Abstract
PURPOSE
Pegaspargase (PEG-ASP) has largely replaced native Escherichia coli asparaginase (L-ASP) in the treatment of acute lymphoblastic leukemia because of its longer half-life and lower immunogenicity. Risk factors for allergic reactions to PEG-ASP remain unclear. Here, we identify risk factors for reactions in a front-line acute lymphoblastic leukemia trial and assess the usefulness of serum antibodies for diagnosing allergy and predicting rechallenge outcome.
PATIENTS AND METHODS
PEG-ASP was administered to 598 patients in St Jude’s Total XVI study. Results were compared with Total XV study (ClinicalTrials.gov identifiers: NCT00549848 and NCT00137111), which used native L-ASP. Serum samples (n = 5,369) were analyzed for anti–PEG-ASP immunoglobulin G by enzyme-linked immunosorbent assay. Positive samples were tested for anti–polyethylene glycol (PEG) and anti–L-ASP. We analyzed potential risk factors for reactions and associations between antibodies and reactions, rechallenge outcomes, and PEG-ASP pharmacokinetics.
RESULTS
Grade 2 to 4 reactions were less common in the Total XVI study with PEG-ASP (81 [13.5%] of 598) than in the Total XV study with L-ASP (169 [41.2%] of 410; P = 1.4 × 10−23). For Total XVI, anti-PEG, not anti–L-ASP, was the predominant component of anti–PEG-ASP antibodies (96%). In a multivariable analysis, more intrathecal therapy (IT) predicted fewer reactions (P = 2.4 × 10−5), which is consistent with an immunosuppressant contribution of IT. Anti–PEG-ASP was associated with accelerated drug clearance (P = 5.0 × 10−6). Failure of rechallenge after initial reactions was associated with anti–PEG-ASP (P = .0078) and was predicted by the occurrence of angioedema with first reaction (P = .01).
CONCLUSION
Less IT therapy was the only independent clinical risk factor for reactions to PEG-ASP. PEG, and not L-ASP, is the major antigen that causes allergic reactions. Anti–PEG-ASP has utility in predicting and confirming clinical reactions to PEG-ASP as well as in identifying patients who are most likely to experience failure with rechallenge.
INTRODUCTION
Asparaginase is used to treat acute lymphoblastic leukemia (ALL) and non-Hodgkin lymphoma. It exploits the insufficiency of asparagine synthesis in lymphoid blasts by depleting asparagine.1 There is increasing interest in its use for treating disease other than leukemia—for example, breast cancer.2
PEGylation, conjugating drugs to polyethylene glycol (PEG), has been used widely in recent drug development.3 PEGylated drugs have longer half-lives and are less immunogenic. PEGylated Escherichia coli l-asparaginase (PEG-ASP) is replacing native E coli l-asparaginase (L-ASP) in ALL treatment regimens4,5 and causes less hypersensitivity.6-9 Nonetheless, reactions to PEG-ASP are not uncommon.10 These reactions are problematic because they cause morbidity11 and inadequate asparaginase activity,12 which necessitates the switch to Erwinia asparaginase (Erwinase), a formulation that requires frequent administration at great expense.13 It is often unclear whether clinical symptoms are indicative of true allergy or some other acute reaction. There is increasing recognition that PEG itself can serve as an allergen,14 but its contributions to PEG-ASP reactions remain poorly characterized, as do risk factors for allergy to PEG-ASP.
We evaluated serum antibodies to PEG-ASP, L-ASP, and PEG itself as well as their performance characteristics in identifying allergic reactions, predicting the success of rechallenge, and predicting asparaginase pharmacokinetics in a front-line clinical trial, the Total XVI study. We also evaluated other risk factors for allergy and compared findings with the predecessor trial Total XV, which used L-ASP instead of PEG-ASP (Data Supplement).
PATIENTS AND METHODS
Patients and Treatment
Children (N = 598) with newly diagnosed ALL were enrolled in St Jude Children’s Research Hospital Total XVI protocol (TXVI) study from September 2007 to March 2017 and were evaluable for reactions to PEG-ASP. Administration of PEG-ASP (as Oncaspar; Enzon Pharmaceuticals, Cranford, NJ) and intrathecal therapy (IT) was confirmed in the research database and the medical record. Use of H1-antihistamines (diphenhydramine and cetirizine), H2-antihistamines (ranitidine), and glucocorticoids (dexamethasone, prednisolone, and hydrocortisone) as premedication was retrieved for patients who experienced reactions to PEG-ASP. Asparaginase-related reactions were prospectively graded using the National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0. Data from 410 patients in the St Jude Total XV protocol (TXV) were revisited.15 Patients on both protocols were assigned to either the low-risk (LR) arm or standard/high-risk (SHR) arm (Data Supplement). Treatment in TXVI differed from that in TXV mainly by the inclusion of a higher number of IT injections during remission induction for higher-risk patients (Data Supplement) and by use of PEG-ASP instead of L-ASP. Informed consent from parents or guardians and patient assent were obtained with oversight by the institutional review board.
Anti-Asparaginase Antibodies
Serum for anti–L-ASP antibodies (TXV)15 and anti–PEG-ASP antibodies (TXVI) was drawn at multiple time points (Data Supplement). Anti–PEG-ASP immunoglobulin G was detected using a modified enzyme-linked immunosorbent assay (ELISA) to avoid Tween-20 (Data Supplement)15,16 and analyzed as a continuous variable (optical density) and dichotomous variable (negative or positive). Samples that were positive for anti–PEG-ASP underwent reflex testing against L-ASP (BioVendor, Brno, Czech Republic) and PEG-bovine catalase (Sigma-Aldrich, St Louis, MO), to determine whether the anti–PEG-ASP was directed against L-ASP or PEG.
Asparaginase Activity, Pharmacokinetics, and Ex Vivo Neutralization
Serum asparaginase activity in TXVI was measured (Data Supplement).17 Pharmacokinetic parameters were estimated with the induction day 3 and continuation week 7 dose (Data Supplement) and the asparaginase activity on day 14 (trough) after a dose of PEG-ASP 2,500 U/m2 was estimated for 582 patients for induction and 495 patients for continuation. Fifty-three samples, of which 36 were positive for anti–PEG-ASP, were used for ex vivo neutralization assay to test whether antibodies inhibited serum asparaginase activity.
Genotyping and Genetic Ancestry
We used genome-wide genotyping of germline DNA to estimate ancestry and assign patients to one of three groups as described (Data Supplement): white; black; and Hispanic, Asian, or other.
Statistical Analysis
We used Wilcoxon rank sum or χ2 tests to identify risk factors for reactions or antibody positivity, to compare antibody positivity by reaction status or age, and to assess predictors of rechallenge outcome. General logistic regression models were used for multivariable analysis, with no model selection procedure.
Sensitivity, specificity, positive predictive value, and negative predictive value of antibodies for reactions were estimated (Data Supplement). Analyses were performed using R3.5.0 (http://www.r-project.org).18 No adjustments to P values were made for multiple comparisons.
RESULTS
Reactions to PEG-ASP Differ From Those to L-ASP
Overall, 81 patients (13.5%) in TXVI developed at least one grade 2 to 4 reaction to PEG-ASP (Fig 1). This percentage is much lower (P = 1.4 × 10−23) than that for L-ASP reactions in TXV (41.2%),15 but a higher proportion of those who experienced a reaction (71.6%) were grade 3 or 4 in TXVI compared with TXV (14.2%; P = 1.5 × 10−19). For TXVI, PEG-ASP reactions predominantly occurred with the first few doses after the 11- to 18-week hiatus after induction, with somewhat differing timing of reactions for TXV (Data Supplement). Patient characteristics at baseline in the two protocols were comparable with the exception of ancestry and a higher number of patients who were classified as high risk for CNS relapse in TXVI than TXV (Data Supplement).
