone.
FIG 3.
Effect of docetaxel alone or in combination with celecoxib on cellular viability in PTGS2-low cell lines (A) MDA-MB-231 and (B) MDA-MB-157 as well as PTGS2-high cell lines (C) BT549 and (D) MDA-MB-436. (*) P < .001; (†) P < .01; (‡) P < .05.
Analyses of the CALGB 30801 trial.
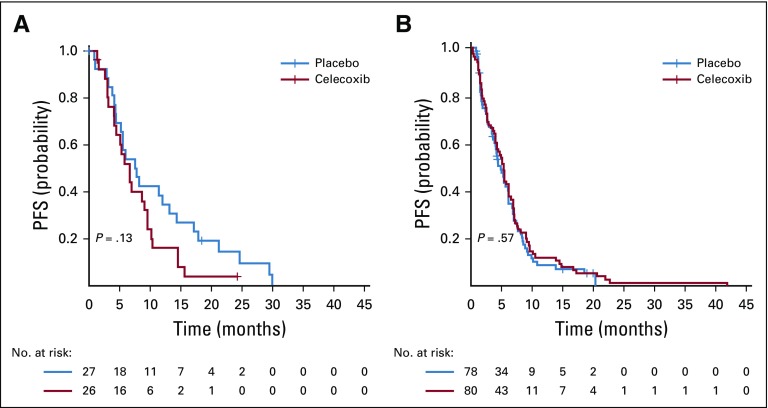
The effect of celecoxib in addition to chemotherapy is associated with a trend toward an impaired progression-free survival in patients with NSCLC who have low values of PGE-M.
In the population of the CALGB 30801 trial with metabolite of prostaglandin E2 (PGE-M) data available, the addition of celecoxib to chemotherapy had no impact on PFS (celecoxib v no celecoxib: HR, 1.08; 95% CI, 0.85 to 1.36; P = .53). In the population with PGE-M values less than quartile 1 (Q1), celecoxib in addition to chemotherapy was associated with a trend toward impaired progression-free survival (PFS) compared with chemotherapy alone (HR, 1.57; 95% CI, 0.87 to 2.84; P = .13). In contrast, for the population with PGE-M values of Q1 or greater, the addition of celecoxib to chemotherapy was not associated with differences in PFS (HR, 0.91; 95% CI, 0.66 to 1.26; P = .57; Appendix Figs A7A and A7B, respectively, online only).
DISCUSSION
In this exploratory analysis of the REMAGUS02 trial, we report an adverse effect of celecoxib use during NAC on survival in patients with breast cancer. The magnitude of this effect was greater in patients with either PTGS2-low tumors or ER-negative tumors, and it was particularly dramatic in the subgroup of patients with ER-negative and PTGS2-low tumors. One might have expected COX-2 inhibitors to act preferentially on tumors cells that express COX-2. Instead, we identified a paradoxical effect on cells with a low expression of PTGS2. The clinical observation was reproduced experimentally by performing translational research in four different breast cancer cell lines. Importantly, this effect was observed only in combination with taxanes and not with celecoxib alone. These results are particularly important because despite the evidence of a potential protective effect of nonsteroidal anti-inflammatory drugs (NSAIDs) against breast cancer in preclinical and epidemiologic data, no randomized trial, to our knowledge, has investigated the addition of any NSAID to NAC in breast cancer. Previous or unpublished randomized trials have been designed using celecoxib alone,26 but evidence is still lacking for the effects of celecoxib in addition to NAC in humans.27 We also found a trend toward a similar effect in an independent cohort derived from a randomized clinical trial, in a different setting, and in another cancer localization (advanced NSCLC).
These results raise concerns about the safety of COX-2 inhibitors during chemotherapy in patients with breast cancer. They are consistent with a recent study11 performed on a cohort of 911 patients with breast cancer, which identified an interaction among COX-2 expression, prognosis, and preoperative NSAID use (Pinteraction = .009). In that study, patients with preoperative NSAID treatment and COX-2-negative tumors had a significantly higher risk of events (HR, 4.51; P < .001) compared with the other patients.
