Abstract
In this review, we highlight the complexities of the natural history, biology, and clinical management of three intermediate connective tissue tumors: desmoid tumor (DT) or aggressive fibromatosis, tenosynovial giant cell tumor (TGCT) or diffuse-type pigmented villonodular synovitis (dtPVNS), and giant cell tumor of bone (GCTB). Intermediate histologies include tumors of both soft tissue and bone origin and are locally aggressive and rarely metastatic. Some common aspects to these tumors are that they can be locally infiltrative and/or impinge on critical organs, which leads to disfigurement, pain, loss of function and mobility, neurovascular compromise, and occasionally life-threatening consequences, such as mesenteric, bowel, ureteral, and/or bladder obstruction. DT, PVNS, and GCTB have few and recurrent molecular aberrations but, paradoxically, can have variable natural histories. A multidisciplinary approach is recommended for optimal management. In DT and PVNS, a course of observation may be appropriate, and any intervention should be guided by symptoms and/or disease progression. A surgical approach should take into consideration the infiltrative nature, difficulty in obtaining wide margins, high recurrence rates, acute and chronic surgical morbidities, and impact on quality of life. There are similar concerns with radiation, which especially relate to optimal field and transformation to high-grade radiation-associated sarcomas. Systemic therapies must be considered carefully in light of acute and chronic toxicities. Although standard and novel therapies are promising, many unanswered questions, such as duration of therapy and optimal end points to evaluate efficacy of drugs in clinical practice and trials, exist. Predictive biomarkers and novel clinical trial end points, such as volumetric measurement, magnetic resonance imaging T2 weighted mapping, nuclear imaging, and patient-reported outcomes, are in development and will require validation in prospective trials.
DESMOID TUMORS
Desmoid tumors (DTs), or aggressive fibromatosis, are classified as benign tumors because of their lack of metastatic potential and low risk of mortality.1-3 The term benign is deceptive, though, because it fails to highlight the impact of the tumor on morbidity as a result of its locally infiltrative nature. DTs can occur in any anatomic location but frequently arise in the abdominal wall, neurovascular bundle of the limb and shoulder girdle, root of the mesentery, and head and neck structures.1 Dependent on location, the presentation can vary from asymptomatic to severe pain, deformity, swelling, loss of function, bowel obstruction or perforation, and/or threat to vital organs (Figs 1A and 1B). A DT is a rare tumor; its estimated annual incidence is 900 patients in the United States. It affects young adults in their 20s and 30s and occurs slightly more often in women. DTs result in significant psychological and economic effects on the lives of young adults, and these effects manifest in chronic use of opioids, social isolation, changes to education and employment, sleeplessness, anxiety, and depression often related to uncertainties in medical management and the unpredictable natural history of the disease.4,5 This unpredictability is illustrated by the rapid growth phase of the tumor and the periods of stabilization followed by a second or third growth phase and spontaneous regressions.6
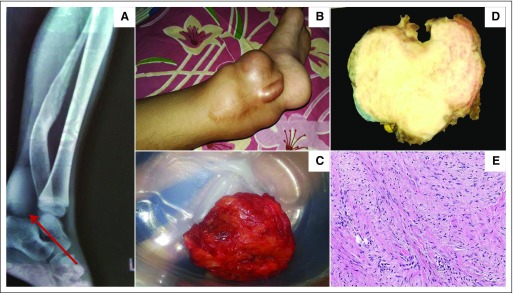
Fig 1.
Desmoid tumor. (A) X-ray of a lower extremity that demonstrates a radiolucent mass. (B) Large protuberant mass that involves the ankle joint. (C) Gross appearance of resected mass. (D) Section of a grossly resected desmoid tumor that show a smooth, glistening white tumor with trabeculations. (E) Hematoxylin and eosin stain that shows uniform elongated spindle cells in a collagenous matrix (magnification, 200×). Panels D and E are courtesy of Meera Hameed, MD, Department of Pathology, Memorial Sloan Kettering Cancer Center, New York, NY.
Given the complexity of this disease, the successful management of the patient with a DT is best undertaken by a multidisciplinary team with sarcoma expertise.7 When this is not feasible, expert guidelines and/or communication with a sarcoma expert may be helpful. Until recently, surgery was the first-line standard of care. Surgery increasingly has fallen out favor; many studies now advocate, when appropriate, an initial period of observation.8-10 A prospective, observational study is ongoing to address this possibility (ClinicalTrials.gov identifier: NCT02547831). Exceptions to observation include life-threatening conditions that are surgically reversible. Surgical or other interventions should be contemplated only if the patient is symptomatic or is having rapid disease progression, or if the disease is threatening vital organs. Given the infiltrative nature, risk of 3-year postsurgical recurrence may be as high as 40% to 50%, as calculated by an online nomogram.11 Interestingly, status of surgical margins (ie, negative or positive) as a prognostic factor to predict risk of recurrence is not predictive. Therefore, enthusiastic attempts to achieve wide margins should be curbed, because this effort may be futile and may result only in significant morbidity. Even minor morbidity from surgery (ie, abdominal wall mesh complications) must be weighed against the morbidity of the disease, which occasionally can be indolent. Radiation may be considered in symptomatic patients if surgery or systemic therapy is contraindicated. Many sarcoma experts tend to avoid this modality in this young population to reduce the risk of radiotherapy-associated malignant sarcoma.