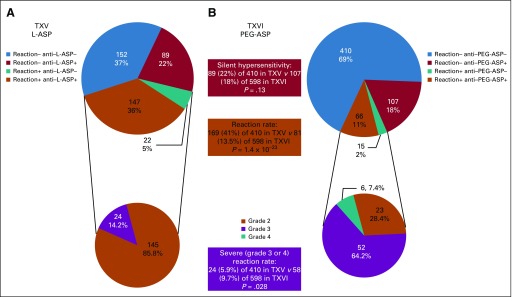
FIG 1.
Reactions by antibody status, preparation, and protocol. (A) Frequency of patients by their anti–Escherichia coli asparaginase (L-ASP) status and reaction to L-ASP in the Total XV study (TXV; upper chart). Distribution of patients who experienced reactions to L-ASP by their reaction grade (lower chart). (B) Frequency of patients by their anti-pegaspargase (PEG-ASP) status and reaction to PEG-ASP in Total XVI study (TXVI; upper chart). Distribution of patients who experienced reactions to PEG-ASP by their reaction grade (lower chart). All reactions are grade 2 or greater. Silent hypersensitivity (antibody positive but reaction negative) occurred in 89 (22%) of 410 and 107 (18%) of 598 patients on TXV versus TXVI (P = .13). Grade 2 to 4 reactions were less common (81 [13.5%] of 598 patients) in TXVI (to PEG-ASP) than in TXV (169 [41%] of 410 patients; to L-ASP; P = 1.4 × 10−23), but a higher proportion were grade 3 or 4 (24 [14.2%] of 169 v 58 [71.6%] of 81; P = 1.5 × 10−19). Overall, there were more grade 3 or 4 reactions with PEG-ASP than L-ASP (P = .028). All P values were generated from χ2 test. Reaction+, patients with allergic reactions to L-ASP (TXV) or PEG-ASP (TXVI); Reaction−, patients who received L-ASP (TXV) or PEG-ASP (TXVI) but did not have reactions to asparaginase.
Risk Factors for PEG-ASP Reactions
All features previously associated with allergy15,19,20 were examined as possible risk factors in addition to the number of intrathecal injections, because it was higher in the TXVI study than in the TXV study and because it was confounded with T-cell immunophenotype, previously identified as protecting against reactions.15,20 Two clinical features, assignment to the LR—as opposed to SHR—therapy arm and non–American Indian ancestry, were associated with L-ASP allergies in TXV.15,19 In contrast, in TXVI, PEG-ASP reactions differed neither by risk arm (P = .84), nor ancestry (P = .30; Table 1). Ancestry also had no association with PEG-ASP reactions when analyses were confined to the SHR arm (P = .71) or to patients with B-cell ALL (P = .21).
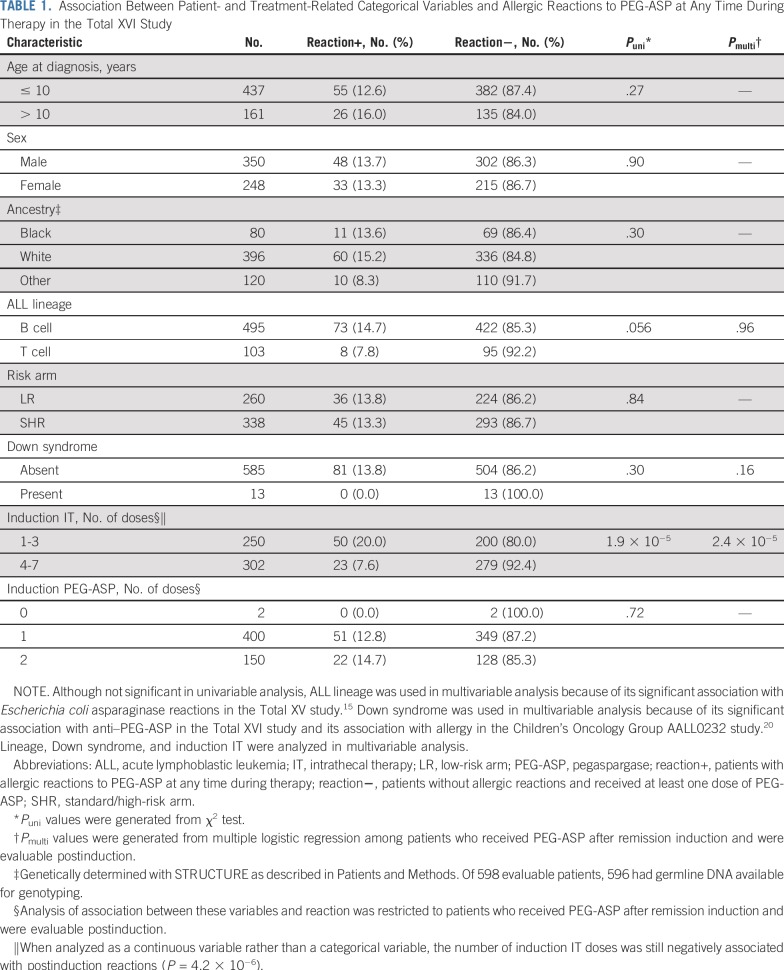
TABLE 1.
Association Between Patient- and Treatment-Related Categorical Variables and Allergic Reactions to PEG-ASP at Any Time During Therapy in the Total XVI Study
Cranial irradiation was omitted and replaced by systemic and intrathecal therapy (IT) in TXV21 and TXVI, which included more IT than TXV for those who were at highest risk of CNS relapse (Data Supplement). During induction, TXV patients received two to four ITs, whereas TXVI patients received one to seven ITs (Data Supplement). The number of induction ITs was negatively associated with PEG-ASP reactions in TXVI (P = 4.2 × 10−6; Table 1), which was also true among patients with B-cell ALL only (P = 4.8 × 10−5). With the lower number of ITs and the lower proportion of patients in TXV who were classified as CNS2 or CNS3 (Data Supplement), there was no significant association between the number of ITs and L-ASP reactions in TXV (P = .082).
Risk Factors for Antibodies and Their Association With Reactions
As described in Patients and Methods and the Data Supplement, the antibody assay for TXVI differed from that of TXV, with Tween-20 removed from all buffers as a result of structural similarity to PEG. Using the modified assay, 618 (11.5%) of 5,369 samples from 598 patients tested positive for anti–PEG-ASP in TXVI. Of the positive samples, 96 (15.5%) were positive for both anti-PEG and anti–L-ASP, 495 (80.1%) were positive for anti-PEG only, and nine (1.5%) were positive for anti–L-ASP only.
Of 81 patients who experienced PEG-ASP reactions, 66 (81.5%) had at least one sample that was positive for anti–PEG-ASP (Fig 1B). This percentage is similar to TXV for anti–L-ASP with L-ASP as the antigen (87.0%),15 indicating similar sensitivity of the modified assay for PEG-ASP as had been true for the prior assay for L-ASP antibodies (P = .25). Furthermore, 79.3% (410 of 517) of nonallergic patients never had a positive sample (Fig 1B), which indicated improved specificity to detect allergy of the modified assay for PEG-ASP antibodies compared with the original assay for L-ASP used in TXV (63.1%; P = 2.0 × 10−6).15 Modifying the assay reagents for use with PEG-ASP instead of L-ASP improved the clinical performance of the ELISA to detect clinical allergic reactions compared with the original assay developed for L-ASP antibodies (Data Supplement).
