Abstract
Auxin response factors (ARFs) are involved in auxin-mediated transcriptional regulation in plants. In this study, we performed functional characterization of SlARF6A in tomato. SlARF6A is located in the nucleus and exhibits transcriptional activator activity. Overexpression of SlARF6A increased chlorophyll contents in the fruits and leaves of tomato plants, whereas downregulation of SlARF6A decreased chlorophyll contents compared with those of wild-type (WT) plants. Analysis of chloroplasts using transmission electron microscopy indicated increased sizes of chloroplasts in SlARF6A-overexpressing plants and decreased numbers of chloroplasts in SlARF6A-downregulated plants. Overexpression of SlARF6A increased the photosynthesis rate and accumulation of starch and soluble sugars, whereas knockdown of SlARF6A resulted in opposite phenotypes in tomato leaves and fruits. RNA-sequence analysis showed that regulation of SlARF6A expression altered the expression of genes involved in chlorophyll metabolism, photosynthesis and sugar metabolism. SlARF6A directly bound to the promoters of SlGLK1, CAB, and RbcS genes and positively regulated the expression of these genes. Overexpression of SlARF6A also inhibited fruit ripening and ethylene production, whereas downregulation of SlARF6A increased fruit ripening and ethylene production. SlARF6A directly bound to the SAMS1 promoter and negatively regulated SAMS1 expression. Taken together, these results expand our understanding of ARFs with regard to photosynthesis, sugar accumulation and fruit development and provide a potential target for genetic engineering to improve fruit nutrition in horticulture crops.
Subject terms: Plant molecular biology, Agricultural genetics
Introduction
Tomato is the world’s second largest vegetable crop rich in nutrients1. Tomato fruit development includes three stages2. The first stage is characterized by an increase in cell number and starch accumulation, followed by cell enlargement with starch degradation and soluble sugar accumulation in the second stage3. Fruit ripening is the last stage, associated with the accumulation of soluble sugars, carotenoids, organic acids, and volatile organic compounds in fruits1.
The chlorophyll accumulation and photosynthetic activity of green fruits influence the nutritional components and flavor of ripening tomato fruits4. Some genes have been reported to affect chlorophyll accumulation, chloroplast development and fruit quality. As negative regulators, DE-ETIOLATED 1/high pigment 2 (DET1/hp2) and UV-DAMAGED DNA-BINDING PROTEIN 1/high pigment 1 (DDB1/hp1) are involved in chloroplast formation and chlorophyll accumulation in tomato fruits5,6. The tomato GOLDEN2-LIKE transcription factors SlGLK1 and SlGLK2 play an important role in chloroplast formation and chlorophyll accumulation7. Evidence suggests that the SlGLK2 gene is predominantly expressed in fruits and that the latitudinal gradient of SlGLK2 expression influences the production of unevenly colored tomato fruits8. Overexpression of the APRR2-LIKE gene, the closest homolog of SlGLK2, increased the size and number of chloroplasts and enhanced chlorophyll accumulation in green tomato fruits9. TKN2 and TKN4, two Class I KNOTTED1-LIKE HOMEOBOX (KNOX) proteins, act as transcriptional activators of SlGLK2 and APRR2-LIKE genes to promote chloroplast development in tomato fruits4. BEL1-LIKE HOMEODOMAIN11 (SlBEL11) also plays an important role in chlorophyll synthesis and chloroplast development in tomato fruits10.
The ripening of tomato is mainly regulated by the ethylene pathway and many transcription factors1,11,12. In the ethylene biosynthetic pathway, S-adenosylmethionine synthetase (SAMS) catalyzes the reaction of ATP and methionine to form S-adenosyl-L-methionine (SAM)13. 1-Aminocyclopropane-1-carboxylic acid (ACC) synthase (ACS) and ACC oxidase (ACO) catalyze the conversion of SAM to ACC and of ACC to ethylene, respectively. The MADS box gene RIPENING INHIBITOR (RIN) controls the early phase of ripening and ethylene production via transcriptional regulation of ACSs and ACOs14. The other ripening regulators affecting ethylene production also include the NAC transcription factor NOR, the SQUAMOSA PROMOTER BINDING protein CNR, the ethylene response factor ERF B3, the AP2/ERF member AP2a, and several MADS box proteins, such as TDR4/SlFUL1, SlFUL2, SlMADS1, TAGL1, and TAG115–21.
Auxin is an important phytohormone involved in flower fertilization, fruit setting, fruit initiation and development22. Auxin is also essential in the regulation of cell division and expansion, controlling final fruit size23. Auxin modulates plant development through transcriptional regulation of auxin-responsive genes, which is primarily mediated by two gene families: the short-lived nuclear protein Aux/IAA family and auxin response factors (ARFs)1,24–26. Most ARFs have an N-terminal DNA-binding domain (B3) required for transcriptional regulation of auxin response genes, a middle region functioning as a repression domain (RD) or activation domain (AD), and a C-terminal dimerization domain (CTD) involved in the formation of homodimers or heterodimers27. ARFs can act as either an activator or a repressor of the transcription of auxin-responsive genes27. Numerous studies have indicated that ARFs are involved in many tomato developmental processes27–32. SlARF4 negatively regulates chlorophyll accumulation and starch biosynthesis in tomato fruit33,34. Our previous study showed that SlARF10 positively regulated chlorophyll accumulation via direct activation of the expression of SlGLK135. Downregulation of ARF6 and ARF8 by overexpression of Arabidopsis microRNA167 results in the failure of pollen germination on the stigma surface and/or growth through the style in tomato36. However, the function of SlARF6 in the regulation of fruit development is still not well understood. In this study, SlARF6A was found to be involved in photosynthesis, sugar accumulation and fruit development in tomato. Our data demonstrate that SlARF6A plays an important role in the regulation of fruit quality and development.
Results
Sequence and expression analysis of SlARF6A gene and subcellular localization and transcriptional activity of SlARF6A protein
The SlARF6A gene has an open reading frame of 2608 bp encoding a putative protein of 869 amino acids. Amino acid sequence analysis revealed that, like SlARF7 and SlARF8, which have typical conserved ARF domains, SlARF6A protein also contained B3-DNA, ARF, and AUX/IAA binding domains (Fig. S1). A phylogenetic tree was constructed to gain insight into the phylogenetic relationship among ARF proteins in Arabidopsis and tomato. ARFs were divided into four major classes: I, II, III, and VI37. SlARF6A along with SlARF6B and AtARF6 were grouped into subclass IIa and are closely related to AtARF8 and SlARF8 (Fig. S2), indicating possible functional similarity among them.
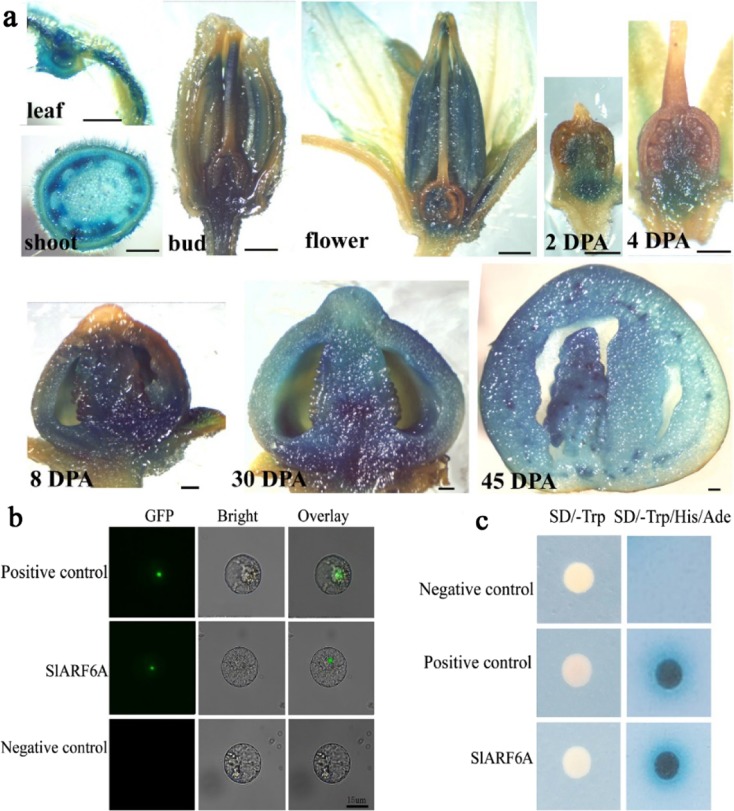
To determine the expression pattern of SlARF6A in planta, a transcriptional fusion was constructed between the SlARF6A promoter and the GUS reporter gene. GUS staining in the transgenic tomato plants was detected in leaves, stems, buds, flowers, and fruits at different developmental stages, an indication of the ubiquitous expression of SlARF6A in all tissues tested. The GUS staining was weak in the early fruits at 2 and 4 days post anthesis (DPA) but became strong at 8, 30 and 45 DPA (Fig. 1a), suggesting possible roles of SlARF6A in the development of tomato fruits.