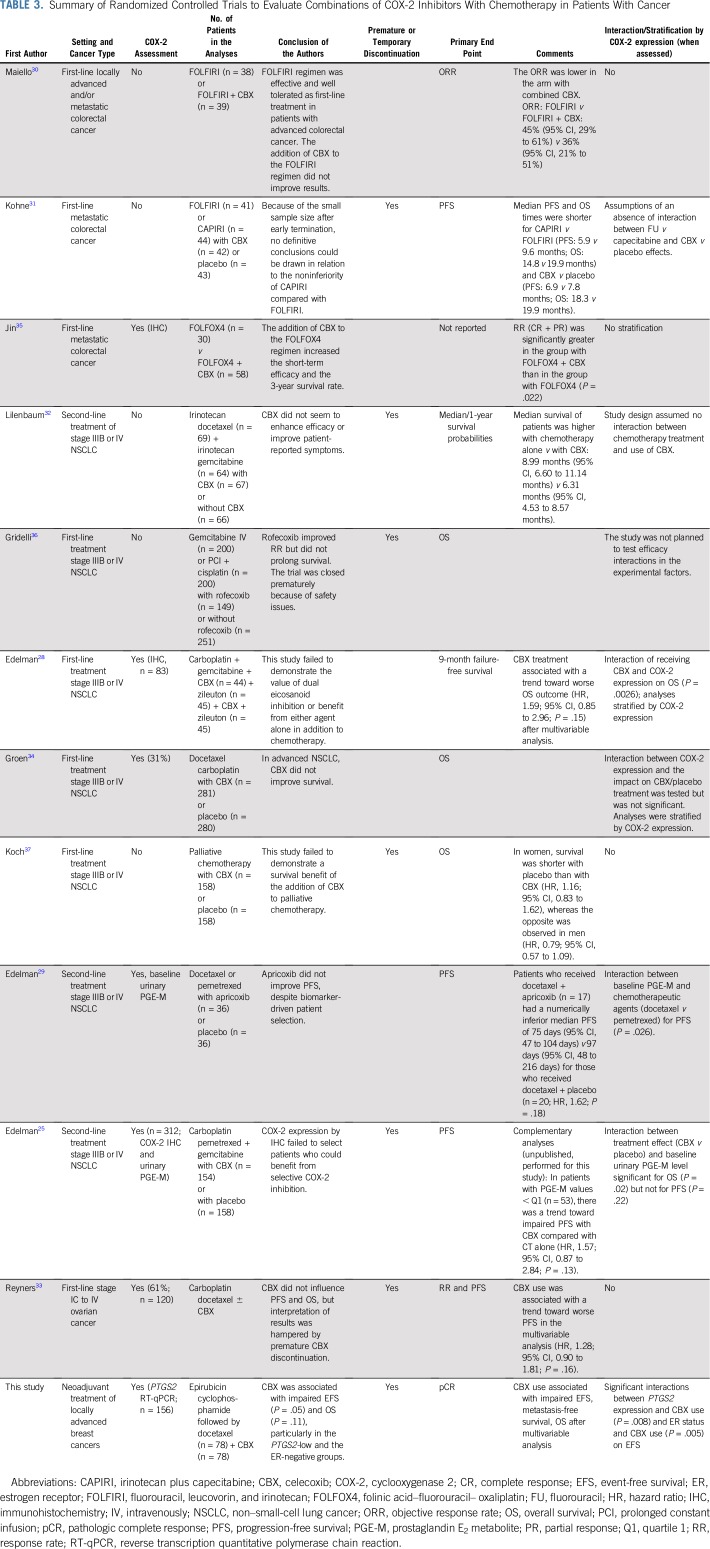
Furthermore, several randomized trials that investigated multiple COX-2 inhibitors in addition to chemotherapy for the treatment of different cancers have reported interactions25,28,29 among COX-2 inhibitor use, COX expression, chemotherapy regimen, and clinical outcome. The findings of these previous studies are listed in Table 3.28-37 In eight of these studies25,28-33 (including this study), COX-2 inhibitor use during chemotherapy was, or tended to be, associated with a poorer outcome than no COX-2 inhibitor use. Of note, seven trials were temporarily or prematurely discontinued because of safety concerns or enrollment failure, which may partially explain underpowered definitive analyses. Although several regimens were used, evidence that the combination of COX-2 inhibitor with chemotherapy might be detrimental was reported in four trials (including this study) that evaluated taxane-based chemotherapy regimens.29,32,33 Finally, only two of 12 randomized trials were stratified for COX-2 expression.28,34 The re-analysis of the 10 remaining trials after stratification by COX-2 expression could unmask a hidden deleterious or beneficial effects in specific subgroups.
TABLE 3.
Summary of Randomized Controlled Trials to Evaluate Combinations of COX-2 Inhibitors With Chemotherapy in Patients With Cancer
This study has limitations. The REMAGUS02 trial was a phase II randomized trial that was only designed to assess the efficacy of celecoxib in the whole population, but analyses stratified by ER or PTGS2 status were not prespecified. Hence, we cannot strictly infer causality for the negative association we report in the subpopulations. However, two arguments suggest that the relevance of these subgroup analyses is not spurious. First, the interactions between COX-2 inhibitors and COX-2 expression has already been demonstrated by multiple teams.25,28,29 Second, both ER status, which is a pivotal biomarker for any breast cancer trial, and PTGS2 (COX-2 expression), which is the very target of the drug tested (ie, celecoxib), have a strong biologic rationale to justify these subgroup analyses. Finally, we cannot derive any information on the safety profile of celecoxib in HER2-positive tumors because of the design of the REMAGUS02 trial (none of the patients with HER2-positive disease received celecoxib). The safety data in HER2-positive tumors could have been informative, because Subbaramaiah et al38 has reported that celecoxib can interrupt HER2 downstream signaling.