In multivariable analyses, the absence of Down syndrome (P = .030) and a lower number of ITs (P = 4.2 × 10−9) were associated with anti–PEG-ASP positivity (Data Supplement).
In the TXVI study as a whole, antibody status was significantly associated with risk for reaction—past or future—at most time points, except for induction day 8 (Fig 2A and Data Supplement), although associations differed in LR versus SHR patients. Thirty patients had positive day 1 samples for anti–PEG-ASP (5.1%; Data Supplement) before the first exposure to PEG-ASP, and antibody positivity correlated with the risk of subsequent reaction (P = .026; Data Supplement). Associations between antibodies and the risk of future reactions (only) are described (Data Supplement).
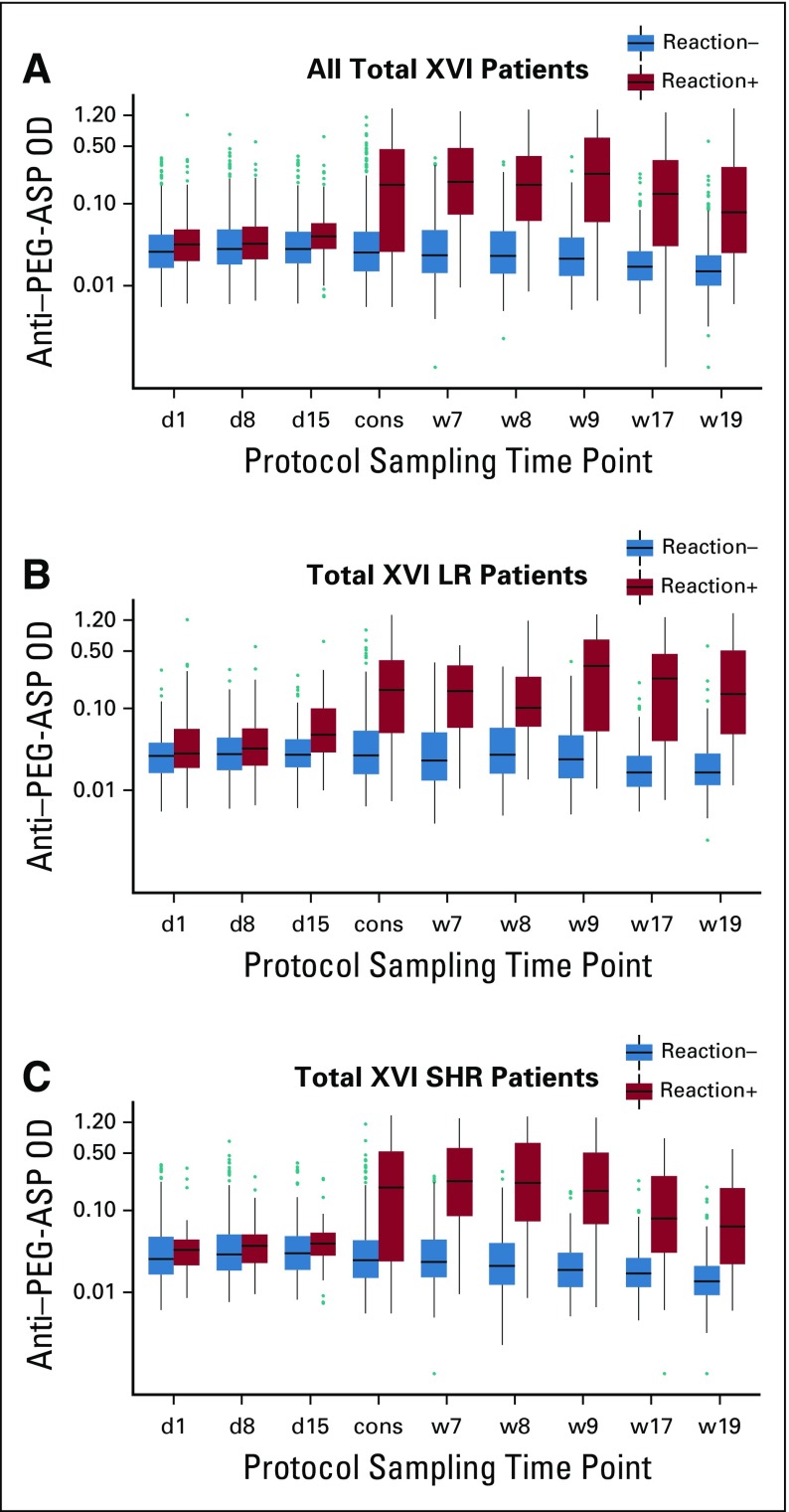
FIG 2.
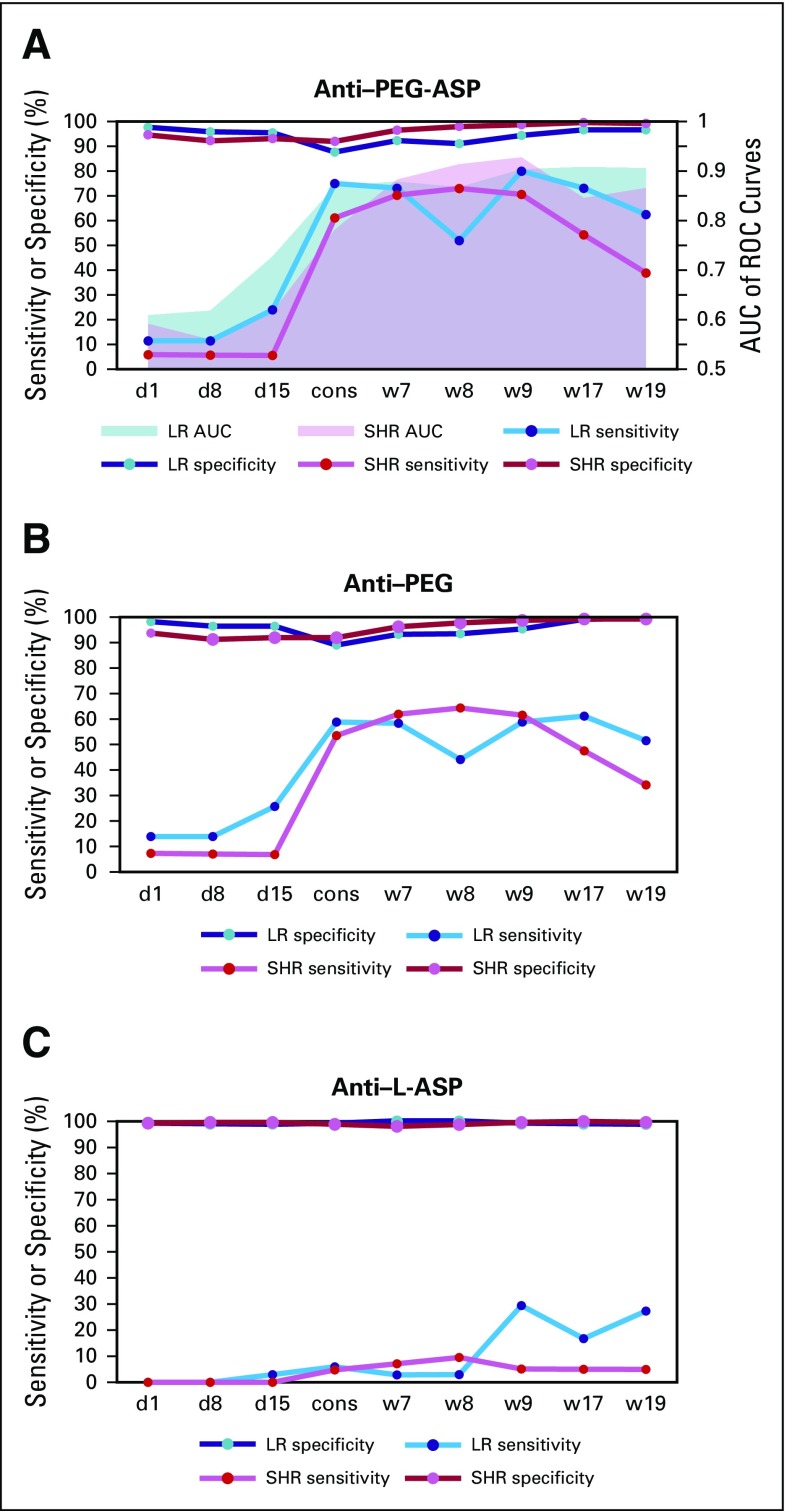
Association between anti-pegaspargase (PEG-ASP) antibody levels at different protocol time points and allergic reactions to PEG-ASP at any time during therapy in all Total XVI study (TXVI) patients (A; P values are .047, .098, 1.1E-4, 3.2E-13, 5.7E-24, 2.8E-25, 1.8E-25, 5.3E-21, and 3.9E-22 comparing reactive patients and nonreactive patients); TXVI low-risk arm (LR) patients (B; P values are .17, .14, 2.5E-4, 3.1E-7, 6.5E-12, 1.5E-10, 1.7E-11, 7.9E-13, and 5.0E-13 comparing reactive and nonreactive patients); and TXVI standard/high-risk arm (SHR) patients (C; P values are .19, .36, .066, 2.6E-7, 1.6E-13, 8.6E-16, 2.5E-15, 6.8E-10, and 1.6E-11 comparing reactive patients and nonreactive patients). cons, consolidation day 1; d1, induction day 1; d8, induction day 8; d15, induction day 15; OD, optical density; Reaction+, patients with allergic reactions at any time during therapy; Reaction−, patients who received PEG-ASP and never developed allergy; w7, continuation week 7; w8, continuation week 8; w9, continuation week 9; w17, continuation week 17; w19, continuation week 19.
The most common time for reactions was during reinduction I for LR and continuation weeks 1 to 6 for SHR patients. In LR patients, anti–PEG-ASP positivity at several time points predicted reinduction I reactions (Data Supplement), with receiver operating characteristic (ROC) curves having area under the curve (AUC) greater than 0.8 at consolidation day 1 (Data Supplement). In SHR patients, only antibody positivity on consolidation day 1 was a significant predictor of continuation weeks 1 to 6 reactions (P = 1.6 × 10−17; Data Supplement), and ROC curve AUCs were not greater than 0.8 until week 7 (Data Supplement), just after the reactions at weeks 1 to 6. The predictive utility of anti-PEG, but not anti–L-ASP, was similar to that of anti–PEG-ASP (Data Supplement).
