Abstract
Renal cancer represents 2% to 3% of all cancers, and its incidence is rising. The increased use of ultrasonography and cross-sectional imaging has resulted in the clinical dilemma of incidentally detected small renal masses (SRMs). SRMs represent a heterogeneous group of tumors that span the full spectrum of metastatic potential, including benign, indolent, and more aggressive tumors. Currently, no composite model or biomarker exists that accurately predicts the diagnosis of kidney cancer before treatment selection, and the use of renal mass biopsy remains controversial. The management of SRMs has changed dramatically over the last two decades as our understanding of tumor biology and competing risks of mortality in this population has improved. In this review, we critically assess published consensus guidelines and recent literature on the diagnosis and management of SRMs, with a focus on patient treatment selection and use of renal mass biopsy, active surveillance, and thermal ablation. Finally, we highlight important opportunities for leveraging recent research discoveries to identify patients with SRMs at high risk for renal cell carcinoma–related mortality and minimize overtreatment and patient morbidity.
EPIDEMIOLOGY OF SMALL RENAL MASSES
Although the true incidence of renal masses (including benign lesions) is unknown, the incidence of renal cell carcinoma (RCC) has steadily increased in the United States and worldwide in recent decades, with stage 1 tumors (≤ 7 cm) now accounting for 40% to 50% of new patients with RCC .1 The increasing incidence of RCC parallels an increase in the use of axial imaging.2 A small renal mass (SRM) is defined as an incidentally detected, contrast-enhancing solid or cystic lesion that is ≤ 4 cm, consistent with clinical stage T1a RCC.3 Among surgically treated SRMs, 80% are malignant; however, most are low-grade, early-stage tumors, and the remaining 20% are benign.4 Despite earlier detection and treatment, epidemiologic studies have demonstrated stable RCC mortality, which suggests possible overdiagnosis and overtreatment.5
PATIENT TREATMENT SELECTION
Because of the early detection of SRMs, patients in contemporary series rarely present with local or systemic symptoms (eg, hematuria).6 The prognosis for a patient with an incidentally detected SRM (pT1a) is favorable, with an estimated 5-year cancer-specific survival (CSS) of 95% to 100%. However, those who subsequently develop metastases (2% of SRMs) face a poor prognosis (5-year CSS of 5% to 10%).7
Differential Diagnosis
Contrast-enhancing solid or cystic SRMs are considered suggestive of RCC. The differential diagnosis also includes benign renal lesions (eg, lipid-poor angiomyolipoma [AML], oncocytoma), and rarely, lymphoma, metastasis from another cancer, and sarcoma. Among the 80% of SRMs that are malignant, 20% are high grade or locally invasive (invasion of perinephric fat or venous structures) and the remainder have limited metastatic potential (eg, low grade, chromophobe, type I papillary RCC).4
Clinical Evaluation
Smoking, obesity, hypertension, and diabetes are documented risk factors for incident RCC and for the metabolic syndrome, cardiovascular disease, and renovascular disease.8 Therefore, clinicians should assess overall health and performance status (eg, Charlson Comorbidity Index). Baseline creatinine and urine dipstick to assess for proteinuria should be used to assign a chronic kidney disease (CKD) stage.
Imaging Characteristics Used to Assign Risk Stratification
Multiphasic enhanced imaging with either magnetic resonance imaging (MRI) or computed tomography (CT) is preferred for initial characterization of SRMs. SRMs should be characterized as either predominantly cystic or solid. Cystic masses are then classified using the Bosniak system (I-IV).9 Overall, cystic RCC displays an indolent course regardless of size and Bosniak category.10 CT and MRI can also reliably discriminate AMLs.11 However, lipid-poor AMLs are difficult to differentiate from clear-cell RCC (ccRCC).12 The vast majority of sporadic AMLs demonstrate a negligible growth rate and are asymptomatic; therefore, AMLs can be safely observed.11 CT and MRI protocols can also help distinguish ccRCC from oncocytoma and papillary and chromophobe RCC; however, the accuracy and generalizability of the results are not entirely reliable.13,14 CT and MRI are also limited in their ability to differentiate which SRMs will be locally invasive (pT3).15,16 The value of [18F]fluorodeoxyglucose–positron emission tomography in RCC remains to be determined.17
Defining Patient Risk Assessment
