Photodynamic therapy (PDT) is a promising approach to treat cancer.1 It involves the use of three individually nontoxic substances to produce a toxic effect. A photosensitizer, when illuminated with light of an appropriate wavelength, will be activated and convert molecular oxygen into reactive oxygen species (ROS), which can then kill tumor cells. The success of PDT depends on its potency as well as the targeting of the photosensitizer to the tumor site.2 For example, in photoimmunotherapy, the photosensitizer is conjugated to monoclonal antibodies that direct the photosensitizer to tumor cells that express the specific antigen recognized by the antibodies, and the tumor cells are killed upon light irradiation of the tumor site.3 This direct cytotoxicity is useful in treating localized tumor; however, it has limited applicability when metastasis occurs. In this regard, the activation of antitumor immunity appears to be more promising because most cancer patients die, not from localized tumor, but from cancer metastasis and relapse.
In addition to killing tumor cells directly, PDT is also known to possess an immunomodulatory effect.4 Using the unsymmetrically substituted bisamino silicon (IV) phthalocyanine (BAM-SiPc) compound that we previously synthesized5 as a photosensitizer, we showed that PDT could not only eradicate mouse-derived CT26 tumors in tumor-bearing BALB/c mice, but also trigger an antitumor immune response that protects the mice against further rechallenge with the same type of tumor cells. Immunohistochemical staining showed the infiltration of dendritic cells, T cells and B cells into the tumor tissue.6 In short, PDT results in antitumor immunity.
The activation of the immune response is believed to be a result of “immunogenic” cell death induced by BAM-SiPc-PDT. In vitro studies showed that BAM-SiPc-PDT led to the secretion of ATP (unpublished) and HMGB1 (unpublished) as well as the exposure of calreticulin, HSP70 and HSP90 on the CT26 tumor cell surface.6 These molecules are damage-associated molecular patterns (DAMPs) and function as “eat me” signals. Originally hidden inside cells, they are released or exposed when cells encounter oxidative stress or endoplasmic reticulum (ER) stress. Their cell surface expression defines immunogenic cell death7, and serves as an important signal for the maturation and activation of dendritic cells. While DAMPs have been extensively studied in the action of chemotherapeutic agents, their involvement in PDT has just emerged in the last few years.
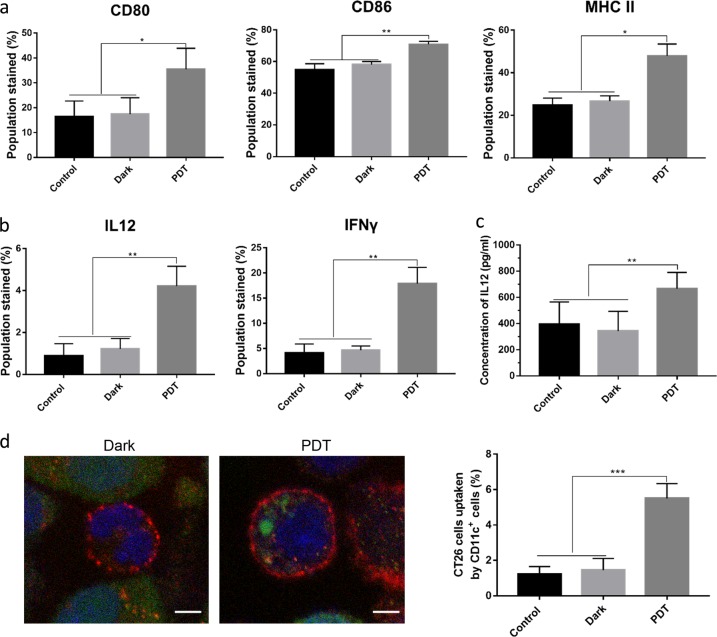
To study the immunogenic properties of BAM-SiPc-PDT-treated DAMP-expressing CT26 cells, we examined their effects on dendritic cells. We isolated and cultured bone marrow monocytes from BALB/c mice and differentiated these cells into dendritic cells. Then, we cocultured the dendritic cells with PDT-treated tumor cells to detect any phenotypic or functional changes in the dendritic cells in vitro. Figure 1a shows that after the coculture, the relative expression of cell surface markers of dendritic cells, including CD80, CD86, and MHC II, increased significantly in the dendritic cells cocultured with the BAM-SiPc-PDT-treated CT26 cells compared with the dendritic cells cocultured the nonilluminated (dark, CT26 cells treated with BAM-SiPc in the absence of light) or control (untreated CT26) cells. In addition to phenotypic changes, functional stimulation of dendritic cells can be demonstrated by the increased production of cytokines. Figure 1b shows the upregulation of interleukin 12 (IL12) and interferon γ (IFNγ) expression in dendritic cells. The increase in IL12 expression was confirmed by ELISA (Fig. 1c). In addition, we found that dendritic cells could recognize and take up PDT-treated CT26 cells (Fig. 1d). Dendritic cells are professional antigen-processing cells that act as messengers between the innate and adaptive immune systems. Activated dendritic cells can cross-present tumor-derived antigens to other immune cells, such as CD4+ helper T cells and CD8+ cytotoxic T cells, which are responsible for killing tumor cells and establishing immunological memory. Taken together, our in vitro studies show that CT26 tumor cells, after BAM-SiPc-PDT, become immunogenic in nature and can activate dendritic cells.