How well did postreaction antibody tests confirm past reactions? Among LR patients, sensitivity of anti–PEG-ASP exceeded 70% and specificity exceeded 90% until week 19, approximately 10 weeks after the reactions during reinduction I (weeks 7 to 9; Data Supplement), and with AUCs of ROC curves greater than 0.9 for weeks 17 and 19 (Data Supplement). Anti-PEG and anti–L-ASP were also confirmative in LR patients, albeit not as markedly (Data Supplement). Among SHR patients, sensitivity of anti–PEG-ASP exceeded 70% and specificity exceeded 90% at weeks 7, 8, and 9, after reactions during continuation weeks 1 to 6 (Data Supplement), and with AUCs for ROC curves greater than 0.9 at weeks 8 and 9 (Data Supplement). Anti-PEG and anti–L-ASP were also confirmative in SHR patients (Data Supplement).
On the basis of the time course of anti–PEG-ASP in those patients who did experience reactions (Data Supplement), the highest anti–PEG-ASP levels occurred approximately 37 days after reactions compared with approximately 50 days for anti–L-ASP after L-ASP reactions in the TXV study.15
Rechallenge
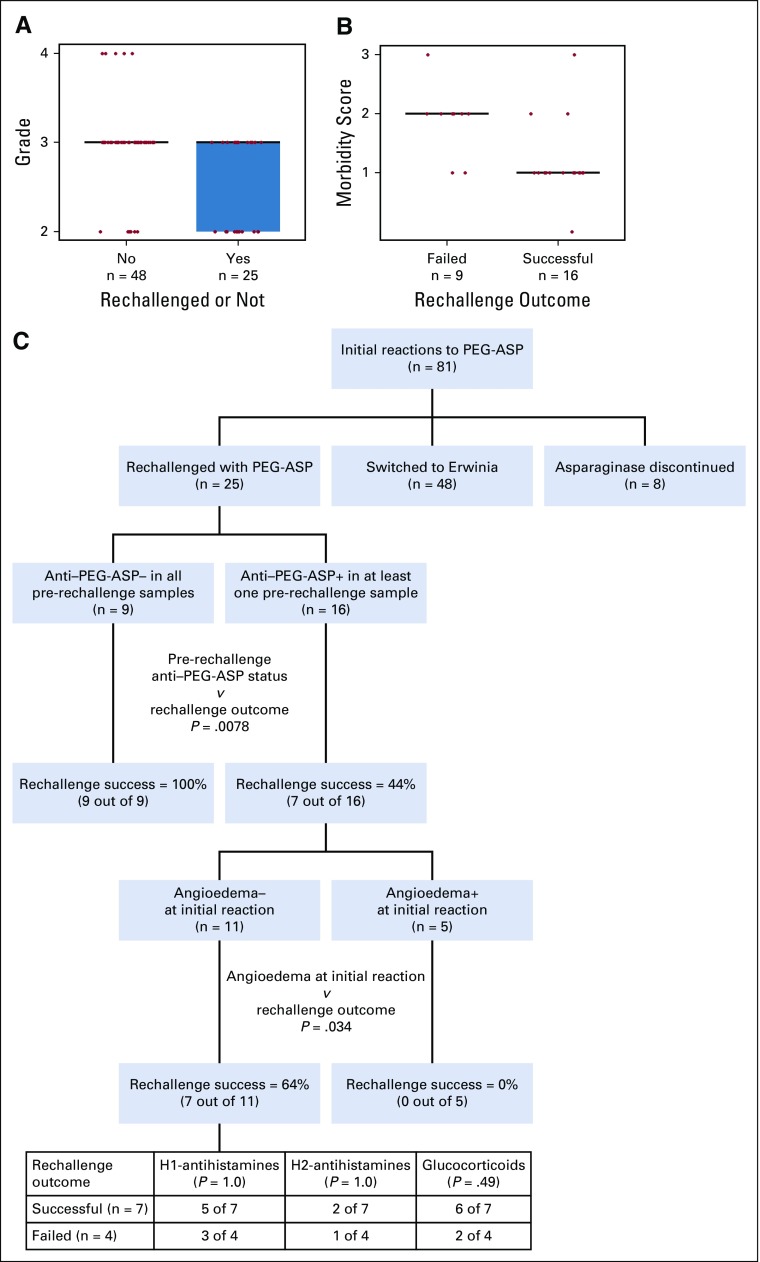
When patients experienced reactions during PEG-ASP, clinicians were often unsure if the reaction was a true allergy. Thus, of the 81 patients who experienced reactions to PEG-ASP, 25 were rechallenged with PEG-ASP instead of switching to Erwinase. This was a biased group: patients with less severe reactions were more likely to be rechallenged (grade 0 to 2 v grade 3 to 4; P = .0041; Fig 3A). Of those who were rechallenged, 16 tolerated rechallenge, whereas nine experienced failure and had another reaction. What predicted the success or failure of rechallenge in this group? The grade of initial reactions did not (grade 0 to 2 v grade 3 to 4; P = .41). Clinical symptoms were classified into five categories: urticarial (flushing, rash, hives, erythema, and pruritus), facial angioedema, respiratory (dyspnea, coughing, wheezing, and bronchospasm), hypotension, and GI symptoms (nausea, vomiting, diarrhea, and abdominal pain), to explore whether categories of symptoms could predict rechallenge outcome. A morbidity score on the basis of the number of symptom categories was assigned and higher morbidity scores were associated with rechallenge failure (P = .0091; Fig 3B). Angioedema (P = .012) and GI symptoms (P = .040) were associated with rechallenge failure (Data Supplement). Anti–PEG-ASP immediately after the reaction (P = .041) or positive anti–PEG-ASP before rechallenge (P = .0078) were associated with rechallenge failure. In multivariable analysis, angioedema and pre-rechallenge anti–PEG-ASP positivity remained associated with rechallenge failure (P = .010 and P = .027; Data Supplement). No association was found with the use of H1-antihistamines (P = 1.0), H2-antihistamines (P = 1.0), or glucocorticoids (P = .20; Fig 3C).