Gross resection of a DT reveals a poorly circumscribed, firm mass that glistens white and has a coarsely trabeculated surface (Figs 1C and 1D).1 Microscopically, elongated, slender, spindle-shaped cells of uniform appearance are present, set in a collagenous matrix that contains prominent blood vessels (Fig 1E). The mitotic rate is variable but lacks cytologic atypia or nuclear hyperchromasia. Immunohistochemistry is positive for nuclear β-catenin (CTNBB1) and is pathognomonic, although only a subpopulation of cells (approximately 30%) is positive. Genomic sequencing reveals trisomies in chromosome 8 and/or 20, but the functional or prognostic significance of this is unclear. Virtually all patients with DTs harbor inactivating mutations in either CTNBB1 (90%, somatic) or APC (9%, germline), which are termed sporadic or hereditary, respectively.12 The presence of these mutations in turn result in aberrant ubiquitin-mediated degradation of β-catenin and subsequent accumulation in the nucleus and constitutive activation of downstream transcription genes.13,14 Genomic studies show few mutations in other genes; the mutations in CTNBBI (T41A, S45F, S45P) and APC are limited to a few hotspot mutations for which prognostic significance is under investigation.15,16 The pathogenesis of DT remains poorly understood.17 Thus, DT may represent an ideal human model system to study the aberrant Wnt signaling pathway.13 This is demonstrated by response in clinical trials with Wnt inhibitors.18
There is no standard of care for systemic therapies in DT. A wide class of drugs has shown variable degrees of activity. These include anti-estrogen or -progesterone treatments, nonsteroidal anti-inflammatory drugs, cytotoxic chemotherapies, tyrosine kinase inhibitors, and novel investigational agents. The choice for first-line systemic therapy should be patient centered and should be weighed carefully against patient convenience, acute and chronic toxicities, and urgency to alleviate symptoms. In patients with symptoms or in those with rapidly progressive disease or threats to vital organs, tyrosine kinase inhibitors (eg, imatinib, sorafenib) or cytotoxic chemotherapies (eg, liposomal doxorubicin, methotrexate, and vinorelbine) all are considered reasonable first choices.4,19-23 For less-aggressive DTs, endocrine therapy (eg, tamoxifen) may be appropriate.24-26 Efforts to correlate estrogen or progesterone receptor status to activity have been unrevealing. These treatment recommendations are informed by single-institution, retrospective, nonrandomized, studies that use small sample sizes. Moreover, the mechanism of action for any of these classes of drugs remains unknown.17 In the past decade, tyrosine kinase inhibitors have come to play an increasingly front-line role because of their convenience and limited long-term toxicities. Preclinical studies showed overexpression of c-KIT and PDGFRA/B, which led to two phase II studies of imatinib that showed response rates of 5% to 19% and a 6-month progression-free survival rate of 63%.21,27 A retrospective study of 24 patients treated with sorafenib showed a response rate of 20% and improvement in symptoms in approximately 70% of patients.4 The first phase III randomized study of sorafenib versus placebo is ongoing, and the results are expected to inform many unanswered questions in the field. This and other prospective studies can help estimate the rate of spontaneous regressions in patients randomly assigned to placebo or observation. Pre- and post- treatment biopsies can help elucidate mechanisms of response to sorafenib. A recent single-arm study of a γ-secretase inhibitor showed promising results in patients with progressive DTs.28,29 The activity of a wide range of drugs is perplexing, and a unifying hypothesis remains elusive.17
GIANT CELL TUMOR OF BONE
Giant cell tumor of bone (GCTB) is a benign, osteolytic tumor that predominantly affects young adults.2 GCTB is a rare condition and accounts for 3% to 5% of all bone tumors.30 The tumors can arise at almost any bony site but most often affect the spine, distal femur, proximal tibia, and distal radius (Fig 2A). GCTB belongs to a family of giant cell rich bony tumors, which includes chondroblastoma, aneurysmal bone cysts, and giant cell granuloma of the jaw.1 Histopathologically, these tumors comprise two cellular populations, a tumorigenic stromal population with osteoblast-like features and a second population of abundant monocyte-derived, multinucleated osteoclastic cells (Fig 2B).31
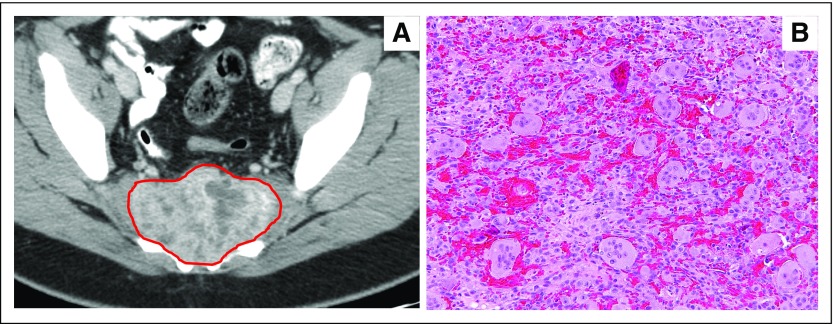
Fig 2.