Fig. 1.
Activation of dendritic cells. Dendritic cells were cocultured with BAM-SiPc-PDT-treated CT26 cells for 24 h before the following analyses were performed. a Cells were collected and stained with anti-CD11c FITC-conjugated, anti-CD80 PE-conjugated, anti-CD86 APC-conjugated, and anti-MHC II PE-conjugated antibodies. The percentage of CD11c-positive dendritic cells with high expression of CD80, CD86, and MHC II was calculated after flow cytometric analysis (N = 5). b Five hours after the addition of Brefeldin A (10 μM), cells were stained with anti-CD11c FITC-conjugated antibodies together with either anti-IL12 APC-conjugated or anti-IFNγ APC-conjugated antibodies before flow cytometry was carried out to examine the expression of these cytokines (N = 4). c Supernatants were collected after coculture to determine the concentration of IL12 by ELISA (N = 3). d The engulfment of CT26 cells by dendritic cells was assessed. PDT-treated CT26 cells were stained with CMFDA (green) and then incubated with dendritic cells for 24 h. The cells were collected and stained with anti-CD11c antibodies (red). The left panel shows the engulfment of the CT26 cells (green) by the dendritic cells (red), which was visualized by confocal microscopy. The nucleus was stained in blue, bar = 5 μm (N = 3). The right panel shows the percentage of CT26 cells engulfed, which was estimated by flow cytometry (N = 5). *p < 0.05; **p < 0.005; ***p < 0.001
An immunogenic response was also demonstrated in an in vivo BALB/c mouse model. BAM-SiPc-PDT-cured mice could resist a further rechallenge with CT26 tumor cells. Intriguingly, serum obtained from the PDT-cured mice could also effectively hinder tumor growth and protect mice against rechallenge in a T-cell-dependent manner, suggesting the involvement of both humoral immunity and cell-mediated immunity in these processes.6 In this regard, it will be interesting to know whether the resistance that develops is tumor cell-type specific or not, i.e., whether a mouse cured of CT26 tumor by PDT can resist challenges with other types of tumor cells. The immunomodulatory properties of the serum could be, at least partially, attributed to its ability to trigger surface DAMP expression on tumor cells.6 It remains unknown, however, whether there exists any specific antigen on the tumor cell surface that is recognized by antibodies.
To strengthen the antitumor immunity induced by PDT, different approaches have been used. Knowing that immunogenic cell death is usually triggered by ER stress, we designed and synthesized an ER-localized photosensitizer by conjugating a BODIPY photosensitizer with the ER-targeting glibenclamide moiety8, and are ready to examine whether it is more potent than BAM-SiPc in terms of antitumor immunity. Enhancement of the immune response has been observed when the photosensitizer Ce6 is encapsulated in a hybrid protein–oxygen nanocarrier to tackle the problem of tumor hypoxia, which affects the ROS generation efficiency.9 Synergistic effects are observed when a photosensitizer is linked to or used together with an immune checkpoint inhibitor.10,11 Activated dendritic cells obtained after incubating with PDT-treated tumor cells have been successfully used in a vaccination approach to inhibit the development of tumors in mice.12 With all these advances, it is believed that PDT will become increasingly important in cancer immunotherapy.