Fig. 1. Molecular properties of SlARF6A.
a Expression pattern of SlARF6A revealed by the expression of the GUS reporter gene driven by the SlARF6A promoter. Gus staining was conducted using leaf, shoot, bud, flower, and fruit tissues from transgenic plants at 2, 4, 8, 30, and 45 days post anthesis (DPA). The bar is 1 mm. b Subcellular localization analysis of SlARF6A protein. The SlARF6A-GFP fusion protein and GFP-positive and GFP-negative controls (PCXDG-GFP) were transiently expressed in tobacco (Nicotiana benthamiana) leaves. Images were taken in a dark field for green fluorescence, while the outline of the cells and the merged image were recorded in a bright field. The bar is 15 μm. c Transcriptional activation activity of SlARF6A protein. The pGBKT7-SlARF6A fusion vector, negative control (Empty pGBKT7 vector) and positive control were transformed into Y2H gold yeast cells. The yeast cells were cultivated on medium without tryptophan (SD-Trp) or without tryptophan, histidine, and adenine (SD-Trp/His/Ade)
To examine its subcellular localization in plants, SlARF6A was fused to GFP and transferred into tobacco protoplasts. Fluorescence microscopy analysis revealed that SlARF6A was specifically localized in the nuclei (Fig. 1b). A GAL4-responsive reporter system in yeast was employed to reveal the transcriptional activity of SlARF6A. SlARF6A was fused to GAL4-BD (DNA binding domain) to form a pGBKT7-SlARF6A fusion plasmid and subsequently transformed into yeast. Yeast transformants harboring the pGBKT7-SlARF6A construct grew well in the medium lacking Trp, His, and Ade (SD-W/H/A), while the yeasts transformed with pGBKT7 vector alone (negative control) could not (Fig. 1c). Assessing transcriptional activity revealed that SlARF6A is a transcriptional activator.
SlARF6A is involved in chlorophyll accumulation and chloroplast development in tomato
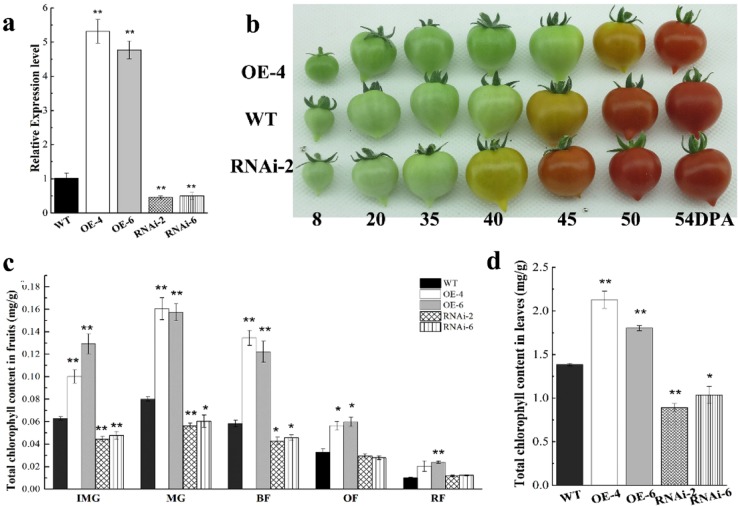
To elucidate the physiological significance of the SlARF6A gene in fruit development, upregulated and downregulated transgenic lines corresponding to independent transformation events were generated in tomato plants. qRT-PCR was used to evaluate the expression level of SlARF6A in all transgenic lines. Compared with the level in the wild type (WT), the expression level of SlARF6A was decreased in RNAi 2 and 6 plants (Fig. 2a) but increased in OE-4 and 6 plants (Fig. 2a).
Fig. 2. Generation of SlARF6A transgenic plants, chlorophyll accumulation, and chloroplast observation in SlARF6A transgenic plants.
a qRT-PCR analysis of the expression of SlARF6A in transgenic lines. The data represent the mean ± SD of four biological replicates. b Fruit phenotypes. WT, wild-type plants; OE, SlARF6A overexpression lines; RNAi, SlARF6A RNAi lines. DPA, days post anthesis. c Chlorophyll contents in fruits of OE-SlARF6A and RNAi-SlARF6A plants. d Chlorophyll contents in leaves of OE-SlARF6A and RNAi-SlARF6A plants. The data represent the mean ± SD of three biological replicates. “*” and “**” are significant differences between transgenic and WT plants at the P < 0.05 and P < 0.01 levels, respectively, as determined by t-test
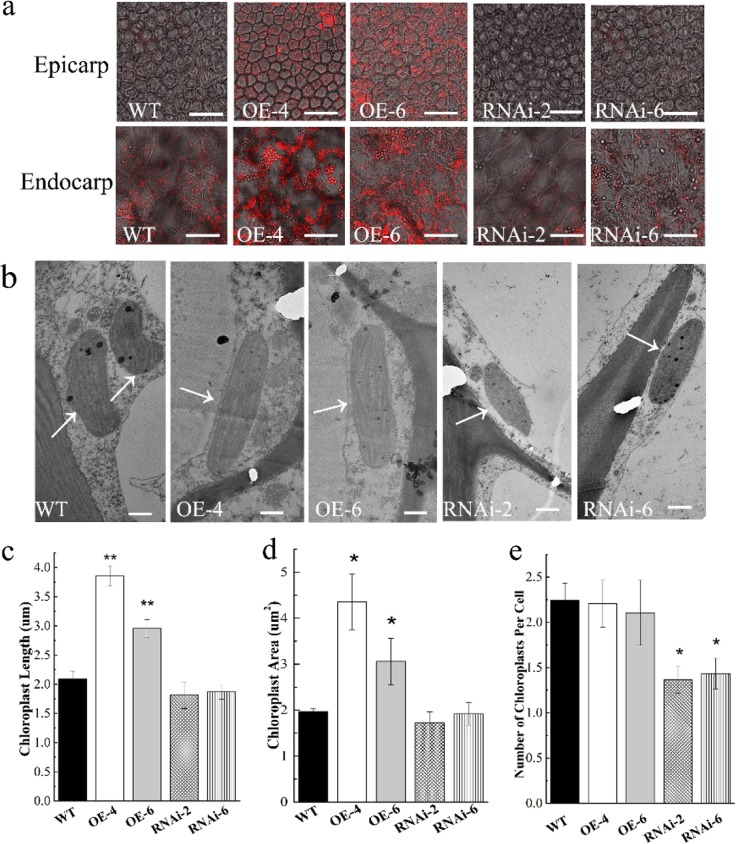
It is noteworthy that altered SlARF6A expression led to a dramatic change in chlorophyll accumulation in transgenic lines. Compared with WT plants, the OE-SlARF6A plants had dark-green fruits at the green fruit stage, whereas the RNAi-SlARF6A plants had light-green fruits (Fig. 2b). The impact of altered SlARF6A expression on chlorophyll accumulation was analyzed by measuring the chlorophyll content in fruits and leaves. The SlARF6A overexpression lines possessed greater accumulation of chlorophyll in the fruits at immature green, mature green, breaker, and orange stages, whereas the RNAi lines had lower chlorophyll accumulation in the fruits at immature green and mature green stages than the WT plants (Fig. 2c). In leaves, the upregulated and downregulated SlARF6A transgenic lines possessed higher and lower chlorophyll levels, respectively, than the WT plants (Fig. 2d). Then, chlorophyll autofluorescence in the pericarp was detected using confocal laser scanning microscopy. OE-SlARF6A plants had stronger chlorophyll autofluorescence, while the RNAi-SlARF6A lines had weaker chlorophyll autofluorescence in both epicarp and endocarp tissues compared with that of the WT plants (Fig. 3a). Then, the chloroplasts were observed using a transmission electron microscope (TEM). The growth of individual chloroplasts in OE-SlARF6A fruits was obviously promoted, with a significant increase in size and length (Fig. 3b). However, the number of chloroplasts per cell in OE-SlARF6A fruits was the same as that in the WT plants. For the RNAi-SlARF6A lines, the number of chloroplasts per cell was obviously decreased, but the size of individual chloroplasts was not changed (Fig. 3c–e).
Fig. 3. Autofluorescence and TEM analysis in transgenic and WT fruits.