This study also has several strengths. As the only randomized trial, to our knowledge, to assess celecoxib in association with NAC in patients with breast cancer, the results show independent, significant, negative associations with EFS, DRFS, and OS, after a long follow-up, both in the intention-to-treat and the per-protocol analyses. A validation phase III trial specifically powered to confirm the deleterious impact of this drug in specified subgroups would be unethical. Thus, these data will remain unique for the foreseeable future. Finally, because there cannot and will not be a confirmatory trial to establish strict causality between celecoxib use during NAC for breast cancer and the risk of adverse outcome, physicians should apply caution and recommend alternatives to prescriptions of celecoxib in patients with ER-negative, HER2-negative breast cancer who are being treated with taxane-containing NAC.
This study has several implications: (1) Given the hypothesis-generating value of these findings, additional research should be performed and may include the post hoc reanalysis of randomized trials that evaluated COX-2 inhibitors in addition to chemotherapy after stratification by COX-2 expression. We also strongly recommend that investigators of clinical trials that evaluate COX-2 inhibitors should provide individual patient data that could be pooled into large meta-analyses. Such an effort is critical to reach robust evidence to derive routine recommendations about the avoidance or the safety of the routine prescription of COX-2 inhibitors during chemotherapy. (2) Evidence for synergy between COX inhibitors and checkpoint blockade immunotherapy is emerging.39,40 On the basis of our results, we recommend the stratification of all future trials that involve these inhibitors according to COX-2 expression status. (3) In the absence of other evidence, we recommend avoidance of celecoxib use and preference for alternative drugs in patients with ER-negative, HER2-negative breast tumors who are receiving docetaxel-containing NAC, unless the expected benefit greatly outweighs the potential risks. Only by carefully addressing these concerns will it be possible to determine the subgroups of patients most likely to benefit from COX inhibitors.
Appendix
Analysis of the REMAGUS02 Trial
Patients.
All of the patients included in this study were informed about the study in advance and gave written consent for participation in the trial and ancillary studies (ISRCTN Registry No. 10059974, French ethics committee Paris-Bicêtre, No. 03-55). The primary outcome measure of the trial was pathologic complete response (pCR), evaluated according to Chevallier criteria.21 The secondary outcome measures were the definition of genomic profiles of success (ie, pCR) or failure for each type of treatment, and these results have been published elsewhere, together with quality-control data.23
Samples.
In total, 156 samples from the 220 patients with breast cancer were available for transcriptomic analyses. The subgroup of 156 patients with available reverse transcription quantitative polymerase chain reaction (RT-qPCR) data did not differ from the remaining 64 patients of the HER2-negative population in terms of age, menopausal status, clinical tumor size, or nodal involvement. However, lobular and grade 1 and 2 tumors were overrepresented in the population without available transcriptome data relative to the population with available transcriptome available (26.6% v 9.6% [P = .004] and 58.3% v 49.3% [P = .03], respectively). Raw data for the patients are provided in the Data Supplement.
Statistical Analysis
Differential expression analysis.
Of 156 patients with RT-qPCR available for PTGS2 expression, 139 had Affymetrix U133A chips (Thermo Fisher Scientific, Waltham, MA) available for analysis. We performed a differential analysis by comparing the mean gene expression of each group according to treatment arm (celecoxib v no celecoxib) using a linear model (limma R package) and retained as differentially expressed genes those for which the mean expression was different with a P value of .05 or lower. The analysis was performed in the whole population and after stratification by PTGS2 status (PTGS2-low, n = 93; PTGS2-high, n = 46).
Experimental Validation
Cell lines.
Human breast cancer cell lines BT-549, MDA-MB-436, MDA-MB-231, and MDA-MB-157 were obtained from the American Type Culture Collection (ATCC, Manassas, VA). The cell lines were authenticated every 20 passages using the GenePrint 10 system kit (B9510; Promega, Madison, WI). All cell lines were cultured in RPMI-1640 medium or DMEM (Thermo Fisher Scientific, Waltham, MA) supplemented with 10% fetal bovine serum (Thermo Fisher Scientific) and 1% antibiotics (penicillin 50 μg/mL, streptomycin 50 μg/mL, neomycin 100 μg/mL; Thermo Fisher Scientific), at 37°C in a humidified atmosphere that contained 5% CO2.
Drugs.
Docetaxel was purchased from Téva laboratory (Courbevoie, France). Celecoxib was purchased from Biogaran laboratory (Colombes, France) and was dissolved in phosphate-buffered saline.
Viability assay.
