Abstract
Purpose
The role of temporary ovarian suppression with gonadotropin-releasing hormone agonists (GnRHa) during chemotherapy as a strategy to preserve ovarian function and fertility in premenopausal women remains controversial. This systematic review and meta-analysis using individual patient–level data was conducted to better assess the efficacy and safety of this strategy in patients with early breast cancer.
Methods
The trials in which premenopausal women with early breast cancer were randomly assigned to receive (neo)adjuvant chemotherapy alone or with concurrent GnRHa were eligible for inclusion. Primary end points were premature ovarian insufficiency (POI) rate and post-treatment pregnancy rate. Disease-free survival and overall survival were secondary end points. Because each study represents a cluster, statistical analyses were performed using a random effects model.
Results
A total of 873 patients from five trials were included. POI rate was 14.1% in the GnRHa group and 30.9% in the control group (adjusted odds ratio, 0.38; 95% CI, 0.26 to 0.57; P < .001). A total of 37 (10.3%) patients had at least one post-treatment pregnancy in the GnRHa group and 20 (5.5%) in the control group (incidence rate ratio, 1.83; 95% CI, 1.06 to 3.15; P = .030). No significant differences in disease-free survival (adjusted hazard ratio, 1.01; 95% CI, 0.72 to 1.42; P = .999) and overall survival (adjusted hazard ratio, 0.67; 95% CI, 0.42 to 1.06; P = .083) were observed between groups.
Conclusion
Our findings provide evidence for the efficacy and safety of temporary ovarian suppression with GnRHa during chemotherapy as an available option to reduce the likelihood of chemotherapy-induced POI and potentially improve future fertility in premenopausal patients with early breast cancer.
INTRODUCTION
Breast cancer is the most commonly diagnosed malignancy in premenopausal women, and its treatment often results in long-term sequelae and impaired quality of life.1 Given the improved prognosis of patients with breast cancer over the past years, survivorship issues are becoming more important.1 The use of anticancer therapies in premenopausal patients with breast cancer is associated with gonadotoxicity.2 Age of the patient at the time of treatment, type of chemotherapy regimen administered, and use of adjuvant endocrine therapy are crucial factors affecting the risk of developing this side effect.2 Chemotherapy-induced premature ovarian insufficiency (POI) can have a substantial negative impact on patients’ quality of life and is associated with several side effects, such as vasomotor symptoms, sexual dysfunction, and fertility-related problems.3
International guidelines recommend to counsel all young patients newly diagnosed with breast cancer about the potential risk of chemotherapy-induced POI and infertility.4,5 Failure to address these issues can negatively influence patients’ psychosocial health and their adherence to the proposed anticancer treatments, potentially affecting disease-related morbidity and mortality.1 Embryo and oocyte cryopreservation are the current standard strategies for fertility preservation in young women with breast cancer.4,5 However, these approaches do not prevent the risk of developing chemotherapy-induced POI. To date, temporary ovarian suppression obtained by administering gonadotropin-releasing hormone agonists (GnRHa) during chemotherapy is the only medical intervention with the potential to preserve ovarian function in premenopausal patients receiving cytotoxic systemic therapy.2 Because of the conflicting results reported in randomized studies, the role of this option remains controversial, and it is still considered an experimental technique by major international guidelines.4,5
In 2015, we performed a systematic review and meta-analysis on the basis of abstracted data from publications to investigate the protective role of temporary ovarian suppression with GnRHa during chemotherapy in premenopausal patients with early breast cancer.6 The use of GnRHa was associated with a significantly reduced risk of chemotherapy-induced POI and amenorrhea 1 year after chemotherapy completion, as well as an increased chance of obtaining a subsequent pregnancy.6 Nevertheless, no final conclusions could be drawn, mainly because of the lack of data on ovarian function beyond 1 year after the end of chemotherapy, the limited information on the safety of this approach, and the lack of availability at that time of the results of the Anglo Celtic Group OPTION (Ovarian Protection Trial in Oestrogen Non-responsive Premenopausal Breast Cancer Patients Receiving Adjuvant or Neo-adjuvant Chemotherapy) trial,7 one of the largest studies in this field. Furthermore, without individual patient–level data, analyses of other efficacy and safety outcomes as well as the evaluation of the association between treatment effect and patient or tumor characteristics were not possible. The current study seeks to provide more conclusive clinical evidence on this controversial topic by conducting a meta-analysis on the basis of individual patient–level data of the randomized trials that investigated the role of temporary ovarian suppression with GnRHa during chemotherapy as a strategy to preserve ovarian function and fertility in premenopausal patients with early breast cancer.
METHODS
This systematic review and meta-analysis of individual patient–level data are reported according to the Preferred Reporting Items for Systematic Reviews and Meta-analysis guidelines.8 A protocol was developed before study initiation and submitted to PROSPERO (registration number CRD42014015638).
Identification of Studies and Collection of Data
Details about the systematic review of the literature were previously reported.6 Briefly, a search using PubMed, Embase, and the Cochrane Library was conducted without any date or language restrictions up to April 30, 2015. Furthermore, conference proceedings presented at the most important international conferences from 2004 onward until April 2015 were searched to identify unpublished studies; cross-referencing from relevant studies and review articles was also conducted to confirm retrieval of all possible pertinent trials. The search strategy was then repeated before final analysis on August 31, 2017 to confirm the retrieval of all possible trials. Eligible studies were randomized trials evaluating the efficacy of adding GnRHa to chemotherapy as a strategy to reduce the occurrence of chemotherapy-induced POI in premenopausal women with early breast cancer receiving (neo)adjuvant chemotherapy.
For all participants enrolled in each of the included trials, individual patient–level data (baseline patient and tumor characteristics, administered treatments, and data on ovarian function after chemotherapy, pregnancies after breast cancer diagnosis, adverse events during treatment, and survival outcomes) were collected. Data from each of the included trials were carefully checked and verified for consistency with their original publications; discrepancies were discussed and resolved with the authors before pooling the data in the final unified database used for analysis.