a Autofluorescence of chlorophylls in the pericarp of tomato fruits, as determined by confocal laser scanning microscopy. The bar is 10 μm. b TEM analysis of chloroplasts in transgenic and WT fruits. The bar is 10 μm. White arrows indicate chloroplasts. c Chloroplast size analysis. d Chloroplast length analysis. e Number of chloroplasts per cell. The data represent the mean ± SD of three biological replicates. “*” indicates significant differences between transgenic and WT plants at P < 0.05 as determined by t-test
SlARF6A positively affects photosynthesis and photosynthate accumulation in tomato
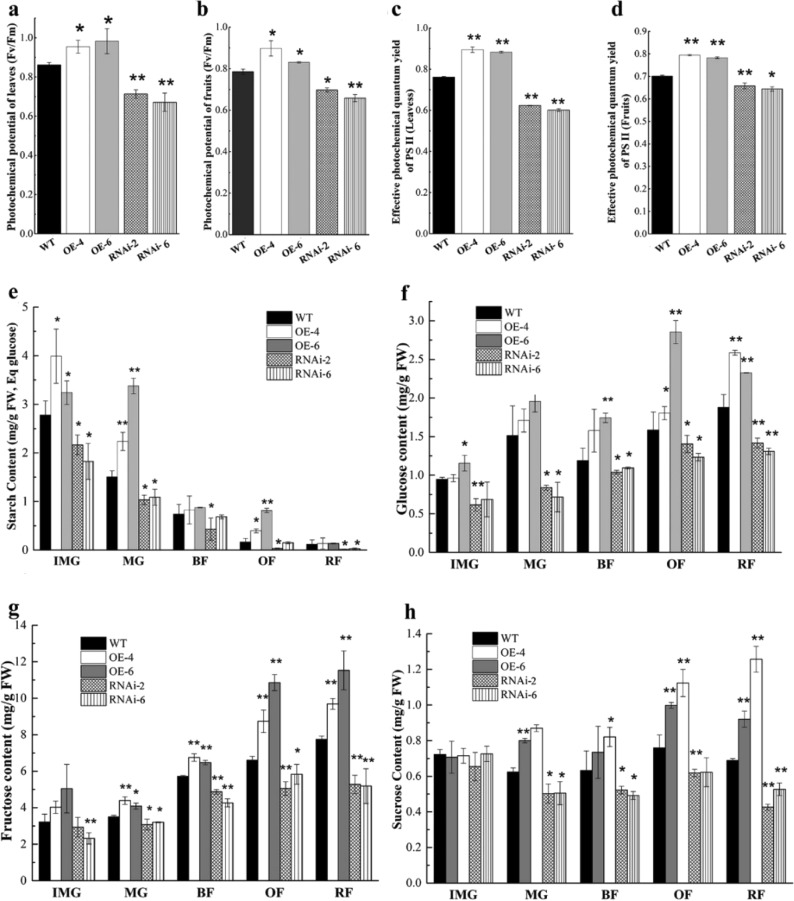
The dark-green phenotype and associated increased chlorophyll content may potentially lead to enhanced photosynthetic performance in tomato plants. The photosynthetic performance in leaves and fruits of SlARF6A transgenic lines was measured. In both leaves and green fruits, the photochemical potential was elevated in OE-SlARF6A lines, whereas the value was decreased in RNAi-SlARF6A plants compared with the WT plants (Fig. 4a, b). The effective photochemical quantum yield of PSII in OE-SlARF6A lines was higher than that of the WT plants in both leaves and fruits, while the values for RNAi-SlARF6A plants were lower than that for the WT plants in both leaves and fruits (Fig. 4c, d). Thus, the SlARF6A gene positively affects photosynthesis in the fruits and leaves of tomato plants.
Fig. 4. Photochemical potential of SlARF6A transgenic plants and accumulation of photosynthetic substances in transgenic fruits.
a Photochemical potential in leaves. b Photochemical potential in fruits. c Effective photochemical quantum yield of PS II in leaves. d, Effective photochemical quantum yield of PS II in fruits. e–h demonstrate the contents of starch (e), glucose (f), fructose (g), and sucrose (h) in transgenic plants, respectively. The data represent the mean ± SD of three biological replicates. “*” and “**” indicate significant differences between the transgenic and WT plants at P < 0.05 and P < 0.01, respectively, as determined by t-test
Sugars are the major products of photosynthesis, so it is essential to evaluate whether the altered chlorophyll level and photosynthetic performance in SlARF6A plants result in altered sugar accumulation. As shown in Fig. 4e, starch levels decreased rapidly throughout fruit development in the transgenic and WT plants. The starch content in OE-SlARF6A fruits was much higher than that in WT fruits at green fruit stages, whereas the starch content in RNAi-SlARF6A fruits was much lower than that in the WT fruits at green stages (Fig. 4e). These data demonstrated that the SlARF6A gene positively affects starch accumulation during green fruit development.
It is well established that starch degradation is the dominant source of soluble sugars in fruits. The contents of fructose, glucose and sucrose were analyzed in SlARF6A transgenic plants. The levels of glucose, fructose and sucrose were significantly higher in the OE-SlARF6A fruits than in the WT fruits, particularly at the orange and red fruit stages (Fig. 4f–h). Compared with the WT fruits, the RNAi-SlARF6A fruits exhibited obviously decreased contents in glucose, fructose and sucrose (Fig. 4f–h). Our data indicated that the SlARF6A gene positively affects the levels of glucose, fructose and sucrose during fruit development.
SlARF6A is involved in fruit ripening and ethylene production in tomato
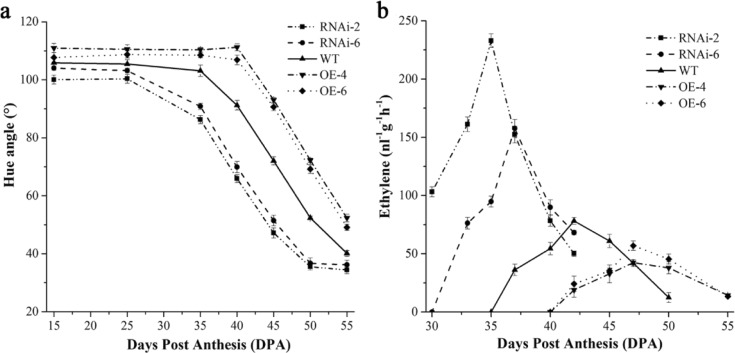
The SlARF6A transgenic plants also exhibited different ripening of fruits than the WT plants. Downregulation of SlARF6A accelerated fruit ripening, with the breaker stage occurring 5 days sooner than that in the WT plants (Fig. 2b), while overexpression delayed the breaker stage by 5 days compared with that of the WT plants (Fig. 2b). The assessment of color change via measurement of the evolution of hue angle values further confirmed the difference between the SlARF6A transgenic lines and WT plants throughout the ripening process (Fig. 5a). The ethylene production was measured using a GC method. When compared with that of the WT plants, the ethylene production of RNAi-SlARF6A plants showed a dramatic induction of ~2-fold and 4-fold at the breaker stage and remained at high levels for 2 and 3 days after the breaker stage, while that of overexpressed lines was inhibited at the breaker stage and remained at low levels for 5 days after the breaker stage compared with the levels in the WT plants (Fig. 5b).
Fig. 5. Altered fruit ripening features of SlARF6A transgenic plants.
a Changes in hue angle in the WT and SlARF6A transgenic plants. b Ethylene production of the WT and SlARF6A transgenic plants at different ripening stages indicated as days post anthesis (DPA). The data represent the means of at least 10 individual fruits. Vertical bars represent SD. In WT plants, 35 DPA corresponds to the mature green (MG) stage, and 40 DPA corresponds to the breaker (BR) stage. In OE-SlARF6A plants, 40 DPA corresponds to the mature green (MG) stage, and 45 DPA corresponds to the breaker (BR) stage. In RNAi-SlARF6A plants, 30 DPA corresponds to the mature green (MG) stage, and 35 DPA corresponds to the breaker (BR) stage
Regulation of SlARF6A expression alters the expression of genes involved in chlorophyll metabolism, photosynthesis and sugar metabolism
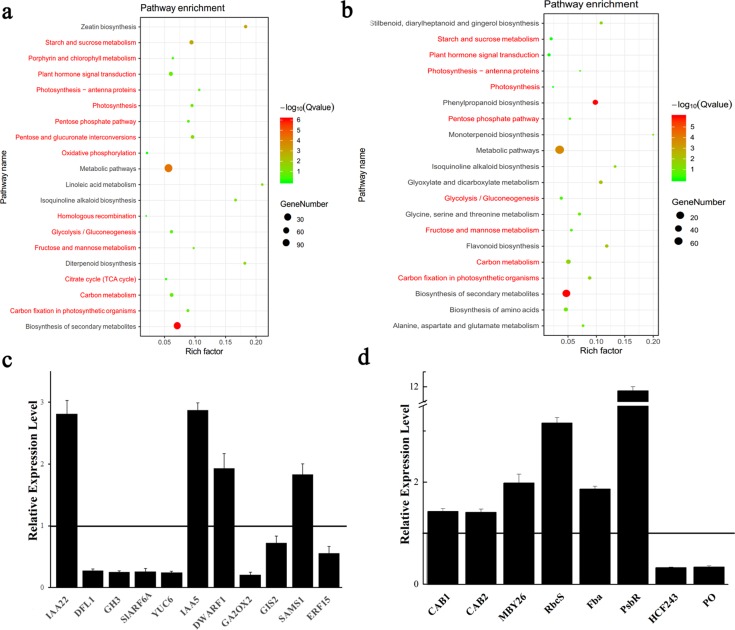
To investigate the molecular mechanism of chlorophyll accumulation, photosynthesis and fruit ripening in SlARF6A transgenic plants, RNA-sequencing (RNA-Seq) was conducted to analyze the differentially expressed genes (DEGs) in OE-SlARF6A and RNAi-SlARF6A plants. Under the criterion of a false discovery rate (FDR) < 0.05, 591 upregulated and 508 downregulated DEGs were identified in 4 DPA ovaries of RNAi-SlARF6A plants, and 254 upregulated and 424 downregulated DEGs were identified in 35 DPA fruits of OE-SlARF6A plants (Table S1). GO function and pathway enrichment analyses showed that knockdown of SlARF6A affected multiple metabolic pathways, including those of porphyrin and chlorophyll metabolism, photosynthesis, photosynthesis-antenna proteins, carbon fixation, starch and sucrose metabolism, fructose and mannose metabolism, and plant hormone signal transduction (Fig. 6a, Table S2). Overexpression of SlARF6A also affected metabolic pathways, including those of photosynthesis, photosynthesis-antenna proteins, carbon fixation, starch and sucrose metabolism, fructose and mannose metabolism, and plant hormone signal transduction (Fig. 6b, Table S3). The expression of two genes encoding chlorophyll A/B binding protein (CAB1 and CAB2) (Solyc02g070950 and Solyc02g071010) was induced in OE-SlARF6A plants. The expression of a gene encoding ribulose bisphosphate carboxylase small chain (RbcS) (Solyc02g085950) was upregulated in OE-SlARF6A plants. Moreover, the expression of a gene encoding SAM synthetase 1 (SAMS1) (Solyc12g099000), which is involved in ethylene biosynthesis, was induced in RNAi-SlARF6A plants. Analysis of the RNA-Seq data also showed that among tomato ARF family genes, only SlARF6A was downregulated in RNAi-SlARF6A plants, indicating the specific knockdown of SlARF6A by the RNAi method. To validate the RNA-Seq results, 11 DEGs in RNAi-SlARF6A plants and 8 DEGs in OE-SlARF6A plants were selected for qRT-PCR analysis, and the results were in accordance with the data from RNA-Seq (Fig. 6c, d), which showed that the results from the RNA-Seq were reproducible and reliable.