A total of 8,000 cells per well were seeded in P96 plates and allowed to adhere for 24 hours at 37°C. Cells were then treated with various concentrations of chemotherapeutic agents and/or celecoxib for 72 hours. Cellular proliferation was measured using the MTS (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium) assay according to the manufacturer’s instructions (Promega, Madison, WI). Absorbance was measured at 490 nm on a 96-well microplate reader (Dynatech Laboratories MRX, Chantilly, VA).
Experimental plan.
We assessed the in vitro antitumor activity of celecoxib in combination with chemotherapeutic agents on the triple-negative breast cancer cell lines. MDA-MB-231 and MDA-MB-157 were defined by qRT-PCR as PTGS2-low cell lines; BT549 and MDA-MB-436, as PTGS2-high cell lines. To this end, we evaluated cellular viability under increasing concentrations of docetaxel in combination or not with celecoxib 25µM.
Analyses of the Cancer and Leukemia Group P 30801 trial.
To confirm our results, we performed a post hoc reanalysis of the Cancer and Leukemia Group B 30801 Alliance trial.21 In this trial, 312 patients with in advanced non–small-cell lung cancer—stage IIIB with pleural effusion or stage IV—were randomly assigned to receive celecoxib or placebo in addition to standard chemotherapy. Only patients with a COX-2 index of two or greater were registered and randomly assigned to treatment. Urinary metabolite of prostaglandin E2 (PGE2), hereafter designated PGE-M, was evaluated at the baseline and on day 8 of the first cycle in 211 patients in the study. Patients were evenly divided into four groups (quartiles) that were based on the quantity of urinary PGE-M at baseline (Q1, 10.09; Q2, 15.38; and Q3, 27.86 ng/mg creatinine). Progression-free survival was analyzed according to celecoxib addition, and the Q1 cutoff was used for PGE-M (PGE-M < Q1 v PGE-M ≥ Q1). Kaplan-Meier curves were used for survival analysis, and the log-rank test was used to assess differences in progression-free survival between PGE-M–defined patient groups.
Per-Protocol Analyses Results: Survival Analyses—The Effect of Celecoxib on Survival is Modified by PTGS2 Expression and Estrogen Receptor Status
Event-free survival analysis.
In the PTGS2-low group, celecoxib use was associated with poorer event-free survival (EFS; hazard ratio [HR], 1.96; 95% CI, 1.02 to 3.76; P = .039; 8-year EFS: 50.5% [95% CI, 37.3% to 68.4%] v 73.1% [95% CI, 62.3% to 85.8%]; Appendix Table A4, online only; Appendix Fig A3A, online only), but the obtained results differed according to ER status (Pinteraction = .011). In ER-negative tumors, celecoxib use was associated with poor EFS (HR, 7.18; 95% CI, 1.5 to 34.3; P = .004, Appendix Fig A3B, online only), whereas it had no such effect on EFS in ER-positive tumors (P = .65; Appendix Fig A3C, online only). In the PTGS2-high group, celecoxib use was not associated with EFS (Appendix Fig A3D, online only), in either the ER-negative (Appendix Fig A3E, online only) or the ER-positive (Appendix Fig A3F, online only) population.
Overall survival analysis.
Similar results were obtained for overall survival (OS; Appendix Table A3, online only; Appendix Figs A4A through A4F, online only). Celecoxib use was associated with poor OS in the PTGS2-low/ER-negative subgroup (HR, 6.81; 95% CI, 1.43 to 32.33; P = .005; 8-year OS, 27.3% [95% CI, 10.4% to 71.6%] v 84.6% [95% CI, 67.1% to 100%]; Appendix Fig A3B online only) but not in the PTGS2-low/ER-positive subgroup (HR, 0.81; 95% CI, 0.26 to 2.56; P = .72; Pinteraction(celecoxib/ER status) = .02; Appendix Fig A4C online only). Finally, Appendix Figure A5 shows the Kaplan-Meier curves for EFS and OS according to PTGS2 expression and the effect of celecoxib in ER-negative tumors in per-protocol analyses.
FIG A1.
Study flow diagram of included patients and tumors samples available for reverse transcription quantitative polymerase chain reaction analysis in the REMAGUS02 (ISRCTN Registry No. 10059974) biologic trial. NAC (neoadjuvant chemotherapy [epirubicin + cyclophosphamide followed by docetaxel]); HER2, human epidermal growth factor receptor 2.
FIG A2.
Kaplan-Meier curves for association between treatment arm (intention-to-treat analyses) and overall survival (OS) according to PTGS2 expression and estrogen receptor (ER) status: (A) PTGS2-low population; (B) PTGS2-low/ER-negative subpopulation; (C) PTGS2-low/ER-positive subpopulation; (D) PTGS2-high population; (E) PTGS2-high/ER-negative subpopulation; and (F) PTGS2-high/ER-positive subpopulation. HR, hazard ratio.
FIG A3.