Outcomes
This study aimed to evaluate both the efficacy (ie, preservation of ovarian function and fertility) and the safety (ie, toxicity and survival outcomes) of temporary ovarian suppression with GnRHa during chemotherapy in premenopausal patients with early breast cancer. Primary end points were POI rate (according to the definition used as primary end point in each trial) and post-treatment pregnancy rate. Secondary end points included amenorrhea rates 1 year and 2 years after the end of chemotherapy, GnRHa-related adverse events (hot flashes, sweating, mood changes, vaginal dryness, and headache), disease-free survival (DFS), and overall survival (OS). Prespecified subgroup analyses investigated the efficacy and safety of temporary ovarian suppression with GnRHa during chemotherapy according to age of the patients, estrogen receptor status, type and duration of chemotherapy administered, and tumor stage.
Statistical Analysis
All analyses were performed including the total number of patients with available information for each specific end point.
For POI rate, the primary end point definition of POI used in each of the included trials was used. To apply a more homogeneous definition, amenorrhea rates (defined as absence of menses) at 1 year and 2 years after the end of chemotherapy were also computed. For post-treatment pregnancy rate, only the first reported pregnancy for each patient was considered independently of its outcome. Adjusted odds ratios (ORs) with 95% CIs were calculated to estimate the effect size of temporary ovarian suppression with GnRHa during chemotherapy. Fisher’s exact test was applied for POI and amenorrhea analyses. Incidence rate ratio (IRR) between the GnRHa and control groups for post-treatment pregnancy rate was computed. A multivariate logistic regression analysis was performed to investigate the effects of GnRHa treatment on the risk of developing POI, 1-year and 2-year amenorrhea adjusting by age of the patients at the time of study entry, estrogen receptor status, type, and duration of chemotherapy administered. Because each trial represents a cluster of allegedly correlated outcomes, a generalized linear mixed model for binary end points with logit link was fitted to the data by adding to the model the random effect of the study. This model allowed estimating the amount of heterogeneity between trials and, accordingly, it provided suitable estimates of the standard errors of the predictors (fixed effects) included in the model.
GnRHa-related adverse events were dichotomized first as no adverse event (grade 0) and adverse event of any grade (1 to 4), and then as no adverse event (grade 0), mild (grade 1 or 2), and severe (grade 3 or 4) adverse events. Toxicity rates were compared using Fisher’s exact test.
The reverse Kaplan-Meier method was used to calculate the median period of follow-up and its interquartile range (IQR). DFS interval was computed as the difference between the date of random assignment and the date of locoregional, contralateral, or distant recurrence, second malignancy, or death, whichever occurred first. OS was defined as the time interval between the date of random assignment and the date of death from any cause. Observation times of patients without the event were censored on the date of their last follow-up visit. DFS and OS probabilities were computed according to the Kaplan-Meier method. To investigate the effect of GnRHa treatment on the risk of developing DFS and OS events adjusting by age of the patients at the time of study entry, estrogen receptor status, type and duration of chemotherapy administered, and tumor stage, a mixed effect Cox proportional hazards model was fitted to the data to take into account the clustering effect of each study. As estimates of treatment effect, adjusted hazard ratios (HRs) with 95% CI were computed. To check for the proportional hazards assumption, the Schoenfeld residuals were examined.
Subgroup analyses of both efficacy and safety end points were performed by means of an interaction test to determine the consistency of the treatment effect on the outcomes according to age of the patients, estrogen receptor status, type and duration of chemotherapy administered, and tumor stage. Likelihood ratio test was applied to test both the main effects and the interaction effects of the covariates included in the statistical models. In addition, a meta-analysis procedure was applied to the data of each individual study for every end point to evaluate the consistency of the results between trials. As overall measure of the effect across studies, we computed the weighted mean of the ORi or HRi estimated from each i-th trial, with weights proportional to the variance of ORi or HRi. The meta-analysis was performed by means of the inverse-variance method transforming the ORi or HRi in its natural logarithm. The heterogeneity between studies was quantified through the Higgins I2 index. In the presence of significant heterogeneity, the random effect model following the method of DerSimonian and Laird was applied, because it is generally more appropriate in such situation.
All statistical tests were two-sided, and P values < .05 were considered statistically significant. Statistical analyses were performed using STATA 14.2 (StataCorp LP, College Station, TX).
RESULTS
Of the 676 entries returned by the initial database search, 662 were excluded because they did not meet the inclusion criteria (Data Supplement). A total of 14 publications corresponding to 13 different randomized trials were considered eligible for this study.7,9-21 Individual patient–level data were available for five major trials (PROMISE-GIM6 [PRevention Of Menopause Induced by chemotherapy: A Study in Early breast cancer patients—Gruppo Italiano Mammella 6],9,10 POEMS [Prevention Of Early Menopause Study]/SWOG S0230,11 Anglo Celtic Group OPTION,7 GBG [German Breast Group]-37 ZORO [ZOladex Rescue of Ovarian function],12 Moffitt-led trial13) including 873 randomly assigned patients. Individual patient–level data from 708 patients included in the remaining eight trials were not available because of refusal to participate for three trials and no success in reaching the principal investigators of the other five trials despite several attempts to contact them.
