Abstract
PURPOSE
Anthracycline-associated risk for subsequent breast cancer in childhood cancer survivors is hypothesized to be mediated by TP53 mutation-related gene-environment interactions. We characterized treatment/genetic risks and the impact of screening for breast cancer in the St Jude Lifetime Cohort.
PATIENTS AND METHODS
Female participants underwent risk-based assessments, prior health event validation, chest radiation dosimetry, and whole genome sequencing. Breast biopsy reports were reviewed. A subgroup (n = 139) underwent both breast magnetic resonance imaging and mammography. Multivariable regression was used to calculate hazard ratios (HRs) and 95% CIs.
RESULTS
Among 1,467 women, 56 developed 68 breast cancers at a median age 38.6 (range, 24.5 to 53.0) years. Cumulative incidences at age 35 years were 1% (no chest radiation) and 8% (≥ 10 Gy of chest radiation). In adjusted models, breast cancer was associated with 20 Gy or more of chest radiation versus none (HR, 7.6; 95% CI, 2.9 to 20.4), anthracycline exposure versus none (1 to 249 mg/m2: HR, 2.6; 95% CI, 1.1 to 6.2; ≥ 250 mg/m2: HR, 13.4, 95% CI, 5.5 to 32.5), and having a breast cancer predisposition gene mutation (HR, 23.0; 95% CI, 7.3 to 72.2). Anthracyclines 250 mg/m2 or greater remained significantly associated with increased risk of breast cancer in models excluding survivors with cancer predisposition gene mutations, chest radiation 10 Gy or greater, or both. Sensitivity/specificity were 53.8%/96.3% for mammography, 69.2%/91.4% for magnetic resonance imaging, and 85.8%/99.7% for dual imaging. Breast cancers detected by imaging and/or prophylactic mastectomy compared with physical findings were more likely to be in situ carcinomas, smaller, without lymph node involvement, and treated without chemotherapy.
CONCLUSION
Higher doses of anthracyclines are associated with increased risk of breast cancer independent of mutations in known cancer predisposition genes. Surveillance imaging identifies breast cancers less likely to require chemotherapy than those detected by physical findings.
INTRODUCTION
Female childhood cancer survivors are at increased risk for breast cancer occurring earlier than in the general population.1-3 There is a dose-dependent association between prior chest radiation and breast cancer.2,4-7 Two studies noted additional risk from anthracycline exposure; however, both observed the highest risk in survivors of cancers potentially associated with Li-Fraumeni syndrome, hypothesizing that this risk resulted from gene-environment interaction.1,3 Neither study assessed anthracycline-related risk within the context of confirmed cancer predisposition genetic mutations.
Recognition of the association between radiation and earlier presentation of subsequent breast cancer in childhood cancer survivors compared with the general population prompted establishment of breast cancer surveillance guidelines specific to childhood, adolescent, and young adult cancer survivors.8 Although mammography represents the gold standard for surveillance in the general population, its use in childhood cancer survivors is limited by increased breast tissue density in younger women.9 The addition of breast magnetic resonance imaging (MRI) improves sensitivity for detecting subsequent breast cancers in survivors with minimal loss of specificity.10,11 Accordingly, existing guidelines suggest imaging with mammography, breast MRI, or both for women at highest risk.12 Studies reporting the clinical efficacy of this approach are sparse.13
Using the clinically assessed St Jude Lifetime Cohort Study (SJLIFE), we sought to provide a comprehensive report on subsequent breast cancer risk, detection, characteristics, and treatment outcomes among female childhood cancer survivors for whom mutations in breast cancer predisposition genes are known and to evaluate whether surveillance imaging affects breast cancer outcomes.
PATIENTS AND METHODS
Study Design
This study was performed in SJLIFE, a retrospective cohort with prospective follow-up and ongoing accrual. The design and methodology for this institutional review board–approved study have been previously described.14
Participants
This analysis included childhood cancer survivors treated at St Jude Children’s Research Hospital, 18 years of age and older, and 10 years or more from diagnosis. Medical events, cumulative treatment exposures, and vital status were abstracted from health records (medical reports, cancer registry follow-up, and next of kin contact, and/or the National Death Index for those lost to follow-up or deceased) by trained research staff.
Study Procedures
Evaluations included physical examination, laboratory assessment, and questionnaires detailing demographics, medical history, and health habits. Breast cancer surveillance was performed, with the recommendation for subsequent annual screening according to consensus guidelines, including paired mammography and MRI for women exposed to 20 Gy or more of chest radiation, beginning at 25 years of age or 8 years or more after exposure, whichever occurred later.12 Shared decision making occurred for women exposed to 10 to 19 Gy of chest radiation.
Radiologists blinded to clinical outcomes retrospectively reviewed images (n = 156) for a subset of individuals (n = 139) for whom both mammography and MRI were performed in parallel. Mammograms were reviewed for size and location of masses, calcifications, architectural distortion, density, margins, and Breast Imaging, Reporting, and Data System (BI-RADS) score, whereas MRIs were reviewed for size and location of masses or enhancement, dynamic enhancement kinetics, margins, and BI-RADS score.15 Images containing lesions with BI-RADS scores of 4 or greater were considered positive. Because blinded reviews occurred months to years after the clinical assessments, post hoc interpretations did not guide biopsy recommendations, which were instead determined by real-time, clinical radiographic interpretations.
Whole genome sequencing (mean coverage per sample, 36.8×) was performed on 1,343 participants using the Illumina HiSeq X Ten System (Illumina, San Diego, CA), as previously described.16 Pathogenic/likely pathogenic mutations in autosomal dominant genes (BRCA1, BRCA2, ATM, CDH1, CHEK2, PALB2, PTEN, STK11, TP53) with high penetrance that have been linked to breast cancer were identified.17
Chest and pelvic radiation doses were estimated from radiation oncology reports using previously described methods.18 Specifically, records were abstracted for dose reconstruction details (ie, prescription, administration dates, dose, orientation, energy, field size, weighting, blocking, and anatomic borders). Maximum prescribed dose for a given region (ie, chest or pelvis) was calculated as the total prescribed dose from overlapping fields within each region. When other regions were the primary target, out-of-field stray dose was estimated based on proximity to treated regions.