FIG 3.
Association between anti-pegaspargase (PEG-ASP) status, reaction severity and clinical symptoms, and PEG-ASP rechallenge. (A) Association between the grade of the initial reaction and whether the patient was rechallenged or not (P = .0041). (B) Association between morbidity score assigned from clinical symptoms and rechallenge outcome (P = .0091). (C) Classification and regression tree of rechallenge outcome prediction.
It is not possible to evaluate the impact of rechallenge on serum asparaginase activity with rechallenge doses for those patients who reacted again, because the second reactions tended to occur so early during infusions that only a small percentage of the dose was administered: nine of nine patients who experienced failure with rechallenge received no more than 12% of the planned dose when they rereacted; thus, serum asparaginase activity was not evaluable. For 13 of 25 rechallenged patients who received the full PEG-ASP dose for continuation week 7 and had serum activity measured within 14 days, median estimated trough serum asparaginase activity was 0.38 U/mL, which was lower than among the 481 patients who did not have any reaction and were evaluable at week 7 (0.54 U/mL; P = .042), but still above the putative threshold for desired activity of 0.1 U/mL for most patients. In fact, only two of 13 rechallenged patients and 18 of 481 non-rechallenged patients had asparaginase levels less than 0.1 U/mL.
Impact of Antibody on Asparaginase Activity
Among the 20 samples that were tested, no association was found between antibody levels—anti–PEG-ASP, anti–L-ASP, or anti-PEG—and neutralization of spiked PEG-ASP activity (Data Supplement). Anti–PEG-ASP did not neutralize L-ASP either (Data Supplement). In contrast, there was an association between the neutralization of spiked L-ASP activity and levels of anti–L-ASP (P < .001 Data Supplement).
In vivo, antibody status was not associated with asparaginase levels during induction (P = .20 for anti–PEG-ASP and anti-PEG; P = .27 for anti–L-ASP; Data Supplement; n = 582), but there was an association during continuation (n = 495; P = 5.0 × 10−6 for anti–PEG-ASP; P = 1.2 × 10−8 for anti–L-ASP; and P = 7.5 × 10−6 for anti-PEG; Data Supplement). The activity-lowering effect of anti–L-ASP was stronger than that of anti–PEG-ASP (P = .0032) during continuation.
DISCUSSION
Antibodies Were Useful at Diagnosing Allergic Reactions and Predicting Rechallenge Failure
There has been controversy over what constitutes PEG-ASP allergic reactions, and it is difficult to make the diagnosis on the basis of symptoms alone.22 The diagnosis is important, because relapse can be minimized by detecting asparaginase allergy and promptly switching to another preparation—for example, Erwinase.23 However, Erwinase requires more frequent administration, has unfavorable pharmacokinetic properties, and is more expensive; therefore, avoiding an incorrect diagnosis of PEG-ASP allergy is also important. Criteria proposed to differentiate true allergy from other reactions have included timing,24 severity and type of symptoms, and low serum asparaginase activity.22 There are few modern trials using PEG-ASP upfront that have included measurements of serum antibodies optimized for PEG-ASP. Studies with native L-ASP followed by PEG-ASP have increased rates of allergy and antibodies that do not reflect the current findings using PEG-ASP upfront.12,25 The strongest clinical evidence of a true allergy is likely to be the recurrence of reactions upon rechallenge. Here, using a modified ELISA assay for anti–PEG-ASP antibodies, antibodies were associated with reaction recurrence upon rechallenge (Fig 3), and they also displayed better sensitivity, specificity, and positive and negative predictive value for the first reaction to PEG-ASP than was true for anti–L-ASP antibodies for the first reaction to L-ASP (Fig 4 and Data Supplement).
FIG 4.
Sensitivity and specificity of antibodies for allergic reactions by risk arm. Sensitivity, specificity and area under the curve (AUC) of receiver operating characteristic (ROC) curves for the association between (A) anti-pegaspargase (PEG-ASP) antibody, (B) anti-PEG antibody, (C) and anti–Escherichia coli asparaginase (L-ASP) antibody measured at different protocol time points and allergic reactions to PEG-ASP at any time during therapy. cons, consolidation day 1; d1, induction day 1; d8, induction day 8; d15, induction day 15; LR, low-risk arm; SHR, standard/high-risk arm; w7, continuation week 7; w8, continuation week 8; w9, continuation week 9; w17, continuation week 17; w19, continuation week 19.
Rechallenge would also be considered a failure if serum asparaginase activity after rechallenge was too low. Although it is not possible to evaluate serum asparaginase activity in those who experience failure with rechallenge and thus stop infusions early, we did compare serum asparaginase activity (estimated after 2,500 U/m2) in those who were rechallenged versus patients at an identical time point who had not suffered reaction. We found that although serum asparaginase was marginally lower (P = .042), it remained greater than a putative desired trough concentration of 0.1 U/mL in 84.6% of rechallenged and 96.3% of nonreacting patients.
Although anti–PEG-ASP was associated with lower serum asparaginase later in therapy, anti–L-ASP was more inhibitory of asparaginase activity, both in vivo and ex vivo, than anti–PEG-ASP (Data Supplement). Thus, anti–PEG-ASP may not directly inhibit the enzymatic activity of PEG-ASP, possibly because of the poor accessibility of the enzyme active site created by PEGylation. It is also possible that anti–PEG-ASP antibodies or accompanying immunologic changes increased the clearance of PEG-ASP without neutralizing PEG-ASP activity, similar to findings from the pegloticase trials.26,27 Because infusions are usually stopped early in the case of a reaction, and because anti-PEG antibodies are not always neutralizing, use of serum asparaginase activity as a method to detect real allergy is not ideal.
Taken together, our data indicate that neither clinical reactions nor the presence of anti–PEG-ASP antibodies can be assumed to indicate inadequate serum asparaginase activity in this setting in which PEG-ASP is used in upfront ALL regimens.