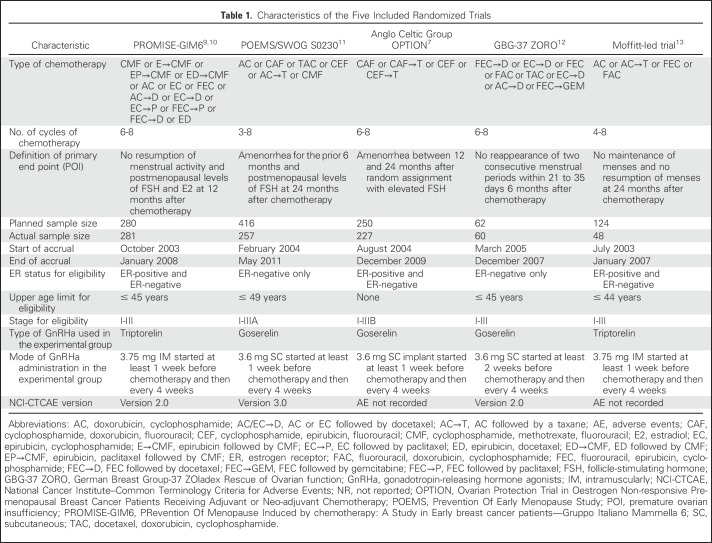
Table 1 summarizes the main characteristics of the five included trials. As per study inclusion criteria, only patients with hormone receptor–negative breast cancer were enrolled in two trials.11,12
Table 1.
Characteristics of the Five Included Randomized Trials
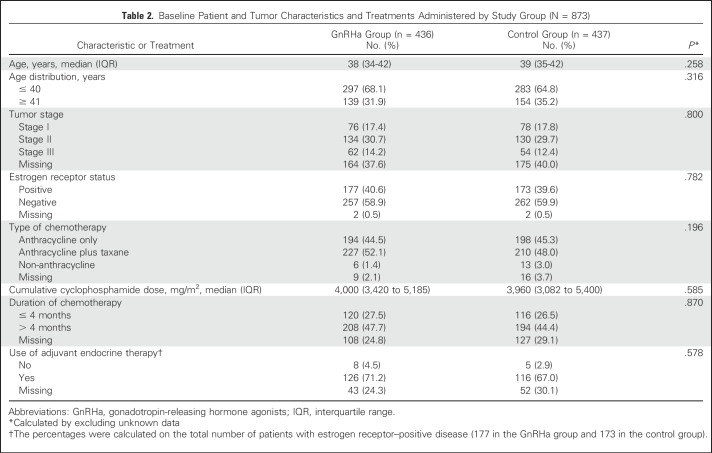
A total of 873 patients were included, of whom 436 were randomly assigned to the GnRHa group and 437 to the control group (Data Supplement). Baseline patient and treatment characteristics were well balanced between the two groups (Table 2). Median age at diagnosis was 38 years (IQR, 34-42 years); 350 patients (40.1%) had estrogen receptor–positive disease.
Table 2.
Baseline Patient and Tumor Characteristics and Treatments Administered by Study Group (N = 873)
Efficacy Results: Preservation of Ovarian Function and Fertility
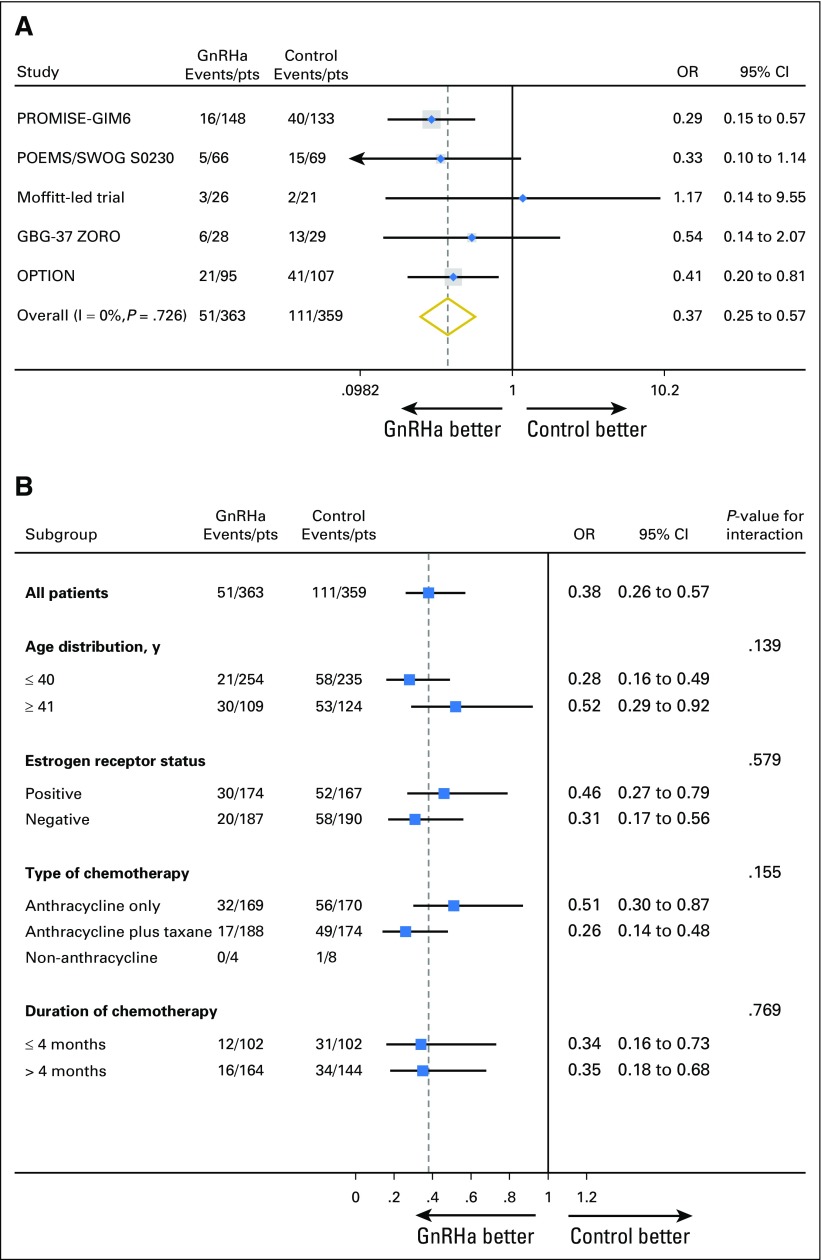
Chemotherapy-induced POI was the primary end point in all trials; different definitions and time points for its evaluation were used (Table 1). POI data were available in 722 (82.7%) of 873 patients. In the GnRHa group, 51 (14.1%) of 363 patients developed POI, as compared with 111 (30.9%) of 359 in the control group (adjusted OR, 0.38; 95% CI, 0.26 to 0.57; P < .001). The meta-analysis approach showed no heterogeneity (I2 = 0%; P = .726; Fig 1A). The effect of GnRHa on reducing the risk of developing chemotherapy-induced POI was homogeneous among the different patient subgroups (Fig 1B). Multivariate analysis showed that only treatment with GnRHa (adjusted OR, 0.38; 95% CI, 0.26 to 0.57; P < .001) and younger age at diagnosis (adjusted OR, 0.35; 95% CI, 0.24 to 0.52; P < .001) were significantly associated with a reduced risk of developing chemotherapy-induced POI (Data Supplement).