Similar to Henderson et al,1 cumulative anthracycline doses were estimated using dose equivalency ratios.19-21 Because conversion ratios associated with breast toxicity do not exist, a secondary analysis similar to that of Teepen et al3 was performed using doxorubicin alone to evaluate the impact of anthracycline conversion on breast cancer risk.
Outcomes
The primary outcome was development of a subsequent breast cancer (invasive and in situ carcinomas). Records were obtained, and breast cancer characteristics were abstracted, including histology, diagnosis date, age at diagnosis, laterality, detection method (physical findings [by survivor or provider], imaging, or prophylactic mastectomy), size, location, nodal involvement, hormone receptor status, intervention (surgery, chemotherapy, hormone therapy, and/or radiation), and occurrence of prophylactic mastectomy. Synchronous, ipsilateral carcinomas, regardless of histology (eg, simultaneously diagnosed invasive and in situ carcinomas) were counted as one. Contralateral cancers, regardless of timing of occurrence or histology, were considered two separate cancers. Subsequent cancers were only considered recurrent (rather than third cancers) when the clinical presentation, location, timing, and/or histologic appearance were strongly suggestive of recurrence.
Statistical Analysis
Descriptive statistics were calculated for demographic and treatment-related variables and compared between women with and without breast cancer using Wilcoxon-Mann-Whitney tests (for median measures) and χ2 (or Fisher’s exact) tests for proportional measures. Breast cancer characteristics and treatment were compared among women with breast cancer by detection method using generalized linear models for continuous variables and Fisher’s exact test for categorical variables. The cumulative incidence function was used to estimate breast cancer risk by age, censoring at age of breast cancer diagnosis or age at SJLIFE assessment for those without breast cancer. Cumulative incidence estimates were stratified by chest radiation and anthracycline exposure, separately. Estimates were compared using Gray’s test for equality of cumulative incidence functions. Multivariable Cox proportional hazards regression was used to evaluate associations between treatment and onset of breast cancer diagnosis, with attained age (time from birth to date of breast cancer or censor date [SJLIFE assessment]) as the time scale. Hazard ratios (HRs) and 95% CIs were calculated for treatment and genetic variables, adjusting for age at diagnosis. A separate model limited to women exposed to less than 10 Gy of chest radiation was performed. Sensitivity and specificity were calculated among women with paired mammograms and MRIs, considering each study independently and both in parallel. Lesions suggestive of cancer (BI-RADS ≥ 4) were considered positive only when pathologically confirmed. Sensitivity was calculated as the number of true positives (biopsy proven) divided by the total number of positive images, whereas specificity was calculated as the number of true negatives (not biopsied or noncancerous biopsy) divided by the total number of negative images (BI-RADS < 4). Parallel sensitivity was calculated considering the scenario in which either test was positive using the formula ([A]sensitivity + [B]sensitivity − [(A)sensitivity × (B)sensitivity]), whereas specificity was calculated considering the scenario in which both tests were negative using the formula ([A]specificity + [B]specificity − [(A)specificity × (B)specificity]).22