Giant cell tumor of bone. (A) Computed tomography scan of an infiltrative mass that involve the sacrum. (B) Osteoclast-like giant cells in a syncytial growth pattern with interspersed mononuclear cells (magnification, 200×). Images courtesy of Robert Lefkowitz, MD, Department of Radiology, and Meera Hameed, MD, Department of Pathology, Memorial Sloan Kettering Cancer Center, New York, NY.
Although notionally benign, the disease is characterized by a broad range of clinical behavior. Presentation is usually accompanied by local pain and swelling, occasionally with pathologic fracture or neurologic symptoms according to the anatomic site. There are three stages of disease: intraosseous lesions with good bony margins; intraosseous lesions with bone thinning; and extraosseous extension.32 A curious feature of benign GCTB is the phenomenon of pulmonary implants, most often observed after instrumentation. These lung deposits are not true metastases and do not necessarily imply malignancy. However, perhaps 1% to 3% of GCTBs will undergo true malignant degeneration and effectively become giant cell–rich osteosarcomas.
Nearly 20 years ago, the signaling pathways by which osteoblasts induce the formation of osteoclasts were identified. Receptor activator of nuclear factor kappa B ligand (RANKL) is a tumor necrosis factor family member secreted by osteoblasts, and it binds via its receptor (ie, RANK) to cells of the monocyte lineage to induce osteoclastic differentiation.33 RANKL expression is increased in stromal cells within GCTBs and is presumed to mediate recruitment of osteoclasts within developing tumors.34 The osteoclastic population is responsible for the osteolysis seen in GCTBs. The primary basis for increased expression of RANKL is not known. Genomic studies have identified recurrent mutations in H3F3A (Gly34Trp or Gly34Leu) in 90% of GCTBs.35 These mutations are found in the stromal population, consistent with their neoplastic origin.
The treatment of localized GCTB is primarily surgical—from intralesional curettage with or without local adjuvants to en bloc resection and even amputation.36 The recurrence rate for surgical resection ranges from 20% to 40%, largely dependent on the extent of surgery, the margins, and the site of disease. At some anatomic sites, such as the sacrum, the surgical morbidity of achieving good margins is major and lifelong. Radiotherapy has been effectively used in situations of multiple recurrences of tumors or surgically intractable GCTB, but has been associated with a variable but significant risk of transformation.37,38 Systemic therapy for GCTB has involved chemotherapy, interferons, and bisphosphonates; these typically are based on case reports and retrospective series.36
Denosumab is a wholly human antibody with high specificity for RANKL. Two prospectively conducted studies have established the clinical efficacy of denosumab in GCTBs. The first of these was a single-arm phase II study of 35 patients with recurrent or locally advanced unresectable GCTB.39 The pathologic complete response rate (defined as the absence of osteoclast-like giant cells) was 85%. Notably, although giant cells were effectively eradicated from most treated tumors, RANKL-positive stromal cells remain visible.40
A second study confirmed these findings in a much larger group of patients with advanced disease.41 This study, which also was a nonrandomized phase II trial, examined the effect of denosumab in the adjuvant and neoadjuvant setting. Convincing data suggested that administration of denosumab before resection resulted in down-staging of the extent of surgery. However, the absence of a control arm and the lack of long-term outcomes precluded insights into whether denosumab reduced relapse rates.
TENOSYNOVIAL GIANT CELL TUMOR
Tenosynovial giant cell tumor (TGCT) is a rare, often locally aggressive neoplasm that affects young patients and tends to affect a single joint, tendon sheath, or bursae (Figs 3A and 3B).42-44 TGCT is characterized by synovial proliferation and the development of tumors that mostly are composed of multinucleated giant cells with osteoclast-like phenotypes, macrophages, histiocytes, and other inflammatory cells (Figs 3C, 3D, and 3E).42,43,45 A neoplastic clone that overexpresses colony-stimulating factor 1 (CSF1), often because of a translocation that involves the CSF1 gene on chromosome 1 (1p13), is present in the majority of TGCTs.46 CSF1 can promote growth of the neoplastic clone in an autocrine fashion.46 However, the neoplastic clone represents only the minority of cells in the tumor; rather, the bulk of a TGCT consists of CSF1-receptor (CSF1R)–bearing cells that are recruited to the joint space. With time, these inflammatory cells can cause repeat hemarthroses, subchondral cystic changes, and bone erosions.45,47 These insults then can lead to joint destruction, significant pain, swelling, decreases in range of motion, and stiffness.