Kaplan-Meier curves for association between treatment arm (per-protocol analyses) and event-free survival (EFS) according to PTGS2 and estrogen receptor (ER) status: (A) PTGS2-low population; (B) PTGS2-low/ER-negative subpopulation; (C) PTGS2-low/ER-positive subpopulation; (D) PTGS2-high population; (E) PTGS2-high/ER-negative subpopulation; and (F) PTGS2-high/ER-positive subpopulation. HR, hazard ratio.
FIG A4.
Kaplan-Meier curves for association between treatment arm (per-protocol analyses) and overall survival (OS) according to PTGS2 expression and estrogen receptor (ER) status: (A) PTGS2-low population; (B) PTGS2-low/ER-negative subpopulation; (C) PTGS2-low/ER-positive subpopulation; (D) PTGS2-high population; (E) PTGS2-high/ER-negative subpopulation; and (F) PTGS2-high/ER-positive subpopulation. HR, hazard ratio.
FIG A5.
Kaplan-Meier combined survival curves for the association between PTGS2 expression and treatment arm (per-protocol analyses) in the estrogen receptor (ER)–negative population: (A) event-free survival (EFS) by PTGS2 expression and celecoxib use; (B) overall survival (OS) by PTGS2 expression and celecoxib use. pp, per protocol.
FIG A6.
PTGS2 expression by reverse transcription quantitative polymerase chain reaction analysis in four triple-negative breast cancer cell lines (MDA-MB-231; MDA-MB-157; BT549; and MDA-MB-436). NE, not expressed.
FIG A7.
Kaplan-Meier curves for association between treatment arm and progression-free survival (PFS) in the Cancer and Leukemia Group B 30801 Alliance trial: (A) population with metabolite prostaglandin E2 (PGE-M) values less than Q1 (less than first quartile); and (B) population with PGE-M values of Q1 or greater (≥ first quartile).
TABLE A1.
Patient and Tumor Characteristics by PTGS2 Expression in the Intention-to-Treat Population
TABLE A2.
Univariable and Multivariable Analyses of Clinical and Pathologic Factors on EFS
TABLE A3.
Univariable and Multivariable Analyses of Clinical and Pathologic Factors on OS
TABLE A4.
EFS and OS HRs in Per-Protocol Analyses by Celecoxib Use and PTGS2 Expression in the Whole Study Population, the ER-Negative Subpopulation, and the ER-Positive Subpopulation
Footnotes
A.-S.H. was supported by an Institut Thématique Multi-Organisme Cancer (ITMO)-Institut National de la Santé et de la Recherche Médicale (INSERM)-Alliance pour les sciences de la vie et de la santé (AVIESAN) cancer translational research grant.
This study was supported by a grant of the French Programme Hospitalier de Recherche Clinique (No. ISRCTN10059974, PHRC: AOM 02 11) and by unrestricted grants from Pfizer France, Roche Pharmaceutical, and Sanofi.
A.-S.H. was a part of the ANR-10-IDEX-0001-02 PSL, the ANR-11-LABX-0043, and the INCa-DGOS-Inserm 12554 units.
AUTHOR CONTRIBUTIONS
Conception and design: Anne-Sophie Hamy, Jean-Yves Pierga, Michel Marty, Bernard Asselain
Collection and assembly of data: Anne-Sophie Hamy, Sandrine Tury, Xiaofei Wang, Junheng Gao, Jean-Yves Pierga, Barbara Pistilli, Marc Espié, Brice Aouchiche
Provision of study material or patients: Jean-Yves Pierga, Sylvie Giacchetti, Etienne Brain, Michel Marty, Marick Laé, Fabien Reyal
Data analysis and interpretation: Anne-Sophie Hamy, Sandrine Tury, Xiaofei Wang, Junheng Gao, Jean-Yves Pierga, Sylvie Giacchetti, Etienne Brain, Gabriel Benchimol, Enora Laas, Marick Laé Bernard Asselain, Martin Edelman, Fabien Reyal
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Celecoxib With Neoadjuvant Chemotherapy for Breast Cancer Might Worsen Outcomes Differentially by COX-2 Expression and ER Status: Exploratory Analysis of the REMAGUS02 Trial
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Jean-Yves Pierga
Honoraria: Amgen, Illumina
Consulting or Advisory Role: Roche, Novartis, Pfizer, Lilly, AstraZeneca, Puma Biotechnology, Celltrion, Sandoz, Ipsen
Research Funding: Roche (Inst), Servier (Inst), Janssen Diagnostics (Inst)
Travel, Accommodations, Expenses: MSD Oncology, Roche, AstraZeneca, Pfizer
Sylvie Giacchetti
Consulting or Advisory Role: Roche, Eisai
Travel, Accommodations, Expenses: Roche, AstraZeneca
