Abstract
PURPOSE
The primary objective was to determine if vaginal cuff brachytherapy and chemotherapy (VCB/C) increases recurrence-free survival (RFS) compared with pelvic radiation therapy (RT) in high-intermediate and high-risk early-stage endometrial carcinoma.
PATIENTS AND METHODS
A randomized phase III trial was performed in eligible patients with endometrial cancer. Eligible patients had International Federation of Gynecology and Obstetrics (2009) stage I endometrioid histology with Gynecologic Oncology Group protocol 33–based high-intermediate–risk criteria, stage II disease, or stage I to II serous or clear cell tumors. Treatment was randomly assigned between RT (45 to 50.4 Gy over 5 weeks) or VCB followed by intravenous paclitaxel 175 mg/m2 (3 hours) plus carboplatin (area under the curve, 6) every 21 days for three cycles.
RESULTS
The median age of the 601 patients was 63 years, and 74% had stage I disease. Histologies included endometrioid (71%), serous (15%), and clear cell (5%). With a median follow-up of 53 months, the 60-month RFS was 0.76 (95% CI, 0.70 to 0.81) for RT and 0.76 (95% CI, 0.70 to 0.81) for VCB/C (hazard ratio, 0.92; 90% confidence limit, 0.69 to 1.23). The 60-month overall survival was 0.87 (95% CI, 0.83 to 0.91) for RT and 0.85 (95% CI, 0.81 to 0.90) for VCB/C (hazard ratio, 1.04; 90% confidence limit, 0.71 to 1.52). Vaginal and distant recurrence rates were similar between arms. Pelvic or para-aortic nodal recurrences were more common with VCB/C (9% v 4%). There was no heterogeneity of treatment effect with respect to RFS or overall survival among clinical or pathologic variables evaluated.
CONCLUSION
Superiority of VCB/C compared with pelvic RT was not demonstrated. Acute toxicity was greater with VCB/C; late toxicity was similar. Pelvic RT alone remains an effective, well-tolerated, and appropriate adjuvant treatment in high-risk early-stage endometrial carcinomas of all histologies.
INTRODUCTION
The American Cancer Society estimates there will be 63,230 new cases of endometrial carcinoma and 11,350 associated deaths in 2018.1 Although postoperative pelvic radiotherapy (RT) has been routinely used for patients with stage I to II disease thought to be at high risk for recurrence, defining the population most at risk is problematic.2-5 Relevant phase III trials have included GOG-99 (Gynecologic Oncology Group), the PORTEC-1, -2, and -3 (Postoperative Radiation Therapy in Endometrial Cancer) studies, although eligibility criteria were skewed to lower-risk patients.4-9
The recently reported PORTEC-3 study included higher-risk patients.10,11 Subgroup analyses in GOG-99 suggested that uterine risk factors (grade 2 to 3, lymphovascular space involvement, and outer third myometrial invasion) previously identified in the prospective surgicopathologic study GOG-33 constituted a high-intermediate–risk group. When stratified by age, these patients derived clinical benefit from RT compared with observation alone (reduction in 4-year recurrence from 27% to 13%).4
The PORTEC-1 trial compared RT with observation after hysterectomy in low-and intermediate-risk patients. Patients with stage IC grade 3 disease were not eligible. Actuarial 15-year locoregional recurrence rate was 6% for pelvic RT and 15.5% for observation. A trend toward improved overall survival (OS) in the RT arm was noted.6,7
As in PORTEC-1, the predominant site of recurrence in GOG-99 was the vaginal cuff, raising the possibility that vaginal cuff brachytherapy (VCB) might be a substitute for RT. Because both studies reported a distant failure rate of approximately 20% to 30% for high-risk patients with observation, adjuvant chemotherapy was considered appropriate for investigation.
Studying the effect of adjuvant systemic chemotherapy in serous tumors is important, because this histologic subgroup is associated with an increase in intra-abdominal and systemic metastases and a disproportionate number of deaths. Better outcomes with adjuvant therapy that includes chemotherapy have been documented in some series, whereas others have failed to identify a favorable impact.9,12-17 GOG-0249 was an open-label phase III trial to study the impact on recurrence-free survival (RFS) of substitution of VCB with chemotherapy (VCB/C) for RT in women with high-risk early-stage endometrial cancer.
PATIENTS AND METHODS
Patients
After hysterectomy, patients with stage I endometrial cancer were eligible if they had endometrioid adenocarcinoma and met study criteria. A bilateral pelvic and para-aortic lymphadenectomy was recommended. Eligibility for patients with endometrioid histology was as follows: age 70 years or older with one uterine risk factor, age 50 years or older with two risk factors, or age 18 years or older with three risk factors. Uterine risk factors included grade 2 or 3 tumor, outer half depth of invasion, and lymphovascular invasion. Positive or negative peritoneal cytology was allowed. Patients with cervical stromal invasion (stage II) were eligible regardless of other risk factors. Patients with serous or clear cell tumors with stage I to II disease and with negative peritoneal cytology were eligible. Retrospective central pathology review confirmed eligibility and established histology. Patients not undergoing node dissection had postoperative computed tomography or magnetic resonance imaging to document the absence of enlarged nodes. Patients had adequate prespecified hematologic and organ function.
Study Design and Treatment
The protocol was approved by the institutional review boards of participating centers; patients provided written informed consent. Study treatment began within 12 weeks of surgery. Patients were randomly assigned at a ratio of one to one to treatment with either RT or VCB/C. Pelvic RT was administered using either standard four-field techniques (three-dimensional conformal RT) or intensity-modulated RT. Treatment plans for patients receiving intensity-modulated RT were constructed and reviewed based on the Radiation Therapy Oncology Group Contouring Atlas. The pelvic RT dose was 45 to 50.4 Gy over 5 to 6 weeks (1.8 Gy per day for 25 to 28 fractions). Patients with cervical involvement or serous or clear cell histology were permitted to receive cuff brachytherapy boosts.
Patients assigned to VCB/C received cuff brachytherapy at a high-dose rate (6 to 7 Gy at 0.5-cm depth for three fractions, 10 to 10.5 Gy at the vaginal surface for three fractions, or 6 Gy at the vaginal surface for five fractions) or low dose-rate (65 to 70 Gy prescribed at the vaginal surface in one to two insertions at a dose rate of 4 to 10 Gy per hour). Vaginal treatment length was not specified but was generally 3 to 5 cm. Chemotherapy began up to 3 weeks from brachytherapy initiation. Paclitaxel was administered intravenously at 175 mg/m2 over 3 hours followed by carboplatin (area under the curve, 6) over 45 minutes, repeated every 21 days for three cycles.