Fig. 6. RNA-Seq analysis of SlARF6A transgenic plants.
a Functional categories of differentially expressed genes (DEGs) between WT and RNAi-SlARF6A plants. b Functional categories of differentially expressed genes (DEGs) between WT and OE-SlARF6A plants. c Transcript levels of the genes identified from the RNA-Seq analysis were validated by qRT-PCR in the RNAi-SlARF6A plants (c) and OE-SlARF6A plants (d). The solid line indicates relative expression levels in the WT. The data represent the mean ± SD of four biological replicates
SlARF6A targets the promoters of CAB, RbcS and SlGLK1 genes and positively regulates the expression of these genes
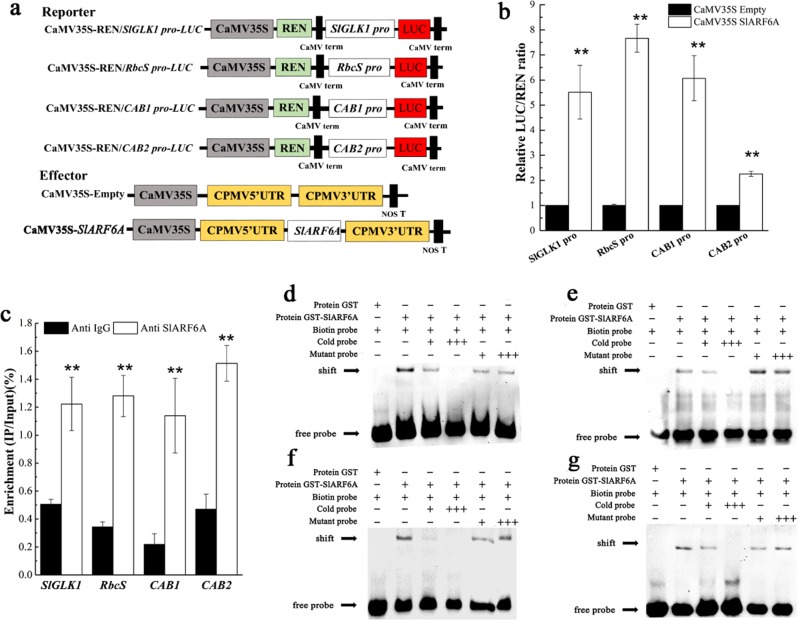
Analysis of the promoter sequences in the CAB and RbcS genes revealed conserved ARF binding sites and TGTCTC boxes. In addition, the chlorophyll phenotypes of SlARF6A overexpression fruits were similar to those in SlGLK overexpressing lines, and the SlGLK1 promoter contained two TGTCTC motifs. qRT-PCR identified that SlARF6A overexpression induced the expression of SlGLK1 and SlGLK2, and knockdown of SlARF6A decreased the expression levels of SlGLK1 and SlGLK2 in fruits and leaves (Fig. S3). Dual-luciferase (LUC) reporter transient expression assays were conducted to examine whether SlARF6A could directly activate or suppress the expression of CAB, RbcS, and SlGLK1 genes. Tobacco leaves were cotransformed with LUC reporter vectors driven by the promoters of CAB, RbcS and SlGLK1 genes together with effector vectors carrying the CaMV35S promoter-driven SlARF6A gene. The results showed that LUC/REN ratios were significantly increased compared with those in the control (Fig. 7a, b). The binding of SlARF6A with the promoters in vivo was verified by ChIP-qPCR analysis. As expected, the promoter sequences containing a motif of TGTCTC in the CAB, RbcS and SlGLK1 genes were significantly enriched with anti-SlARF6A compared with the negative control anti-IgG (Fig. 7c). Furthermore, the direct binding of SlARF6A protein to the promoters of CAB, RbcS, and SlGLK1 was verified by an electrophoretic mobility shift assay (EMSA). We generated a recombinant glutathione S-transferase (GST) fusion protein with truncated SlARF6A (GST-tSlARF6A) (Fig. S4). The purified GST-tSlARF6A fusion protein bound to biotin-labeled probes containing the TGTCTC motif from the promoters of CAB, RbcS, and SlGLK1 and caused a mobility shift. When unlabeled promoter fragments were used as competitors, the mobility shift was efficiently abrogated in a dose-dependent manner (Fig. 7d–g). In addition, as a negative control, the mobility shift was also abolished when biotin-labeled probes were incubated with GST only (Fig. 7d–g). This result demonstrated that SlARF6A targets the promoters of CAB, RbcS, and SlGLK1 genes and positively regulates chlorophyll accumulation, chloroplast development and photosynthesis.
Fig. 7. SlARF6A binds to the promoters of SlGLK1, CAB and RbcS genes and promotes the transcription of these genes.
a Diagrams of the reporter and effector constructs used in the dual-luciferase reporter assay. b In vivo interactions of SlARF6A with the promoters obtained from transient assays in tobacco leaves. The ratio of LUC/REN of the empty vector plus promoter was used as a calibrator (set as 1). Each value represents the mean ± SD of six biological replicates. c ChIP-qPCR assay for direct binding of SlARF6A to the promoters. Values are the percentage of DNA fragments that coimmunoprecipitated with anti-FLAG antibodies or nonspecific antibodies (anti-IgG) relative to the input DNA. The data represent the mean ± SD of four biological replicates. d, e, f, g EMSA showing the binding of SlARF6A to the promoters of CAB1, CAB2, RbcS, and SlGLK1, respectively. Biotin-labeled DNA probes from native promoters or mutants were incubated with GST-SlARF6A protein, and the DNA-protein complexes were separated on 6% native polyacrylamide gels
SlARF6A directly targets the SAMS1 promoter and negatively regulates SAMS1 expression
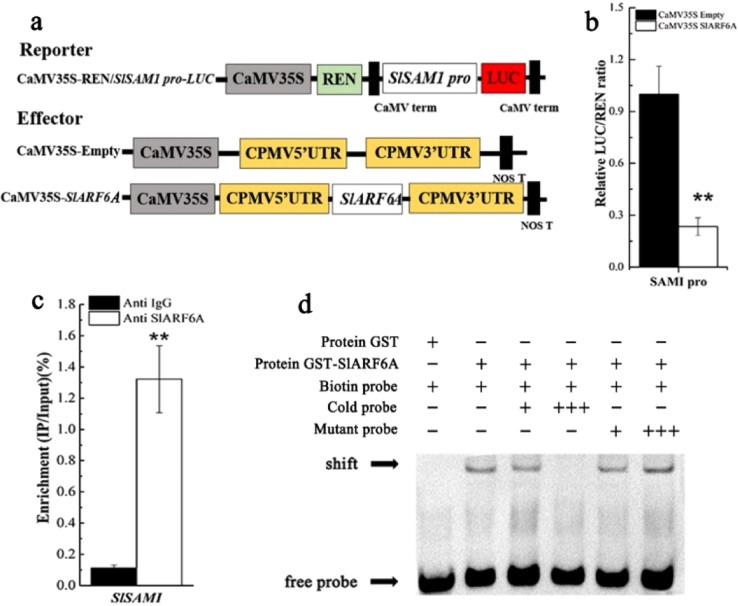
SAMS1 is the key enzyme catalyzing the synthesis of SAM in the ethylene biosynthesis pathway. Motif analysis showed that the SAMS1 promoter contains the conserved ARF binding site, the TGTCTC box. The transient expression assays showed that the LUC/REN ratios were significantly decreased compared with that of the control, suggesting that SlARF6A negatively regulates the expression of SAMS1 genes (Fig. 8a, b). ChIP-qPCR was carried out to confirm the binding of SlARF6A with the SAMS1 promoter in vivo, and the results showed that the promoter sequences containing the TGTCTC of SAMS1 were significantly enriched compared with those with the negative control anti-IgG (Fig. 8c).
Fig. 8. SlARF6A binds to the SAMS1 promoter and inhibits SAMS1 transcription.
a Diagrams of the reporter and effector constructs used in the dual-luciferase reporter assay. b In vivo interactions of SlARF6A with the promoter obtained from transient assays in tobacco leaves. The ratio of LUC/REN of the empty vector plus promoter was used as a calibrator (set as 1). Each value represents the mean ± SD of six biological replicates. c ChIP-PCR assay for direct binding of SlARF6A to the SAMS1 promoter. Values are the percentage of DNA fragments that coimmunoprecipitated with anti-FLAG antibodies or nonspecific antibodies (anti-IgG) relative to the input DNA. The data represent the mean ± SD of four biological replicates. d EMSA showing the binding of SlARF6A to the SAMS1 promoter
The direct binding of SlARF6A protein to the SAMS1 promoter was further verified by EMSA. The results indicated that the SlARF6A protein directly bound to the TGTCTC motif in the SAMS1 promoter (Fig. 8d). Taken together, SlARF6A can target the SAMS1 promoter and negatively regulate the expression of SAMS1 genes. The data demonstrate that SlARF6A plays an important role in ethylene production and fruit ripening.