Fig 1.
Premature ovarian insufficiency (A) by trial, and (B) by patient subgroup. GBG-37 ZORO, German Breast Group-37 ZOladex Rescue of Ovarian function; GnRHa, gonadotropin-releasing hormone agonists; OPTION, Ovarian Protection Trial in Oestrogen Non-responsive Premenopausal Breast Cancer Patients Receiving Adjuvant or Neo-adjuvant Chemotherapy; OR, odds ratio; POEMS, Prevention Of Early Menopause Study; PROMISE-GIM6, PRevention Of Menopause Induced by chemotherapy: a Study in Early breast cancer patients—Gruppo Italiano Mammella 6; pts, patients.
One-year amenorrhea data were available in 760 (87.1%) of 873 patients. In the GnRHa group, 142 (36.8%) of 386 patients developed 1-year amenorrhea as compared with 151 (40.4%) of 374 in the control group (adjusted OR, 0.92; 95% CI, 0.66 to 1.28; P = .623; Data Supplement).
Two-year amenorrhea data were available in 424 (48.6%) of 873 patients; this end point was not collected in the PROMISE-GIM6 study,9,10 and in patients who developed DFS and/or OS events between 1 and 2 years. In the GnRHa group, 39 (18.2%) of 214 patients developed 2-year amenorrhea, as compared with 63 (30.0%) of 210 in the control group (adjusted OR, 0.51; 95% CI, 0.31 to 0.85; P = .009; Data Supplement).
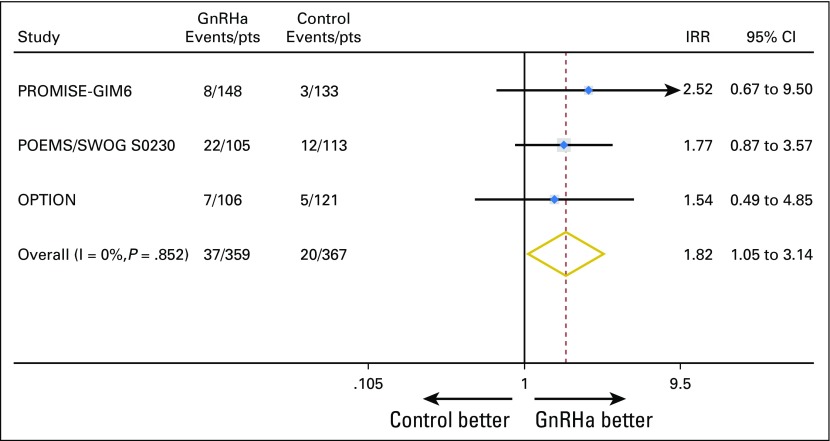
The three largest trials reported post-treatment pregnancies7,9-11; preservation of fertility was a preplanned secondary end point in only one trial.11 Information on post-treatment pregnancies was available in 726 (83.2%) of 873 patients. In the GnRHa group, 37 (10.3%) of 359 women had at least one post-treatment pregnancy, as did 20 (5.5%) of 367 in the control group (IRR, 1.83; 95% CI, 1.06 to 3.15; P = .030; Data Supplement). The meta-analysis approach showed no heterogeneity (I2 = 0%; P = .852; Fig 2). All pregnancies occurred in patients with ≤ 40 years of age at the time of diagnosis; 49 (86.0%) and eight (14.0%) of 57 were observed in women with estrogen receptor–negative and estrogen receptor–positive disease, respectively (Data Supplement).
Fig 2.
Post-treatment pregnancies by trial. GnRHa, gonadotropin-releasing hormone agonists; IRR, incidence rate ratio; OPTION, Ovarian Protection Trial in Oestrogen Non-responsive Premenopausal Breast Cancer Patients Receiving Adjuvant or Neo-adjuvant Chemotherapy; POEMS Prevention Of Early Menopause Study; PROMISE-GIM6, PRevention Of Menopause Induced by chemotherapy: a Study in Early breast cancer patients—Gruppo Italiano Mammella 6; pts, patients.
Safety Results: Toxicity and Survival Outcomes
GnRHa-related adverse events of any grade were recorded in three studies,9-12 and two of them reported their severity by grade.9-11 Concurrent administration of GnRHa and chemotherapy was associated with a significantly higher incidence of hot flashes and sweating, of grade 1 or 2 in the majority of the cases (Data Supplement). No significant difference was observed in the incidence of mood changes, vaginal dryness, and headache between the GnRHa and control groups.
Survival outcomes were collected in all included trials but one.13 Median follow-up time was 5.0 years (IQR, 3.0-6.3 years).