Study Procedure
Patients were evaluated weekly for toxicity. Adverse events were graded and categorized according to the Common Terminology Criteria for Adverse Events (version 3). After treatment, patients were seen initially within 4 weeks, quarterly for 2 years, semiannually for 3 years, and then annually for 10 years. Pelvic abdominal imaging (computed tomography or magnetic resonance) and chest x-ray were performed within 4 weeks after study therapy, semiannually for 2 years, and then annually for 3 years. RFS was defined as the period from study entry until disease recurrence, death, or date of last contact.
Patients were asked to complete a quality-of-life (QOL) and patient-reported outcome (PRO) survey at baseline, 4 and 11 weeks, and 8 and 14 months. Tools included the Trial Outcome Index of the Functional Assessment of Cancer Therapy (FACT) for endometrial cancer for measuring QOL, the FACT/GOG neurotoxicity (FACT/GOG-Ntx) four-item subscale for measuring chemotherapy-induced neurotoxic symptoms, and the fatigue subscale (13 items) of the Functional Assessment of Chronic Illness Therapy (FACIT) for measuring fatigue. QOL as measured with the Trial Outcome Index of the FACT for endometrial cancer will be reported separately.
Statistical Analysis
Treatment was stratified before randomization by intent to use VCB in the pelvic RT arm and by lymphadenectomy versus no lymphadenectomy. This stratification was meant to balance treatment assignments. The primary objective was to determine if VCB/C increases RFS compared with RT. Secondary objectives included comparisons of OS, patterns of failure, and frequency/severity of adverse events between the treatment arms.
Death before recurrence in the target population for this study was less common compared with GOG-0099. Reduction in recurrence in the high-intermediate–risk subgroup of GOG-099 was 58%. A relative reduction of 49% was thought to be achievable in this population. On the basis of the GOG experience, 85% of patients treated with pelvic RT were expected to be alive and recurrence free at 36 months. Reduction in the recurrence/death hazard of 49% would increase the 36-month RFS to 92% (absolute difference, 7%). Observation of 77 events was needed for 90% power with type I error set at 0.05 for a one-tailed test of RFS. With 2 years of follow-up, a sample size of 562 patients was targeted. The independence of RFS and OS with treatment was evaluated by a log-rank test in an intention-to-treat analysis. Interim analyses were scheduled at 44% and 68% of the expected number of recurrences and deaths, respectively. Stratification factors were not thought to be prognostic for the primary end point; a stratified analysis was not planned.
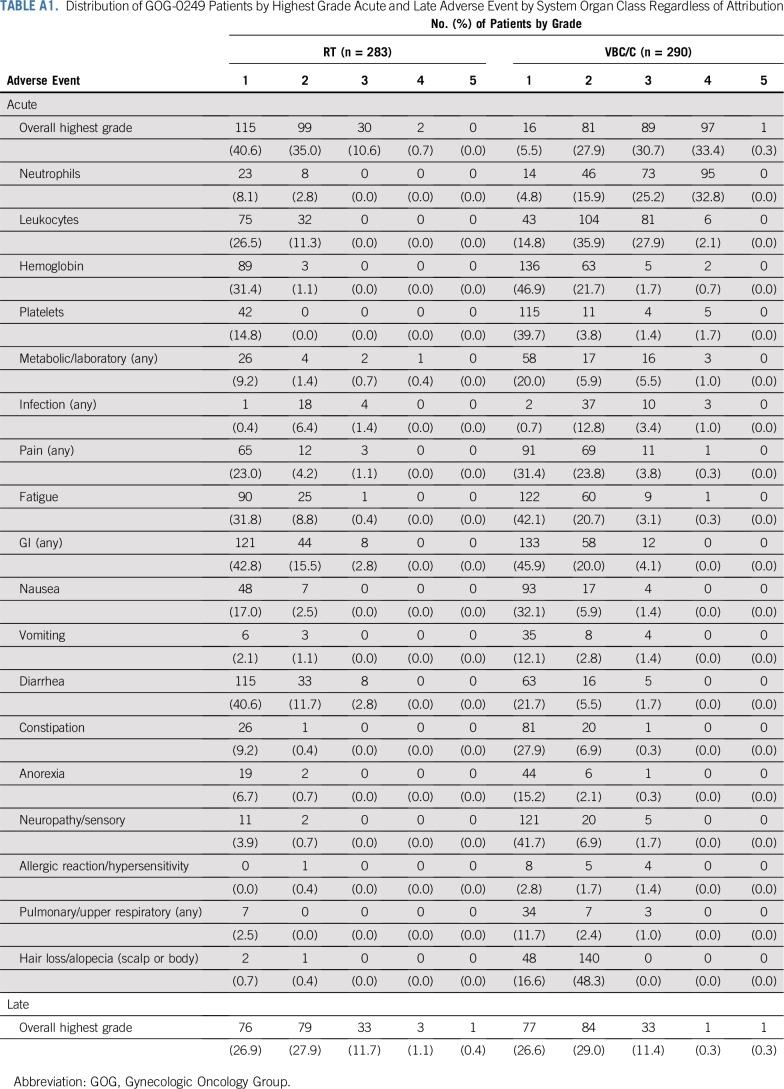
Frequencies of the maximum grade of acute and late adverse events within each system organ class, each adverse event term, and overall adverse event terms, regardless of attribution, were tabulated by treatment arm for all eligible patients for whom study therapy was initiated (Appendix Table A1, online only). Important adverse events were selected for reporting in the main article.
Competing risk analyses were carried out for three types of recurrences: first vaginal, first pelvic/para-aortic node, and first distant. More than one recurrence type could be reported for each patient. Death before a specific type of recurrence was considered a competing event.
Patient-reported neurotoxicity and fatigue as measured with the FACT/GOG-Ntx subscale and the FACIT fatigue subscale were analyzed with a linear mixed model adjusting for pretreatment score, treatment assignment, and age at enrollment. Patients were classified by their randomly assigned regimen rather than treatment received. Assessment time points were treated as categorical, because they were not equally spaced. The covariance matrix among the repeated PRO scores reported by the same patient was assumed to be unstructured. To reflect the observed covariance pattern of the repeated measures, the empiric variance was used in the precision of parameter estimates. The denominator df for testing treatment effects was computed using Satterthwaite’s approximation. Interactions between time points and treatment were tested for the constant differential treatment effects over time. If the interaction effect was not statistically significant, an overall effect was estimated by a weighted average of estimates from each time point. If testing for interaction was rejected, treatment comparison was then performed for each assessment time. Hochberg’s step-up method was used to adjust for P values to test the least squares mean differences between treatment groups obtained from the fitted mixed model over assessment time points. To ensure an overall type I error of 5%, the significant level of each QOL/PRO was set at 1.7% using the Sidak method.