Etienne Brain
Honoraria: AstraZeneca, Roche, Pfizer, Mylan, Bristol-Myers Squibb
Consulting or Advisory Role: Puma Biotechnology, Pfizer, Clinigen Group, Bristol-Myers Squibb, Samsung
Research Funding: HalioDx (Inst)
Travel, Accommodations, Expenses: Roche, Pfizer, Mylan, Pierre Fabre, AstraZeneca
Barbara Pistilli
Consulting or Advisory Role: Puma Biotechnology
Research Funding: Pfizer (Inst), Puma Biotechnology (Inst), Merus (Inst)
Michel Marty
Honoraria: Sanofi, Roche, Genentech, Pfizer, Pierre Fabre
Consulting or Advisory Role: Sanofi, Roche, Pfizer, Pierre Fabre
Travel, Accommodations, Expenses: Roche, Genentech
Bernard Asselain
Honoraria: Bristol-Myers Squibb, Roche, AstraZeneca, Ipsen
Consulting or Advisory Role: Bristol-Myers Squibb, Roche, AstraZeneca, Ipsen
Brice Aouchiche
Employment: AbbVie (I)
Martin Edelman
Stock and Other Ownership Interests: Biomarker Strategies
Consulting or Advisory Role: Lilly, Bristol-Myers Squibb, Boehringer Ingelheim, WIndMIL, ARMO BioSciences, BerGenBio, Syndax
Research Funding: Bristol-Myers Squibb (Inst), Apexigen (Inst), Nektar (Inst)
Travel, Accommodations, Expenses: Bristol-Myers Squibb, ARMO BioSciences, BerGenBio
Other Relationship: AstraZeneca, MedImmune, Lilly, Boehringer Ingelheim, Takeda
Fabien Reyal
Honoraria: Agendia
Consulting or Advisory Role: Agendia
Travel, Accommodations, Expenses: Roche
No other potential conflicts of interest were reported.
REFERENCES
- 1.Zhou YY, Hu ZG, Zeng FJ, et al. Clinical profile of cyclooxygenase-2 inhibitors in treating non-small cell lung cancer: A meta-analysis of nine randomized clinical trials. PLoS One. 2016;11:e0151939. doi: 10.1371/journal.pone.0151939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arber N. Cyclooxygenase-2 inhibitors in colorectal cancer prevention: Point. Cancer Epidemiol Biomarkers Prev. 2008;17:1852–1857. doi: 10.1158/1055-9965.EPI-08-0167. [DOI] [PubMed] [Google Scholar]
- 3.Singh-Ranger G, Salhab M, Mokbel K. The role of cyclooxygenase-2 in breast cancer: Review. Breast Cancer Res Treat. 2008;109:189–198. doi: 10.1007/s10549-007-9641-5. [DOI] [PubMed] [Google Scholar]
- 4.Arun B, Goss P. The role of COX-2 inhibition in breast cancer treatment and prevention. Semin Oncol. 2004;31:22–29. doi: 10.1053/j.seminoncol.2004.03.042. [DOI] [PubMed] [Google Scholar]
- 5.Howe LR. Inflammation and breast cancer: Cyclooxygenase/prostaglandin signaling and breast cancer. Breast Cancer Res. 2007;9:210. doi: 10.1186/bcr1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Masferrer JL, Leahy KM, Koki AT, et al. Antiangiogenic and antitumor activities of cyclooxygenase-2 inhibitors. Cancer Res. 2000;60:1306–1311. [PubMed] [Google Scholar]
- 7.Denkert C, Winzer K-J, Müller B-M, et al. Elevated expression of cyclooxygenase-2 is a negative prognostic factor for disease-free survival and overall survival in patients with breast carcinoma. Cancer. 2003;97:2978–2987. doi: 10.1002/cncr.11437. [DOI] [PubMed] [Google Scholar]
- 8.Kim HS, Moon H-G, Han W, et al. COX2 overexpression is a prognostic marker for stage III breast cancer. Breast Cancer Res Treat. 2012;132:51–59. doi: 10.1007/s10549-011-1521-3. [DOI] [PubMed] [Google Scholar]
- 9.Ristimäki A, Sivula A, Lundin J, et al. Prognostic significance of elevated cyclooxygenase-2 expression in breast cancer. Cancer Res. 2002;62:632–635. [PubMed] [Google Scholar]
- 10.Chikman B, Vasyanovich S, Lavy R, et al. COX2 expression in high-grade breast cancer: Evidence for prognostic significance in the subset of triple-negative breast cancer patients. Med Oncol. 2014;31:989. doi: 10.1007/s12032-014-0989-1. [DOI] [PubMed] [Google Scholar]
- 11.Simonsson M, Björner S, Markkula A, et al. The prognostic impact of COX-2 expression in breast cancer depends on oral contraceptive history, preoperative NSAID use, and tumor size. Int J Cancer. 2017;140:163–175. doi: 10.1002/ijc.30432. [DOI] [PubMed] [Google Scholar]