Antibodies Were Primarily Directed at PEG
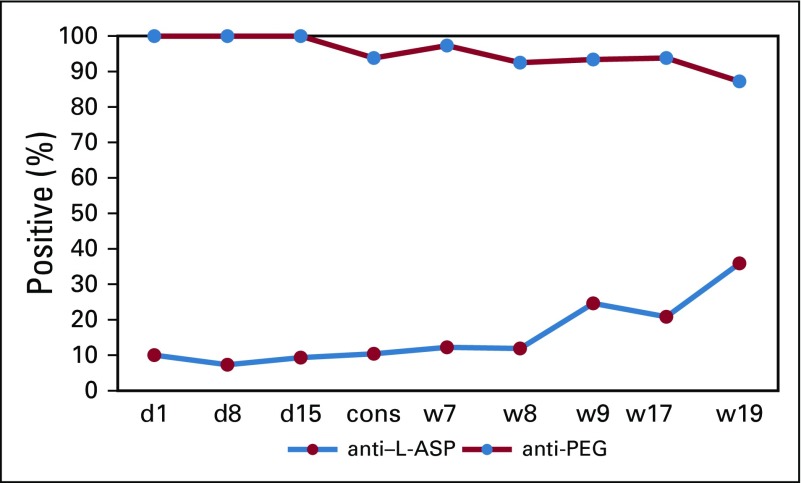
Consistent with the modest neutralization of PEG-ASP activity in vivo, antibodies against PEG-ASP were primarily directed against PEG, not L-ASP. Although PEGylation decreased immunogenicity compared with native L-ASP (Fig 1), PEG itself is becoming increasingly recognized as an antigen.14,28 Anti-PEG was the predominant component (96%) of anti–PEG-ASP (Fig 5 and Data Supplement), as was reported for pegloticase for gout.29 Prevalence of anti-PEG in patients with ALL has not been extensively reported, but in the general population it has been reported to be as high as 10% to 30%.30,31 In fact, pretreatment anti-PEG was present in our cohort; therefore, patients can experience a reaction to the first dose of PEG-ASP. The 5.1% of patients with pretreatment anti-PEG were at higher risk of subsequent reactions to PEG-ASP (P = .026; Data Supplement). We reported two patients who experienced reactions to PEG-ASP and were successfully switched to L-ASP without additional reaction, which indicates that their reactions were likely mediated by anti-PEG and not anti–L-ASP.32 At the time of that report, the ELISA used had not been optimized for the detection of anti–PEG-ASP. One of those two patients was enrolled in the TXVI study. In retrospect, using our modified ELISA, the patient was positive for anti–PEG-ASP and anti-PEG, but negative for anti–L-ASP, which explains the success of L-ASP substitution. The success of the L-ASP substitution in this single patient as well as the underlying antibody profile support the idea that it would be helpful if L-ASP or alternative non-PEGylated formulations were commercially available for use in selected patients who have become sensitized to PEG itself. These findings also support the importance of our assay, which can distinguish antibodies to PEG versus those to the asparaginase formulation. Patients who are allergic to PEG-ASP experienced failure with PEGylated Erwinase and suffered cross-reactions, likely to PEG.33 Of note, our patient with the highest preexisting levels of anti–PEG-ASP and anti-PEG in the TXVI study experienced a reaction to his or her first dose of PEG-ASP. Similar cases have been reported for pegloticase and pegnivacogin.34,35 Of interest, we found that age was associated with higher preexisting anti–PEG-ASP levels (P = 8.6 × 10−10; Data Supplement), possibly because as children mature, there is increasing exposure to PEG-containing products, such as laxatives (Miralax), eye drops, tablet coatings, topicals, and food.
FIG 5.
Of samples that were positive for anti-pegaspargase, shown are the percent positive for anti-PEG and anti–Escherichia coli asparaginase (L-ASP) at each protocol time point in the Total XVI study. cons, consolidation day 1; d1, induction day 1; d8, induction day 8; d15, induction day 15; w7, continuation week 7; w8, continuation week 8; w9, continuation week 9; w17, continuation week 17; w19, continuation week 19.
Consistent with our previous analysis from the TXV study with L-ASP,15 asparaginase activity was lower in those few samples from PEG-ASP–treated patients with higher anti–L-ASP levels. Thus, it may be important to differentiate anti–PEG-ASP from anti–L-ASP in patients treated with PEG-ASP.
Clinical Features Predicted Rechallenge Failure
Although we acknowledge that patients with the most severe reactions to PEG-ASP were not rechallenged, of those who were rechallenged, anti–PEG-ASP positivity associated with rechallenge failure, which provides additional evidence that our antibody assay distinguished real PEG-ASP allergy from nonallergy infusion reactions. Of interest, angioedema and GI reactions were the symptom classes associated with rechallenge failure, whereas reaction severity grade was not predictive. In multivariable analysis, angioedema and anti–PEG-ASP were the strongest predictors of rechallenge failure (Data Supplement). All nine rechallenged patients without anti–PEG-ASP positive samples before rechallenge were successfully rechallenged and tolerated all subsequent PEG-ASP doses. Furthermore, eight of these nine patients never developed any anti–PEG-ASP antibodies. Premedication did not affect rechallenge outcome, similar to some studies,36,37 although we acknowledge that rechallenge was biased against those with the most impressive initial reactions in our group and that the immunosuppression of our ALL regimen may differ from that of others.
Some Clinical Features Predicted Allergy, and Allergy to PEG-ASP Differed From That to L-ASP
We reported fewer reactions to L-ASP among patients with T-cell ALL.15 Here, we observed fewer reactions in patients with T-cell ALL in the TXVI study in univariable analysis among SHR patients (P = .044), but this association disappeared (P = .49) in a multivariable analysis that included the number of ITs, a novel risk factor reported herein. IT number was the only factor associated with reactions in all patients (P = 2.4 × 10−5; Table 1). Among the three drugs in ITs, methotrexate is immunosuppressive and IT methotrexate can reach cytotoxic levels in serum, which has systemic effects.38-40 Admittedly, other systemic chemotherapy administered during induction was also immunosuppressive, but it did not differ significantly from patient to patient as much as the number of ITs (Data Supplement); therefore, other chemotherapy was unlikely to differentiate those at higher versus lower risk of reactions. We hypothesize that IT use may not have been a risk factor in other trials because it was not evaluated, and most trials do not have as many ITs, nor as much variability in the number of ITs among patients.41 In addition, a higher proportion of patients in the TXVI study (Data Supplement) were CNS positive and thus received more ITs compared with prior St Jude trials and many other ALL trials. Representing a small subset (13 of 598), Down syndrome was associated with the absence of anti–PEG-ASP (P = .030; Data Supplement), which is consistent with our previous report.20
Although reactions to PEG-ASP were less common than reactions to L-ASP, which was consistent with a previous report,1 they were more severe (Fig 1), similar to other trials.42-44 Unlike in the TXV study with L-ASP, neither the risk nor the timing of reaction differed by risk arm in TXVI using PEG-ASP. Patients who experienced reactions received a median of only three doses of PEG-ASP before their reaction (Data Supplement), similar to other studies.43,44
In summary, in the TXVI study with PEG-ASP as the primary formulation, the majority of patients (81.5%) with allergy had antibodies to PEG-ASP, similar to the percent of allergic patients who had anti–L-ASP in the TXV study. With PEG-ASP, the primary antigen was PEG, not L-ASP. Patients who received fewer ITs had a higher risk of reaction. Some patients who have an apparent allergic reaction to PEG-ASP can be successfully rechallenged. Predictors of successful rechallenge include a lack of anti–PEG-ASP antibodies and lack of angioedema. Measurement of anti–PEG-ASP, especially anti-PEG, could be of use in managing patients who are treated with PEG-ASP and other PEGylated therapeutics.