RESULTS
Study Population
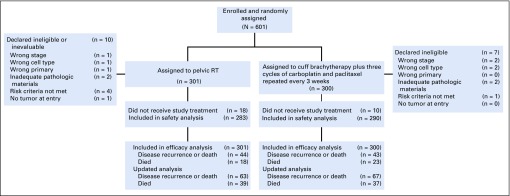
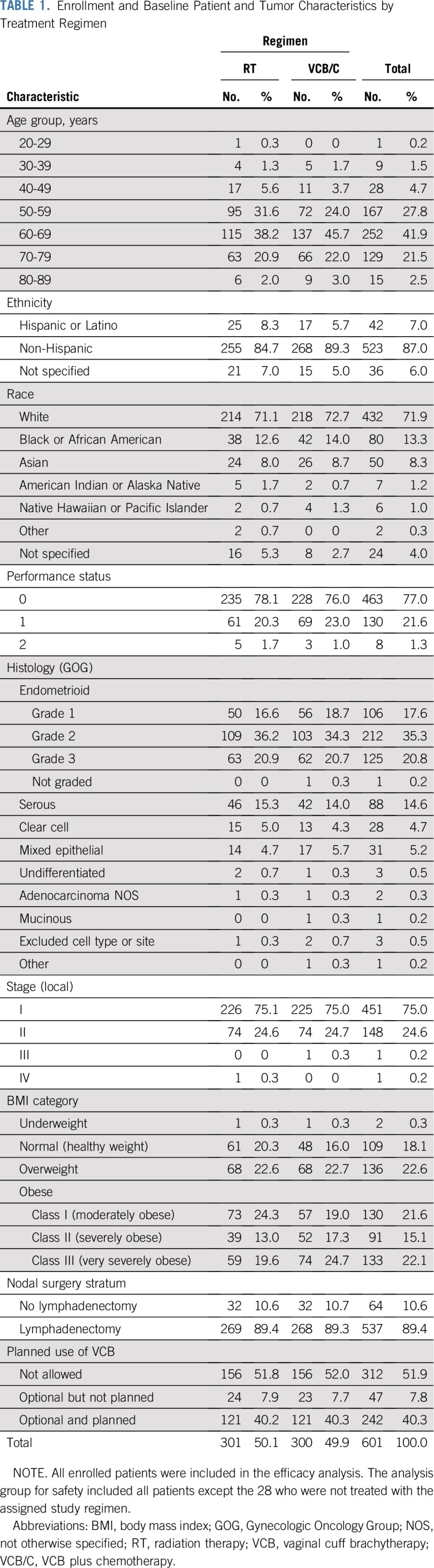
From 2009 to 2013, 601 patients were enrolled. Figure 1 shows the CONSORT diagram. Patient characteristics were similar between treatment arms (Table 1). Among patients with stage I endometrioid histology, 8% were older than age 70 with one risk factor, 51% were older than age 50 with two risk factors, and 31% had three risk factors.
FIG 1.
CONSORT diagram. RT, radiation therapy.
TABLE 1.
Enrollment and Baseline Patient and Tumor Characteristics by Treatment Regimen
Treatment
Of the 301 patients assigned to RT, 91% completed therapy. The median RT dose was 45 Gy. Of patients assigned to RT, 32% also received brachytherapy. Of the 300 patients assigned to VCB/C, 87% completed therapy. Recurrence during therapy was rare, at rates of 0.3% (n = 1) and 1.3% (n = 4) with RT and VCB/C, respectively.
Toxicity
Acute adverse events were more frequent and severe in the VCB/C arm. There was little difference between the arms when evaluating distribution of late toxicity.
RFS and OS
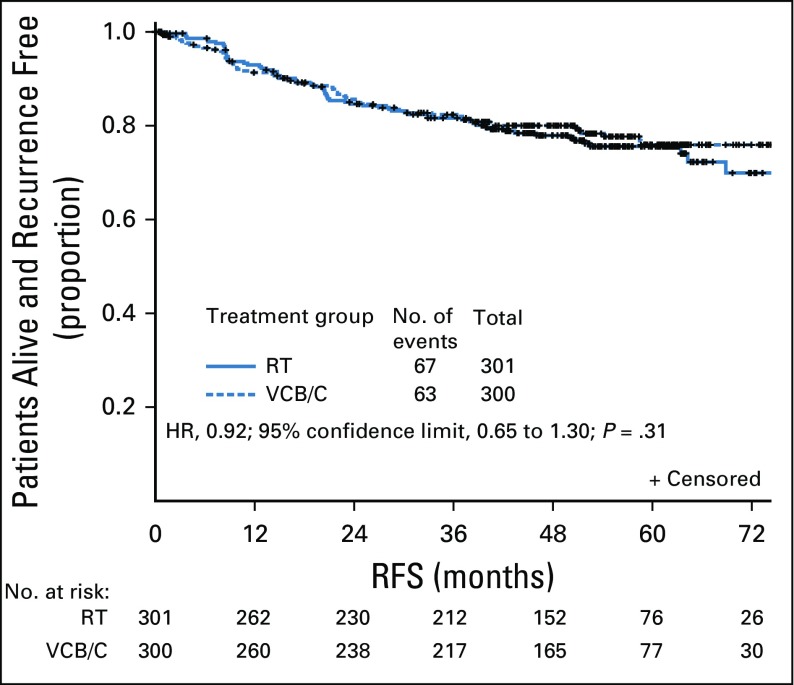
Neither interim analysis resulted in study termination. The primary report of RFS in 2013 included 87 events with a median follow-up of 28 months. The updated analysis occurred with a median follow-up of 53 months and 130 events, including 16 deaths in the absence of known recurrence. With a median follow-up of 53 months, the 60-month RFS was 0.76 (95% CI, 0.70 to 0.81) for RT and 0.76 (95% CI, 0.70 to 0.81) for VCB/C (hazard ratio [HR], 0.92; 90% confidence limit, 0.69 to 1.23). The 60-month OS was 0.87 (95% CI, 0.83 to 0.91) for RT and 0.85 (95% CI, 0.81 to 0.90) for VCB/C (HR, 1.04; 90% confidence limit, 0.71 to 1.52). For RFS, the estimated treatment HR was 0.92 (P = .31) for VCB/C relative to RT (Fig 2). Estimates of proportion of patients alive and recurrence free at 12, 24, and 36 months were 0.93, 0.85, and 0.82 for RT and 0.91, 0.86, and 0.82 for VCB/C.