Acknowledgements
This work was supported by a grant from the Research Grants Council of the Hong Kong Special Administrative Region China [Project no. CUHK 14171717].
Competing interests
The authors declare no competing interests.
References
- 1.Fong Wing-Ping, Yeung Hing-Yuen, Lo Pui-Chi, Ng Dennis K. P. Handbook of Photonics for Biomedical Engineering. Dordrecht: Springer Netherlands; 2014. Photodynamic Therapy; pp. 1–20. [Google Scholar]
- 2.Abrahamse H, Hamblin MR. New photosensitizers for photodynamic therapy. Biochem J. 2016;473:347–364. doi: 10.1042/BJ20150942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siddiqui MR, et al. Targeting epidermal growth factor receptor (EGFR) and human epidermal growth factor receptor 2 (HER2) expressing bladder cancer using combination photoimmunotherapy (PIT) Sci. Rep. 2019;9:2084. doi: 10.1038/s41598-019-38575-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Preise D, et al. Systemic antitumor protection by vascular-targeted photodynamic therapy involves cellular and humoral immunity. Cancer Immunol. Immunother. 2009;58:71–84. doi: 10.1007/s00262-008-0527-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lo PC, et al. New amphiphilic silicon(IV) phthalocyanines as efficient photosensitizers for photodynamic therapy: synthesis, photophysical properties, and in vitro photodynamic activities. Chem. Eur. J. 2004;10:4831–4838. doi: 10.1002/chem.200400462. [DOI] [PubMed] [Google Scholar]
- 6.Yeung HY, Lo PC, Ng DK, Fong WP. Anti-tumor immunity of BAM-SiPc-mediated vascular photodynamic therapy in a BALB/c mouse model. Cell Mol. Immunol. 2017;14:223–234. doi: 10.1038/cmi.2015.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galluzzi L, Buqué A, Kepp O, Zitvogel L, Kroemer G. Immunogenic cell death in cancer and infectious disease. Nat. Rev. Immunol. 2017;17:97–111. doi: 10.1038/nri.2016.107. [DOI] [PubMed] [Google Scholar]
- 8.Zhou Y, et al. Endoplasmic reticulum-localized two-photon-absorbing boron dipyrromethenes as advanced photosensitizers for photodynamic therapy. J. Med. Chem. 2018;61:3952–3961. doi: 10.1021/acs.jmedchem.7b01907. [DOI] [PubMed] [Google Scholar]
- 9.Chen Z, et al. Bioinspired hybrid protein oxygen nanocarrier amplified photodynamic therapy for eliciting anti-tumor immunity and abscopal effect. ACS Nano. 2018;12:8633–8645. doi: 10.1021/acsnano.8b04371. [DOI] [PubMed] [Google Scholar]
- 10.Song W, et al. Enhanced immunotherapy based on photodynamic therapy for both primary and lung metastasis tumor eradication. ACS Nano. 2018;12:1978–1989. doi: 10.1021/acsnano.7b09112. [DOI] [PubMed] [Google Scholar]
- 11.O'Shaughnessy MJ, et al. Systemic antitumor immunity by PD-1/PD-L1 inhibition is potentiated by vascular-targeted photodynamic therapy of primary tumors. Clin. Cancer Res. 2018;24:592–599. doi: 10.1158/1078-0432.CCR-17-0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zheng Y, et al. Photodynamic-therapy activates immune response by disrupting immunity homeostasis of tumor cells, which generates vaccine for cancer therapy. Int J. Biol. Sci. 2016;12:120–132. doi: 10.7150/ijbs.12852. [DOI] [PMC free article] [PubMed] [Google Scholar]