Footnotes
Funded by US National Institutes of Health Grants No. P50-GM115279, R01-CA142665, R37-CA36401, and P30-CA21765; Sigma Tau; Baxalta; Shire; and American Lebanese Syrian Associated Charities.
Clinical trial information: NCT00549848.
AUTHOR CONTRIBUTIONS
Conception and design: Yiwei Liu, William E. Evans, Sima Jeha, Mary V. Relling
Provision of study materials or patients: Ching-Hon Pui, Sima Jeha
Collection and assembly of data: Yiwei Liu, Colton A. Smith, John C. Panetta, Lauren E. Thompson, Alejandro R. Molinelli, Deqing Pei, Nancy M. Kornegay, Kristine R. Crews, Ching-Hon Pui, Sima Jeha
Data analysis and interpretation: Yiwei Liu, Colton A. Smith, John C. Panetta, Wenjian Yang, Jacob P. Counts, Alejandro R. Molinelli, Nancy M. Kornegay, Hope Swanson, Cheng Cheng, Seth E. Karol, William E. Evans, Hiroto Inaba, Ching-Hon Pui
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Antibodies Predict Pegaspargase Allergic Reactions and Failure of Rechallenge
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Hiroto Inaba
Research Funding: Servier
Ching-Hon Pui
Honoraria: Amgen, Bristol-Myers Squibb
Consulting or Advisory Role: Adaptive Biotechnologies
Mary V. Relling
Research Funding: Servier
No other potential conflicts of interest were reported.
REFERENCES
- 1.Hijiya N, van der Sluis IM. Asparaginase-associated toxicity in children with acute lymphoblastic leukemia. Leuk Lymphoma. 2016;57:748–757. doi: 10.3109/10428194.2015.1101098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Knott SRV, Wagenblast E, Khan S, et al. Asparagine bioavailability governs metastasis in a model of breast cancer. Nature. 2018;554:378–381. doi: 10.1038/nature25465. [Erratum: Nature 556:135, 2018] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mishra P, Nayak B, Dey RK. PEGylation in anti-cancer therapy: An overview. Asian Journal of Pharmaceutical Sciences. 2016;11:337–348. [Google Scholar]
- 4.Advani AS, Sanford B, Luger S, et al. Frontline-treatment of acute lymphoblastic leukemia (ALL) in older adolescents and young adults (AYA) using a pediatric regimen is feasible: Toxicity results of the prospective US Intergroup Trial C10403 (Alliance) Blood. 2013;122:3903. [Google Scholar]
- 5.Pieters R, de Groot-Kruseman H, Van der Velden V, et al. Successful therapy reduction and intensification for childhood acute lymphoblastic leukemia based on minimal residual disease monitoring: Study ALL10 from the Dutch Childhood Oncology Group. J Clin Oncol. 2016;34:2591–2601. doi: 10.1200/JCO.2015.64.6364. [DOI] [PubMed] [Google Scholar]
- 6.Avramis VI, Sencer S, Periclou AP, et al. A randomized comparison of native Escherichia coli asparaginase and polyethylene glycol conjugated asparaginase for treatment of children with newly diagnosed standard-risk acute lymphoblastic leukemia: A Children’s Cancer Group study. Blood. 2002;99:1986–1994. doi: 10.1182/blood.v99.6.1986. [DOI] [PubMed] [Google Scholar]
- 7.Kim HJ, Ock CY, Kim TM, et al. Comparison of native Escherichia coli L-asparaginase versus pegylated asparaginase, in combination with ifosfamide, methotrexate, etoposide, and prednisolone, in extranodal NK/T-cell lymphoma, nasal type. Cancer Res Treat. 2018;50:670–680. doi: 10.4143/crt.2017.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kurtzberg J, Asselin B, Bernstein M, et al. Polyethylene glycol-conjugated L-asparaginase versus native L-asparaginase in combination with standard agents for children with acute lymphoblastic leukemia in second bone marrow relapse: A Children’s Oncology Group Study (POG 8866) J Pediatr Hematol Oncol. 2011;33:610–616. doi: 10.1097/MPH.0b013e31822d4d4e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stock W, Douer D, DeAngelo DJ, et al. Prevention and management of asparaginase/pegasparaginase-associated toxicities in adults and older adolescents: Recommendations of an expert panel. Leuk Lymphoma. 2011;52:2237–2253. doi: 10.3109/10428194.2011.596963. [DOI] [PubMed] [Google Scholar]
- 10.Ko RH, Jones TL, Radvinsky D, et al. Allergic reactions and antiasparaginase antibodies in children with high-risk acute lymphoblastic leukemia: A Children’s Oncology Group report. Cancer. 2015;121:4205–4211. doi: 10.1002/cncr.29641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Browne EK, Moore C, Sykes A, et al. Clinical characteristics of intravenous PEG-asparaginase hypersensitivity reactions in patients undergoing treatment for acute lymphoblastic leukemia. J Pediatr Oncol Nurs. 2018;35:103–109. doi: 10.1177/1043454217741868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Armstrong JK, Hempel G, Koling S, et al. Antibody against poly(ethylene glycol) adversely affects PEG-asparaginase therapy in acute lymphoblastic leukemia patients. Cancer. 2007;110:103–111. doi: 10.1002/cncr.22739. [DOI] [PubMed] [Google Scholar]
- 13.Tong WH, van der Sluis IM, Alleman CJ, et al. Cost-analysis of treatment of childhood acute lymphoblastic leukemia with asparaginase preparations: The impact of expensive chemotherapy. Haematologica. 2013;98:753–759. doi: 10.3324/haematol.2012.073510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wenande E, Garvey LH. Immediate-type hypersensitivity to polyethylene glycols: A review. Clin Exp Allergy. 2016;46:907–922. doi: 10.1111/cea.12760. [DOI] [PubMed] [Google Scholar]
- 15.Liu C, Kawedia JD, Cheng C, et al. Clinical utility and implications of asparaginase antibodies in acute lymphoblastic leukemia. Leukemia. 2012;26:2303–2309. doi: 10.1038/leu.2012.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang B, Hak LJ, Relling MV, et al. ELISA to evaluate plasma anti-asparaginase IgG concentrations in patients with acute lymphoblastic leukemia. J Immunol Methods. 2000;239:75–83. doi: 10.1016/s0022-1759(00)00182-4. [DOI] [PubMed] [Google Scholar]
- 17.Fernandez CA, Cai X, Elozory A, et al. High-throughput asparaginase activity assay in serum of children with leukemia. Int J Clin Exp Med. 2013;6:478–487. [PMC free article] [PubMed] [Google Scholar]
- 18.R Development Core Team . R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; [Google Scholar]
- 19.Chen SH, Pei D, Yang W, et al. Genetic variations in GRIA1 on chromosome 5q33 related to asparaginase hypersensitivity. Clin Pharmacol Ther. 2010;88:191–196. doi: 10.1038/clpt.2010.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fernandez CA, Smith C, Yang W, et al. Genome-wide analysis links NFATC2 with asparaginase hypersensitivity. Blood. 2015;126:69–75. doi: 10.1182/blood-2015-02-628800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pui CH, Campana D, Pei D, et al. Treating childhood acute lymphoblastic leukemia without cranial irradiation. N Engl J Med. 2009;360:2730–2741. doi: 10.1056/NEJMoa0900386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burke MJ, Rheingold SR. Differentiating hypersensitivity versus infusion-related reactions in pediatric patients receiving intravenous asparaginase therapy for acute lymphoblastic leukemia. Leuk Lymphoma. 2017;58:540–551. doi: 10.1080/10428194.2016.1213826. [DOI] [PubMed] [Google Scholar]