Survival by detection method was estimated using Kaplan-Meier curves. Follow-up started at breast cancer diagnosis, and censoring occurred at the date of death or data collection end date (June 30, 2015). Survival estimates were compared using the log-rank test. SAS version 9.4 (SAS Institute, Cary, NC) was used to conduct all analyses.
RESULTS
Participant Characteristics
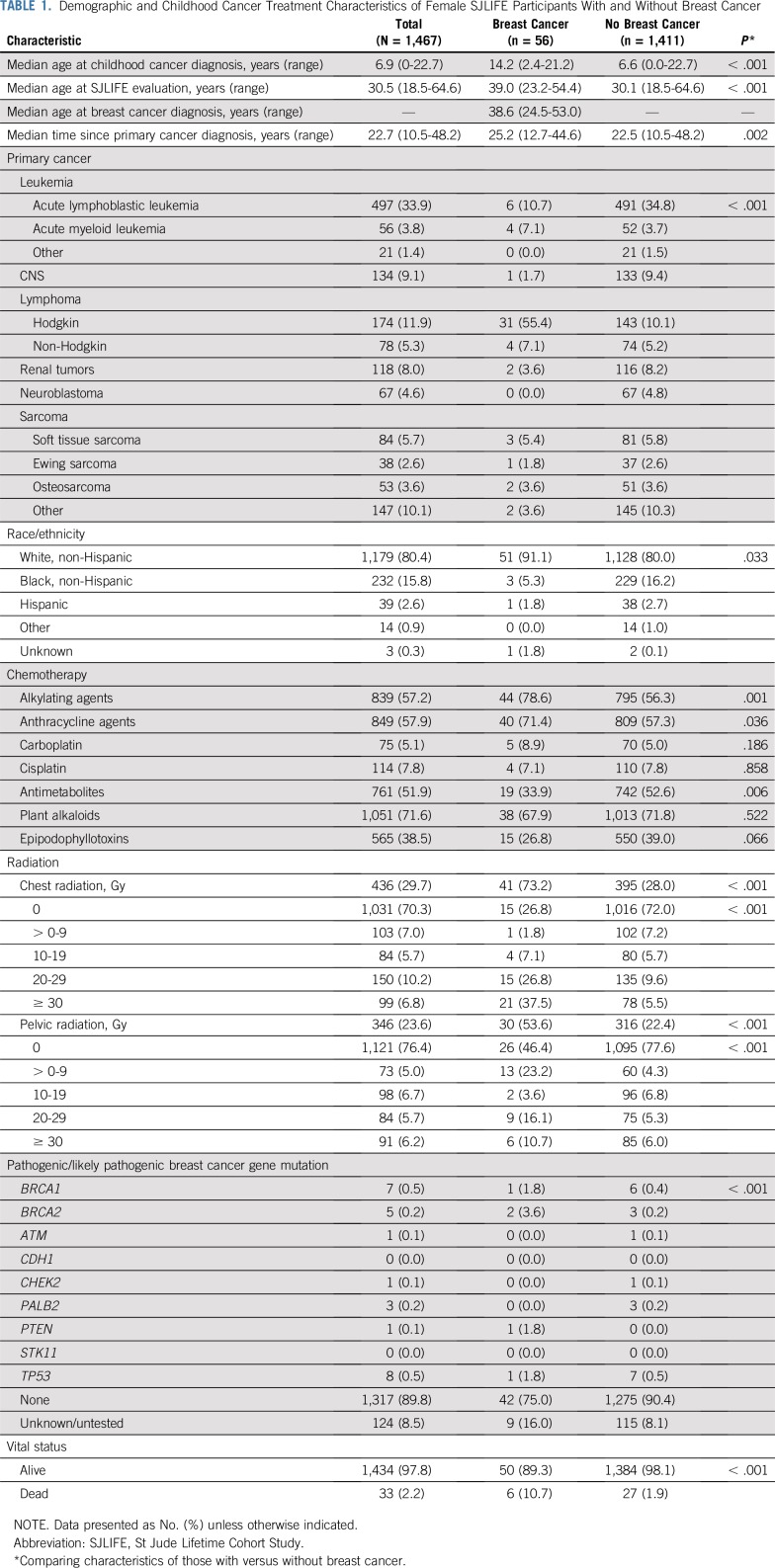
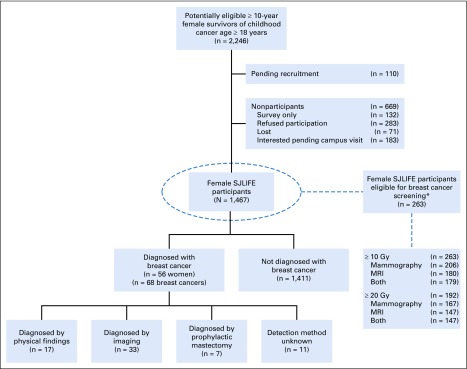
Eligible female SJLIFE participants (n = 1,467; Appendix Fig A1, online only) had a median age of 6.9 years (range, 0 to 22.7 years) at childhood cancer diagnosis and 30.5 years (range, 18.5 to 64.6 years) at evaluation (Table 1). Among these, 976 (66.5%) total and 37 (66.1%) with breast cancer were also participants in the Childhood Cancer Survivor Study.23,24 Fifty-six women developed 68 breast cancers at a median age of 38.6 years (range, 24.5 to 53.0 years). Compared with survivors without breast cancer, those with breast cancer were older at childhood cancer diagnosis (14.2 v 6.6 years; P < .001) and at evaluation (39.0 v 30.1 years; P < .001), more commonly diagnosed with Hodgkin lymphoma (55.4% v 10.1%; P < .001), and more likely to be white non-Hispanic (91.1% v 80.0%; P = .033). A higher proportion of survivors with subsequent breast cancer received alkylating agents (78.6% v 56.3%; P = .001), anthracyclines (71.4% v 57.3%; P = .036), and/or radiation (73.2% v 28%; P < .001) compared with survivors without breast cancer. Among the females with whole genome sequencing, a pathogenic/likely pathogenic mutation was present in 10.6% of survivors compared with 1.6% of those without a subsequent breast cancer.
TABLE 1.
Demographic and Childhood Cancer Treatment Characteristics of Female SJLIFE Participants With and Without Breast Cancer
Breast Cancer Characteristics
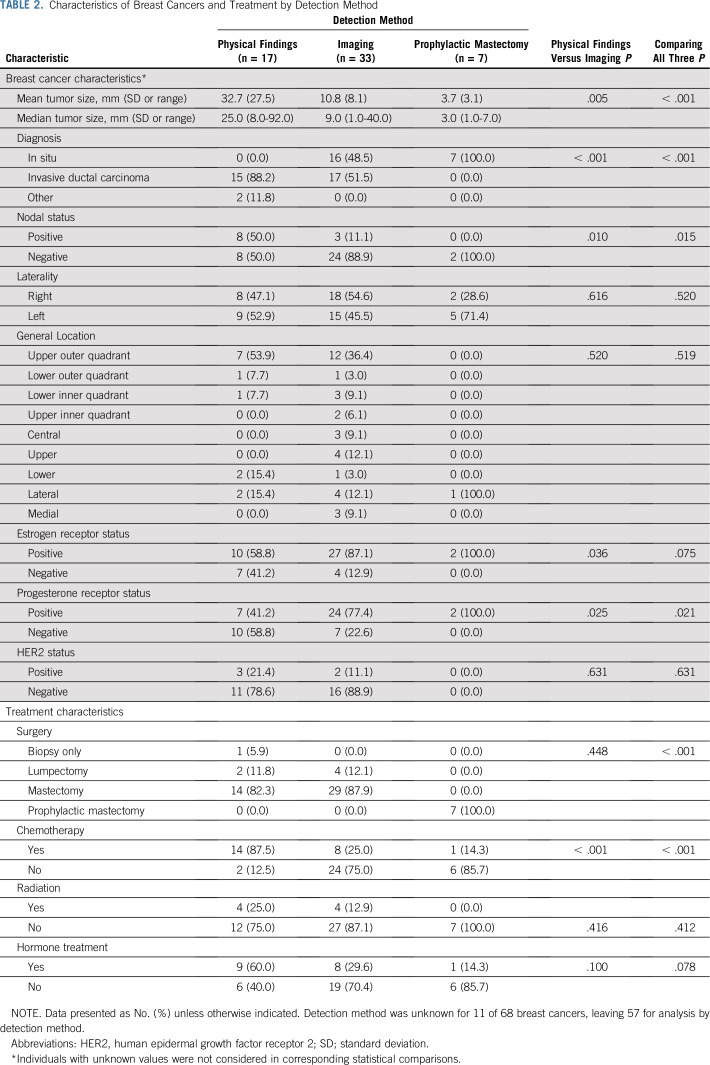
Among 68 confirmed breast cancers, there were 38 invasive ductal carcinomas, two infiltrating lobular carcinomas, one mucinous carcinoma, one combined secretory carcinoma and ductal carcinoma in situ, and 26 in situ carcinomas (22 ductal, two lobular, and two combined ductal and lobular). Compared with those detected by physical findings (n = 17), breast cancers detected by imaging (n = 33) and prophylactic mastectomy (n = 7) were more likely to be in situ carcinomas, smaller, progesterone receptor positive, and without lymph node involvement (Table 2). Detection method could not be determined for 11 breast cancers. Breast cancers diagnosed by imaging or prophylactic mastectomy were more likely to be treated without chemotherapy than those diagnosed by physical findings, but did not differ with respect to radiation or hormone therapy.
TABLE 2.