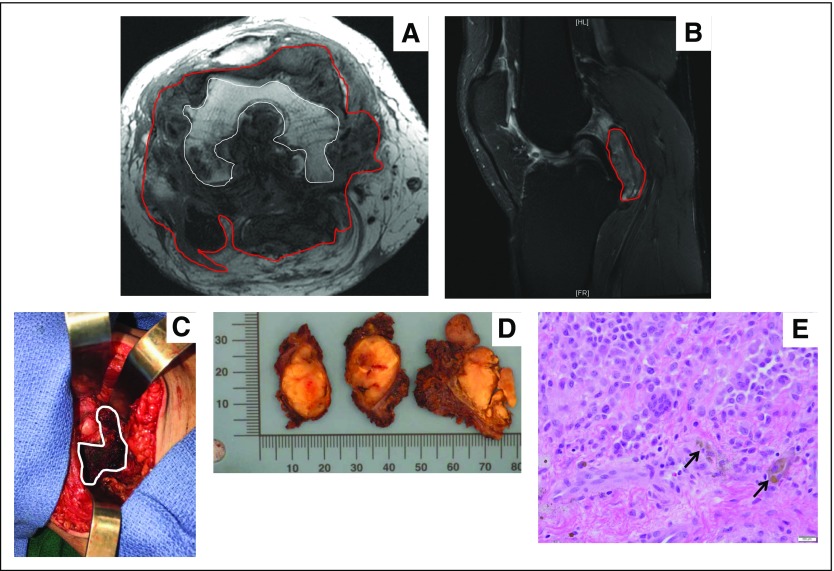
Fig 3.
Pigmented villonodular synovitis.(A) Axial T1-weighted magnetic resonanceI image that shows lobulated PVNS (red line) throughout joint and extensive subjacent erosions of femoral condyles (white outline). (B) Sagittal T2 fat-saturated magnetic resonance image that shows a multilobulated intra-articular mass abutting the posterior cruciate ligament, tibial plateau, and posterior knee joint capsule. (C) Intraoperative imaging that shows a brownish pigmented mass. (D) Gossly resected specimens that show multiple yellowish to brown masses with no encapsulation. (E) Randomly distributed multinucleated osteoclast-like giant cells accompanied by mononuclear cells, histiocytes, and hemosiderin-laden macrophages (black arrows; magnification, 200×). Images courtesy of David Panicek, MD, Department of Radiology, Meera Hameed, MD, Department of Pathology, and Nicola Fabbri, MD, Department of Orthopedic Oncology Surgery, Memorial Sloan Kettering Cancer Center, New York, NY.
There are two types of TGCTs: localized (nodular) type and the more clinically aggressive and infiltrative diffuse type (dtTGCT).43 dtTGCT mostly affects the knee, but it can be seen in any joint, including the hip, ankle, elbow, and jaw.47,48 dtTGCT is not a malignancy per se and is rarely life threatening. However, it can confer significant morbidity and can greatly alter an individual’s functionality because of significant pain, medication use, dysmotility, disability, and lost work hours.49 In the past, few treatment options were available for patients with dtTGCTs. Patients were treated symptomatically and frequently underwent recurrent surgeries that were often incomplete and that had recurrence rates of 30% to 40%.44
The identification of the role of CSF1 in the pathogenesis and propagation of dtTGCT has been revolutionization of treatments for patients with this rare neoplasm. The first case report to demonstrate activity of imatinib mesylate, a multitargeted tyrosine kinase that also inhibits CSF1R, in a patient with dtTGCT showed complete tumor response.50 Subsequent data with imatinib and nilotinib only showed modest activity.48,51 Somewhat simultaneously to the discovery of the role of CSF1 in dtTGCT, a newer generation of specific and highly potent inhibitors of the CSF1/CSF1R axis were developed to exploit these drugs in immuno-oncology. Two potent and specific CSF1R inhibitors, pexidartinib and emactuzumab, are in varying stages of clinical trials.42,52 These trials are demonstrating dramatic and durable responses with symptomatic improvement in dtTGCTs. A pivotal, randomized, double-blinded, placebo-controlled study with pexidartinib (NCT02371369) is ongoing. Early data suggest that CSF1R inhibitors do not yet appear curative for the majority of patients. Patients also can have variable decreases in tumor burden—some with dramatic responses within the first few months of therapy. Regardless of radiographic response, many patients have a significant reduction in symptoms with the initiation of therapy that, along with joint preservation, may arguably be one of the most important aspects of treatment.42,48,50-52
All of these situations highlight continued questions about the proper application of CSF1R inhibitors in dtTGCTs. Moving forward, establishment of the timing for treatment initiation will be paramount. Considerations could include earlier application before the development of a substantial tumor burden or before joint destruction occurs.
CHALLENGES AND FUTURE DIRECTIONS
The ability to pursue drug development in DT, PVNS, and GCTB has unmasked some interesting dilemmas in these rare diseases. Essentially, the presence of active drugs that have a regulatory approval strategy has tested the medical community about the true knowledge of these diseases to inform clinical trial end points. This knowledge included an understanding of the natural history and variable clinical course of the disease, how to appropriately apply the medications (and in which setting and patient population), how to judge meaningful efficacy, and how to establish appropriate risk tolerance for physicians and patients alike.
Overall survival is the gold-standard primary end point in oncology clinical trials. In a disease like DT, PVNS, and/or GCTB, this is not an appropriate end point because of the low mortality. Therefore, alternate surrogate end points for drug efficacy include response rates, progression-free survival, and avoidance of morbid therapies. It is critical to understand that these end points are surrogates for something that is clinically meaningful, such as improvement in quality of life. The challenges are to identify the most relevant symptoms for each of these diseases and to develop tools that are capable of quantifying these changes to assess clinical benefit. A challenge in the use of existing generic oncology tools for quality of life as a primary or key end point is that these tools are not validated for these specific diseases and, therefore, are not acceptable to regulatory agencies. Therefore, it is essential to develop and incorporate patient-reported outcome (PRO) tools to use as novel end points in clinical trials.