FIG 2.
Intention-to-treat analysis of recurrence-free survival (RFS) by randomized treatment. There were 130 events reported as of December 11, 2016, with a median follow-up time of 53 months. There was insufficient evidence to reject the null hypothesis of no superiority of vaginal cuff brachytherapy plus three cycles of carboplatin and paclitaxel chemotherapy (VCB/C) over pelvic radiation therapy (RT). The log-rank test statistic for a true intention-to-treat analysis was 2.75 (one-tailed test P = .31). The estimated treatment hazard ratio (HR) was 0.92 (regimen II relative to regimen I). The (1-α) × 100% Wald CI was 0.651 to 1.296 for a two-sided α = 0.05 (0.025 in each tail) and 0.688 to 1.226 for a two-sided α = 0.10 (0.05 in each tail). Analysis was repeated to assess the sensitivity of results to different patient groups. When all ineligible patients were removed or when all ineligible or untreated patients were removed, the results were similar.
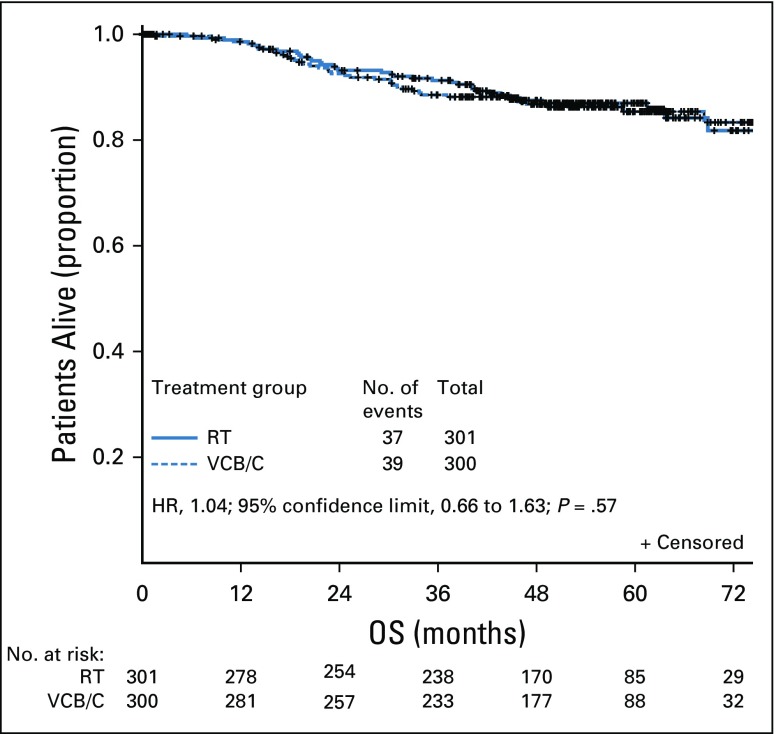
In our analysis, 76 deaths were reported (Fig 3). Estimated treatment HR for death was 1.04 (VCB/C v pelvic RT; P = .57). Estimates of proportion of patients alive at 12, 24, and 36 months were 0.99, 0.93, and 0.91 for RT and 0.99, 0.93, and 0.88 for VCB/C, respectively.
FIG 3.
Intention-to-treat analysis of overall survival (OS) by randomly assigned treatment. As of December 11, 2016, 76 deaths were reported. The median follow-up time was estimated to be 53 months. There was insufficient evidence to reject the null hypothesis of no superiority of vaginal cuff brachytherapy plus three cycles of carboplatin and paclitaxel chemotherapy (VCB/C) over radiation therapy (RT) with respect to OS. The log-rank test statistic was −0.756 (one-tailed test P = .57). The estimated treatment hazard ratio (HR) was 1.04 (regimen II relative to regimen I). The (1-α) × 100% Wald CI was 0.664 to 1.632 for a two-sided α = 0.05 (0.025 in each tail) and 0.713 to 1.518 for a two-sided α = 0.10 (0.05 in each tail). An effect size of 0.51 (49% decrease in hazard) was not contained in these CIs.
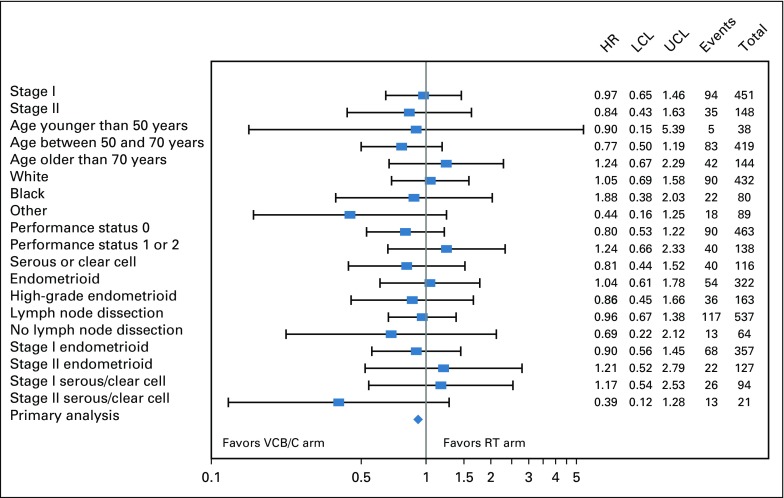
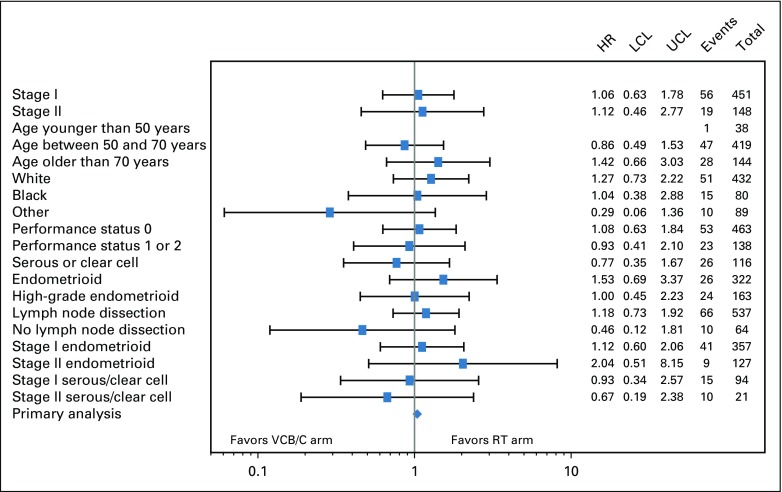
Subgroup analyses evaluating for treatment effect by stage, age, race, performance status, histology, and lymphadenectomy on RFS and OS are shown in Figures 4 and 5. There was no statistically significant evidence of heterogeneity with respect to RFS or OS among the variables tested.