Because of competing risks of mortality, especially among elderly and comorbid patients, nomograms have been developed to improve risk prediction of non-RCC mortality. Kutikov et al18,19 demonstrated that as age and Charlson Comorbidity Index increase among patients with localized RCC (T1-4N0M0), the risk of noncancer-specific mortality increases significantly. These predictive nomograms were generated using an older (≥ 66 years) and surgically treated population, making them less applicable to younger and less comorbid patients. Despite improvements in risk stratification, population-based studies show that only a small proportion of older patients (≥ 70 years old) diagnosed with SRMs are managed with active surveillance (AS) or watchful waiting.20
Approximately 10% to 52% of patients with localized RCC have CKD (estimated glomerular filtration rate [eGFR] < 60 mL/min/1.73m2) at the time of diagnosis. Protein detected on urine dipstick (2+ on dipstick) should trigger quantitative measurement with 24-urine protein or albumin-to-creatinine ratio. Patients with preexisting CKD or proteinuria have decreased overall survival (OS) and are at increased risk for progressive decline in renal function after treatment.21 Guidelines suggest pretreatment referral to nephrology for patients at high risk for CKD progression (eGFR < 45, confirmed proteinuria, patients with diabetes with preexisting CKD, or if eGFR after surgery will be ≤ 30 mL/min/1.73 m2).22 Clinicians can also consider split renal function assessment with renal scintigraphy for patients with compromised renal function or multiple/bilateral tumors.23
Defining Oncologic and Treatment Risk
Pretreatment nomograms have been developed to improve risk prediction of malignancy, histology, morbidity, and survival. In a recent Agency for Healthcare Research and Quality (AHRQ) comprehensive review and meta-analysis (clinical stage T1 to T2), the strongest factors predictive of malignant pathology were tumor size and sex.24 Among 12 studies (n = 9,401), each centimeter increase in tumor size was associated with a 33% increased risk of malignancy.24 On the basis of tumor size alone, the risk of malignancy and metastases varies significantly within the category of an SRM.7 Among 16 studies (n = 10,475), men were more likely to harbor malignant pathology (odds ratio, 2.7; 95% CI, 2.39 to 3.02). The strength of evidence was low for symptoms at presentation, age, and body mass index.24 Using clinical (age, sex, smoking) and radiographic (tumor size) characteristics, Lane et al25 created a preoperative nomogram (n = 851) that achieved a concordance index of 0.64 for benign pathology in tumors ≤ 7 cm. Among 2,517 patients with localized RCC (median size, 5.3 cm; range, 0.5 to 20 cm), Raj et al.26 demonstrated that using preoperative imaging (lymphadenopathy, necrosis, tumor size) and clinical (sex, mode of presentation [incidental v systemic]) characteristics achieved a concordance index of 0.80 for 12-year metastases-free survival. Anatomic classification scores have also been used to help standardize treatment reporting, assist in treatment selection, and predict complications (eg, R.E.N.A.L nephrometry score27). Increasing R.E.N.A.L nephrometry score has been shown to improve risk prediction of malignancy, grade, and pathologic upstaging.27
RENAL MASS BIOPSY
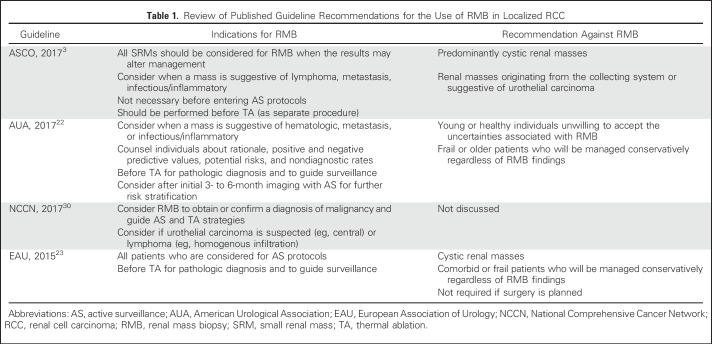
The role of renal mass biopsy (RMB) in the management of SRMs remains controversial because of concerns over diagnostic accuracy, safety, and capacity to affect clinical management.28 The perceived benefit of RMB is to inform risk stratification, prevent overtreatment of benign/low-grade lesions, and guide treatment selection. The use of RMB has increased over time, with the highest use demonstrated in thermal ablation (TA) or systemic therapy and approximately one in five patients undergoing radical nephrectomy (RN) or partial nephrectomy (PN).29 The current guideline recommendations for RMB are listed in Table 1.
Table 1.
Review of Published Guideline Recommendations for the Use of RMB in Localized RCC
Technical Aspects
RMB can be performed under ultrasound or CT guidance with similar diagnostic yield.23 Core biopsy (CB) has superior diagnostic rates compared with fine-needle aspiration alone; however, there does seem to be added diagnostic utility in the combination of a fine-needle aspiration smear with CB.31,32 Guidelines recommend multiple cores (two to three) with a 16- to 18-gauge core needle.22 However, an ex vivo comparison of 14-, 18-, and 20-gauge needle biopsies demonstrated that a minimum of an 18-gauge needle resulted in the most accurate histologic diagnosis.33 Overall, protocols and definitions of success in RMB series vary, making studies difficult to compare.