The first prospectively developed PRO tool in DTs has been developed and has identified 11 symptoms and 17 psychosocial parameters as critical to the patient experience.5 This tool is undergoing validation in prospective trials. Similarly, a dtTGCT-specific PRO instrument was created. This was applied initially in the dtTGCT expansion cohort of the pexidartinib phase I trial.42,49 It also was incorporated into the pivotal phase III study of pexidartinib as a close secondary end point along with other subjective and objective assessment tools, including the Brief Pain Inventory, Worst Pain Numeric Rating Scale, the PRO Measurement Information System Physical Function Scale, the Worst Stiffness Numeric Rating Scale, and independent and blinded goniometry testing.
Response rates and progression-free survival may serve as surrogate end points for overall survival in certain malignancies. Given the infiltrative nature of DT, PVNS, and GCTB, tumor shrinkage can have a direct impact on PRO parameters and other clinical benefits, such as avoidance of mutilation from surgeries and avoidance of threats to vital organs. Therefore, accurate radiographic measurements by existing standards, such as RECIST version 1.1 or WHO, is critical when response rates are used as key end points. It is abundantly clear that it is challenging to accurately and reproducibly measure tumors that are asymmetric and that infiltrate multiple layers of fascia, neurovascular bundles, and complex joint spaces. Therefore, it is critical to develop and validate appropriate imaging techniques that can accurately assess the clinical efficacy of drugs. In a retrospective study of 79 patients with DTs who were treated with sorafenib, the response rates varied between RECIST (18%) and WHO (26%) criteria.53 Choice of the correct imaging criteria has profound implications on trial end points and success and failure of drugs. After these issues were recognized, a novel imaging modality was created specifically for dtTGCT and was incorporated into the phase I and ongoing phase III pexidartinib trials. The tumor volume score (TVS), an magnetic resonance imaging–based tool, calculates tumor volume as a percentage of the entire synovium.42 Beyond dimensional measurements, other imaging characteristics may serve as novel end points for drug effect. Patients with DT who undergo effective treatment may have RECIST-stable disease but can show changes in the magnetic resonance imaging T2 signal, which suggests a transformation from a cellular to a collagenous mass or scar tissue (Figs 4A and 4B).
Fig 4.
Challenges in accurate imaging of tumors to access efficacy of drugs. (A) Baseline pretreatment magnetic resonance imaging (MRI) scan shows high T1 signal indicative of an active, cellular tumor. (B) Post-sorafenib MRI scan that shows a minimal decrease in tumor size but a significant decrease in T1 signal, which suggests a decrease in cellularity and an increase in collagen or tissue scarring.
Denosumab, pexidartinib, and sorafenib or liposomal doxorubicin have shown promising signals, including high response rates and/or prolonged progression-free survival, in GCTB, PVNS, and DT (Fig 5).42 However, these drugs do not appear curative, and there is emerging evidence that cessation of drugs may result in recurrence.53 Therefore, these drugs currently are used continuously and indefinitely. Because these are generally non–life-threatening diseases, an answer to the question of treatment duration is essential. The long-term physiologic, psychological, and financial impacts on young adults, who may be treated for decades, must be weighed against the morbidity of the disease. Because of the number and kinetics of the neoplastic clone, it is uncertain whether DT, PVNS, and GCTB are susceptible to the selective pressure of continued and prolonged use of these inhibitors. In addition, given the high response and progression-free survival rates, the question is whether a therapeutic ceiling has been hit in PVNS or GCTB. Given this question, newer agents need to demonstrate superior efficacy, improved cure rates, lower acute and chronic toxicities, convenience in dosing schedules, and more. Although preoperative use of tyrosine kinases inhibitors or chemotherapy does appear to facilitate surgical approaches in large tumors at difficult sites, there are little prospective data to guide their use in the neoadjuvant and/or adjuvant setting.
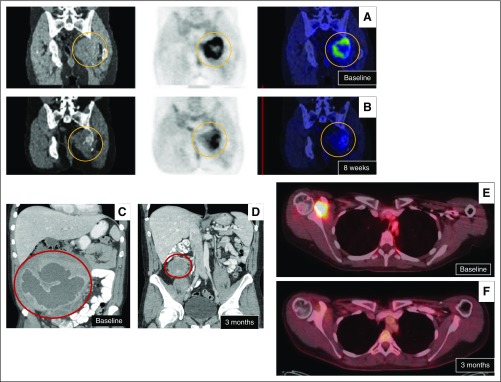
Fig 5.
Treatment response. Positron emission tomography (PET)/computed tomography (CT) scan of giant cell tumor of bone (A) at presentation (ie, baseline) and (B) after 8 weeks of denosumab, which shows minimal decrease in size but significant decrease in fluorodeoxyglucose (FDG) avidity. (C) A deep mesenteric and abdominal desmoid tumor (C) at baseline experienced (D) a dramatic response to sequential sorafenib (5 weeks, discontinued for hypertension) followed by liposomal doxorubicin (two cycles). PET/CT of recurrent pigmented villonodular synovitis (E) at presentation and (F) after 3 months of imatinib that shows significant decrease in tumor size and FDG avidity. Images courtesy of David Thomas, MBBS, PhD, Garvan Institute of Medical Research, Darlinghurst, Australia; Robert Lefkowitz, MD, Department of Radiology, and Marylou Keohan, MD, Department of Medical Oncology, Memorial Sloan Kettering Cancer Center, New York, NY; and Vinod Ravi, MD, Department of Medical Oncology, MD Anderson Cancer Center, Houston, TX.