FIG 4.
Forest plot of recurrence-free survival (RFS) by treatment of selected subgroups. RFS treatment hazard ratio (HR) estimates on the basis of a Cox proportional hazards model for selected subgroups are displayed in a forest plot and plotted on the log scale with a 95% CI. The HR estimate is represented graphically by a vertical dash; the CI is represented by a horizontal line. The relative HR estimates and variance of the log HR are listed. The HRs are relative to the radiation therapy (RT) arm and vary around 1.0. The vertical line at 1.0 represents no difference in the hazard rates; to the right favors WPRT and to the left favors the arm with vaginal cuff brachytherapy plus chemotherapy (VCB/C). None of the CIs exclude 1.0. LCL, lower confidence limit; UCL, upper confidence limit.
FIG 5.
Forest plot of overall survival (OS) by treatment of selected subgroups. OS treatment hazard ratio (HR) estimates on the basis of a Cox proportional hazards model for selected subgroups are displayed in a forest plot and plotted on the log scale with a 95% CI. The HR estimate is represented graphically by a vertical dash; the CI is represented by a horizontal line. The relative HR estimates and variance of the log HR are listed. The HRs are relative to the radiation therapy (RT) arm and vary around 1.0. The vertical line at 1.0 represents no difference in the hazard rates; to the right favors WPRT and to the left favors the arm with vaginal cuff brachytherapy plus chemotherapy (VCB/C). None of the CIs exclude 1.0. LCL, lower confidence limit; UCL, upper confidence limit
Sites of Recurrence
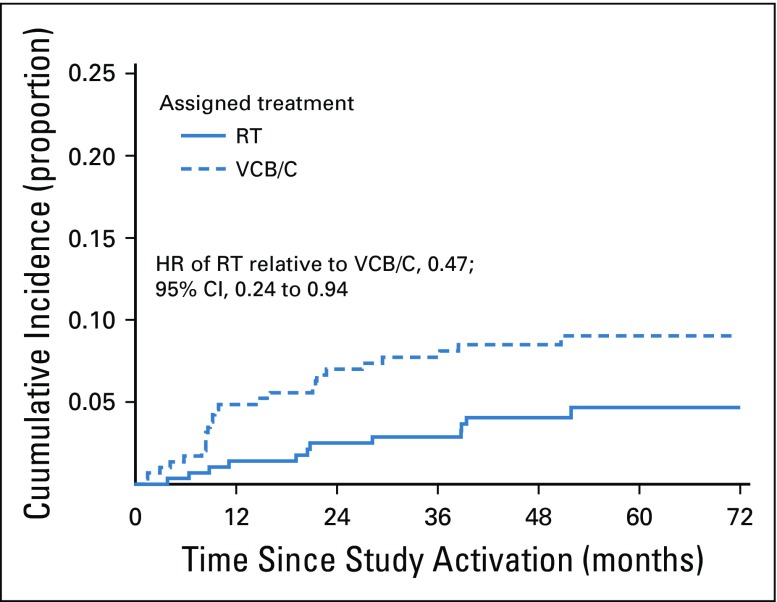
There was a significant differential in the cumulative incidence of para-aortic nodal or pelvic recurrences between treatment arms (HR of RT relative to VCB/C, 0.47; 95% CI, 0.24 to 0.94). The cumulative incidence proportion of these types of recurrences was 4% for RT and 9% for VCB/C within 5 years of entry (Fig 6). Pelvic and para-aortic recurrences were roughly numerically equivalent between those with serous carcinomas and those with grade 2 to 3 endometrial carcinomas. No pelvic or para-aortic recurrences were noted among patients with clear cell histologies. There was no difference in incidence of vaginal recurrences (HR of RT relative to VCB/C, 1.0; 95% CI, 0.33 to 3.16) or distant recurrences (HR of RT relative to VCB/C, 1.0; 95% CI, 0.68 to 1.52). Approximately 2.5% and 18% of patients developed vaginal or distant recurrences, respectively, within 5 years.
FIG 6.
Cumulative incidence of pelvic or para-aortic recurrence (competing event is death before recurrence of interest). Three competing risk analyses were carried out for three different types of recurrences: any vaginal, any pelvic or any para-aortic nodes, and any distant. More than one recurrence type could be reported for each patient. Death before a specific type of recurrence was considered a competing event. There was a significant differential in the cumulative incidence of pelvic or para-aortic node recurrences between the treatment arms (hazard ratio [HR] of radiation therapy [RT] relative to vaginal cuff brachytherapy plus chemotherapy [VCB/C], 0.472; 95% CI, 0.24 to 0.94). The 52-month cumulative incidence proportion of these types of recurrences was 0.044 for the RT arm and 0.092 for the VCB/C arm. There were no differences in the incidence of vaginal or distant recurrences between the two treatment arms. Approximately 2.5% and 18% of patients will have a vaginal or distant recurrence, respectively, within 5 years of treatment.
PROs
As measured with the FACIT fatigue subscale score, fatigue recovered to pretreatment levels at 11 weeks in the RT arm but not in the VCB/C arm. The largest treatment difference was observed at 11 weeks, with patients in the VCB/C group reporting 3.7 points lower (98.3% CI, −5.9 to 1.6; P < .001) than those in the RT group. The VCB/C arm returned to baseline at 8 months.
Patients in the VCB/C arm reported increased neurotoxicity, measured with the FACT/GOG-Ntx subscale, when compared with those in the RT group. Significant treatment differences were observed at 4 and 11 weeks and at 8 months after treatment. The largest treatment difference was at 11 weeks (98.3% CI, −2.9 to 1.5; P < .001). The VCB/C arm returned to baseline at 14 months.