Accuracy
Two recent reviews have comprehensively evaluated the accuracy and harms of RMB.24,31 Marconi et al31 evaluated 57 studies (n = 5,228) and reported a median rate of diagnostic RMBs (diagnosis of malignancy) of 92% (interquartile range, 80% to 96.8%). For CB, the recent AHRQ review (> 18 studies; n = 2,203),24 demonstrated a sensitivity of 97.5%, specificity of 96.2%, and positive predictive value of 99.8% but a poor negative predictive value of 68.5%. Nondiagnostic rates ranged from 0% to 22.6%.24 The majority of nondiagnostic biopsies corresponded with malignant surgical pathology (90.4%). Repeat RMB after an initial nondiagnostic biopsy yields a higher diagnostic rate (83% to 100%) but may be underutilized.34 Predictors of nondiagnostic RMB in SRMs are smaller size, cystic masses, nonenhancing (≤ 20 HU), and skin-to-tumor distance of ≥ 13 cm.35
Histologic determination of RCC subtype is highly accurate, but the accuracy for grade is less reliable. In the AHRQ review, concordance between histologic subtype and surgical specimens was 96% among SRMs.31 Oncocytoma remains a challenge, because close to one quarter of patients have RCC on surgical pathology.36 Fuhrman nuclear grading accuracy compared with surgical specimens is poor, but can be improved using a simplified two-tiered system (high- v low-grade).31,37 Approximately 20% of RMB-determined low-grade (1 to 2) cancers are upgraded to high grade (3 to 4) on surgical pathology.24 The difficulty in assigning a grade determination is likely a reflection of intratumoral grade heterogeneity previously documented in RCC.38
Safety
Overall, complications secondary to RMB are infrequent and include perinephric hematoma, clinically significant pain, gross hematuria, pneumothorax, and hemorrhage.24 The largest RMB series (n = 529) to date reported a 2% rate of Clavien grade ≥ 2 complications.34 Clinically significant bleeding after RMB is unusual and generally self-limited.31 Needle biopsy tract seeding historically has been a concern for clinicians; however, with modern biopsy techniques using a coaxial sheath method, this risk is nearly negligible. All but one case report of needle tract seeding was without the use of a coaxial sheath, and although there is one recent report in the literature, this event remains exceedingly rare.39,40
Summary
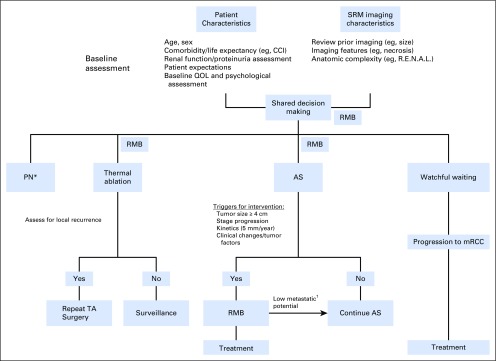
RMB demonstrates a reliable ability to determine the presence of malignancy and characterize histology in SRMs but is limited for grade. Notably, one third of patients with a nondiagnostic biopsy will harbor malignancy on surgical pathology. These findings led guideline panels (Table 1) to suggest offering RMB as an adjunctive option in the evaluation of patients with localized RCC. A recently proposed algorithm41 suggests that RMB can be selectively used to improve risk stratification in patients where the clinical management may change on the basis of the results of the biopsy (eg, multiple renal masses or hereditary RCC syndromes). In Figure 1, we summarize the possible clinical scenarios in which RMB may be used for the management of SRMs.
Fig 1.
Suggested algorithm for the management of small renal masses (SRMs). Renal mass biopsy (RMB) depicts clinical scenarios in which RMB can be considered. (*) When technically feasible. (†) Benign pathology, chromophobe, papillary type 1, or Fuhrman grade 1 to 2 metastatic renal cell carcinoma (mRCC). AS, active surveillance; CCI, Charlson Comorbidity Index; PN, partial nephrectomy; QOL, quality of life; RCC, renal cell carcinoma; TA, thermal ablation.
ACTIVE SURVEILLANCE
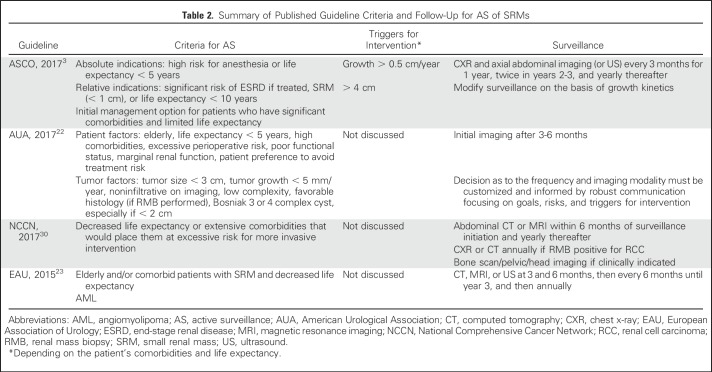
PN is considered the gold standard treatment of patients with clinical stage T1a tumors. Other management options include AS, TA, and surgery (PN or RN). A summary of the guideline recommendations for AS criteria, triggers for delayed intervention (DI), and follow-up protocols are listed in Table 2. Several recent reviews have summarized existing retrospective AS studies for SRMs.42
Table 2.
Summary of Published Guideline Criteria and Follow-Up for AS of SRMs
Survival Outcomes
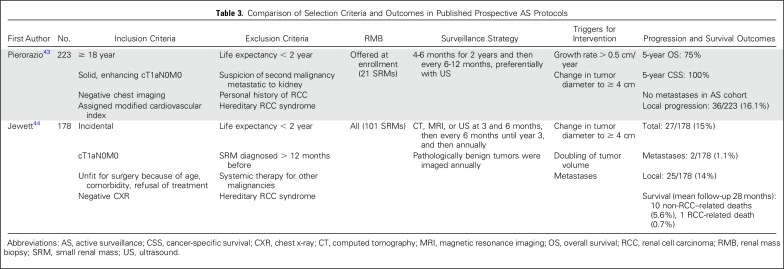
To date, only two studies have evaluated AS protocols prospectively.43,44 The details of these two studies are listed in Table 3. Overall, both retrospective and prospective studies have reported cancer-specific and metastasis-free survival of 98% to 100%.42 OS has ranged from 69% to 94%, reflective of an overall older and comorbid population. In an update on the Delayed Intervention and Surveillance for Small Renal Masses (DISSRM) trial (N = 271), investigators reported a mean growth rate of 0.09 (standard deviation, ± 1.51) cm per year, with the highest variability noted within the first year and decreasing with longer follow-up.45 Notably, patients choosing AS in the DISSRM trial who required DI were still eligible for nephron-sparing approaches.43
Table 3.