Characteristics of Breast Cancers and Treatment by Detection Method
Cumulative Incidence of and Risk Factors for Breast Cancer
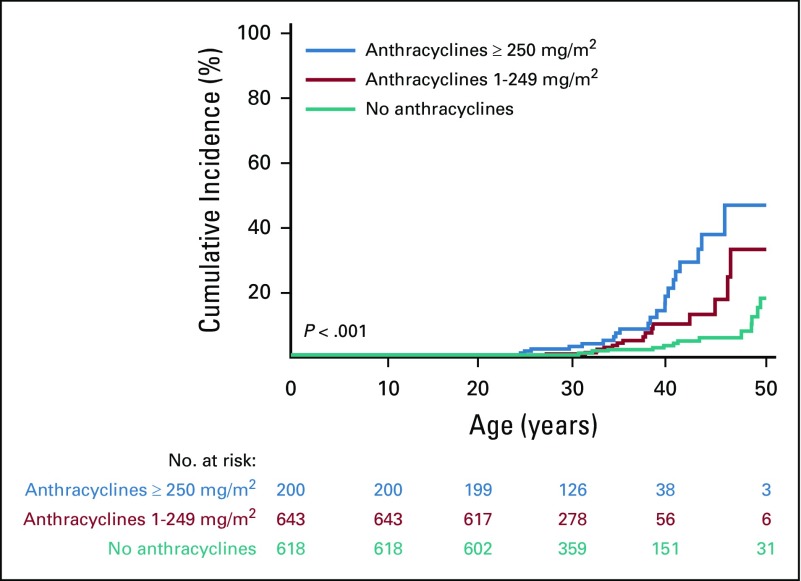
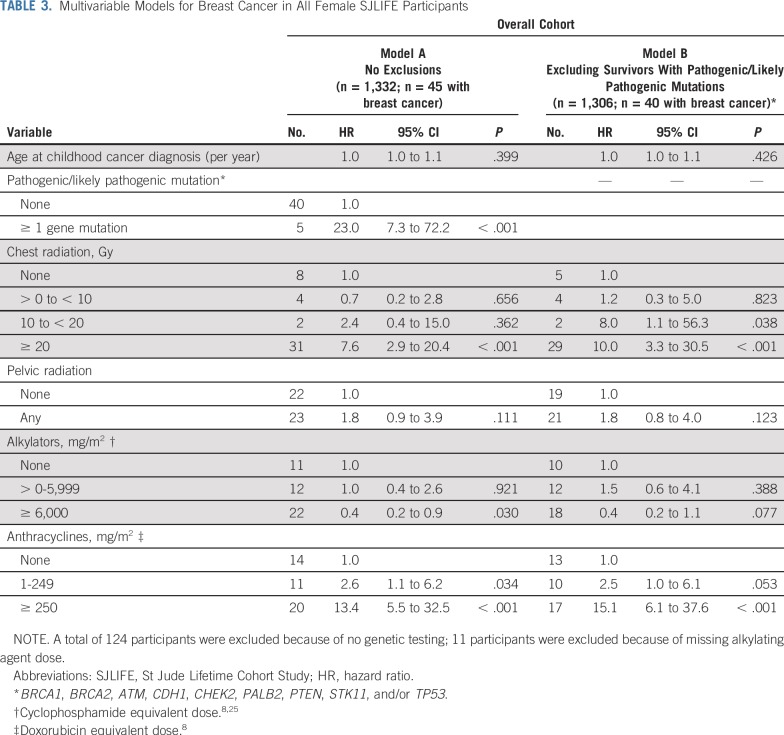
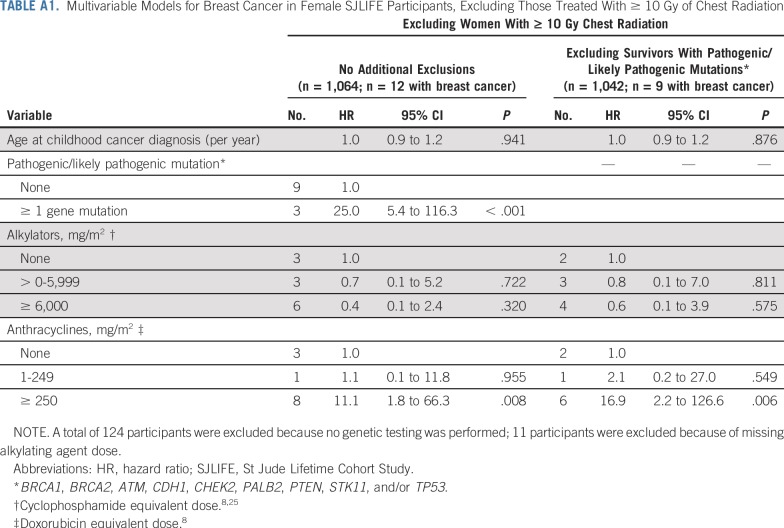
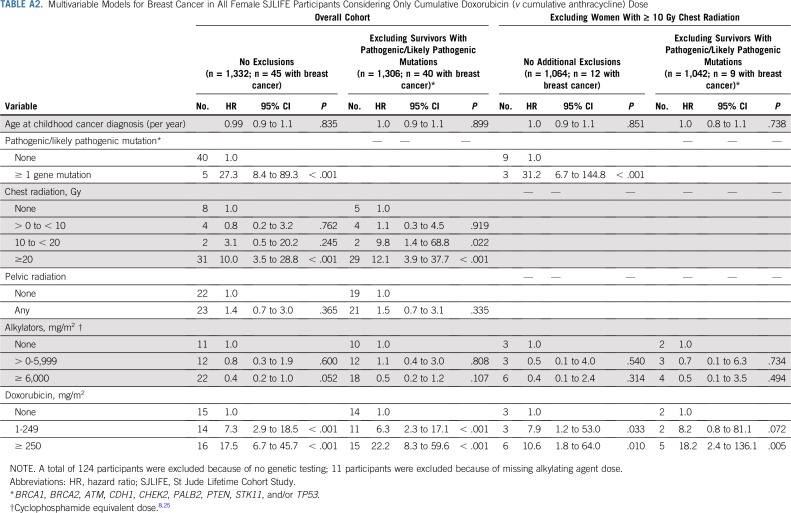
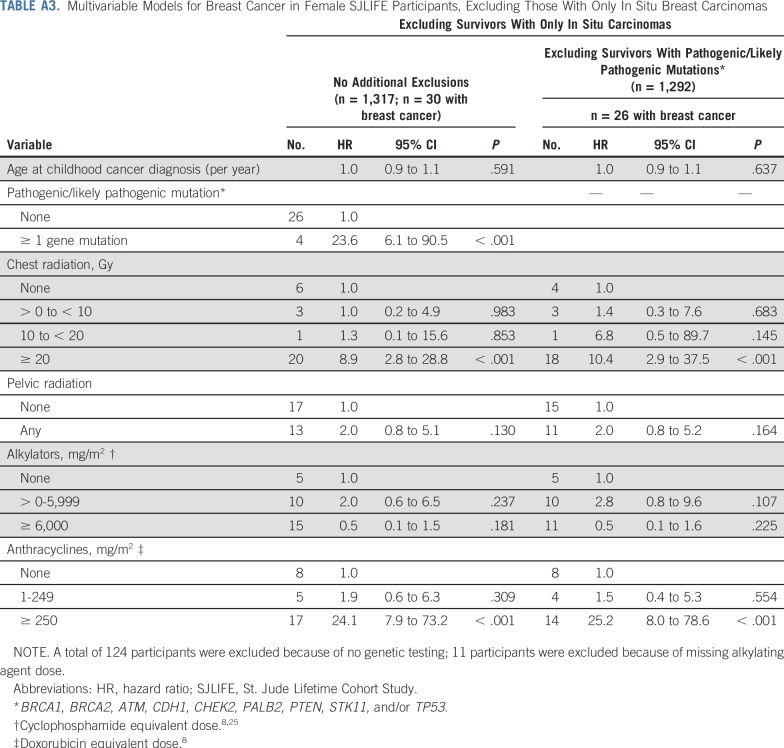
The cumulative incidence of breast cancer in women unexposed to chest radiation was 1% at 35 years of age and 15% at 50 years of age. Among those treated with 10 Gy or greater of chest radiation, rates were 8% and 41% at 35 and 50 years of age, respectively. Similarly, the cumulative incidence in women who did not receive anthracyclines was 2% at 35 years of age and 15% at 50 years of age, whereas in those treated with 250 mg/m2 or greater, the rates for those 35 years of age and 50 years of age were 7% and 46%, respectively (Fig 1). Multivariable cox proportional hazards model results are listed in Table 3. Having received 20 Gy or more of chest radiation (HR, 7.6; 95% CI, 2.9 to 20.4; P < .001), anthracycline doses of 1 to 249 mg/m2 (HR, 2.6; 95% CI, 1.1 to 6.2; P = .034) and 250 mg/m2 or greater (HR, 13.4; 95% CI, 5.5 to 32.5; P < .001), and having a known pathogenic/likely pathogenic cancer predisposition gene mutation (HR, 23.0; 95% CI, 7.3 to 72.2; P < .001) were associated with increased risk of subsequent breast cancer. Cumulative cyclophosphamide equivalent alkylating agent doses of 6,000 mg/m2 or greater were associated with decreased risk of breast cancer (HR, 0.4; 95% CI, 0.2 to 0.9; P = .030; Table 3, Model A). Restricting the model to women who did not have pathogenic/likely pathogenic mutations in known cancer predisposition genes, we observed similar associations with radiation and anthracyclines (Table 3, Model B). To further explore this anthracycline association, we restricted the models to exclude women who received chest radiation of 10 Gy or greater. Anthracyclines 250 mg/m2 or greater remained significantly associated with an increased risk of breast cancer