DISCUSSION
In this study of patients with high-intermediate– and high-risk early-stage endometrial carcinoma, a postoperative adjuvant strategy of VCB followed by three cycles of paclitaxel and carboplatin chemotherapy was not superior to RT and was associated with more frequent and severe acute toxicity. Assuming proportional hazards, the data are consistent with the hypothesis that the hazard of VCB/C is as much as 30% greater than that of RT alone. This effect size is comparable to increasing the probability of recurrence at 3 years by 5%. Given this, and the fact that the study did not prespecify a noninferiority region, a conclusion of equivalence or noninferiority in the absence of a statistically significant increase in RFS was not possible. Analysis was repeated to assess the sensitivity of results to different patient groups. When all ineligible and/or untreated patients were removed, the conclusions were similar. Pelvic and para-aortic nodal failure was higher in patients who received adjuvant VCB/C compared with RT. Absence of benefit when adjuvant chemotherapy is added to RT in patients with stage I to II disease was also observed in PORTEC-3.11
Two other randomized trials comparing RT with chemotherapy have been conducted, showing comparable results. Maggi et al18 compared RT with five cycles of platinum-based chemotherapy in a population that included 62% with stage IIIA to IIIC disease. Five-year RFS was 63% in both arms. In the Japanese GOG study, in which 24% of patients had stage IIIA to IIIC disease, RT was compared with platinum-based chemotherapy.19 The 5-year RFS was 84% versus 82% for pelvic RT and chemotherapy, respectively.
Acute adverse events were more common and severe in the VCB/C arm compared with the RT arm, consistent with reports on toxicity and QOL from PORTEC-3, comparing pelvic RT alone with chemotherapy and RT plus four cycles of chemotherapy.10 At completion of RT and 6 months later, quantitative assessment found that physical functioning and QOL measures were significantly worse in chemotherapy and RT arm. Adverse events of grade 2 or greater were found in 94% of patients receiving chemotherapy and RT versus 44% of those randomly assigned to RT. At 24 months, sensory neuropathy of grade 2 or greater was significantly worse in the chemotherapy and RT arm at 10% versus less than 1% with RT. Detailed analyses of PRO and QOL comparisons are forthcoming in future articles.
This study benefits from a well-defined patient population of patients with high-intermediate– and high-risk early-stage endometrial carcinoma, including high-risk histologies. Careful pathologic review and RT quality assurance were undertaken. Paclitaxel and carboplatin was the chemotherapy regimen used and seems justified as the most active/tolerable regimen.20 The decision to use three cycles of chemotherapy was based on several factors, including toxicity concerns in a population of patients in whom a majority are cured without adjuvant therapy, the high incidence of comorbid diseases in this group, the reality that patients enrolled in endometrial studies using six to eight cycles of chemotherapy often receive fewer and still see a benefit,16 and historical precedent.9,19
Many patients meeting study criteria are cured without adjuvant therapy. In GOG-99, 73% of high-intermediate–risk patients treated with surgery alone remained recurrence free at 48 months.4 Therefore, it is important to improve selection for adjuvant treatment to limit treatment to patients not cured by surgery alone. The high-intermediate–risk criteria as defined in GOG-99 do seem to constitute a high-risk group of patients with recurrence rates of 15% to 20%, even with adjuvant treatment.
By including high-risk histologies (serous and clear cell) and cervical involvement (stage II), our study included patients with a higher risk profile than those in the GOG-99 population. Patients with serous histology accounted for 15% of accruals and 29% of recurrences, confirming this assertion. Although systemic therapy is often recommended for patients with serous histology, the benefit of chemotherapy in our study was less clear. Hogberg et al9 compared RT with or without chemotherapy and found the addition of chemotherapy to be associated with an observed 36% reduction in recurrence/death (HR, 0.63; P = .009). However, in a subgroup of 140 patients with serous or clear cell tumors treated with pelvic RT and chemotherapy, the benefit of chemotherapy was less clear.
Similar to earlier studies in which local therapy (either vaginal brachytherapy or pelvic RT) was administered, vaginal recurrences were uncommon. However, pelvic/nodal failures were higher in the absence of pelvic RT. In the high-intermediate group of patients in GOG-99 and PORTEC-1, the risk of locoregional recurrence in the observation arms was approximately 13% and 16%, respectively.4,6 In our study, locoregional failures were even higher, reflecting the higher-risk population. As seen in earlier studies, distant failure remains a common failure pattern and varies by risk group.4,6-8 Distant recurrences were equal between the two arms (18%). Some studies support the efficacy of systemic therapy in the highest risk groups.9,12-17
Our study did not demonstrate superiority of VCB/C over RT in high-intermediate– and high-risk early-stage endometrial carcinoma with respect to RFS and OS, nor did it demonstrate equivalence. The incidences of vaginal and distant cumulative recurrences were not statistically significantly different in the arms, but pelvic and para-aortic nodal failures were significantly higher in the VCB/C arm. Acute toxicity was substantially higher with VCB/C, although differences in late toxicity were minimal.
Pelvic RT remains an appropriate treatment for high-risk early-stage endometrial carcinoma. The benefit of adjuvant chemotherapy with regard to OS in stage I to II disease remains to be demonstrated in a prospective manner. Distant recurrence remains the predominant failure pattern when local therapy is administered. Avenues of exploration designed to limit systemic failure through identification of predictive biomarkers that govern the immunogenic molecular cascade in the tumor microenvironment are under investigation. Novel combinations, dose intensification, and additional translational research represent potential paths forward.