Finally, we must continue to advance our understanding of the biology of these infiltrative connective tissue tumors. Genetic studies have shown few molecular aberrations beyond CTBNN1, H3F3A, or CSF1R and, in fact, these tumors have the lowest tumor mutational burdens among all connective tissue tumors.54 These diseases serve as good human models to study these specific pathways. Conversely, despite a single molecular driver, the variability in clinical behavior suggests that additional signal pathways or growth factors may be involved. Additional studies can establish prognostic and predictive markers and enhance therapeutic options either through combination strategies or through the development of novel drugs.
Footnotes
Supported by Memorial Sloan Kettering Cancer Center Core Grant No. P30 CA008748.
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Collection and assembly of data: Mrinal M. Gounder
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Locally Aggressive Connective Tissue Tumors
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Mrinal M. Gounder
Honoraria: Amgen, Daiichi Sankyo, Karyopharm Therapeutics, Tracon Pharma, Epizyme
Consulting or Advisory Role: Amgen, Daiichi Sankyo, Karyopharm Therapeutics, Epizyme
Speakers' Bureau: Amgen
Travel, Accommodations, Expenses: Amgen, Daiichi Sankyo, Karyopharm Therapeutics
David M. Thomas
Speakers' Bureau: Roche
Research Funding: AstraZeneca, Pfizer, Roche, Eisai, Loxo
William D. Tap
Consulting or Advisory Role: EMD Serono, PLexxikon, Janssen, Lilly, Daiichi Sankyo, Novartis, Eisai, Immune Design, Adaptimmune, Tracon Pharma, Loxo
REFERENCES
- 1. World Health Organization: International Agency for Research on Cancer: WHO Classification of Tumours of Soft Tissue and Bone, 4th ed. Lyon, France, IARC Press, 2013. [Google Scholar]
- 2. Brennan MF, Antonescu CR, Alektiar K, et al: Management of Soft Tissue Sarcoma (ed 2). Switzerland, Springer, 2016.
- 3.Fiore M, MacNeill A, Gronchi A, et al. : Desmoid-type fibromatosis: Evolving treatment standards. Surg Oncol Clin N Am 25:803-826, 2016 [DOI] [PubMed] [Google Scholar]
- 4.Gounder MM, Lefkowitz RA, Keohan ML, et al. : Activity of sorafenib against desmoid tumor/deep fibromatosis. Clin Cancer Res 17:4082-4090, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paty J, Maddux L, Gounder MM: Prospective development of a patient reported outcomes (PRO) tool in desmoid tumors: A novel clinical trial endpoint. J Clin Oncol 35:11022-11022, 2017 [Google Scholar]
- 6.Bonvalot S, Ternès N, Fiore M, et al. : Spontaneous regression of primary abdominal wall desmoid tumors: More common than previously thought. Ann Surg Oncol 20:4096-4102, 2013 [DOI] [PubMed] [Google Scholar]
- 7.von Mehren M, Randall RL, Benjamin RS, et al. : Soft tissue sarcoma, version 2.2016: NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 14:758-786, 2016 [DOI] [PubMed] [Google Scholar]
- 8.Penel N, Le Cesne A, Bonvalot S, et al. : Surgical versus non-surgical approach in primary desmoid-type fibromatosis patients: A nationwide prospective cohort from the French Sarcoma Group. Eur J Cancer 83:125-131, 2017 [DOI] [PubMed] [Google Scholar]
- 9.Fiore M, Rimareix F, Mariani L, et al. : Desmoid-type fibromatosis: A front-line conservative approach to select patients for surgical treatment. Ann Surg Oncol 16:2587-2593, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Bonvalot S, Eldweny H, Haddad V, et al. : Extra-abdominal primary fibromatosis: Aggressive management could be avoided in a subgroup of patients. Eur J Surg Oncol 34:462-468, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Crago AM, Denton B, Salas S, et al. : A prognostic nomogram for prediction of recurrence in desmoid fibromatosis. Ann Surg 258:347-353, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crago AM, Chmielecki J, Rosenberg M, et al. : Near universal detection of alterations in CTNNB1 and Wnt pathway regulators in desmoid-type fibromatosis by whole-exome sequencing and genomic analysis. Genes Chromosomes Cancer 54:606-615, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nusse R, Clevers H: Wnt/β-catenin signaling, disease, and emerging therapeutic modalities. Cell 169:985-999, 2017 [DOI] [PubMed] [Google Scholar]
- 14.Salas S, Chibon F, Noguchi T, et al. : Molecular characterization by array comparative genomic hybridization and DNA sequencing of 194 desmoid tumors. Genes Chromosomes Cancer 49:560-568, 2010 [DOI] [PubMed] [Google Scholar]