Discussion
In this study, we functionally characterized the transcription factor SlARF6A in tomato. However, there are two very similar SlARF6 genes in the tomato genome, namely, SlARF6A and SlARF6B. We also examined the function of SlARF6B using genetic approaches and found no obvious phenotypes in the transgenic RNAi and overexpression tomato plants (data not shown). This may be related to the fact that SlARF6B lacks the AUX/IAA domain in the C-terminus of the protein (Fig. S1).
SlARF6A regulates photosynthesis in tomato
Previous studies reported that chlorophyll accumulation increased in Arabidopsis roots when they were detached from shoots, which was repressed by auxin treatment38. Mutant analyses showed that auxin inhibits the accumulation of chlorophyll through the function of IAA14, ARF7, and ARF19 in Arabidopsis38. In tomato, SlARF4 plays an important role as an inhibitor in chlorophyll biosynthesis and sugar accumulation via transcriptional inhibition of SlGLK1 expression in tomato33,34. In this study, overexpression of SlARF6A resulted in enhanced chlorophyll accumulation and chloroplast development, whereas downregulation of SlARF6A decreased chlorophyll accumulation and chloroplast number in tomato (Fig. 3). These results demonstrate that SlARF6A positively regulates chlorophyll accumulation and chloroplast number in tomato. Our study also showed that SlARF6A directly targeted the SlGLK1 promoter and positively regulated SlGLK1 expression (Fig. 7). Nguyen et al. (2014) reported that overexpression of SlGLK1 and SlGLK2 produced dark-green fruits and increased chlorophyll accumulation and chloroplast development8. The fact that the phenotypes of SlGLK1 overexpression plants resembled those described in the OE-SlARF6A plants further suggests that SlARF6A positively regulates SlGLK1 to improve chlorophyll accumulation and chloroplast development in tomato leaves and fruits.
Although SlGLK1 and SlGLK2 have similar functions, SlGLK1 functions largely in leaves, while SlGLK2 functions in fruits8. However, SlGLK2 does not account for the chlorophyll phenotypes in OE and RNAi-SlARF6A plants because the ‘Micro-Tom’ variety possesses two null alleles of SlGLK239. In our study, downregulation of SlARF6A reduced SlGLK1 expression and chlorophyll accumulation, whereas overexpression of SlARF6A increased SlGLK1 expression and chlorophyll accumulation in leaves and fruits of tomato plants (Figs. 2 and 3). The data demonstrate that SlGLK1 may be involved in chlorophyll accumulation not only in tomato leaves but also in fruits. Further study is needed to elucidate the important role of SlGLK1 in tomato fruit using CRISPR/Cas9 technologies.
The chlorophyll a/b-binding proteins (CABs) are the apoproteins of the light-harvesting complex of photosystem II (PSII). CABs are normally complexed with xanthophylls and chlorophyll, functioning as the antenna complex, and are involved in photosynthetic electron transport40. Meng et al. (2018) reported that SlBEL11 directly acted on the promoter of CABs to suppress their transcription10. Silencing of SlBEL11 increased the expression of CAB genes, resulting in enhanced chlorophyll accumulation and stability in thylakoid membranes of chloroplasts in green tomato fruit10. In our study, SlARF6A targeted the promoter of CABs, which positively regulated chlorophyll accumulation, chloroplast development and photosynthesis in tomato (Figs. 2, 3, 4 and 6). Our data further demonstrate important roles of CABs in chloroplast activity and photosynthesis in tomato.
Rubisco, a key enzyme in the fixation of CO2, is the rate-limiting factor in the photosynthesis pathway under conditions of saturating light and atmospheric CO241. The RbcL and RbcS genes encode two subunits that form the Rubisco enzyme42. The RbcL and RbcS genes are localized to the chloroplasts and to the nucleus, respectively43. Our study showed that overexpression of SlARF6A increased the expression of the RbcS gene. Moreover, SlARF6A directly targeted the RbcS promoter and positively regulated RbcS expression (Fig. 7). In addition, SlARF6A positively affected photosynthesis in the fruits and leaves of tomato plants (Fig. 4). Our study demonstrates that SlARF6A has important roles in photosynthesis via the direct regulation of the RbcS gene in tomato.
Interestingly, RNA-Seq data showed that the expression levels of SlARF4 and SlARF10 genes were not altered in RNAi-SlARF6A and OE-SlARF6A plants, suggesting that SlARF6A may act on chlorophyll accumulation independently of SlARF4 and SlARF10. However, studies indicate that ARFs must form dimers on palindromic TGTCTC AuxREs to form a stable complex, leading to the possibility that SlARF6A, SlARF4 and SlARF10 could form dimers with each other to regulate chlorophyll metabolism27. Further study could focus on the interactions among SlARF6A, SlARF4, and SlARF10 to comprehensively elucidate the effects of the transcriptional regulation of ARFs on chlorophyll accumulation in tomato.
SlARF6A regulates photosynthate accumulation in tomato
Downregulation of SlARF4 increased the photosynthesis rate and enhanced the accumulation of starch, glucose and fructose in tomato fruits8. In this study, the increased chlorophyll accumulation and photosynthesis rate in OE-SlARF6A plants resulted in the increased contents of starch and soluble sugars in fruits (Fig. 4). Starch is a dominant factor in the nutrients and flavor of fruits8. AGPase catalyzes the first regulatory step in starch synthesis, converting glucose-1-phosphate and ATP into ADP-glucose44,45. This critical catalytic reaction is also a limiting step during starch biosynthesis in potato (Solanum tuberosum) tubers46. Knockdown of SlARF4 increases the expression of AGPase genes and starch content8. In this study, SlARF6A was positively correlated with the expression of AGPase genes (Fig. S3), suggesting the important role of AGPase genes in starch biosynthesis in tomato. However, the EMSA failed to detect any binding between SlARF6A and the promoters of AGPase genes, even though auxin-responsive motifs were detected in the promoters of AGPase S1 and AGPase S2 genes.
Evidence suggests that sucrose induces the expression of AGPase genes in leaves and fruits in tomato47. In this study, overexpression of SlARF6A led to increased sucrose content in tomato fruits, while the RNAi-SlARF6A fruits displayed decreased sucrose accumulation (Fig. 4). The altered accumulation of starch in OE-SlARF6A and RNAi-SlARF6A lines may be explained by the altered expression of AGPase genes influenced by sucrose in tomato. Overexpression of SlARF6A also resulted in increased glucose and fructose content, which was likely due to the increased starch content degraded into increased contents of soluble sugars in tomato fruits. Our results are consistent with the notion that incipient starch content determines soluble sugars in the process of fruit development48,49. Our study also provides a valuable method to improve the nutritional value of tomato fruits via regulation of SlARF6A expression.
SlARF6A is involved in ethylene production and fruit ripening in tomato
The tomato ARF2A gene was reported to positively regulate fruit ripening50. Overexpression of ARF2A in tomato resulted in blotchy ripening, and silencing of ARF2A led to retarded fruit ripening50. Overexpression of ARF2A in tomato promoted early production of ethylene and expression of ethylene biosynthesis and receptor genes. In this study, SlARF6A negatively regulated fruit ripening and ethylene biosynthesis in tomato fruit (Fig. 5). S-adenosyl-L-methionine (SAM), synthesized by SAM synthetase from ATP and methionine, is a substrate for ethylene biosynthesis (Roje, 2006). SAM is converted to ACC by the ACS enzyme, and ACC is then converted to ethylene by ACO51,52. The level of SAM is tightly controlled to integrate developmental signals into the hormonal control of plant development47,53. In Arabidopsis, overexpression of SAMS1 increases the SAM and ethylene levels, whereas sam1/2 mutants show the opposite phenotype in seedlings54. Similarly, in tomato plants, overexpression of SAMS1 results in higher concentrations of ACC and ethylene compared with those in WT plants55. These data indicate the important role of the SAMS1 gene in ethylene biosynthesis in plants. In this study, SlARF6A directly targeted the SAMS1 promoter and negatively regulated SAMS1 expression (Fig. 8). The regulatory mechanism by which SlARF6A affects fruit ripening and ethylene production in tomato fruits can be explained by the interaction between SlARF6A and the SAMS1 promoter.
It is interesting that ethylene and auxin interact with each other to control some plant developmental processes. For example, ethylene controls root growth through regulation of auxin biosynthesis, transport and signaling56,57, while the formation of hypocotyl apical hooks is also regulated in a similar fashion in Arabidopsis58. In tomato, knockdown of IAA3 results in both auxin and ethylene phenotypes, suggesting that IAA3 might be the molecular connection between ethylene and auxin59. Liu et al. (2018) reported that the ethylene response factor SlERFB3 integrated ethylene and auxin signaling through direct regulation of the Aux/IAA27 gene in tomato59. Our results indicate that SlARF6A negatively regulates ethylene biosynthesis and that the interaction of SlARF6A and SAMS1 represents an important integrative hub mediating ethylene-auxin cross-talk in tomato.
In summary, our results demonstrate that SlARF6A regulates chlorophyll level and chloroplast development by directly binding to the promoters of the SlGLK1, CAB1, and CAB2 genes. SlARF6A also directly targets the RbcS gene promoter, activating RbcS expression and increasing the photosynthetic rate. The increased chlorophyll accumulation and chloroplast activity improve photosynthesis, resulting in the increased accumulation of starch and soluble sugars in tomato. In addition, SlARF6A can act directly on the promoter of SAMS1 and negatively regulate its expression, thereby influencing ethylene production and fruit ripening. The present study provides new insight into the link between auxin signaling, chloroplast activity, and ethylene biosynthesis during tomato fruit development. Our data also provide an effective way to improve fruit nutrition of horticulture crops via regulation of chlorophyll accumulation and photosynthetic activity.