Comparison of Selection Criteria and Outcomes in Published Prospective AS Protocols
Triggers for Delayed Intervention
Triggers for DI while receiving AS have been assessed retrospectively. Smaldone et al46 recently performed a systematic review of 18 series (880 patients, 936 masses) and found that increasing age, initial tumor diameter/volume, and linear/volumetric growth rates were significantly different between those who experienced disease progression (n = 18) compared with those who did not. Among the prospective cohorts, triggers for DI included tumor size > 4 cm, increasing tumor complexity, symptoms (eg, hematuria), infiltrative appearance, patient preference, and/or interval growth (> 0.5 cm/year). Among 447 patients, McIntosh et al47 demonstrated that 38% of renal masses (median size, 2.1 cm; interquartile range, 1.5 to 3.1) exhibited no initial growth (< 1 mm/year); however, an initial high longitudinal growth rate (> 10 mm/year) was associated with a higher cumulative risk of DI. Growth alone may not be an indication of histology, because both benign and malignant lesions can grow at similar rates and different histologies of RCC demonstrate varied risks of metastases by tumor size alone.48 However, the development of metastases in AS protocols seems to be preceded by rapid local growth or multiple growth periods.46,49 Among RMB-diagnosed oncocytic neoplasms, AS has been demonstrated to be safe.50
THERMAL ABLATION
To date, no randomized prospective studies have compared TA techniques with surgery (PN or RN) or compared each TA modality (cryoablation v radiofrequency ablation). The CONSERVE trial (ClinicalTrials.gov identifier: NCT01608165) was terminated early due to poor accrual to study PN versus TA in masses amenable to both modalities in healthy individuals. A prospective, nonrandomized study evaluating ultrasound-guided percutaneous microwave ablation versus laparoscopic PN for SRMs is currently ongoing (Clinicaltrials.gov identifier: NCT03094949). Most data available are retrospective, include an older and comorbid population, and vary in the rates of use and timing of RMB.
Survival Outcomes
CSS and OS rates are similar across management strategies for T1a RCC, but TA is associated with a higher local recurrence rate.24 Guidelines generally recommend considering percutaneous TA as an option for clinical T1a masses ≤ 3 cm.22,23 Both cryoablation and radiofrequency ablation are options and demonstrate no difference in complications, local recurrences, metastatic progression, or CSS.51 In a recent systematic review and meta-analysis comparing TA (n = 3,974) and PN (n = 2,519), all-cause mortality and CSS was higher among patients undergoing TA and there was no significant difference in local recurrence rate or risk of metastasis.52 Notably, survival outcomes may depend on histology where ccRCC has a worse prognosis compared with papillary RCC.53 Options for the management of local recurrence for TA include PN or repeat TA. PN for the treatment of recurrent RCC after TA can be technically challenging, therefore increasing the risk of conversion to RN and complications.54
Complications
In a recent systematic review and meta-analysis (n = 3,974), complication rates were lower for TA (odds ratio, 0.49; 95% CI 0.25 to 0.94) compared with PN.52 Specifically, TA has demonstrated decreased blood loss and transfusion rates compared with PN or RN. TA demonstrates improved renal functional outcomes compared with PN or RN.52 Not surprisingly, length of hospital stay is shorter with TA compared with PN or RN. Acute kidney injury and minor/major Clavien complication rates are similar among techniques (TA, PN, RN).55 Renal mass location and complexity need to be considered to prevent complications related to proximity to ureters, ureteropelvic junction, small or large bowel, and nerves. R.E.N.A.L nephrometry scores can help estimate the probability of local tumor progression and potential complications.56
RENAL FUNCTIONAL OUTCOMES
Numerous variables can influence renal functional outcomes, including the amount of parenchyma removed/ablated, ischemia type/duration, patient age, comorbidities, and presence of preexisting CKD. Renal functional outcomes favor TA and AS over PN or RN.36 After PN or RN, eGFR can improve over time, with approximately 40% of patients returning to their baseline renal function after 1 year.57 Overall, RN has been associated with worse renal functional outcomes compared with either nephron-sparing technique: TA or PN. TA may not adversely affect renal function and therefore may be particularly suited for patients needing maximum conservation of renal parenchyma.
FUTURE DIRECTIONS
Patient Decision Aids
Currently, no published decision aids have been evaluated for kidney cancer. Patients face a range of complex decisions regarding treatment options for SRMs and, often, there is no best treatment choice. Golan et al58 recently evaluated patient (n = 73) and physician (n = 59) perspectives on RMB and found that both physicians and patients were most uncomfortable about the negative predictive value of RMB. However, most patients (approximately 60%) would opt for RMB after being informed of the imperfect accuracy of the procedure. More studies are needed to help patients navigate the complex options surrounding the management of SRMs.