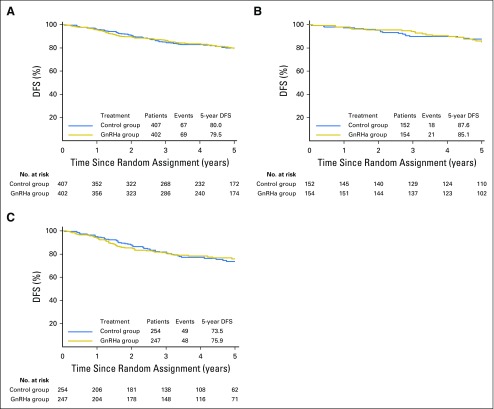
Among the 809 (92.7%) of 873 patients evaluable for DFS, 136 events (16.8%) were observed, 69 (17.2%) of 402 in the GnRHa group and 67 (16.5%) of 407 in the control group. Five-year DFS was 79.5% (95% CI, 74.7% to 83.5%) in the GnRHa group and 80.0% (95% CI, 75.2% to 83.9%) in the control group (adjusted HR, 1.01; 95% CI, 0.72 to 1.42; P = .999; Fig 3A). The meta-analysis approach showed low heterogeneity (I2 = 3.1%; P = .377; Data Supplement). Subgroup analysis according to estrogen receptor status showed no significant interaction (Pinteraction = .867); the adjusted HRs were 1.17 (95% CI, 0.62 to 2.20) and 0.95 (95% CI, 0.64 to 1.42) in patients with estrogen receptor–positive (Fig 3B) and estrogen receptor–negative (Fig 3C) disease, respectively. The Data Supplement reports the multivariate analysis for DFS.
Fig 3.
Disease-free survival (DFS) in (A) the whole study population, (B) patients with estrogen receptor–positive disease, and (C) patients with estrogen receptor–negative disease. GnRHa, gonadotropin-releasing hormone agonists.
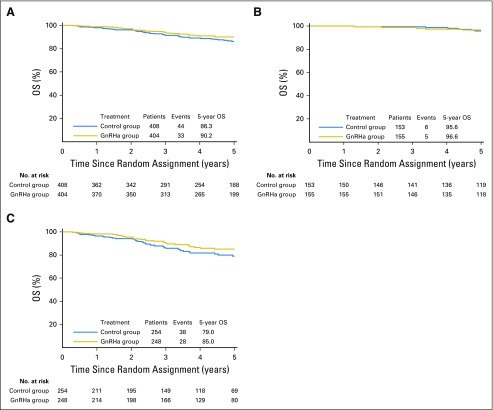
Among the 812 (93.0%) of 873 patients evaluable for OS, 77 events (9.5%) were observed, 33 (8.2%) of 404 in the GnRHa group and 44 (10.8%) of 408 in the control group. Five-year OS was 90.2% (95% CI, 86.4% to 92.9%) in the GnRHa group and 86.3% (95% CI, 82.0% to 89.7%) in the control group (adjusted HR, 0.67; 95% CI, 0.42 to 1.06; P = .083; Fig 4A). The meta-analysis approach showed that heterogeneity was rather high (I2 = 51.1%; P = .105; Data Supplement). Subgroup analysis according to estrogen receptor status showed no significant interaction (Pinteraction = .762); the adjusted HRs were 0.79 (95% CI, 0.24 to 2.59) and 0.65 (95% CI, 0.39 to 1.07) in patients with estrogen receptor–positive (Fig 4B) and estrogen receptor–negative (Fig 4C) disease, respectively. The Data Supplement reports the multivariate analysis for OS.
Fig 4.
Overall survival (OS) in (A) the whole study population, (B) patients with estrogen receptor–positive disease, and (C) patients with estrogen receptor–negative disease. GnRHa, gonadotropin-releasing hormone agonists.
DISCUSSION
This meta-analysis included individual patient–level data from five major trials that investigated the role of temporary ovarian suppression with GnRHa during chemotherapy as a strategy to preserve ovarian function and fertility in premenopausal women with early breast cancer. Concurrent administration of GnRHa and chemotherapy significantly reduced the risk of developing chemotherapy-induced POI and was associated with a higher number of post-treatment pregnancies. No heterogeneity of treatment effect between subgroups was shown. Similar DFS and OS were observed between groups irrespective of the estrogen receptor status of the disease.
The avoidance of symptoms associated with chemotherapy-induced loss of gonadal function represents an important goal to be achieved in young survivors of breast cancer, even in patients without the desire to have a subsequent pregnancy.22 Our study supports the protective gonadal effect of GnRHa administration during chemotherapy, with a significant 16.8% absolute reduction in the incidence of chemotherapy-induced POI (adjusted OR, 0.38; 95% CI, 0.26 to 0.57; P < .001). The efficacy of this strategy was consistent across all subgroups analyzed, including in patients with estrogen receptor–positive disease, and independently of their age at the time of treatment. The different definition and time point of evaluation used in the trials highlights the current lack of standardized definition of chemotherapy-induced POI. Nevertheless, the availability of individual patient–level data allowed analysis of ovarian function recovery on the basis of more homogeneous definitions. Using 1-year amenorrhea, the reduced absolute 3.6% difference favoring the GnRHa was not statistically significant (adjusted OR, 0.92; 95% CI, 0.66 to 1.28; P = .623). On the contrary, our prior meta-analysis on the basis of abstracted data showed a significant reduction in the risk of 1-year amenorrhea (OR, 0.55; 95% CI, 0.41 to 0.73; P < .001) when considering the eight trials that reported this end point.6 The more limited number of trials included in the current analysis may explain this discrepancy. Nevertheless, the benefit of concurrent administration of GnRHa and chemotherapy became clearly evident at a longer time point, 2 years after the end of chemotherapy (11.8% absolute reduction; adjusted OR, 0.51; 95% CI, 0.31 to 0.85; P = .009). However, this end point could be evaluated in a more limited number of patients as compared with 1-year amenorrhea. This is likely attributable to longer-term evaluation not being originally planned for most of these studies and the difficulty faced by investigators in collecting information on end points like menstrual function. These findings also highlight that even if the majority of patients experience menstrual function recovery in the first 12 months after chemotherapy, this can also occur beyond 1 year. Hence, an evaluation of chemotherapy-induced POI too close to the end of chemotherapy might not have captured the protective effect of GnRHa that became more evident at a longer time point. These findings also support the recent expert opinion–based suggestion to define menopausal status after chemotherapy not earlier than 2 years after the end of treatment.1