APPENDIX
The following Gynecologic Oncology Group institutions participated in this study: University of Oklahoma Health Sciences Center, Cancer Trials Support Unit, Seoul National University Hospital, Ohio State University Comprehensive Cancer Center, Women and Infants Hospital, Georgia Center for Oncology Research and Education, Yale University, Women’s Cancer Center of Nevada, University of Hawaii, Fox Chase Cancer Center, University of North Carolina at Chapel Hill, University of Colorado Cancer Center–Anschutz Cancer Pavilion, University of California at Los Angeles Health System, Saint Joseph’s Hospital and Medical Center, University of Minnesota Medical Center–Fairview, University of New Mexico, Case Western Reserve University, Duke University Medical Center, University of California Medical Center at Irvine–Orange Campus, Washington University School of Medicine, Stony Brook University Medical Center, MD Anderson Cancer Center, University of Chicago, Abington Memorial Hospital, Walter Reed National Military Medical Center, State University of New York Downstate Medical Center, Cooper Hospital University Medical Center, Roswell Park Cancer Institute, University of Texas Southwestern Medical Center, University of Massachusetts Memorial Health Care, The Hospital of Central Connecticut, Aurora Women’s Pavilion of Aurora West Allis Medical Center, University of Iowa Hospitals and Clinics, University of Cincinnati, Wake Forest University Health Sciences, University of Pittsburgh Cancer Institute, Memorial Sloan Kettering Cancer Center, Iowa-Wide Oncology Research Coalition National Cancer Institute Community Oncology Research Program (NCORP), University of Alabama at Birmingham, University of Kentucky, Wayne State University/Karmanos Cancer Institute, Abramson Cancer Center of the University of Pennsylvania, Indiana University Hospital/Melvin and Bren Simon Cancer Center, Cancer Research for the Ozarks NCORP, Geisinger Medical Center, Greenville Health System Cancer Institute/Greenville Community Clinical Oncology Program (CCOP), University of Mississippi Medical Center, Cleveland Clinic Foundation, Mayo Clinic, University of Texas–Galveston, Cancer Research Consortium of West Michigan NCORP, Wichita CCOP, Fred Hutchinson Cancer Research Center, Moffitt Cancer Center and Research Institute, University of Wisconsin Hospital and Clinics, Kalamazoo CCOP, Northern Indiana Cancer Research Consortium, Penn State Milton S. Hershey Medical Center, Rush University Medical Center, Gynecologic Oncology of West Michigan, University of California San Francisco–Mount Zion, Carolinas Medical Center/Levine Cancer Institute, Froedtert and the Medical College of Wisconsin, Evanston CCOP–North Shore University Health System, Central Illinois CCOP, Delaware/Christiana Care CCOP, Upstate Carolina CCOP, Mainline Health CCOP, Meharry Medical College Minority-Based CCOP, and Colorado Cancer Research Program NCORP.
TABLE A1.
Distribution of GOG-0249 Patients by Highest Grade Acute and Late Adverse Event by System Organ Class Regardless of Attribution
Footnotes
Supported by National Cancer Institute grants to the Gynecologic Oncology Group Administrative Office (CA 27469), the Gynecologic Oncology Group Statistical and Data Center (CA 37517), NRG Oncology (1 U10 CA180822), and NRG Operations (U10CA180868) and in part by Memorial Sloan Kettering Cancer Center Support Grant No. P30 CA008748 (C.A.A.).
Clinical trial information: NCT00807768.
See accompanying Oncology Grand Rounds on page 1778
AUTHOR CONTRIBUTIONS
Conception and design: Marcus E. Randall, Virginia Filiaci, D. Scott McMeekin, Catheryn M. Yashar, Robert S. Mannel, Nick M. Spirtos, William Small, Carol A. Aghajanian, David S. Miller
Administrative support: Marcus E. Randall, Robert S. Mannel, David S. Miller
Provision of study material or patients: Marcus E. Randall, Catheryn M. Yashar, Robert S. Mannel, Paul A. DiSilvestro, James J. Burke, Nick M. Spirtos, Keith Terada, Penny R. Anderson, David S. Miller
Collection and assembly of data: Marcus E. Randall, Virginia Filiaci, D. Scott McMeekin, Helen Huang, Jae-Weon Kim, Ritu Salani, Paul A. DiSilvestro, James J. Burke, Thomas Rutherford, Keith Terada, Wendy R. Brewster, William Small, David S. Miller
Data analysis and interpretation: Marcus E. Randall, Virginia Filiaci, Vivian von Gruenigen, Helen Huang, Catheryn M. Yashar, David S. Miller
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Phase III Trial: Adjuvant Pelvic Radiation Therapy Versus Vaginal Brachytherapy Plus Paclitaxel/Carboplatin in High-Intermediate and High-Risk Early-Stage Endometrial Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Marcus E. Randall
Honoraria: Isoray
Virginia Filiaci
Travel, Accommodations, Expenses: Regeneron, VBL Therapeutics
Other Relationship: GOG Foundation, GOG Foundation (I)
Vivian von Gruenigen
Employment: US Acute Care Solutions (I)
Stock and Other Ownership Interests: US Acute Care Solutions (I)
Robert S. Mannel
Consulting or Advisory Role: Clovis Oncology (Inst), Tesaro (Inst)
Ritu Salani
Honoraria: Clovis Oncology, AstraZeneca, Tesaro, Johnson & Johnson, Genmab
Consulting or Advisory Role: Genentech
Speakers’ Bureau: Genentech
Paul A. DiSilvestro
Consulting or Advisory Role: AstraZeneca, Tesaro
Research Funding: Janssen Oncology (Inst), Tesaro (Inst), AstraZeneca (Inst), Genentech (Inst), AbbVie (Inst)
Expert Testimony: Johnson & Johnson
James J. Burke
Employment: HCA Healthcare
Stock and Other Ownership Interests: Allergan, Allergan (I)
Nick M. Spirtos
Research Funding: AbbVie (Inst), AstraZeneca (Inst), Genentech/Roche (Inst), Clovis Oncology (Inst)
William Small
Honoraria: Carl Zeiss Meditec
Consulting or Advisory Role: Varian Medical Systems
Speakers’ Bureau: Carl Zeiss Meditec
Research Funding: Carl Zeiss Meditec
Travel, Accommodations, Expenses: Carl Zeiss Meditec
Carol A. Aghajanian
Consulting or Advisory Role: Clovis Oncology, Tesaro, Mateon Therapeutics, Immunogen
Research Funding: Genentech/Roche (Inst), AbbVie (Inst), Clovis Oncology (Inst), AstraZeneca (Inst)
David S. Miller