Materials and methods
Plant materials and growth conditions
Tomato plants (Solanum lycopersicum ‘Micro-Tom’) were used in this study. ‘Micro-Tom’ is a popular variety because of its fast turnaround time and easy transformation. The plants were grown on soil under standard greenhouse conditions with a 14-h-day/10-h night cycle, 25 °C/20 °C day/night temperature, 60% relative humidity and 250 mol m–2 s–1 intense light. Transgenic seeds of T1, T2 and T3 generations were screened by sterilizing, rinsing in sterile water, and then transfer into Magenta vessels containing 40 mL of 1/2-strength Murashige and Skoog medium with R3 vitamin (100 mg L–1 kanamycin, 0.5 mg L–1 thiamine, 0.5 mg L–1 pyridoxine and 0.25 mg L–1 nicotinic acid), 0.8% (w/v) agar, and 1.5% (w/v) sucrose, pH 5.9.
Plasmid construction and generation of transgenic plants
DNA fragments, the SlARF6A (Solyc12g006340) promoter, the full-length SlARF6A coding sequence and a partial SlARF6A coding sequence were amplified using tomato genomic DNA or cDNA. The PCR primers used for amplification are detailed in Supplementary Table S3. The SlARF6A promoter sequence was cloned into a pLP100 vector containing the GUS reporter gene. To obtain overexpressed SlARF6A vector, the ORF sequence of SlARF6A was cloned into plant binary vector pLP100 in the sense orientation under the transcriptional control of a cauliflower mosaic virus (CaMV) 35 S promoter. For construction of the RNAi vector, the 200 bp sequences of SlARF6A were amplified and inserted in pCAMIBA2301 under control of the (CaMV) 35S promoter and a nopaline synthase terminator. The resulting transgenic plant was obtained by Agrobacterium tumefaciens-mediated transformation according to Jones et al. (2002)33. All experiments were performed using homozygous lines from the T3 generation.
qRT-PCR analysis
Tomato total RNA was extracted using an RNeasy Plant Mini Kit (Qiagen, Valencia, CA, USA), and qRT-PCR was carried out using All-in-One™ qPCR Mix (GeneCopoeia, Rockville, MD, USA) with a CFX96 real-time PCR detection system (Bio-Rad, Hercules, CA, USA) according to Zhang et al. (2015)32. The relative expression levels of genes were calculated from ΔΔCt values using ubiquitin gene expression as an internal control. The primer sequences used for qRT-PCR are listed in Supplementary Table S3.
GUS staining and analysis
Tissues from SlARF6A promoter-GUS plants were collected and submerged in GUS staining solution (0.1 M sodium phosphate buffer, pH 7.2, 10 mM EDTA). After being infiltrated with GUS staining solution under vacuum for 15 min twice, tissues were incubated in the solution at 37 °C overnight. Then, the samples were washed via a graded ethanol series and observed under a light microscope.
Subcellular localization and transcriptional activation activity of SlARF6A
The ORF sequence of SlARF6A was cloned into a PCX-DG vector to generate the SlARF6A-GFP fusion expression vector. Specific primer sequences are listed in Supplementary Table S1. Suspension-cultured tobacco (Nicotiana tabacum cv. Bright Yellow-2) cells were used to obtain protoplasts that were transfected with the SlARF6A-GFP fusion expression vector. Transformation assays were carried out according to the procedures described by Chaabouni et al. (2009)60.
The ORF sequence of SlARF6A was amplified and fused to the GAL4 DNA-binding (DB) domain to obtain the pGBKT7-SlARF6A fusion construct (DB-SlARF6A). The pGBKT7-SlARF6A vectors were transformed into Y2H gold yeast cells and cultivated on plates in minimal medium without tryptophan (SD-Trp) or without tryptophan, histidine, and adenine (SD-Trp/His/Ade). The transcriptional activation activity was analyzed based on the growth status and α-galactosidase (α-gal) activity.
Chlorophyll analysis and chlorophyll fluorescence parameter measurements
For chlorophyll content determination, the fruits at different developmental stages and leaf tissues were collected and examined based on the methods described by Powell et al. (2012)39. To determine chlorophyll autofluorescence, pericarp was peeled off tomato fruits and observed with a TCS SP2 laser confocal microscope (Leica, Germany). For transmission electron microscopy, pericarp tissues were examined with an FEI Tecnai T12 twin transmission electron microscope according to the method described by Nguyen et al. (2014)8.
For measurements of photosynthesis rates, the green mature fruits and leaves were measured via a PAM-2500 pulse-amplitude modulation fluorometer (Heinz Walz, Effeltrich, Germany). The chlorophyll fluorescence parameter was measured based on the method described by Maury et al. (1996)61.
Extraction and assay of metabolites
For sugar extraction, 1 g of fruit tissue was collected and ground under liquid nitrogen. Subsequently, 10 mL of 80% (v/v) ethanol was used for extraction three times at 80℃ for 30 min. After centrifugation, samples were completely evaporated under vacuum and then dissolved in 4 mL of distilled water. Using the dissolved samples, HPLC was carried out to determine the content of sucrose, fructose and glucose. Starch content determination was performed using fruit pellets. Four milliliters of 0.2 M KOH was used to dissolve the pellet by incubating the sample in a boiling water bath for 30 min. Then, 1.48 mL of 1 M acetic acid (pH 4.5) with 7 units of amyloglucosidase was employed to hydrolyze each sample for 45 min. Finally, 10 mL of distilled water was adopted to dissolve the sample, and then the dissolved sample was used for starch content measurement.
For metabolite measurement, HPLC analysis was conducted using an Agilent 1260 Series liquid chromatograph system (Agilent Technologies, Palo Alto, CA, USA) with a vacuum degasser, an autosampler, a binary pump, and a diode array detector (DAD) controlled by Agilent ChemStation software. A precolumn (Waters XBridge BEH Amide column, 3.9 × 5 mm i.d., 3.5 μm) and a Waters XBridge Amide column (4.6 × 150 mm i.d., 3.5 μm) were used for analysis. The separation was performed via an isocratic solvent system with solvent A (0.2% triethylamine water solution) and solvent B (acetonitrile), while the mobile phase was maintained at 75% B for 15 min for elution. The column temperature was maintained at 38 °C, and the solvent flow rate was 0.6 mL/min. Meanwhile, the injection volume was 10 μL for each sample. With a drift tube temperature at 80 °C, the detection system for HPLC was an ELSD 2000, and air was used as the carrier gas with a flow rate of 2.2 L/min. Finally, the contents of glucose, fructose, sucrose and starch in tomato fruits were determined based on the methods described by Geigenberger et al. (1996)62.
RNA-Seq analysis
The ovaries (4 DPA) of WT and RNAi-SlARF6A plants and the mature green fruits (35 DPA) of WT and OE-SlARF6A plants were collected for RNA-Seq analysis. Total RNA was isolated using a DNeasy Plant Mini Kit (Qiagen, Valencia, CA, USA). RNA-Seq was carried out at the Shanghai Majorbio Biopharm Technology Co., Ltd., as described by Zhang et al. (2015)32. The Illumina HiSeqTM 2000 platform was used according to the manufacturer’s instructions. All clean reads were aligned to the tomato genome (http://solgenomics.net/organism/Solanum_lycopersicum/genome) using TopHat (http://tophat.cbcb.umd). Transcript abundance was normalized by the fragments per kilobase of exon per million mapped reads (FRKM) method using Cuffdiff software (http://cole-trapnell-lab.github.io/cufflinks/). A false discovery rate (FDR) of less than 0.05 was used as the threshold for differentially expressed genes (DEGs). GO functional enrichment and KEGG pathway analysis were carried out using goatools (https://github.com/tanghaibao/goatools) and KOBAS (http://kobas.cbi.pku.edu.cn/home.do). Pathway enrichment was analyzed using the Benjamini and Hochberg correction method with FDR < 0.05.
Promoter analysis and dual-luciferase transient expression assay
For promoter analysis, PLACE signal scan search software (http://www.dna.affrc.go.jp/PLACE/signalscan.html) was used to analyze the motifs of target genes. A dual-luciferase transient expression assay for SlARF6A was carried out using tobacco leaves (Nicotiana benthamiana). For effector vector construction, the full coding sequence of SlARF6A was amplified and then cloned into the pGreenII 62-SK vector63. For reporter vector construction, the promoters of SlGLK1, CAB, RbcS, and SAMS1 genes were cloned into a pGreenII 0800-LUC vector (Hellens et al., 2005)63. The primer sequences used for the vector construct are shown in Supplementary Table S3. A dual-luciferase assay kit (Promega, USA) was employed to measure the activities of LUC and REN luciferase according to the manufacturer’s instructions via a Luminoskan Ascent microplate luminometer (Thermo Fisher Scientific, USA). For each pair of vectors, six biological repeats were performed.