Approximately 50% of young patients with breast cancer are concerned about the possible risk of infertility as a consequence of chemotherapy use and desire to have children after the end of treatment.23 Nevertheless, these patients are, among survivors of cancer, those with the lowest chances of subsequent pregnancies.4 In our analysis, less than 10% of the patients had a post-treatment pregnancy (57 patients, 7.9%), in line with the available data in the literature of a pregnancy rate in survivors of breast cancer ranging between 4% and 7%.24 Although the absolute numbers remain low and the trials were not designed to address pregnancy as primary end point, a statistically significant higher number of patients who underwent temporary ovarian suppression with GnRHa during chemotherapy had a subsequent pregnancy as compared with those who received cytotoxic therapy alone (37 v 20; IRR, 1.83; 95% CI, 1.06 to 3.15; P = .030). This suggests the potential role of this strategy also as a fertility preservation procedure. Embryo and oocyte cryopreservation remain the first options to be proposed to women interested in fertility preservation.4,5 Nevertheless, being not mutually exclusive, temporary ovarian suppression with GnRHa during chemotherapy can also be used in this setting after cryopreservation procedures to increase the chances of a subsequent pregnancy as well as in patients who do not have access to assisted reproductive options.25 Notably, for patients receiving temporary ovarian suppression with GnRHa during chemotherapy after prior controlled ovarian stimulation for embryo/oocyte cryopreservation, the timing for administering the long-acting GnRHa needs to be further explored, considering its potential use as trigger of final follicular maturation instead of chorionic gonadotropin or short-acting GnRHa. In addition, future research efforts should aim at clarifying the fertility outcomes of patients who undergo temporary ovarian suppression with GnRHa during chemotherapy after cryopreservation procedures as compared with those of women who access only one of the two strategies.25
The main adverse events associated with GnRHa administration are vasomotor symptoms and sexual problems.26 Nevertheless, these side effects are mainly of grade 1 or 2 and are reversible at the time of treatment completion. Importantly, preservation of ovarian function with GnRHa use during chemotherapy may help avoid menopausal symptoms, including loss of bone density, in the long term; this is of crucial importance also in women not interested in fertility preservation.
In the past, two major safety concerns on the use of temporary ovarian suppression with GnRHa during chemotherapy were raised for women with estrogen receptor–positive disease: a potential antagonism with concurrent administration of antiestrogen therapy and cytotoxic systemic therapy, and the possible detrimental effect on prognosis of the lack of chemotherapy-induced POI.27 These concerns have been recently dispelled by the results of the TEXT (Tamoxifen and Exemestane Trial) and SOFT (Suppression of Ovarian Function Trial) trials showing no survival difference between patients who received GnRHa concurrently or sequentially to chemotherapy.28 Our study confirms the safety of concurrent administration of GnRHa and chemotherapy in all patients with breast cancer, irrespective of the estrogen receptor status of their disease. Nevertheless, of note, the majority of patients with estrogen receptor–positive disease included in this analysis derive from the PROMISE-GIM6 trial.9,10 In this study, approximately 70% of the patients with estrogen receptor–positive disease who had resumed ovarian function after chemotherapy were treated with GnRHa as part of adjuvant endocrine therapy.10 This further supports that the safety of ovarian function preservation in patients with hormone receptor–positive disease should be considered in the context of subsequent ovarian function suppression as part of adjuvant endocrine therapy.29
Some limitations of the current study should be acknowledged. It was possible to include individual patient–level data from only five major randomized trials, corresponding to 55.2% (873 of 1,581) of the eligible population for this study. Nevertheless, for all end points that could be evaluated also in our previous meta-analysis of abstracted data, similar results were observed, with the exception of 1-year amenorrhea.6 This may suggest that the inclusion of all studies would not have modified the overall findings. For the efficacy end points, major limitations are the lack of data on the extent of ovarian function preservation using more sensitive biomarkers like the anti-Müllerian hormone, the limited information on patients’ wish to have a pregnancy, and on the number of women with more than one post-treatment pregnancy. Finally, a few baseline characteristics and end point data were missing in some trials and for some patients also in studies that had originally planned to collect them. Nevertheless, there is no evidence of imbalance for these missing data or a differential drop-out between the GnRHa and control groups; thus, it is unlikely that they may have influenced the comparisons between randomly assigned groups.
In conclusion, our systematic review and meta-analysis of individual patient–level data provides evidence for the efficacy and safety of temporary ovarian suppression with GnRHa during chemotherapy in premenopausal patients with early breast cancer. Given the findings of our study, this strategy should be considered as an available option to reduce the likelihood of chemotherapy-induced POI and potentially improve future fertility in premenopausal patients with early breast cancer undergoing (neo)-adjuvant chemotherapy.
ACKNOWLEDGMENT
Matteo Lambertini acknowledges the support from the European Society for Medical Oncology (ESMO) for a Translational Research Fellowship at Institut Jules Bordet in Brussels (Belgium); he received a San Antonio Breast Cancer Symposium Clinical Scholar Award for presenting the results of this systematic review and meta-analysis at the 2017 San Antonio Breast Cancer Symposium. The authors thank all the patients and the investigators from the Gruppo Italiano Mammella (GIM) study group, the SWOG, the Anglo Celtic Group, the German Breast Group (GBG), and the Moffitt-led study who participated in the five included trials.
Footnotes
Supported in part by the Italian Association for Cancer Research (AIRC) Grant No. 2013:14272.
The financial sponsor of the study (AIRC) had no role in study design, data collection, or analysis, interpretation, or writing of the report, and it had no access to the individual patient–level data.
Presented at the 2017 San Antonio Breast Cancer Symposium, San Antonio, TX, December 7, 2017.
Processed as a Rapid Communication manuscript.
A.H.P. and L.D.M. are co-last authors.
Clinical trial information: PROSPERO registration number CRD42014015638.