Criteria for AS
Currently, no standard composite model is used to assess the appropriateness of AS for a given patient. However, existing nomograms are consistent in finding tumor size to be an important predictor of malignancy. Therefore, within the SRM category, there exists a range of risk on the basis of tumor size alone.4 The importance of assessing competing risks in this population warrants exploration of novel markers of overall frailty and performance status. For example, frailty index has been evaluated across multiple cancer types and is a strong marker for treatment morbidity and OS.59 Composite nomograms, also incorporating tissue-based biomarkers, are needed to assist clinicians and patients in deciding whether to pursue AS.
Biomarkers
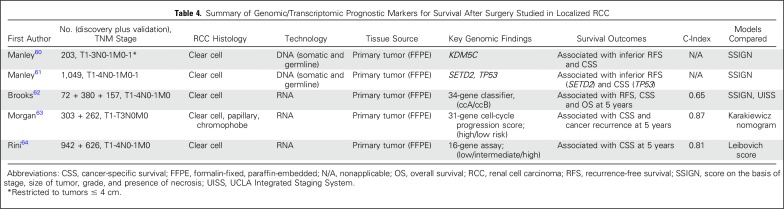
The aim of tissue markers in the clinical scenario of an SRM would be to (1) aid in histologic diagnosis, (2) detect genomic/transcriptomic markers associated with aggressive disease, and (3) diagnosis of benign tumors. Table 4 lists current tissue biomarkers available for localized RCC that are used to risk stratify patients after treatment. Notably, these biomarkers are derived from formalin-fixed paraffin-embedded tissue from the primary tumor and may be evaluated from RMB specimens. The use of tissue-based biomarkers must be balanced with the reality that they may be costly. One potential solution for this would be the use of immunohistochemistry or radiomics (combining the mutational status of specific genes with radiographic findings). Finally, other clinical host biomarkers, such as body composition and neutrophil-to-lymphocyte ratio have been associated with a poor prognosis in localized RCC and should be explored among patients with SRMs.23,65
Table 4.
Summary of Genomic/Transcriptomic Prognostic Markers for Survival After Surgery Studied in Localized RCC
Imaging
Novel imaging modalities to improve the characterization of SRMs are urgently needed. Recent reports describe using 99mTc-sestamibi single-photon emission CT to differentiate renal oncocytomas and hybrid oncocytic/chromophobe tumors from RCC; however, although promising, these methods still require further validation.66 Prostate-specific membrane antigen–targeted 18F-DCFPyL positron emission tomography/CT has also demonstrated activity in patients with RCC and requires further evaluation.67 Radiomics can be used along with clinical and radiographic findings and is a growing field of interest.68 Improvements in CT and MRI-based sequencing also need to be explored (eg, postcontrast time-attenuation curves and lesion homogeneity on CT can be used to differentiate different histologies).69 Fusion technology akin to what is used in prostate cancer for fusion biopsies is also being explored.70
In conclusion, with a rising incidence of SRMs and negligible improvement in mortality, clinicians and researchers are challenged to improve risk prediction and explore novel diagnostic avenues to improve patient care. Recognizing the importance of competing risks in this comorbid population has led to the increased use of AS and nephron-sparing approaches. Use of RMB remains controversial and requires standardization of definitions and protocols for proper prospective assessment. Furthermore, improved methods for risk prediction of OS and CSS that combine patient performance status, clinical and radiographic features, and tissue-based markers are urgently needed. These efforts should also be coupled with measures to improve imaging diagnostic techniques and tools to assist patients in navigating complex decision making surrounding SRMs.
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Collection and assembly of data: All authors
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Current Management of Small Renal Masses, Including Patient Selection, Renal Tumor Biopsy, Active Surveillance, and Thermal Ablation
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Alejandro Sanchez
No relationship to disclose
Adam S. Feldman
Honoraria: Olympus America, Janssen
Consulting or Advisory Role: Myriad Genetics, Olympus
Research Funding: Myriad Genetics
Travel, Accommodations, Expenses: Olympus, Myriad Genetics
A. Ari Hakimi
No relationship to disclose
REFERENCES
- 1.Kane CJ, Mallin K, Ritchey J, et al. : Renal cell cancer stage migration: Analysis of the National Cancer Data Base. Cancer 113:78-83, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Welch HG, Skinner JS, Schroeck FR, et al. : Regional variation of computed tomographic imaging in the United States and the risk of nephrectomy. JAMA Intern Med 178:221-227, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Finelli A, Ismaila N, Bro B, et al. : Management of small renal masses: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 35:668-680, 2017 [DOI] [PubMed] [Google Scholar]