- 15.Colombo C, Belfiore A, Paielli N, et al. : β-Catenin in desmoid-type fibromatosis: Deep insights into the role of T41A and S45F mutations on protein structure and gene expression. Mol Oncol 11:1495-1507, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salas S, Brulard C, Terrier P, et al. : Gene expression profiling of desmoid tumors by cDNA microarrays and correlation with progression-free survival. Clin Cancer Res 21:4194-4200, 2015 [DOI] [PubMed] [Google Scholar]
- 17.Gounder MM: Notch inhibition in desmoids: “Sure it works in practice, but does it work in theory?” Cancer 121:3933-3937, 2015 [DOI] [PubMed] [Google Scholar]
- 18.Jimeno A, Gordon MS, Chugh R, et al. : A first-in-human phase I study of anticancer stem-cell agent OMP-54F28 (FZD8-Fc), decoy receptor for WNT ligands, in patients with advanced solid tumors. J Clin Oncol 32:2505-2505, 2014 [DOI] [PubMed] [Google Scholar]
- 19.de Camargo VP, Keohan ML, D’Adamo DR, et al. : Clinical outcomes of systemic therapy for patients with deep fibromatosis (desmoid tumor). Cancer 116:2258-2265, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palassini E, Frezza AM, Mariani L, et al. : Long-term efficacy of methotrexate plus vinblastine/vinorelbine in a large series of patients affected by desmoid-type fibromatosis. Cancer J 23:86-91, 2017 [DOI] [PubMed] [Google Scholar]
- 21.Chugh R, Wathen JK, Patel SR, et al. : Efficacy of imatinib in aggressive fibromatosis: Results of a phase II multicenter Sarcoma Alliance for Research through Collaboration (SARC) trial. Clin Cancer Res 16:4884-4891, 2010 [DOI] [PubMed] [Google Scholar]
- 22.Skapek SX, Ferguson WS, Granowetter L, et al. : Vinblastine and methotrexate for desmoid fibromatosis in children: Results of a Pediatric Oncology Group phase II trial. J Clin Oncol 25:501-506, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Benech N, Walter T, Saurin JC: Desmoid tumors and celecoxib with sorafenib. N Engl J Med 376:2595-2597, 2017 [DOI] [PubMed] [Google Scholar]
- 24.Skapek SX, Anderson JR, Hill DA, et al. : Safety and efficacy of high-dose tamoxifen and sulindac for desmoid tumor in children: Results of a Children’s Oncology Group (COG) phase II study. Pediatr Blood Cancer 60:1108-1112, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kasper B, Baumgarten C, Bonvalot S, et al. : Management of sporadic desmoid-type fibromatosis: A European consensus approach based on patients’ and professionals’ expertise—A sarcoma patients EuroNet and European Organisation for Research and Treatment of Cancer/Soft Tissue and Bone Sarcoma Group initiative. Eur J Cancer 51:127-136, 2015 [DOI] [PubMed] [Google Scholar]
- 26.Fiore M, Colombo C, Radaelli S, et al. : Hormonal manipulation with toremifene in sporadic desmoid-type fibromatosis. Eur J Cancer 51:2800-2807, 2015 [DOI] [PubMed] [Google Scholar]
- 27.Kasper B, Gruenwald V, Reichardt P, et al. : Imatinib induces sustained progression arrest in RECIST progressive desmoid tumours: Final results of a phase II study of the German Interdisciplinary Sarcoma Group (GISG). Eur J Cancer 76:60-67, 2017 [DOI] [PubMed] [Google Scholar]
- 28.Kummar S, O’Sullivan Coyne G, Do KT, et al. : Clinical activity of the γ-secretase inhibitor PF-03084014 in adults with desmoid tumors (aggressive fibromatosis). J Clin Oncol 35:1561-1569, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Messersmith WA, Shapiro GI, Cleary JM, et al. : A phase I, dose-finding study in patients with advanced solid malignancies of the oral γ-secretase inhibitor PF-03084014. Clin Cancer Res 21:60-67, 2015 [DOI] [PubMed] [Google Scholar]
- 30.Larsson SE, Lorentzon R, Boquist L: Giant-cell tumor of bone: A demographic, clinical, and histopathological study of all cases recorded in the Swedish Cancer Registry for the years 1958 through 1968. J Bone Joint Surg Am 57:167-173, 1975 [PubMed] [Google Scholar]
- 31.Atkins GJ, Haynes DR, Graves SE, et al. : Expression of osteoclast differentiation signals by stromal elements of giant cell tumors. J Bone Miner Res 15:640-649, 2000 [DOI] [PubMed] [Google Scholar]
- 32.Campanacci M, Baldini N, Boriani S, et al. : Giant-cell tumor of bone. J Bone Joint Surg Am 69:106-114, 1987 [PubMed] [Google Scholar]