Protein expression and EMSA
The nucleotide sequence of the putative DNA-binding domain of SlARF6A (from 1 to 978 bp) was amplified and fused to that of the glutathione S-transferase (GST) tag in a pGEX-4T-1 bacterial expression vector (GE Healthcare Life Science, China) and expressed using Escherichia coli strain BM Rosetta (DE3). Isopropyl-β-D-thiogalactopyranoside (1 mM) was used to induce recombinant protein expression, and a GST-Tagged Protein Purification Kit (Clontech, USA) was used to purify the protein. Purified recombinant proteins and biotin-labeled fragments of the target promoters were used to conduct EMSA with a LightShift Chemiluminescent EMSA kit (Thermo Fisher Scientific, USA) based on the method described in detail by Han et al. (2016)64. The Pierce Biotin 3’ End DNA Labeling Kit (Thermo Fisher Scientific, USA) was employed to label the probe containing the TGTCTC sequence with biotin. The unlabeled same sequence was used in the assay as a competitor. To generate the mutant probe, the TGTCTC DNA fragment was changed to AAAAAA. Biotin-labeled DNA was assayed via a ChemiDoc™ MP Imaging System (Bio-Rad, USA) based on the manufacturer’s procedures. All primers for the EMSA are listed in Supplementary Table S3.
Chromatin immunoprecipitation
A ChIP-qPCR assay was carried out based on the method described in detail by Qin et al. (2012)65. All primer sequences used in this analysis are listed in Table S3.
Supplementary information
Acknowledgements
This work was supported by the National Key R&D Program of China (2016YFD0400100), the Project of Chongqing Science and Technology Commission (CSTC2015JCYJA80018) and the National Natural Science Foundation of China (31272165). We greatly appreciate Prof. Jianye Chen and Prof. Jianfei Kuang for consulting on some experimental design and providing instrumentation. We greatly appreciate Prof. Daqi Fu for helpful discussion and comments and Ayla Norris for revising the manuscript.
Author contributions
W.D., Y.Y., X.X., C.J. and Z.G. contributed to the experimental design and data analysis; F.Y., Y.T., Z.X. and X.H. contributed to chlorophyll analysis; Q.Z. and F.Y. contributed to sugar measurement; and W.Z., L.M. and Y.L. contributed to RNA-Seq data analysis. All authors edited the combined manuscript. W.D., Y.Y. and C.J. finalized the article.
Conflict of interst
The authors declare that they have no conflict of interest.
Supplementary information
Supplementary Information accompanies this paper at (10.1038/s41438-019-0167-x).
References
- 1.Klee HJ, Giovannoni JJ. Genetics and control of tomato fruit ripening and quality attributes. Annu. Rev. Genet. 2011;45:41–59. doi: 10.1146/annurev-genet-110410-132507. [DOI] [PubMed] [Google Scholar]
- 2.Ho, L. C. & Hewitt, J. D. Fruit development. (eds. Atherton, J. G. & Rudich, J.) The Tomato Crop. 201–240 (Chapman and Hall, London, 1986).
- 3.Schaffer AA, Petreikov M. Sucrose-to-starch metabolism in tomato fruit undergoing transient starch accumulation. Plant Physiol. 1997;113:739–746. doi: 10.1104/pp.113.3.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nadakuduti SS, Holdsworth WL, Klein CL, Barry CS. KNOX genes influence a gradient of fruit chloroplast development through regulation of GOLDEN2-LIKE expression in tomato. Plant J. 2014;78:1022–1033. doi: 10.1111/tpj.12529. [DOI] [PubMed] [Google Scholar]
- 5.Kolotilin I, et al. Transcriptional profiling of high pigment 2(dg) tomato mutant links early fruit plastid biogenesis with its overproduction of phytonutrients. Plant Physiol. 2007;145:389–401. doi: 10.1104/pp.107.102962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rohrmann J, et al. Combined transcription factor profiling, microarray analysis and metabolite profiling reveals the transcriptional control of metabolic shifts occurring during tomato fruit development. Plant J. 2011;68:999–1013. doi: 10.1111/j.1365-313X.2011.04750.x. [DOI] [PubMed] [Google Scholar]
- 7.Waters MT, Moylan EC, Langdale JA. GLK transcription factors regulate chloroplast development in a cell-autonomous manner. Plant J. 2008;56:432–444. doi: 10.1111/j.1365-313X.2008.03616.x. [DOI] [PubMed] [Google Scholar]
- 8.Nguyen CV, et al. Tomato Golden 2-like (GLK) transcription factors reveal molecular gradients functioning during fruit development and ripening. Plant Cell. 2014;26:585–601. doi: 10.1105/tpc.113.118794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pan Y, et al. Network inference analysis identifies an APRR2-like gene linked to pigment accumulation in tomato and pepper fruits. Plant Physiol. 2013;161:1476–1485. doi: 10.1104/pp.112.212654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meng LH, et al. BEL1-LIKE HOMEODOMAIN11 regulates chloroplast development and chlorophyll synthesis in tomato fruit. Plant J. 2018;94:1126–1140. doi: 10.1111/tpj.13924. [DOI] [PubMed] [Google Scholar]
- 11.Li S, et al. The RIN-MC fusion of MADS-box transcription factors has transcriptional activity and modulates expression of many ripening genes. Plant Physiol. 2018;176:891–909. doi: 10.1104/pp.17.01449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao, Y. et al. A NAC transcription factor, NOR-like1, is a new positive regulator of tomato fruit ripening. Hortic. Res. 5, 75 (2018). [DOI] [PMC free article] [PubMed]
- 13.Roje S. S-Adenosyl-L-methionine: beyond the universal methyl group donor. Phytochemistry. 2006;67:1686–1698. doi: 10.1016/j.phytochem.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 14.Barry CS, Llop-Tous MI, Grierson D. The regulation of 1-aminocyclopropane-1-carboxylic acid synthase gene expression during the transition from system-1 to system-2 ethylene synthesis in tomato. Plant Physiol. 2000;123:979–986. doi: 10.1104/pp.123.3.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manning K, et al. A naturally occurring epigenetic mutation in a gene encoding an SBP-box transcription factor inhibits tomato fruit ripening. Nat. Genet. 2006;38:948–952. doi: 10.1038/ng1841. [DOI] [PubMed] [Google Scholar]
- 16.Itkin M, et al. TOMATO AGAMOUS-LIKE 1 is a component of the fruit ripening regulatory network. Plant J. 2009;60:1081–1095. doi: 10.1111/j.1365-313X.2009.04064.x. [DOI] [PubMed] [Google Scholar]
- 17.Chung MY, et al. A tomato (Solanum lycopersicum) APETALA2/ERF gene, SlAP2a, is a negative regulator of fruit ripening. Plant J. 2010;64:936–947. doi: 10.1111/j.1365-313X.2010.04384.x. [DOI] [PubMed] [Google Scholar]
- 18.Karlova R, et al. Transcriptome and metabolite profiling show that APETALA2a is a major regulator of tomato fruit ripening. Plant Cell. 2011;23:923–941. doi: 10.1105/tpc.110.081273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bemer M, et al. The tomato FRUITFULL homologs TDR4/FUL1 and MBP7/FUL2 regulate ethylene-independent aspects of fruit ripening. Plant Cell. 2012;24:4437–4451. doi: 10.1105/tpc.112.103283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao Y, et al. Diversity and redundancy of the ripening regulatory networks revealed by the fruitENCODE and the new CRISPR/Cas9 CNR and NOR mutants. Hortic. Res. 2019;6:39. doi: 10.1038/s41438-019-0122-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu M, et al. The chimeric repressor version of an Ethylene Response Factor (ERF) family member, Sl-ERF.B3, shows contrasting effects on tomato fruit ripening. New Phytol. 2014;203:206–218. doi: 10.1111/nph.12771. [DOI] [PubMed] [Google Scholar]
- 22.De Jong M, Wolters-Arts M, Feron R, Mariani C, Vriezen WH. The Solanum lycopersicum auxin response factor 7 (SlARF7) regulates auxin signaling during tomato fruit set and development. Plant J. 2009;57:160–170. doi: 10.1111/j.1365-313X.2008.03671.x. [DOI] [PubMed] [Google Scholar]
- 23.Devoghalaere F, et al. A genomics approach to understanding the role of auxin in apple (Malus x domestica) fruit size control. BMC Plant Biol. 2012;12:7. doi: 10.1186/1471-2229-12-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ulmasov T, Hagen G, Guifoyle T. Dimerization and DNA binding of auxin response factors. Plant J. 1999;19:309–319. doi: 10.1046/j.1365-313X.1999.00538.x. [DOI] [PubMed] [Google Scholar]
- 25.Guilfoyle TJ, Hagen G. Auxin response factors. Curr. Opin. Plant. Biol. 2007;10:453–460. doi: 10.1016/j.pbi.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 26.Guilfoyle TJ, Hagen G. Getting a grasp on domain III/IV responsible for Auxin Response Factor–IAA protein interactions. Plant Sci. 2012;190:82–88. doi: 10.1016/j.plantsci.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 27.Zouine M, et al. Characterization of the tomato ARF gene family uncovers a multi-levels post-transcriptional regulation including alternative splicing. PLoS ONE. 2014;9:e84203. doi: 10.1371/journal.pone.0084203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Y, et al. Diversification, phylogeny and evolution of auxin response factor (ARF) family: insights gained from analyzing maize ARF genes. Mol. Biol. Rep. 2012;39:2401–2415. doi: 10.1007/s11033-011-0991-z. [DOI] [PubMed] [Google Scholar]