- 4.Bhindi B, Lohse CM, Mason RJ, et al. : Are we using the best tumor size cut-points for renal cell carcinoma staging? Urology 109:121-126, 2017 [DOI] [PubMed] [Google Scholar]
- 5.Znaor A, Lortet-Tieulent J, Laversanne M, et al. : International variations and trends in renal cell carcinoma incidence and mortality. Eur Urol 67:519-530, 2015 [DOI] [PubMed] [Google Scholar]
- 6.Nguyen MM, Gill IS, Ellison LM: The evolving presentation of renal carcinoma in the United States: Trends from the Surveillance, Epidemiology, and End Results program. J Urol 176:2397-2400, 2006; discussion 2400 [DOI] [PubMed] [Google Scholar]
- 7.Umbreit EC, Shimko MS, Childs MA, et al. : Metastatic potential of a renal mass according to original tumour size at presentation. BJU Int 109:190-194, 2012; discussion 194 [DOI] [PubMed] [Google Scholar]
- 8.Chow WH, Dong LM, Devesa SS: Epidemiology and risk factors for kidney cancer. Nat Rev Urol 7:245-257, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Warren KS, McFarlane J: The Bosniak classification of renal cystic masses. BJU Int 95:939-942, 2005 [DOI] [PubMed] [Google Scholar]
- 10. doi: 10.1016/j.juro.2017.09.078. Chandrasekar T, Ahmad AE, Fadaak K, et al: Natural history of complex renal cysts: Clinical evidence supporting active surveillance. J Urol 199:633-640, 2018. [DOI] [PubMed] [Google Scholar]
- 11.Bhatt JR, Richard PO, Kim NS, et al. : Natural history of renal angiomyolipoma (AML): Most patients with large AMLs > 4cm can be offered active surveillance as an initial management strategy. Eur Urol 70:85-90, 2016 [DOI] [PubMed] [Google Scholar]
- 12.Kay FU, Pedrosa I: Imaging of solid renal masses. Radiol Clin North Am 55:243-258, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosenkrantz AB, Hindman N, Fitzgerald EF, et al. : MRI features of renal oncocytoma and chromophobe renal cell carcinoma. AJR Am J Roentgenol 195:W421-W427, 2010 [DOI] [PubMed] [Google Scholar]
- 14.Young JR, Margolis D, Sauk S, et al. : Clear cell renal cell carcinoma: Discrimination from other renal cell carcinoma subtypes and oncocytoma at multiphasic multidetector CT. Radiology 267:444-453, 2013 [DOI] [PubMed] [Google Scholar]
- 15.Kim C, Choi HJ, Cho KS: Diagnostic value of multidetector computed tomography for renal sinus fat invasion in renal cell carcinoma patients. Eur J Radiol 83:914-918, 2014 [DOI] [PubMed] [Google Scholar]
- 16.Hedgire SS, Elmi A, Nadkarni ND, et al. : Preoperative evaluation of perinephric fat invasion in patients with renal cell carcinoma: Correlation with pathological findings. Clin Imaging 37:91-96, 2013 [DOI] [PubMed] [Google Scholar]
- 17.Wang HY, Ding HJ, Chen JH, et al. : Meta-analysis of the diagnostic performance of [18F]FDG-PET and PET/CT in renal cell carcinoma. Cancer Imaging 12:464-474, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kutikov A, Egleston BL, Canter D, et al. : Competing risks of death in patients with localized renal cell carcinoma: A comorbidity based model. J Urol 188:2077-2083, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kutikov A, Egleston BL, Wong YN, et al. : Evaluating overall survival and competing risks of death in patients with localized renal cell carcinoma using a comprehensive nomogram. J Clin Oncol 28:311-317, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim SP, Gross CP, Meropol N, et al. : National treatment trends among older patients with T1-localized renal cell carcinoma. Urol Oncol 35:113.e15-113.e21, 2017 [DOI] [PubMed] [Google Scholar]
- 21.Lane BR, Demirjian S, Derweesh IH, et al. : Survival and functional stability in chronic kidney disease due to surgical removal of nephrons: Importance of the new baseline glomerular filtration rate. Eur Urol 68:996-1003, 2015 [DOI] [PubMed] [Google Scholar]
- 22.Campbell S, Uzzo RG, Allaf ME, et al. : Renal mass and localized renal cancer: AUA guideline. J Urol 198:520-529, 2017 [DOI] [PubMed] [Google Scholar]
- 23.Ljungberg B, Bensalah K, Canfield S, et al. : EAU guidelines on renal cell carcinoma: 2014 update. Eur Urol 67:913-924, 2015 [DOI] [PubMed] [Google Scholar]
- 24.Pierorazio PM, Johnson MH, Patel HD, et al. : Management of renal masses and localized renal cancer. Comparative Effectiveness Review No. 167. JHU Evidence-based Practice Center Contract No. 290-2012-00007-I. AHRQ Publication No. 16-EHC001-EF Rockville, MD, Agency for Healthcare Research and Quality; February; 2016. [PubMed] [Google Scholar]
- 25.Lane BR, Babineau D, Kattan MW, et al. : A preoperative prognostic nomogram for solid enhancing renal tumors 7 cm or less amenable to partial nephrectomy. J Urol 178:429-434, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Raj GV, Thompson RH, Leibovich BC, et al. : Preoperative nomogram predicting 12-year probability of metastatic renal cancer. J Urol 179:2146-2151, 2008; discussion 2151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kutikov A, Smaldone MC, Egleston BL, et al. : Anatomic features of enhancing renal masses predict malignant and high-grade pathology: A preoperative nomogram using the RENAL nephrometry score. Eur Urol 60:241-248, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barwari K, de la Rosette JJ, Laguna MP: The penetration of renal mass biopsy in daily practice: A survey among urologists. J Endourol 26:737-747, 2012 [DOI] [PubMed] [Google Scholar]