- 12.Xu F, Li M, Zhang C, et al. Clinicopathological and prognostic significance of COX-2 immunohistochemical expression in breast cancer: A meta-analysis. Oncotarget. 2017;8:6003–6012. doi: 10.18632/oncotarget.13990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Evans JF, Kargman SL. Cancer and cyclooxygenase-2 (COX-2) inhibition. Curr Pharm Des. 2004;10:627–634. doi: 10.2174/1381612043453126. [DOI] [PubMed] [Google Scholar]
- 14.Regulski M, Regulska K, Prukała W, et al. COX-2 inhibitors: A novel strategy in the management of breast cancer. Drug Discov Today. 2015 doi: 10.1016/j.drudis.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 15.Tfayli A, Yang J, Kojouri K, et al. Neoadjuvant therapy with celecoxib to women with early-stage breast cancer. Neoplasma. 2008;55:122–126. [PubMed] [Google Scholar]
- 16.Aristarco V, Serrano D, Gandini S, et al. A randomized, placebo-controlled, phase II, presurgical biomarker trial of celecoxib versus exemestane in postmenopausal breast cancer patients. Cancer Prev Res (Phila) 2016;9:349–356. doi: 10.1158/1940-6207.CAPR-15-0311. [DOI] [PubMed] [Google Scholar]
- 17.Lustberg MB, Povoski SP, Zhao W, et al. Phase II trial of neoadjuvant exemestane in combination with celecoxib in postmenopausal women who have breast cancer. Clin Breast Cancer. 2011;11:221–227. doi: 10.1016/j.clbc.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Falandry C, Canney PA, Freyer G, et al. Role of combination therapy with aromatase and cyclooxygenase-2 inhibitors in patients with metastatic breast cancer. Ann Oncol. 2009;20:615–620. doi: 10.1093/annonc/mdn693. [DOI] [PubMed] [Google Scholar]
- 19.Gasparini G, Longo R, Sarmiento R, et al. Inhibitors of cyclo-oxygenase 2: A new class of anticancer agents? Lancet Oncol. 2003;4:605–615. doi: 10.1016/s1470-2045(03)01220-8. [DOI] [PubMed] [Google Scholar]
- 20.Csiki I, Johnson DH. Did targeted therapy fail cyclooxygenase too? J Clin Oncol. 2006;24:4798–4800. doi: 10.1200/JCO.2006.08.0622. [DOI] [PubMed] [Google Scholar]
- 21.Pierga J-Y, Delaloge S, Espié M, et al. A multicenter randomized phase II study of sequential epirubicin/cyclophosphamide followed by docetaxel with or without celecoxib or trastuzumab according to HER2 status, as primary chemotherapy for localized invasive breast cancer patients. Breast Cancer Res Treat. 2010;122:429–437. doi: 10.1007/s10549-010-0939-3. [DOI] [PubMed] [Google Scholar]
- 22.Giacchetti S, Hamy A-S, Delaloge S, et al. Long-term outcome of the REMAGUS 02 trial, a multicenter randomised phase II trial in locally advanced breast cancer patients treated with neoadjuvant chemotherapy with or without celecoxib or trastuzumab according to HER2 status. Eur J Cancer. 2017;75:323–332. doi: 10.1016/j.ejca.2017.01.008. [DOI] [PubMed] [Google Scholar]
- 23.de Cremoux P, Valet F, Gentien D, et al. Importance of pre-analytical steps for transcriptome and RT-qPCR analyses in the context of the phase II randomised multicentre trial REMAGUS02 of neoadjuvant chemotherapy in breast cancer patients. BMC Cancer. 2011;11:215. doi: 10.1186/1471-2407-11-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hamy AS, Bieche I, Lehmann-Che J, et al. BIRC5 (survivin): A pejorative prognostic marker in stage II/III breast cancer with no response to neoadjuvant chemotherapy. Breast Cancer Res Treat. 2016;159:499–511. doi: 10.1007/s10549-016-3961-2. [DOI] [PubMed] [Google Scholar]
- 25.Edelman MJ, Wang X, Hodgson L, et al. Phase III randomized, placebo-controlled, double-blind trial of celecoxib in addition to standard chemotherapy for advanced non–small-cell lung cancer with cyclooxygenase-2 overexpression: CALGB 30801 (Alliance) J Clin Oncol. 2017;35:2184–2192. doi: 10.1200/JCO.2016.71.3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Strasser-Weippl K, Higgins MJ, Chapman JW, et al. Effects of celecoxib and low-dose aspirin on outcomes in adjuvant aromatase inhibitor-treated patients: CCTG MA.27. J Natl Cancer Inst. 2018;110:1003–1008. doi: 10.1093/jnci/djy017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hawk E, Maresso KC, Brown P. NSAIDs to prevent breast cancer recurrence? An unanswered question. J Natl Cancer Inst. 2018;110:927–928. doi: 10.1093/jnci/djy049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Edelman MJ, Watson D, Wang X, et al. Eicosanoid modulation in advanced lung cancer: Cyclooxygenase-2 expression is a positive predictive factor for celecoxib + chemotherapy—Cancer and Leukemia Group B Trial 30203. J Clin Oncol. 2008;26:848–855. doi: 10.1200/JCO.2007.13.8081. [DOI] [PubMed] [Google Scholar]