- 23.Vrooman LM, Stevenson KE, Supko JG, et al. Postinduction dexamethasone and individualized dosing of Escherichia coli L-asparaginase each improve outcome of children and adolescents with newly diagnosed acute lymphoblastic leukemia: Results from a randomized study—Dana-Farber Cancer Institute ALL Consortium Protocol 00-01. J Clin Oncol. 2013;31:1202–1210. doi: 10.1200/JCO.2012.43.2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kloos RQ, Pieters R, Escherich G, et al. Allergic-like reactions to asparaginase: Atypical allergies without asparaginase inactivation. Pediatr Blood Cancer. 2016;63:1928–1934. doi: 10.1002/pbc.26123. [DOI] [PubMed] [Google Scholar]
- 25.Tong WH, Pieters R, Kaspers GJ, et al. A prospective study on drug monitoring of PEGasparaginase and Erwinia asparaginase and asparaginase antibodies in pediatric acute lymphoblastic leukemia. Blood. 2014;123:2026–2033. doi: 10.1182/blood-2013-10-534347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lipsky PE, Calabrese LH, Kavanaugh A, et al. Pegloticase immunogenicity: The relationship between efficacy and antibody development in patients treated for refractory chronic gout. Arthritis Res Ther. 2014;16:R60. doi: 10.1186/ar4497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chirmule N, Jawa V, Meibohm B. Immunogenicity to therapeutic proteins: Impact on PK/PD and efficacy. AAPS J. 2012;14:296–302. doi: 10.1208/s12248-012-9340-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stone CA, Jr, Liu Y, Relling M.V., et al. Immediate hypersensitivity to polyethylene glycols and polysorbates: More common than we have recognized. J Allergy Clin Immunol Pract. doi: 10.1016/j.jaip.2018.12.003. 10.1016/j.jaip.2018.12.003 [Epub ahead of print on December 14, 2018] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hershfield MS, Ganson NJ, Kelly SJ, et al. Induced and pre-existing anti-polyethylene glycol antibody in a trial of every 3-week dosing of pegloticase for refractory gout, including in organ transplant recipients. Arthritis Res Ther. 2014;16:R63. doi: 10.1186/ar4500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen BM, Su YC, Chang CJ, et al. Measurement of pre-existing IgG and IgM antibodies against polyethylene glycol in healthy individuals. Anal Chem. 2016;88:10661–10666. doi: 10.1021/acs.analchem.6b03109. [DOI] [PubMed] [Google Scholar]
- 31.Yang Q, Jacobs TM, McCallen JD, et al. Analysis of pre-existing IgG and IgM antibodies against polyethylene glycol (PEG) in the general population. Anal Chem. 2016;88:11804–11812. doi: 10.1021/acs.analchem.6b03437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fernandez CA, Stewart E, Panetta JC, et al. Successful challenges using native E. coli asparaginase after hypersensitivity reactions to PEGylated E. coli asparaginase. Cancer Chemother Pharmacol. 2014;73:1307–1313. doi: 10.1007/s00280-014-2464-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rau RE, Dreyer Z, Choi MR, et al. Outcome of pediatric patients with acute lymphoblastic leukemia/lymphoblastic lymphoma with hypersensitivity to pegaspargase treated with PEGylated Erwinia asparaginase, pegcrisantaspase: A report from the Children’s Oncology Group. Pediatr Blood Cancer. 2018;65:e26873. doi: 10.1002/pbc.26873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abbott LS, Zakova M, Shaikh F, et al. Allergic reactions associated with intravenous versus intramuscular pegaspargase: A retrospective chart review. Paediatr Drugs. 2015;17:315–321. doi: 10.1007/s40272-015-0129-1. [DOI] [PubMed] [Google Scholar]
- 35.Ganson NJ, Povsic TJ, Sullenger BA, et al. Pre-existing anti-polyethylene glycol antibody linked to first-exposure allergic reactions to pegnivacogin, a PEGylated RNA aptamer. J Allergy Clin Immunol. 2016;137:1610.e7–1613.e7. doi: 10.1016/j.jaci.2015.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sahiner UM, Yavuz ST, Gökce M, et al. Anaphylactic reaction to polyethylene-glycol conjugated-asparaginase: Premedication and desensitization may not be sufficient. Pediatr Int. 2013;55:531–533. doi: 10.1111/ped.12131. [DOI] [PubMed] [Google Scholar]
- 37.Wu HL. Retrospective evaluation of a rechallenge protocol in patients experiencing hypersensitivity reactions with prior chemotherapy in a tertiary hospital. J Oncol Pharm Pract. doi: 10.1177/1078155218796190. 10.1177/1078155218796190 [Epub ahead of print on September 4, 2018] [DOI] [PubMed] [Google Scholar]
- 38.Thyss A, Suciu S, Bertrand Y, et al. Systemic effect of intrathecal methotrexate during the initial phase of treatment of childhood acute lymphoblastic leukemia. J Clin Oncol. 1997;15:1824–1830. doi: 10.1200/JCO.1997.15.5.1824. [DOI] [PubMed] [Google Scholar]
- 39.Kose F, Abali H, Sezer A, et al. Little dose, huge toxicity: Profound hematological toxicity of intrathecal methotrexate. Leuk Lymphoma. 2009;50:282–283. doi: 10.1080/10428190802603169. [DOI] [PubMed] [Google Scholar]
- 40.Gregory RE, Pui CH, Crom WR. Raised plasma methotrexate concentrations following intrathecal administration in children with renal dysfunction. Leukemia. 1991;5:999–1003. [PubMed] [Google Scholar]
- 41.Winick N, Devidas M, Chen S, et al. Impact of initial CSF findings on outcome among patients with National Cancer Institute standard- and high-risk B-cell acute lymphoblastic leukemia: A report from the Children’s Oncology Group. J Clin Oncol. 2017;35:2527–2534. doi: 10.1200/JCO.2016.71.4774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Place AE, Stevenson KE, Vrooman LM, et al. Intravenous pegylated asparaginase versus intramuscular native Escherichia coli L-asparaginase in newly diagnosed childhood acute lymphoblastic leukaemia (DFCI 05-001): A randomised, open-label phase 3 trial. Lancet Oncol. 2015;16:1677–1690. doi: 10.1016/S1470-2045(15)00363-0. [DOI] [PubMed] [Google Scholar]
- 43.Henriksen LT, Harila-Saari A, Ruud E, et al. PEG-asparaginase allergy in children with acute lymphoblastic leukemia in the NOPHO ALL2008 protocol. Pediatr Blood Cancer. 2015;62:427–433. doi: 10.1002/pbc.25319. [DOI] [PubMed] [Google Scholar]
- 44.Salzer W, Burke MJ, Larsen EC, et al. Incidence of allergic reactions to pegaspargase (PEG) administered intramuscularly versus intravenously (IM vs. IV) in children and young adults with high risk B-lymphoblastic leukemia (HR B-ALL): Results of Children's Oncology Group (COG) studies AALL0232/AALL1131. Blood. 2015;126:1303. [Google Scholar]