- 29.Leppert JT, Hanley J, Wagner TH, et al. : Utilization of renal mass biopsy in patients with renal cell carcinoma. Urology 83:774-779, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Motzer RJ, Jonasch E, Agarwal N, et al. : Kidney cancer, version 2.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 15:804-834, 2017 [DOI] [PubMed] [Google Scholar]
- 31.Marconi L, Dabestani S, Lam TB, et al. : Systematic review and meta-analysis of diagnostic accuracy of percutaneous renal tumour biopsy. Eur Urol 69:660-673, 2016 [DOI] [PubMed] [Google Scholar]
- 32.Chen AL, Brown PA, Sweeney BJ, et al. : Smears are important for adequate cytologic diagnosis of kidney lesions. J Am Soc Cytopathol 6:162-169, 2017 [DOI] [PubMed] [Google Scholar]
- 33.Breda A, Treat EG, Haft-Candell L, et al. : Comparison of accuracy of 14-, 18- and 20-G needles in ex-vivo renal mass biopsy: A prospective, blinded study. BJU Int 105:940-945, 2010 [DOI] [PubMed] [Google Scholar]
- 34.Richard PO, Jewett MA, Bhatt JR, et al. : Renal tumor biopsy for small renal masses: A single-center 13-year experience. Eur Urol 68:1007-1013, 2015 [DOI] [PubMed] [Google Scholar]
- 35.Leveridge MJ, Finelli A, Kachura JR, et al. : Outcomes of small renal mass needle core biopsy, nondiagnostic percutaneous biopsy, and the role of repeat biopsy. Eur Urol 60:578-584, 2011 [DOI] [PubMed] [Google Scholar]
- 36.Patel HD, Pierorazio PM, Johnson MH, et al. : Renal functional outcomes after surgery, ablation, and active surveillance of localized renal tumors: A systematic review and meta-analysis. Clin J Am Soc Nephrol 12:1057-1069, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Halverson SJ, Kunju LP, Bhalla R, et al. : Accuracy of determining small renal mass management with risk stratified biopsies: Confirmation by final pathology. J Urol 189:441-446, 2013 [DOI] [PubMed] [Google Scholar]
- 38.Ball MW, Bezerra SM, Gorin MA, et al. : Grade heterogeneity in small renal masses: Potential implications for renal mass biopsy. J Urol 193:36-40, 2015 [DOI] [PubMed] [Google Scholar]
- 39.Viswanathan A, Ingimarsson JP, Seigne JD, et al. : A single-centre experience with tumour tract seeding associated with needle manipulation of renal cell carcinomas. Can Urol Assoc J 9:E890-E893, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mullins JK, Rodriguez R: Renal cell carcinoma seeding of a percutaneous biopsy tract. Can Urol Assoc J 7:E176-E179, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kutikov A, Smaldone MC, Uzzo RG, et al. : Renal mass biopsy: Always, sometimes, or never? Eur Urol 70:403-406, 2016 [DOI] [PubMed] [Google Scholar]
- 42.Ristau BT, Kutikov A, Uzzo RG, et al. : Active surveillance for small renal masses: When less is more. Eur Urol Focus 2:660-668, 2016 [DOI] [PubMed] [Google Scholar]
- 43.Pierorazio PM, Johnson MH, Ball MW, et al. : Five-year analysis of a multi-institutional prospective clinical trial of delayed intervention and surveillance for small renal masses: The DISSRM registry. Eur Urol 68:408-415, 2015 [DOI] [PubMed] [Google Scholar]
- 44.Jewett MA, Mattar K, Basiuk J, et al. : Active surveillance of small renal masses: Progression patterns of early stage kidney cancer. Eur Urol 60:39-44, 2011 [DOI] [PubMed] [Google Scholar]
- 45. doi: 10.1016/j.juro.2017.09.087. Uzosike AC, Patel HD, Alam R, et al: Growth kinetics of small renal masses on active surveillance: Variability and results from the DISSRM registry. J Urol199:641-648, 2017. [DOI] [PubMed] [Google Scholar]
- 46.Smaldone MC, Kutikov A, Egleston BL, et al. : Small renal masses progressing to metastases under active surveillance: A systematic review and pooled analysis. Cancer 118:997-1006, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McIntosh AG, Ristau BT, Ruth K, et al. : Active surveillance for localized renal masses: Tumor growth, delayed intervention rates, and > 5-yr clinical outcomes. Eur Urol 74:157-164, 2018 [DOI] [PubMed] [Google Scholar]
- 48.Daugherty M, Sedaghatpour D, Shapiro O, et al. : The metastatic potential of renal tumors: Influence of histologic subtypes on definition of small renal masses, risk stratification, and future active surveillance protocols. Urol Oncol 35:153.e15-153.e20, 2017 [DOI] [PubMed] [Google Scholar]
- 49. doi: 10.1111/bju.14051. Jang A, Patel HD, Riffon M, et al: Multiple growth periods predict unfavourable pathology in patients with small renal masses. BJU Int 121:732-736, 2017. [DOI] [PubMed] [Google Scholar]