in women with and without predisposition gene mutations (Appendix Table A1, online only). To explore the differential effect of cumulative doxorubicin equivalent anthracycline dose conversions, we performed the same models incorporating only doxorubicin and observed similar findings (Appendix Table A2, online only). Last, we excluded women with in situ breast cancers only and found similar incidences and risk estimates (Appendix Table A3, online only).
FIG 1.
Cumulative incidence of breast cancer in female childhood cancer survivors by anthracycline exposure.
TABLE 3.
Multivariable Models for Breast Cancer in All Female SJLIFE Participants
Sensitivity and Specificity of Imaging
Among 263 women eligible for screening, 206 (78%) completed a mammogram, 180 (68%) completed an MRI, and 179 (68%) completed both. Among those exposed to 20 Gy or more of radiation affecting the breast (n = 192), 167 (87%) had a mammogram, 147 (77%) had an MRI, and 147 (77%) had both. For sensitivity/specificity analyses, 139 of 179 (78%) women (mean age ± standard deviation, 36.9 ± 7.8 years; mean chest radiation dose, 26.4 ± 9.79 Gy) with paired images (n = 156) were available at the time of blinded radiographic reviews. Among 33 lesions with a BI-RADS score of 4 or greater on blinded interpretations, biopsies were obtained for 19 patients (57.6%). Among 14 lesions not biopsied, 11 had a BI-RADS score of less than 4 on clinical interpretations (in contrast to blinded, retrospective research related); therefore, no real-time biopsy was recommended. Among the remaining three patients for which biopsy was clinically recommended, one died of heart failure before biopsy, and two were nonadherent. Sensitivity and specificity were 53.8% (95% CI, 26.8% to 80.9%) and 96.3% (95% CI, 94.1% to 98.4%) for mammography, 69.2% (95% CI, 44.1% to 94.3%) and 91.4% (95% CI, 88.1% to 94.6%) for MRI, and 85.8% (95% CI, 72.4% to 99.2%; either image positive) and 99.7% (95% CI, 99.3% to 100.0%; both images negative) for parallel dual imaging, respectively.