- 33.Dougall WC, Glaccum M, Charrier K, et al. : RANK is essential for osteoclast and lymph node development. Genes Dev 13:2412-2424, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morgan T, Atkins GJ, Trivett MK, et al. : Molecular profiling of giant cell tumor of bone and the osteoclastic localization of ligand for receptor activator of nuclear factor kappaB. Am J Pathol 167:117-128, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Behjati S, Tarpey PS, Presneau N, et al. : Distinct H3F3A and H3F3B driver mutations define chondroblastoma and giant cell tumor of bone. Nat Genet 45:1479-1482, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thomas DM: RANKL, denosumab, and giant cell tumor of bone. Curr Opin Oncol 24:397-403, 2012 [DOI] [PubMed] [Google Scholar]
- 37.Leggon RE, Zlotecki R, Reith J, et al. : Giant cell tumor of the pelvis and sacrum: 17 cases and analysis of the literature. Clin Orthop Relat Res 423:196-207, 2004 [DOI] [PubMed] [Google Scholar]
- 38.Schwartz LH, Okunieff PG, Rosenberg A, et al. : Radiation therapy in the treatment of difficult giant cell tumors. Int J Radiat Oncol Biol Phys 17:1085-1088, 1989 [DOI] [PubMed] [Google Scholar]
- 39.Thomas D, Henshaw R, Skubitz K, et al. : Denosumab in patients with giant-cell tumour of bone: An open-label, phase 2 study. Lancet Oncol 11:275-280, 2010 [DOI] [PubMed] [Google Scholar]
- 40.Branstetter DG, Nelson SD, Manivel JC, et al. : Denosumab induces tumor reduction and bone formation in patients with giant-cell tumor of bone. Clin Cancer Res 18:4415-4424, 2012 [DOI] [PubMed] [Google Scholar]
- 41.Chawla S, Henshaw R, Seeger L, et al. : Safety and efficacy of denosumab for adults and skeletally mature adolescents with giant cell tumour of bone: Interim analysis of an open-label, parallel-group, phase 2 study. Lancet Oncol 14:901-908, 2013 [DOI] [PubMed] [Google Scholar]
- 42.Tap WD, Wainberg ZA, Anthony SP, et al. : Structure-guided blockade of CSF1R kinase in tenosynovial giant-cell tumor. N Engl J Med 373:428-437, 2015 [DOI] [PubMed] [Google Scholar]
- 43.Brahmi M, Vinceneux A, Cassier PA: Current systemic treatment options for tenosynovial giant cell tumor/pigmented villonodular synovitis: Targeting the CSF1/CSF1R axis. Curr Treat Options Oncol 17:10, 2016 [DOI] [PubMed] [Google Scholar]
- 44.Palmerini E, Staals EL, Maki RG, et al. : Tenosynovial giant cell tumour/pigmented villonodular synovitis: Outcome of 294 patients before the era of kinase inhibitors. Eur J Cancer 51:210-217, 2015 [DOI] [PubMed] [Google Scholar]
- 45.Darling JM, Goldring SR, Harada Y, et al. : Multinucleated cells in pigmented villonodular synovitis and giant cell tumor of tendon sheath express features of osteoclasts. Am J Pathol 150:1383-1393, 1997 [PMC free article] [PubMed] [Google Scholar]
- 46.West RB, Rubin BP, Miller MA, et al. : A landscape effect in tenosynovial giant-cell tumor from activation of CSF1 expression by a translocation in a minority of tumor cells. Proc Natl Acad Sci USA 103:690-695, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ravi V, Wang WL, Lewis VO: Treatment of tenosynovial giant cell tumor and pigmented villonodular synovitis. Curr Opin Oncol 23:361-366, 2011 [DOI] [PubMed] [Google Scholar]
- 48.Cassier PA, Gelderblom H, Stacchiotti S, et al. : Efficacy of imatinib mesylate for the treatment of locally advanced and/or metastatic tenosynovial giant cell tumor/pigmented villonodular synovitis. Cancer 118:1649-1655, 2012 [DOI] [PubMed] [Google Scholar]
- 49.Gelhorn HL, Tong S, McQuarrie K, et al. : Patient-reported symptoms of tenosynovial giant cell tumors. Clin Ther 38:778-793, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Blay JY, El Sayadi H, Thiesse P, et al. : Complete response to imatinib in relapsing pigmented villonodular synovitis/tenosynovial giant cell tumor (PVNS/TGCT). Ann Oncol 19:821-822, 2008 [DOI] [PubMed] [Google Scholar]
- 51. Gelderblom H, Perol D, Chevreau C, et al: An open-label international multicentric phase II study of nilotinib in progressive pigmented villo-nodular synovitis (PVNS) not amenable to a conservative surgical treatment. J Clin Oncol 31, 2013 (suppl; abstr 10516)
- 52.Cassier PA, Italiano A, Gomez-Roca CA, et al. : CSF1R inhibition with emactuzumab in locally advanced diffuse-type tenosynovial giant cell tumours of the soft tissue: A dose-escalation and dose-expansion phase I study. Lancet Oncol 16:949-956, 2015 [DOI] [PubMed] [Google Scholar]
- 53.Munhoz RR, Lefkowitz RA, Kuk D, et al. : Efficacy of sorafenib in patients with desmoid-type fibromatosis. J Clin Oncol 34:11065-11065, 2016 [Google Scholar]
- 54. Gounder MM, Ali SM, Robinson V, et al: Impact of next-generation sequencing (NGS) on diagnostic and therapeutic options in soft-tissue and bone sarcoma. J Clin Oncol 35, 2017 (suppl; abstr 11001)