- 50.Richard PO, Jewett MA, Bhatt JR, et al. : Active surveillance for renal neoplasms with oncocytic features is safe. J Urol 195:581-586, 2016 [DOI] [PubMed] [Google Scholar]
- 51.Pierorazio PM, Patel HD, Johnson MH, et al. : Distinguishing malignant and benign renal masses with composite models and nomograms: A systematic review and meta-analysis of clinically localized renal masses suspicious for malignancy. Cancer 122:3267-3276, 2016 [DOI] [PubMed] [Google Scholar]
- 52.Rivero JR, De La Cerda J, III, Wang H, et al. : Partial nephrectomy versus thermal ablation for clinical stage t1 renal masses: Systematic review and meta-analysis of more than 3,900 patients. J Vasc Interv Radiol 29:18-29, 2018 [DOI] [PubMed] [Google Scholar]
- 53.Lay AH, Faddegon S, Olweny EO, et al. : Oncologic efficacy of radio frequency ablation for small renal masses: Clear cell vs papillary subtype. J Urol 194:653-657, 2015 [DOI] [PubMed] [Google Scholar]
- 54.Jiménez JA, Zhang Z, Zhao J, et al. : Surgical salvage of thermal ablation failures for renal cell carcinoma. J Urol 195:594-600, 2016 [DOI] [PubMed] [Google Scholar]
- 55.Hui GC, Tuncali K, Tatli S, et al. : Comparison of percutaneous and surgical approaches to renal tumor ablation: Metaanalysis of effectiveness and complication rates. J Vasc Interv Radiol 19:1311-1320, 2008 [DOI] [PubMed] [Google Scholar]
- 56.Reyes J, Canter D, Putnam S, et al. : Thermal ablation of the small renal mass: Case selection using the R.E.N.A.L.-Nephrometry Score. Urol Oncol 31:1292-1297, 2013 [DOI] [PubMed] [Google Scholar]
- 57. doi: 10.1016/j.juro.2017.10.027. Zabor EC, Furberg H, Lee B, et al: Long-term renal function recovery following radical nephrectomy for kidney cancer: Results from a multicenter confirmatory study. J Urol 199:921-926, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. doi: 10.1016/j.euf.2016.11.003. Golan S, Lotan P, Tapiero S, et al: Diagnostic needle biopsies in renal masses: Patient and physician perspectives. Eur Urol Focus 10.1016/j.euf.2016.11.003 [epub ahead of print on November 23, 2016] [DOI] [PubMed] [Google Scholar]
- 59.Shah R, Attwood K, Arya S, et al. : Association of frailty with failure to rescue after low-risk and high-risk inpatient surgery. JAMA Surg 153:e180214, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. doi: 10.1016/j.urolonc.2017.10.012. Manley BJ, Reznik E, Ghanaat M, et al: Characterizing recurrent and lethal small renal masses in clear cell renal cell carcinoma using recurrent somatic mutations. Urol Oncol . [epub ahead of print on November 10, 2017] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Manley BJ, Zabor EC, Casuscelli J, et al. : Integration of recurrent somatic mutations with clinical outcomes: A pooled analysis of 1049 patients with clear cell renal cell carcinoma. Eur Urol Focus 3:421-427, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brooks SA, Brannon AR, Parker JS, et al. : ClearCode34: A prognostic risk predictor for localized clear cell renal cell carcinoma. Eur Urol 66:77-84, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. doi: 10.1016/j.eururo.2017.12.002. Morgan TM, Mehra R, Tiemeny P, et al: A multigene signature based on cell cycle proliferation improves prediction of mortality within 5 yr of radical nephrectomy for renal cell carcinoma. Eur Urol 73:763-769, 2017. [DOI] [PubMed] [Google Scholar]
- 64.Rini B, Goddard A, Knezevic D, et al. : A 16-gene assay to predict recurrence after surgery in localised renal cell carcinoma: Development and validation studies. Lancet Oncol 16:676-685, 2015 [DOI] [PubMed] [Google Scholar]
- 65.Psutka SP, Boorjian SA, Moynagh MR, et al. : Decreased skeletal muscle mass is associated with an increased risk of mortality after radical nephrectomy for localized renal cell cancer. J Urol 195:270-276, 2016 [DOI] [PubMed] [Google Scholar]
- 66.Gorin MA, Rowe SP, Baras AS, et al. : Prospective evaluation of (99m)Tc-sestamibi SPECT/CT for the diagnosis of renal oncocytomas and hybrid oncocytic/chromophobe tumors. Eur Urol 69:413-416, 2016 [DOI] [PubMed] [Google Scholar]
- 67.Jones KM, Solnes LB, Rowe SP, et al. : Use of quantitative SPECT/CT reconstruction in 99mTc-sestamibi imaging of patients with renal masses. Ann Nucl Med 32:87-93, 2018 [DOI] [PubMed] [Google Scholar]
- 68.Karlo CA, Di Paolo PL, Chaim J, et al. : Radiogenomics of clear cell renal cell carcinoma: Associations between CT imaging features and mutations. Radiology 270:464-471, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sheir KZ, El-Azab M, Mosbah A, et al. : Differentiation of renal cell carcinoma subtypes by multislice computerized tomography. J Urol 174:451-455, 2005; discussion 455 [DOI] [PubMed] [Google Scholar]
- 70.Amalou H, Wood BJ: Multimodality fusion with MRI, CT, and ultrasound contrast for ablation of renal cell carcinoma. Case Rep Urol 2012:390912, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]