Survival by Detection Method
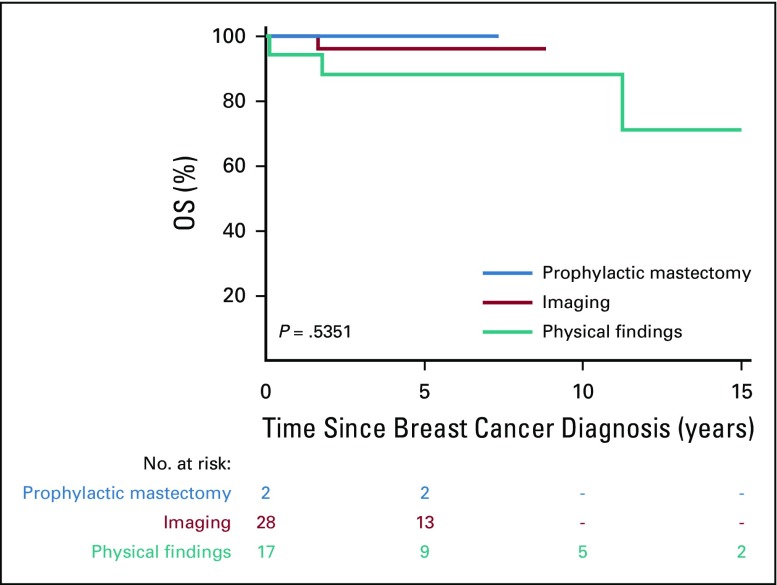
Figure 2 depicts overall survival from first subsequent breast cancer, by detection method, for the 47 women with known detection status. Although differences did not reach statistical significance (P = .535), we observed decreasing 5-year overall survival rates for those detected by prophylactic mastectomy ( n = 2, 100%), imaging (n = 28, 96.0%; 95% CI, 74.8% to 99.4%), and physical findings (n = 17, 87.8%; 95% CI, 59.5% to 96.8%).
FIG 2.
Overall survival (OS) after the diagnosis of subsequent breast cancer by detection method.
DISCUSSION
Using the clinically assessed and prospectively followed SJLIFE cohort, we comprehensively report on subsequent breast cancer risk, detection, characteristics, and outcomes among women treated for childhood cancer. Female survivors exposed to higher doses of anthracyclines are at comparable risk to those exposed to chest radiation and women with BRCA1 mutations in the general population.26 In addition, breast cancers detected by imaging were more likely to be localized, noninvasive tumors that did not require systemic chemotherapy.
We observed a greater than 13-fold risk for breast cancer in women exposed to 250 mg/m2 or more of anthracyclines compared with none. By comparison, Henderson et al1 and Teepen et al3 reported a 2.5- to six-fold increased risk of breast cancer in women exposed to anthracycline chemotherapies1,3; however, both studies showed attenuation of risk in individuals with primary cancers not reported to occur in association with Li-Fraumeni syndrome. The authors hypothesized that this association was driven largely by gene-environment interaction. Neither was able to test this hypothesis directly via the use of available sequencing data. Conversely, SJLIFE survivors were prospectively evaluated for subsequent breast cancer,14 chest dosimetry was systematically estimated (as opposed to prescribed field dose),18 and breast cancer predisposition gene mutations were identified by whole genome and exome sequencing.16 We therefore demonstrated that treatment-related risk occurs independently of autosomal dominantly inherited cancer predisposition gene mutations (including TP53). We recently reported that combinations of rare and common genetic variants associated with breast cancer in the general population increase subsequent breast cancer risk in childhood cancer survivors.27 Future investigations are needed to understand anthracycline-associated risk, not only in the context of variants associated with breast cancer, but other factors such as anthracycline metabolism, as well. Collectively, these studies suggest that women exposed to higher doses of anthracyclines are at substantially increased risk for breast cancer, yet to date, survivorship guidelines only address women who received chest radiation, likely missing a subgroup for whom routine surveillance would be equally beneficial.
Studies have suggested that dual imaging provides a sensitive and specific approach to detect subsequent breast cancer.10,11,28 We confirmed these findings in the large, well-characterized SJLIFE cohort. Our specificity of 99.7% for dual imaging required both images to be negative and identified a group of women for which a normal mammogram and MRI are quite reassuring regarding the absence of occult malignancies. These findings align with our clinical practice of not biopsying abnormalities of BI-RADS less than 4 on either mammography or MRI.
Although screening and early detection have reduced mortality in the general population,29 similar studies are limited in childhood cancer survivors.13 We observed that cancers identified by surveillance and/or prophylactic mastectomy were more likely to be localized and less likely to require treatment with systemic chemotherapy compared with those detected by physical findings, a promising finding in this often heavily pretreated population. However, emerging evidence suggests that women with subsequent breast cancers are at increased risk for inferior outcomes because of both breast cancer and nonbreast cancer–related morbidity and mortality.30-32 Notably, those diagnosed with early-stage subsequent breast cancers seem disproportionately affected by nonbreast cancer–related mortality.30 Collectively, these findings prioritize a need for collaborative investigations powered to better understand the potential benefits of surveillance strategies.
A number of limitations must be considered when interpreting these results. First, dosimetry estimations were limited to the chest, rather than breast. Although risk estimates using breast dosimetry will be a focus in SJLIFE, they are currently unavailable. Second, we included in situ carcinomas, which may have affected our incidence and risk estimates. We are reassured, however, that this was not evident in sensitivity analyses excluding those with in situ carcinomas. Our 65% participation rate introduced the possibility of bias; however, we previously demonstrated a lack of substantive differences between the SJLIFE and source population.33 We performed an additional sensitivity analysis, including all nonparticipant females exposed to 10 Gy or more of chest radiation or anthracyclines 250 mg/m2 or more in our cumulative incidence estimates. Assuming none of these women developed breast cancer, our estimates did not change; therefore, we feel confident that potential participation bias from women with breast cancer did not significantly alter our findings. In addition, not all lesions suggestive of cancer underwent biopsy, perhaps leading to underestimation of breast cancer incidence and imaging sensitivity. This reflects that biopsy recommendations were made for lesions with a BI-RADS score of 4 or greater on the basis of clinical, real-time radiographic interpretations of questionable lesions. Sensitivity and specificity estimations were calculated from retrospective reviews by radiologists blinded to clinical outcomes and therefore were at times incongruent with clinical, real-time assessments. Because we required both images to be negative for specificity calculations, and biopsy would not be recommended for such patients, this estimation would not be expected to be affected by adherence to biopsy recommendations. In addition, because our sensitivity and specificity estimates were based on baseline screening assessments, they are likely representative of prevalent rather than incident lesions. Last, despite the relatively large number of survivors in our cohort, the relative infrequency of subsequent breast cancers limits the power of our study to robustly explore outcomes (eg, survival differences) by detection method. However, our findings remain provocative and prompt follow-up investigation.