- 29.Guan XX, et al. Temporal and spatial distribution of auxin response factor genes during tomato flower abscission. J. Plant. Growth Regul. 2013;33:17–327. [Google Scholar]
- 30.Liu X, et al. AUXIN RESPONSE FACTOR 3 integrates the functions of AGAMOUS and APETALA2 in floral meristem determinacy. Plant J. 2014;80:629–641. doi: 10.1111/tpj.12658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ckurshumova W, Smirnova T, Marcos D, Zayed Y, Berleth T. Irrepressible MONOPTEROS/ARF5 promotes de novo shoot formation. New Phytol. 2014;204:556–566. doi: 10.1111/nph.13014. [DOI] [PubMed] [Google Scholar]
- 32.Zhang XL, et al. Auxin response gene SlARF3 plays multiple roles in tomato development and is involved in the formation of epidermal cells and trichomes. Plant Cell Physiol. 2015;56:2110–2124. doi: 10.1093/pcp/pcv136. [DOI] [PubMed] [Google Scholar]
- 33.Jones B, et al. Down-regulation of DR12, an auxin-response-factor homolog, in the tomato results in a pleiotropic phenotype including dark green and blotchy ripening fruit. Plant J. 2002;32:603–613. doi: 10.1046/j.1365-313X.2002.01450.x. [DOI] [PubMed] [Google Scholar]
- 34.Sagar M, et al. Sl-ARF4, an auxin response factor involved in the control of sugar metabolism during tomato fruit development. Plant Physiol. 2013;161:1362–1374. doi: 10.1104/pp.113.213843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yuan YJ, et al. SlARF10, an auxin response factor, is involved in chlorophyll and sugar accumulation during tomato fruit development. J. Exp. Bot. 2018;69:5507–5518. doi: 10.1093/jxb/ery328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu N, et al. Down-regulation of AUXIN RESPONSE FACTORS 6 and 8 by microRNA 167 leads to floral development defects and female sterility in tomato. J. Exp. Bot. 2014;65:2507–2520. doi: 10.1093/jxb/eru141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krogan NT, Ckurshumova W, Marcos D, Caragea AE, Berleth T. Deletion of MP/ARF5 domains III and IV reveals a requirement for Aux/IAA regulation in Arabidopsis leaf vascular patterning. New Phytol. 2011;194:391–401. doi: 10.1111/j.1469-8137.2012.04064.x. [DOI] [PubMed] [Google Scholar]
- 38.Kobayashi K, et al. Regulation of root greening by light and auxin/cytokinin signaling in Arabidopsis. Plant Cell. 2012;24:1081–1095. doi: 10.1105/tpc.111.092254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Powell ALT, et al. Uniform ripening encodes a Golden 2-like transcription factor regulating tomato fruit chloroplast development. Science. 2012;336:1711–1715. doi: 10.1126/science.1222218. [DOI] [PubMed] [Google Scholar]
- 40.Jansson S. The light-harvesting chlorophyll a/b-binding proteins. BBA-Biomembr. 1994;1184:1–19. doi: 10.1016/0005-2728(94)90148-1. [DOI] [PubMed] [Google Scholar]
- 41.Patel M, Berry JO. Rubisco gene expression in C4 plants. J. Exp. Bot. 2008;59:1625–1634. doi: 10.1093/jxb/erm368. [DOI] [PubMed] [Google Scholar]
- 42.Spreitzer ME, Salvucci RJ. Rubisco: structure, regulatory interactions, and possibilities for a better enzyme. Ann. Rev. Plant Biol. 2002;53:449–475. doi: 10.1146/annurev.arplant.53.100301.135233. [DOI] [PubMed] [Google Scholar]
- 43.Sasanuma T. Characterization of the rbcS multigene family in wheat: subfamily classification, determination of chromosomal location and evolutionary analysis. Mol. Gen. Genet. 2001;265:161–171. doi: 10.1007/s004380000404. [DOI] [PubMed] [Google Scholar]
- 44.Stark DM, Timmerman KP, Barry GF, Preiss J, Kishore GM. Regulation of the amount of starch in plant tissues by ADP glucose pyrophosphorylase. Science. 1992;258:287–292. doi: 10.1126/science.258.5080.287. [DOI] [PubMed] [Google Scholar]
- 45.Yin YG, et al. Salinity induces carbohydrate accumulation and sugar-regulated starch biosynthetic genes in tomato (Solanum lycopersicum L. cv.‘Micro-Tom’) fruits in an ABA-and osmotic stress-independent manner. J. Exp. Bot. 2009;61:563–574. doi: 10.1093/jxb/erp333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tiessen A, et al. Starch synthesis in potato tubers is regulated by post-translational redox modification of ADP-glucose pyrophosphorylase: A noble regulatory mechanism linking starch synthesis to the sucrose supply. Plant Cell. 2002;14:2191–2213. doi: 10.1105/tpc.003640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sauter M, Moffatt B, Saechao MC, Hell R, Wirtz M. Methionine salvage and S-adenosylmethionine: essential links between sulfur, ethylene and polyamine biosynthesis. Biochem. J. 2013;451:145–154. doi: 10.1042/BJ20121744. [DOI] [PubMed] [Google Scholar]
- 48.Schaffer AA, et al. ADP glucose pyrophosphorylase activity and starch accumulation in immature tomato fruit: the effect of a Lycopersicon hirsutum-derived introgression encoding for the large subunit. Plant Sci. 2000;152:135–144. doi: 10.1016/S0168-9452(99)00224-1. [DOI] [Google Scholar]
- 49.Baxter CJ, et al. Fruit carbohydrate metabolism in an introgression of tomato with increased fruit soluble solids. Plant Cell Physiol. 2005;46:425–437. doi: 10.1093/pcp/pci040. [DOI] [PubMed] [Google Scholar]
- 50.Breitel DA, et al. AUXIN RESPONSE FACTOR 2 intersects hormonal signals in the regulation of tomato fruit ripening. PLoS. Genet. 2016;12:e1005903. doi: 10.1371/journal.pgen.1005903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang KL, Li H, Ecker JR. Ethylene biosynthesis and signaling networks. Plant Cell. 2002;14:S131–S151. doi: 10.1105/tpc.001768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang SF, Hoffman NE. Ethylene biosynthesis and its regulation in higher plants. Ann. Rev. Plant Physiol. 1984;35:155–189. doi: 10.1146/annurev.pp.35.060184.001103. [DOI] [Google Scholar]
- 53.Van de Poel B, et al. Targeted systems biology profiling of tomato fruit reveals coordination of the Yang cycle and a distinct regulation of ethylene biosynthesis during post-climacteric ripening. Plant Physiol. 2012;160:1498–1514. doi: 10.1104/pp.112.206086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mao D, et al. FERONIA receptor kinase interacts with S-adenosylmethionine synthetase and suppresses S-adenosylmethionine production and ethylene biosynthesis in Arabidopsis. Plant Cell Environ. 2015;38:2566–2574. doi: 10.1111/pce.12570. [DOI] [PubMed] [Google Scholar]
- 55.Gong B, et al. Overexpression of S-adenosyl-L-methionine synthetase increased tomato tolerance to alkali stress through polyamine metabolism. Plant Biotechnol. J. 2014;12:694–708. doi: 10.1111/pbi.12173. [DOI] [PubMed] [Google Scholar]
- 56.Ruzicka K, et al. Ethylene regulates root growth through effects on auxin biosynthesis and transport-dependent auxin distribution. Plant Cell. 2007;19:2197–2212. doi: 10.1105/tpc.107.052126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stepanova AN, Yun J, Likhacheva AV, Alonso JM. Multilevel interactions between ethylene and auxin in Arabidopsis roots. Plant Cell. 2007;19:2169–2185. doi: 10.1105/tpc.107.052068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Muday GK, Rahman A, Binder BM. Auxin and ethylene: collaborators or competitors? Trends Plant. Sci. 2012;17:181–195. doi: 10.1016/j.tplants.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 59.Liu M, et al. The tomato Ethylene Response Factor Sl-ERF.B3 integrates ethylene and auxin signaling via direct regulation of Sl-Aux/IAA27. New Phytol. 2018;219:631–640. doi: 10.1111/nph.15165. [DOI] [PubMed] [Google Scholar]
- 60.Chaabouni S, et al. Sl-IAA3, a tomato Aux/IAA at the crossroads of auxin and ethylene signaling involved in differential growth. J. Exp. Bot. 2009;60:1349–1362. doi: 10.1093/jxb/erp009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Maury P, Mojayad F, Berger M, Planchon C. Photochemical response to drought acclimation in two sunflower genotypes. Physiol. Plant. 1996;98:57–66. doi: 10.1111/j.1399-3054.1996.tb00675.x. [DOI] [Google Scholar]
- 62.Geigenberger P, Lerchi J, Stitt M, Sonnewald U. Phloem-specific expression of pyrophosphatase inhibits long distance transport of carbohydrates and amino acids in tobacco plants. Plant Cell Environ. 1996;19:43–55. doi: 10.1111/j.1365-3040.1996.tb00225.x. [DOI] [Google Scholar]
- 63.Hellens RP, et al. Transient expression vectors for functional genomics, quantification of promoter activity and RNA silencing in plants. Plant. Methods. 2005;1:13. doi: 10.1186/1746-4811-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Han YC, et al. Banana transcription factor MaERF11 recruits histone deacetylase MaHDA1 and represses the expression of MaACO1 and expansins during fruit ripening. Plant Physiol. 2016;171:1070–1084. doi: 10.1104/pp.16.00301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Qin G, Wang Y, Cao B, Wang W, Tian S. Unraveling the regulatory network of the MADS box transcription factor RIN in fruit ripening. Plant J. 2012;70:243–255. doi: 10.1111/j.1365-313X.2011.04861.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.