Consulting or Advisory Role: Genentech, Tesaro, Eisai, AstraZeneca, Guardant Health, Janssen Oncology, Alexion Pharmaceuticals, Karyopharm Therapeutics, Incyte, Janssen, Clovis Oncology, Merck Sharp & Dohme (Inst)
Speakers’ Bureau: Clovis Oncology, Genentech
Research Funding: US Biotest (Inst), Advenchen Laboratories (Inst), Millennium Pharmaceuticals (Inst), Tesaro (Inst), Xenetic Biosciences (Inst), Advaxis (Inst), Janssen (Inst), Aeterna Zentaris (Inst), TRACON Pharma (Inst), Pfizer (Inst), Immunogen (Inst), Mateon Therapeutics (Inst), Merck Sharp & Dohme (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.Aalders J, Abeler V, Kolstad P, et al. Postoperative external irradiation and prognostic parameters in stage I endometrial carcinoma: Clinical and histopathologic study of 540 patients. Obstet Gynecol. 1980;56:419–427. [PubMed] [Google Scholar]
- 3.Creutzberg CL, van Putten WL, Koper PC, et al. Surgery and postoperative radiotherapy versus surgery alone for patients with stage-1 endometrial carcinoma: Multicentre randomised trial—PORTEC Study Group: Post operative radiation therapy in endometrial carcinoma. Lancet. 2000;355:1404–1411. doi: 10.1016/s0140-6736(00)02139-5. [DOI] [PubMed] [Google Scholar]
- 4.Keys HM, Roberts JA, Brunetto VL, et al. A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma: A Gynecologic Oncology Group study. Gynecol Oncol. 2004;92:744–751. doi: 10.1016/j.ygyno.2003.11.048. [DOI] [PubMed] [Google Scholar]
- 5.Kong A, Johnson N, Kitchener HC, et al. Adjuvant radiotherapy for stage I endometrial cancer: An updated Cochrane systematic review and meta-analysis. J Natl Cancer Inst. 2012;104:1625–1634. doi: 10.1093/jnci/djs374. [DOI] [PubMed] [Google Scholar]
- 6.Nout RA, van de Poll-Franse LV, Lybeert MLM, et al. Long-term outcome and quality of life of patients with endometrial carcinoma treated with or without pelvic radiotherapy in the post operative radiation therapy in endometrial carcinoma 1 (PORTEC-1) trial. J Clin Oncol. 2011;29:1692–1700. doi: 10.1200/JCO.2010.32.4590. [DOI] [PubMed] [Google Scholar]
- 7. Creutzberg CL, Nout RA, Lybeert ML, et al: Fifteen-year radiotherapy outcomes of the randomized PORTEC-1 trial for endometrial carcinoma. Int J Radiat Oncol Biol Phys 81:e631-e638, 2011. [DOI] [PubMed] [Google Scholar]
- 8.Nout RA, Smit VT, Putter H, et al. Vaginal brachytherapy versus pelvic external beam radiotherapy for patients with endometrial cancer of high-intermediate risk (PORTEC-2): An open-label, non-inferiority, randomised trial. Lancet. 2010;375:816–823. doi: 10.1016/S0140-6736(09)62163-2. [DOI] [PubMed] [Google Scholar]
- 9.Hogberg T, Signorelli M, de Oliveira CF, et al. Sequential adjuvant chemotherapy and radiotherapy in endometrial cancer: Results from two randomised studies. Eur J Cancer. 2010;46:2422–2431. doi: 10.1016/j.ejca.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Boer SM, Powell ME, Mileshkin L, et al. Toxicity and quality of life after adjuvant chemoradiotherapy versus radiotherapy alone for women with high-risk endometrial cancer (PORTEC-3): An open-label, multicentre, randomised, phase 3 trial. Lancet Oncol. 2016;17:1114–1126. doi: 10.1016/S1470-2045(16)30120-6. [DOI] [PubMed] [Google Scholar]
- 11.de Boer SM, Powell ME, Mileshkin L, et al. Adjuvant chemoradiotherapy versus radiotherapy alone for women with high-risk endometrial cancer (PORTEC-3): Final results of an international, open-label, multicentre, randomised, phase 3 trial. Lancet Oncol. 2018;19:295–309. doi: 10.1016/S1470-2045(18)30079-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fader AN, Nagel C, Axtell AE, et al. Stage II uterine papillary serous carcinoma: Carboplatin/paclitaxel chemotherapy improves recurrence and survival outcomes. Gynecol Oncol. 2009;112:558–562. doi: 10.1016/j.ygyno.2008.11.016. [DOI] [PubMed] [Google Scholar]
- 13.Goldberg H, Miller RC, Abdah-Bortnyak R, et al. Outcome after combined modality treatment for uterine papillary serous carcinoma: A study by the Rare Cancer Network (RCN) Gynecol Oncol. 2008;108:298–305. doi: 10.1016/j.ygyno.2007.10.037. [DOI] [PubMed] [Google Scholar]
- 14.Havrilesky LJ, Secord AA, Bae-Jump V, et al. Outcomes in surgical stage I uterine papillary serous carcinoma. Gynecol Oncol. 2007;105:677–682. doi: 10.1016/j.ygyno.2007.01.041. [DOI] [PubMed] [Google Scholar]
- 15.Viswanathan AN, Macklin EA, Berkowitz R, et al. The importance of chemotherapy and radiation in uterine papillary serous carcinoma. Gynecol Oncol. 2011;123:542–547. doi: 10.1016/j.ygyno.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 16.Randall ME, Filiaci VL, Muss H, et al. Randomized phase III trial of whole-abdominal irradiation versus doxorubicin and cisplatin chemotherapy in advanced endometrial carcinoma: A Gynecologic Oncology Group study. J Clin Oncol. 2006;24:36–44. doi: 10.1200/JCO.2004.00.7617. [DOI] [PubMed] [Google Scholar]
- 17.McMeekin DS, Filiaci VL, Thigpen JT, et al. The relationship between histology and outcome in advanced and recurrent endometrial cancer patients participating in first-line chemotherapy trials: A Gynecologic Oncology Group study. Gynecol Oncol. 2007;106:16–22. doi: 10.1016/j.ygyno.2007.04.032. [DOI] [PubMed] [Google Scholar]
- 18.Maggi R, Lissoni A, Spina F, et al. Adjuvant chemotherapy vs radiotherapy in high-risk endometrial carcinoma: Results of a randomised trial. Br J Cancer. 2006;95:266–271. doi: 10.1038/sj.bjc.6603279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Susumu N, Sagae S, Udagawa Y, et al. Randomized phase III trial of pelvic radiotherapy versus cisplatin-based combined chemotherapy in patients with intermediate- and high-risk endometrial cancer: A Japanese Gynecologic Oncology Group study. Gynecol Oncol. 2008;108:226–233. doi: 10.1016/j.ygyno.2007.09.029. [DOI] [PubMed] [Google Scholar]
- 20.Miller D, Filiaci V, Fleming G, et al. Randomized phase III noninferiority trial of first line chemotherapy for metastatic or recurrent endometrial carcinoma: A Gynecologic Oncology Group study. Gynecol Oncol. 2012;125:771. (abstr) [Google Scholar]