- 29.Edelman MJ, Tan MT, Fidler MJ, et al. Randomized, double-blind, placebo-controlled, multicenter phase II study of the efficacy and safety of apricoxib in combination with either docetaxel or pemetrexed in patients with biomarker-selected non–small-cell lung cancer. J Clin Oncol. 2015;33:189–194. doi: 10.1200/JCO.2014.55.5789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maiello E, Giuliani F, Gebbia V, et al. FOLFIRI with or without celecoxib in advanced colorectal cancer: A randomized phase II study of the Gruppo Oncologico dell’Italia Meridionale (GOIM) Ann Oncol. 2006;17:vii55–vii59. doi: 10.1093/annonc/mdl952. [DOI] [PubMed] [Google Scholar]
- 31.Köhne C-H, De Greve J, Hartmann JT, et al. Irinotecan combined with infusional 5-fluorouracil/folinic acid or capecitabine plus celecoxib or placebo in the first-line treatment of patients with metastatic colorectal cancer: EORTC study 40015. Ann Oncol. 2008;19:920–926. doi: 10.1093/annonc/mdm544. [DOI] [PubMed] [Google Scholar]
- 32.Lilenbaum R, Socinski MA, Altorki NK, et al. Randomized phase II trial of docetaxel/irinotecan and gemcitabine/irinotecan with or without celecoxib in the second-line treatment of non–small-cell lung cancer. J Clin Oncol. 2006;24:4825–4832. doi: 10.1200/JCO.2006.07.4773. [DOI] [PubMed] [Google Scholar]
- 33.Reyners AKL, de Munck L, Erdkamp FLG, et al. A randomized phase II study investigating the addition of the specific COX-2 inhibitor celecoxib to docetaxel plus carboplatin as first-line chemotherapy for stage IC to IV epithelial ovarian cancer, fallopian tube or primary peritoneal carcinomas: The DoCaCel study. Ann Oncol. 2012;23:2896–2902. doi: 10.1093/annonc/mds107. [DOI] [PubMed] [Google Scholar]
- 34.Groen HJM, Sietsma H, Vincent A, et al. Randomized, placebo-controlled phase III study of docetaxel plus carboplatin with celecoxib and cyclooxygenase-2 expression as a biomarker for patients with advanced non–small-cell lung cancer: The NVALT-4 study. J Clin Oncol. 2011;29:4320–4326. doi: 10.1200/JCO.2011.35.5214. [DOI] [PubMed] [Google Scholar]
- 35.Jin C-H, Wang A-H, Chen J-M, et al. Observation of curative efficacy and prognosis following combination chemotherapy with celecoxib in the treatment of advanced colorectal cancer. J Int Med Res. 2011;39:2129–2140. doi: 10.1177/147323001103900609. [DOI] [PubMed] [Google Scholar]
- 36.Gridelli C, Gallo C, Ceribelli A, et al. Factorial phase III randomised trial of rofecoxib and prolonged constant infusion of gemcitabine in advanced non-small-cell lung cancer: the GEmcitabine-COxib in NSCLC (GECO) study. Lancet Oncol. 2007;8:500–512. doi: 10.1016/S1470-2045(07)70146-8. [DOI] [PubMed] [Google Scholar]
- 37.Koch A, Bergman B, Holmberg E, et al. Effect of celecoxib on survival in patients with advanced non-small cell lung cancer: a double blind randomised clinical phase III trial (CYCLUS study) by the Swedish Lung Cancer Study Group. Eur J Cancer Oxf Engl. 2011;1990 47:1546–1555. doi: 10.1016/j.ejca.2011.03.035. [DOI] [PubMed] [Google Scholar]
- 38.Subbaramaiah K, Norton L, Gerald W, et al. Cyclooxygenase-2 is overexpressed in HER2/neu-positive breast cancer: Evidence for involvement of AP-1 and PEA3. J Biol Chem. 2002;277:18649–18657. doi: 10.1074/jbc.M111415200. [DOI] [PubMed] [Google Scholar]
- 39.Hennequart M, Pilotte L, Cane S, et al. Constitutive IDO1 expression in human tumors is driven by cyclooxygenase-2 and mediates intrinsic immune resistance. Cancer Immunol Res. 2017;5:695–709. doi: 10.1158/2326-6066.CIR-16-0400. [DOI] [PubMed] [Google Scholar]
- 40.Zelenay S, van der Veen AG, Böttcher JP, et al. Cyclooxygenase-dependent tumor growth through evasion of immunity. Cell. 2015;162:1257–1270. doi: 10.1016/j.cell.2015.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]