In conclusion, female childhood cancer survivors who received 250 mg/m2 or more of doxorubicin equivalent anthracycline chemotherapy are at high risk for subsequent breast cancer independent of prior chest radiation or known genetic predisposition. We recommend screening survivors treated with higher doses of anthracyclines in a manner consistent with those who have received radiation affecting the breast and/or have a known breast cancer predisposition mutation (eg, BRCA1/2). In addition, dual imaging with mammography and breast MRI is a sensitive and specific approach to identifying breast cancers that require less aggressive therapy than those detected by physical findings and should be considered the standard of care for childhood cancer survivors.
APPENDIX
FIG A1.
Flow diagram of recruitment of female childhood cancer survivors. SJLIFE, St Jude Lifetime cohort; MRI, magnetic resonance imaging. (*) Radiation 10 Gy or greater potentially affecting the breast, age of 25 years or older, and 8 years or more from radiation exposure.8
TABLE A1.
Multivariable Models for Breast Cancer in Female SJLIFE Participants, Excluding Those Treated With ≥ 10 Gy of Chest Radiation
TABLE A2.
Multivariable Models for Breast Cancer in All Female SJLIFE Participants Considering Only Cumulative Doxorubicin (v cumulative anthracycline) Dose
TABLE A3.
Multivariable Models for Breast Cancer in Female SJLIFE Participants, Excluding Those With Only In Situ Breast Carcinomas
Footnotes
Preliminary results presented at the 50th Congress of the International Society of Paediatric Oncology, Kyoto, Japan, November 16-19, 2018.
Supported by a Cancer Center Support (CORE) Grant (P30 CA21765) to St Jude Children’s Research Hospital (U01 CA195547 1; multiple principal investigators: M.M.H. and L.L.R.) and the American Lebanese Syrian Associated Charities, Memphis, TN.
AUTHOR CONTRIBUTIONS
Conception and design: Matthew J. Ehrhardt, Malek J. Baassiri, Deokumar Srivastava, Jinghui Zhang, Leslie L. Robison, Kirsten K. Ness, Melissa M. Hudson
Financial support: Jinghui Zhang, Leslie L. Robison, Melissa M. Hudson
Administrative support: Matthew J. Ehrhardt, Jinghui Zhang, Leslie L. Robison, Melissa M. Hudson
Provision of study materials or patients: Matthew J. Ehrhardt, Jinghui Zhang, Leslie L. Robison, Melissa M. Hudson
Collection and assembly of data: Matthew J. Ehrhardt, Karen Hale, Malek J. Baassiri, Carol Rodriguez, Carmen L. Wilson, Surekha S. Joshi, Sheila Shope, Rebecca M. Howell, Jinghui Zhang, Leslie L. Robison, Kirsten K. Ness, Melissa M. Hudson
Data analysis and interpretation: Matthew J. Ehrhardt, Carrie R. Howell, Malek J. Baassiri, Carol Rodriguez, Thomas C. Lemond, Zhaoming Wang, Deokumar Srivastava, Daniel A. Mulrooney, Jinghui Zhang, Leslie L. Robison, Kirsten K. Ness, Melissa M. Hudson
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Subsequent Breast Cancer in Female Childhood Cancer Survivors in the St Jude Lifetime Cohort Study (SJLIFE)
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Melissa M. Hudson
Consulting or Advisory Role: Coleman Supportive Oncology Initiative for Children with Cancer, Oncology Research Information Exchange Network, Princess Máxima Center
No other potential conflicts of interest were reported.
REFERENCES
- 1.Henderson TO, Moskowitz CS, Chou JF, et al. Breast cancer risk in childhood cancer survivors without a history of chest radiotherapy: A report from the Childhood Cancer Survivor Study. J Clin Oncol. 2016;34:910–918. doi: 10.1200/JCO.2015.62.3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moskowitz CS, Chou JF, Wolden SL, et al. Breast cancer after chest radiation therapy for childhood cancer. J Clin Oncol. 2014;32:2217–2223. doi: 10.1200/JCO.2013.54.4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Teepen JC, van Leeuwen FE, Tissing WJ, et al. Long-term risk of subsequent malignant neoplasms after treatment of childhood cancer in the DCOG LATER Study Cohort: Role of chemotherapy. J Clin Oncol. 2017;35:2288–2298. doi: 10.1200/JCO.2016.71.6902. [DOI] [PubMed] [Google Scholar]
- 4.Inskip PD, Robison LL, Stovall M, et al. Radiation dose and breast cancer risk in the childhood cancer survivor study. J Clin Oncol. 2009;27:3901–3907. doi: 10.1200/JCO.2008.20.7738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhatia S, Robison LL, Oberlin O, et al. Breast cancer and other second neoplasms after childhood Hodgkin’s disease. N Engl J Med. 1996;334:745–751. doi: 10.1056/NEJM199603213341201. [DOI] [PubMed] [Google Scholar]
- 6.Guibout C, Adjadj E, Rubino C, et al. Malignant breast tumors after radiotherapy for a first cancer during childhood. J Clin Oncol. 2005;23:197–204. doi: 10.1200/JCO.2005.06.225. [DOI] [PubMed] [Google Scholar]
- 7.Travis LB, Hill DA, Dores GM, et al. Breast cancer following radiotherapy and chemotherapy among young women with Hodgkin disease. JAMA. 2003;290:465–475. doi: 10.1001/jama.290.4.465. [DOI] [PubMed] [Google Scholar]
- 8. Children’s Oncology Group: Long-term follow-up guidelines for survivors of childhood, adolescent, and young adult cancers: Version 4.0 – October 2013. http://www.survivorshipguidelines.org/pdf/LTFUGuidelines_40.pdf.