See accompanying Editorial on page 1895
AUTHOR CONTRIBUTIONS
Conception and design: Matteo Lambertini, Ann H. Partridge, Lucia Del Mastro
Provision of study materials or patients: Matteo Lambertini, Halle C.F. Moore, Robert C.F. Leonard, Sibylle Loibl, Pamela Munster, Richard A. Anderson, Susan Minton, Francesca Poggio, Kathy S. Albain, Douglas J.A. Adamson, Bernd Gerber, Gianfilippo Bertelli, Sabine Seiler, Amy Cripps, Ann H. Partridge, Lucia Del Mastro
Collection and assembly of data: Matteo Lambertini, Halle C.F. Moore, Robert C.F. Leonard, Sibylle Loibl, Pamela Munster, Marco Bruzzone, Luca Boni, Joseph M. Unger, Richard A. Anderson, Keyur Metha, Francesca Poggio, Kathy S. Albain, Douglas J.A. Adamson, Bernd Gerber, Gianfilippo Bertelli, Sabine Seiler, Marcello Ceppi, Ann H. Partridge, Lucia Del Mastro
Data analysis and interpretation: Matteo Lambertini, Marco Bruzzone, Marcello Ceppi, Ann H. Partridge, Lucia Del Mastro
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Gonadotropin-Releasing Hormone Agonists During Chemotherapy for Preservation of Ovarian Function and Fertility in Premenopausal Patients With Early Breast Cancer: A Systematic Review and Meta-Analysis of Individual Patient–Level Data
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Matteo Lambertini
Consulting or Advisory Role: Teva
Travel, Accommodations, Expenses: Astellas Pharma
Halle C.F. Moore
Research Funding: Puma Biotechnology (Inst), AbbVie (Inst)
Robert C.F. Leonard
No relationship to disclose
Sibylle Loibl
Research Funding: Pfizer, Roche, Celgene, Amgen, Novartis (Inst)
Pamela Munster
Honoraria: Sanofi, Amgen (I), AstraZeneca, EMD Serono
Consulting or Advisory Role: OncoSec
Research Funding: Merck (Inst), Pfizer (Inst), Novartis (Inst), GlaxoSmithKline (Inst), OncoMed (Inst), Celgene (Inst), Andes Biotechnologies (Inst), Incyte (Inst), Ignyta (Inst), CBT Pharmaceuticals (Inst), Merrimack (Inst), Genentech (Inst), OncoSec (Inst), Bristol-Myers Squibb (Inst), Plexxikon (Inst), Piramal Life Science (Inst), Andes Biotechnologies (Inst), Immune Design (Inst), Biomarin (Inst)
Marco Bruzzone
No relationship to disclose
Luca Boni
No relationship to disclose
Joseph M. Unger
No relationship to disclose
Richard A. Anderson
Consulting or Advisory Role: Roche Diagnostics, HRA Pharma, NeRRe Therapeutics
Speakers' Bureau: IBSA, Merck
Research Funding: Ferring Pharmaceuticals
Keyur Metha
No relationship to disclose
Susan Minton
No relationship to disclose
Francesca Poggio
No relationship to disclose
Kathy S. Albain
Consulting or Advisory Role: Novartis, Pfizer, Myriad Genetics, Genentech
Other Relationship: Puma Biotechnology
Douglas J.A. Adamson
Stock or Other Ownership: GlaxoSmithKline (I)
Research Funding: Roche (Inst), Aventis Pharma (Inst), Novartis (Inst), Amgen (Inst), Pfizer (Inst), Bayer (Inst), Sanofi (Inst), Boehringer Ingelheim (Inst), Immodulon Therapeutics (Inst), Schering-Plough (Inst), Biocompatibles (Inst), AstraZeneca (Inst)
Bernd Gerber
Travel, Accommodations, Expenses: AstraZeneca, Roche
Amy Cripps
Employment: NexGen Oncology
Travel, Accommodations, Expenses: Foundation Medicine
Gianfilippo Bertelli
Consulting or Advisory Role: Roche, Novartis Pharmaceuticals UK, Genomic Health
Travel, Accommodations, Expenses: Roche
Sabine Seiler
Consulting or Advisory Role: Hexal, Roche, Amgen, Novartis
Travel, Accommodations, Expenses: Hexal, Roche, Novartis, Amgen
Marcello Ceppi
No relationship to disclose
Ann H. Partridge
No relationship to disclose
Lucia Del Mastro
Honoraria: Roche, Novartis, Takeda Pharmaceuticals, Ipsen, Eisai, Pfizer
Consulting or Advisory Role: Eli Lilly
REFERENCES
- 1.Paluch-Shimon S, Pagani O, Partridge AH, et al. : ESO-ESMO 3rd international consensus guidelines for breast cancer in young women (BCY3). Breast 35:203-217, 2017 [DOI] [PubMed] [Google Scholar]
- 2.Lambertini M, Goldrat O, Clatot F, et al. : Controversies about fertility and pregnancy issues in young breast cancer patients: Current state of the art. Curr Opin Oncol 29:243-252, 2017 [DOI] [PubMed] [Google Scholar]
- 3.Howard-Anderson J, Ganz PA, Bower JE, et al. : Quality of life, fertility concerns, and behavioral health outcomes in younger breast cancer survivors: A systematic review. J Natl Cancer Inst 104:386-405, 2012 [DOI] [PubMed] [Google Scholar]
- 4.Peccatori FA, Azim HA, Jr, Orecchia R, et al. : Cancer, pregnancy and fertility: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 24:vi160-vi170, 2013. (suppl 6) [DOI] [PubMed] [Google Scholar]
- 5.Loren AW, Mangu PB, Beck LN, et al. : Fertility preservation for patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 31:2500-2510, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lambertini M, Ceppi M, Poggio F, et al. : Ovarian suppression using luteinizing hormone-releasing hormone agonists during chemotherapy to preserve ovarian function and fertility of breast cancer patients: A meta-analysis of randomized studies. Ann Oncol 26:2408-2419, 2015 [DOI] [PubMed] [Google Scholar]
- 7.Leonard RCF, Adamson DJA, Bertelli G, et al. : GnRH agonist for protection against ovarian toxicity during chemotherapy for early breast cancer: The Anglo Celtic Group OPTION trial. Ann Oncol 28:1811-1816, 2017 [DOI] [PubMed] [Google Scholar]
- 8.Moher D, Liberati A, Tetzlaff J, et al. : Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339:b2535, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Del Mastro L, Boni L, Michelotti A, et al. : Effect of the gonadotropin-releasing hormone analogue triptorelin on the occurrence of chemotherapy-induced early menopause in premenopausal women with breast cancer: A randomized trial. JAMA 306:269-276, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Lambertini M, Boni L, Michelotti A, et al. : Ovarian suppression with triptorelin during adjuvant breast cancer chemotherapy and long-term ovarian function, pregnancies, and disease-free survival: A randomized clinical trial. JAMA 314:2632-2640, 2015 [DOI] [PubMed] [Google Scholar]
- 11.Moore HCF, Unger JM, Phillips K-A, et al. : Goserelin for ovarian protection during breast-cancer adjuvant chemotherapy. N Engl J Med 372:923-932, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gerber B, von Minckwitz G, Stehle H, et al. : Effect of luteinizing hormone-releasing hormone agonist on ovarian function after modern adjuvant breast cancer chemotherapy: The GBG 37 ZORO study. J Clin Oncol 29:2334-2341, 2011 [DOI] [PubMed] [Google Scholar]
- 13.Munster PN, Moore AP, Ismail-Khan R, et al. : Randomized trial using gonadotropin-releasing hormone agonist triptorelin for the preservation of ovarian function during (neo)adjuvant chemotherapy for breast cancer. J Clin Oncol 30:533-538, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Badawy A, Elnashar A, El-Ashry M, et al. : Gonadotropin-releasing hormone agonists for prevention of chemotherapy-induced ovarian damage: Prospective randomized study. Fertil Steril 91:694-697, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Sverrisdottir A, Nystedt M, Johansson H, et al. : Adjuvant goserelin and ovarian preservation in chemotherapy treated patients with early breast cancer: Results from a randomized trial. Breast Cancer Res Treat 117:561-567, 2009 [DOI] [PubMed] [Google Scholar]
- 16.Elgindy EA, El-Haieg DO, Khorshid OM, et al. : Gonadatrophin suppression to prevent chemotherapy-induced ovarian damage: A randomized controlled trial. Obstet Gynecol 121:78-86, 2013 [DOI] [PubMed] [Google Scholar]
- 17.Song G, Gao H, Yuan Z: Effect of leuprolide acetate on ovarian function after cyclophosphamide-doxorubicin-based chemotherapy in premenopausal patients with breast cancer: Results from a phase II randomized trial. Med Oncol 30:667, 2013 [DOI] [PubMed] [Google Scholar]
- 18.Karimi-Zarchi M, Forat-Yazdi M, Vafaeenasab MR, et al. : Evaluation of the effect of GnRH agonist on menstrual reverse in breast cancer cases treated with cyclophosphamide. Eur J Gynaecol Oncol 35:59-61, 2014 [PubMed] [Google Scholar]
- 19.Sun J, Ren Y, Li W: Effect of zoladex administered before chemotherapy on menstruation of patients with breast cancer. China Disability Medicine 19:15-16, 2011 [Google Scholar]
- 20. Li JW, Liu GY, Yu KD, et al: Effect of using LHRH analog during chemotherapy (CT) on premature ovarian failure and prognosis in premenopausal patients with early-stage, hormone receptor-positive breast cancer: The primary analysis of a randomized controlled phase III trial. Cancer Res 75: P1-12-02, 2015 (abstr)
- 21.Li M, Huang H, Liang Y, et al. : Effect of zoladex administered before chemotherapy on menstruation of patients with breast cancer. Chinese Journal of Clinical Oncology 35:905-907, 2008 [Google Scholar]
- 22.Rosenberg SM, Tamimi RM, Gelber S, et al. : Treatment-related amenorrhea and sexual functioning in young breast cancer survivors. Cancer 120:2264-2271, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ruddy KJ, Gelber SI, Tamimi RM, et al. : Prospective study of fertility concerns and preservation strategies in young women with breast cancer. J Clin Oncol 32:1151-1156, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Azim HA, Jr, Kroman N, Paesmans M, et al. : Prognostic impact of pregnancy after breast cancer according to estrogen receptor status: A multicenter retrospective study. J Clin Oncol 31:73-79, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lambertini M, Cinquini M, Moschetti I, et al. : Temporary ovarian suppression during chemotherapy to preserve ovarian function and fertility in breast cancer patients: A GRADE approach for evidence evaluation and recommendations by the Italian Association of Medical Oncology. Eur J Cancer 71:25-33, 2017 [DOI] [PubMed] [Google Scholar]
- 26.Ribi K, Luo W, Bernhard J, et al. : Adjuvant tamoxifen plus ovarian function suppression versus tamoxifen alone in premenopausal women with early breast cancer: Patient-reported outcomes in the suppression of ovarian function trial. J Clin Oncol 34:1601-1610, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rugo HS, Rosen MP: Reducing the long-term effects of chemotherapy in young women with early-stage breast cancer. JAMA 306:312-314, 2011 [DOI] [PubMed] [Google Scholar]
- 28.Regan MM, Walley BA, Francis PA, et al. : Concurrent and sequential initiation of ovarian function suppression with chemotherapy in premenopausal women with endocrine-responsive early breast cancer: An exploratory analysis of TEXT and SOFT. Ann Oncol 28:2225-2232, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burstein HJ, Lacchetti C, Anderson H, et al. : Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: American Society of Clinical Oncology clinical practice guideline update on ovarian suppression. J Clin Oncol 34:1689-1701, 2016 [DOI] [PubMed] [Google Scholar]