- 9.Murphy CD, Lee JM, Drohan B, et al. The American Cancer Society guidelines for breast screening with magnetic resonance imaging: An argument for genetic testing. Cancer. 2008;113:3116–3120. doi: 10.1002/cncr.23913. [DOI] [PubMed] [Google Scholar]
- 10.Ng AK, Garber JE, Diller LR, et al. Prospective study of the efficacy of breast magnetic resonance imaging and mammographic screening in survivors of Hodgkin lymphoma. J Clin Oncol. 2013;31:2282–2288. doi: 10.1200/JCO.2012.46.5732. [DOI] [PubMed] [Google Scholar]
- 11.Tieu MT, Cigsar C, Ahmed S, et al. Breast cancer detection among young survivors of pediatric Hodgkin lymphoma with screening magnetic resonance imaging. Cancer. 2014;120:2507–2513. doi: 10.1002/cncr.28747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mulder RL, Kremer LC, Hudson MM, et al. Recommendations for breast cancer surveillance for female survivors of childhood, adolescent, and young adult cancer given chest radiation: A report from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Oncol. 2013;14:e621–e629. doi: 10.1016/S1470-2045(13)70303-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. doi: 10.1093/jnci/djw010. Hodgson DC, Cotton C, Crystal P, et al: Impact of early breast cancer screening on mortality among young survivors of childhood Hodgkin’s lymphoma. J Natl Cancer Inst 108:djw010, 2016. [DOI] [PubMed] [Google Scholar]
- 14.Hudson MM, Ness KK, Nolan VG, et al. Prospective medical assessment of adults surviving childhood cancer: Study design, cohort characteristics, and feasibility of the St. Jude Lifetime Cohort study. Pediatr Blood Cancer. 2011;56:825–836. doi: 10.1002/pbc.22875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D’Orsi CJ, Sickles EA, Mendelson EB, et al. ACR Bi-RADS Atlas, Breast Imaging Reporting and Data System. Reston VA, American College of Radiology; 2013. [Google Scholar]
- 16.Wang Z, Wilson CL, Easton J, et al. Genetic risk for subsequent neoplasms among long-term survivors of childhood cancer. J Clin Oncol. 2018;36:2078–2087. doi: 10.1200/JCO.2018.77.8589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. doi: 10.1038/gim.2016.190. Kalia SS, Adelman K, Bale SJ, et al: Recommendations for reporting of secondary findings in clinical exome and genome sequencing, 2016 update (ACMG SF v2.0): A policy statement of the American College of Medical Genetics and Genomics. Genet Med 19:249-255, 2017 [Erratum: Genet Med 19:484, 2017] [DOI] [PubMed] [Google Scholar]
- 18.Stovall M, Weathers R, Kasper C, et al. Dose reconstruction for therapeutic and diagnostic radiation exposures: Use in epidemiological studies. Radiat Res. 2006;166:141–157. doi: 10.1667/RR3525.1. [DOI] [PubMed] [Google Scholar]
- 19.Allen A. The cardiotoxicity of chemotherapeutic drugs. Semin Oncol. 1992;19:529–542. [PubMed] [Google Scholar]
- 20.Launchbury AP, Habboubi N. Epirubicin and doxorubicin: A comparison of their characteristics, therapeutic activity and toxicity. Cancer Treat Rev. 1993;19:197–228. doi: 10.1016/0305-7372(93)90036-q. [DOI] [PubMed] [Google Scholar]
- 21.Tolba KA, Deliargyris EN. Cardiotoxicity of cancer therapy. Cancer Invest. 1999;17:408–422. doi: 10.3109/07357909909021433. [DOI] [PubMed] [Google Scholar]
- 22.Weinstein S, Obuchowski NA, Lieber ML. Clinical evaluation of diagnostic tests. AJR Am J Roentgenol. 2005;184:14–19. doi: 10.2214/ajr.184.1.01840014. [DOI] [PubMed] [Google Scholar]
- 23.Robison LL, Armstrong GT, Boice JD, et al. The Childhood Cancer Survivor Study: A National Cancer Institute-supported resource for outcome and intervention research. J Clin Oncol. 2009;27:2308–2318. doi: 10.1200/JCO.2009.22.3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robison LL, Mertens AC, Boice JD, et al. Study design and cohort characteristics of the Childhood Cancer Survivor Study: A multi-institutional collaborative project. Med Pediatr Oncol. 2002;38:229–239. doi: 10.1002/mpo.1316. [DOI] [PubMed] [Google Scholar]
- 25.Green DM, Nolan VG, Goodman PJ, et al. The cyclophosphamide equivalent dose as an approach for quantifying alkylating agent exposure: A report from the Childhood Cancer Survivor Study. Pediatr Blood Cancer. 2014;61:53–67. doi: 10.1002/pbc.24679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuchenbaecker KB, Hopper JL, Barnes DR, et al. Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. JAMA. 2017;317:2402–2416. doi: 10.1001/jama.2017.7112. [DOI] [PubMed] [Google Scholar]
- 27.Wang Z, Liu Q, Wilson CL, et al. Polygenic determinants for subsequent breast cancer risk in survivors of childhood cancer: The St Jude Lifetime Cohort Study (SJLIFE) Clin Cancer Res. 2018;24:6230–6235. doi: 10.1158/1078-0432.CCR-18-1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chiarelli AM, Prummel MV, Muradali D, et al. Effectiveness of screening with annual magnetic resonance imaging and mammography: Results of the initial screen from the Ontario High Risk Breast Screening Program. J Clin Oncol. 2014;32:2224–2230. doi: 10.1200/JCO.2013.52.8331. [DOI] [PubMed] [Google Scholar]
- 29.Berry DA, Cronin KA, Plevritis SK, et al. Effect of screening and adjuvant therapy on mortality from breast cancer. N Engl J Med. 2005;353:1784–1792. doi: 10.1056/NEJMoa050518. [DOI] [PubMed] [Google Scholar]
- 30. Moskowitz CS, Chou JF, Neglia JP, et al: Mortality following breast cancer in survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. J Clin Oncol 36, 2018 (suppl; abstr 10511) [Google Scholar]
- 31.Elkin EB, Klem ML, Gonzales AM, et al. Characteristics and outcomes of breast cancer in women with and without a history of radiation for Hodgkin’s lymphoma: A multi-institutional, matched cohort study. J Clin Oncol. 2011;29:2466–2473. doi: 10.1200/JCO.2010.32.4079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Milano MT, Li H, Gail MH, et al. Long-term survival among patients with Hodgkin’s lymphoma who developed breast cancer: A population-based study. J Clin Oncol. 2010;28:5088–5096. doi: 10.1200/JCO.2010.29.5683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ojha RP, Oancea SC, Ness KK, et al. Assessment of potential bias from non-participation in a dynamic clinical cohort of long-term childhood cancer survivors: Results from the St. Jude Lifetime Cohort Study. Pediatr Blood Cancer. 2013;60:856–864. doi: 10.1002/pbc.24348. [DOI] [PMC free article] [PubMed] [Google Scholar]