Abstract
Many childhood cancer survivors carry a significant risk for late morbidity and mortality, a consequence of the numerous therapeutic exposures that contribute to their cure. Focused surveillance for late therapy-related complications provides opportunities for early detection and implementation of health-preserving interventions. The substantial body of research that links therapeutic exposures used during treatment of childhood cancer to adverse outcomes among survivors enables the characterization of groups at the highest risk for developing complications related to specific therapies; however, methods available to optimize screening strategies to detect these therapy-related complications are limited. Moreover, the feasibility of conducting clinical trials to test screening recommendations for childhood cancer survivors is limited by requirements for large sample sizes, lengthy study periods, prohibitive costs, and ethical concerns. In addition, the harms of screening should be considered, including overdiagnosis and psychological distress. Experts in several countries have developed guideline recommendations for late effects surveillance and have collaborated to harmonize these recommendations internationally to enhance long-term follow-up care and quality of life for childhood cancer survivors. Methods used in these international efforts include systematic literature searches, development of evidence-based summaries, rigorous evaluation of the evidence, and formulation of consensus-based surveillance recommendations for each late complication. Alternate methods to refine recommendations, such as cumulative burden assessment and risk prediction and cost-effectiveness modeling, may provide novel approaches to guide survivorship care in this vulnerable population and, thus, represents a worthy objective for future international survivorship collaborations.
INTRODUCTION
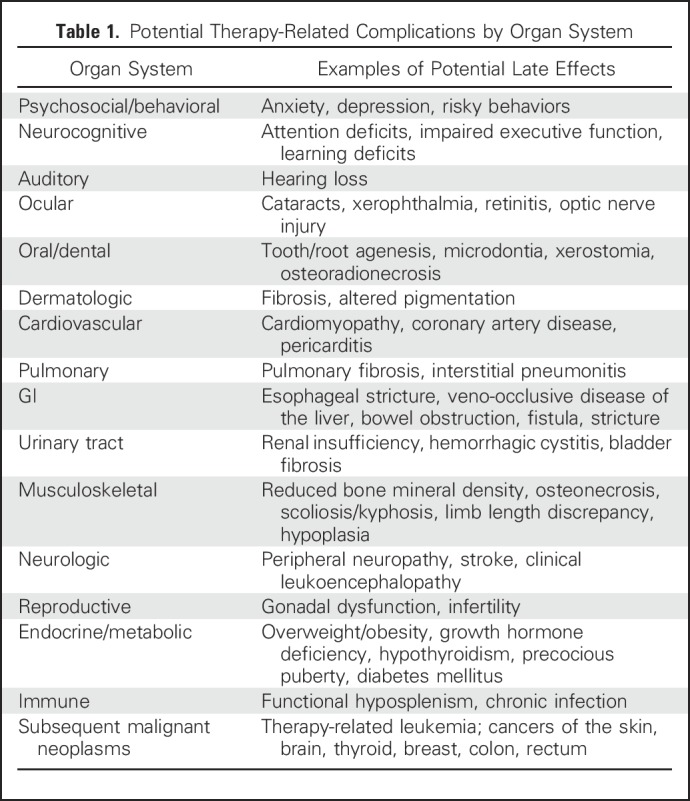
With continued advancements in the treatment of pediatric cancer, the population of long-term survivors continues to grow.1,2 Many of these childhood cancer survivors, however, carry a significant risk for late morbidity and mortality, an untoward consequence of numerous therapeutic exposures used to achieve cure in pediatric oncology.1,3-5 Therapy-related late complications may affect multiple organ systems, and many of these late effects are potentially life-threatening or life-altering6 (Table 1).
Table 1.
Potential Therapy-Related Complications by Organ System
Focused surveillance for late therapy-related complications provides opportunities for early detection and implementation of health-preserving interventions.6,7 Conversely, identification of surveillance testing that may be unnecessary or inadvisable because of the potential for overdiagnosis and inappropriate interventions is important. Implementation of specialized screening recommendations for survivors is based on the rationale that the magnitude of risk for developing each targeted complication exceeds that of the general population and that early detection will be associated with reduced morbidity. Timing, intensity, and duration of recommended screening requires attention to the period of risk, latency from exposure, and subpopulations at highest risk.
Significant challenges exist to implementing randomized clinical trials that would define optimal screening strategies, given the relatively small population of survivors, the heterogeneity of their treatment exposures, and the delayed presentation of many late effects. Nevertheless, a substantial body of research links therapeutic exposures used during childhood cancer treatment to certain adverse outcomes among survivors, which enables characterization of groups at the highest risk for developing therapy-specific complications.8
We review current pediatric oncology survivorship guideline initiatives that span several countries wherein knowledge from late effects research has been translated into recommendations for follow-up care. We also highlight international efforts currently in progress to harmonize long-term follow-up guidelines for survivors; address survivorship research deficits; and review emerging new methods, such as cumulative burden assessment and risk prediction and cost-effectiveness modeling, for use in guiding survivorship care.
CLINICAL PRACTICE GUIDELINE INITIATIVES
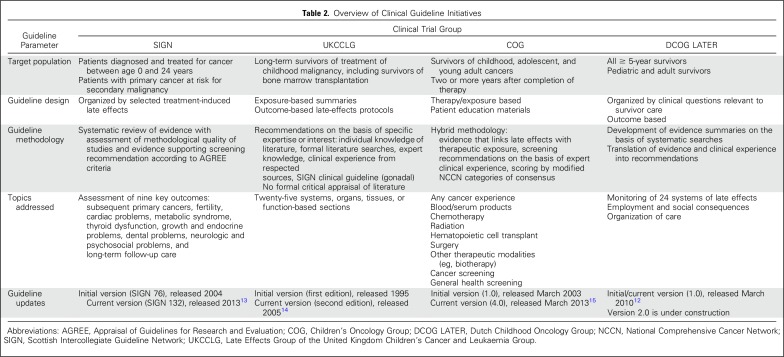
Several countries and clinical trials groups across North America and Europe have developed initiatives to guide the care of long-term childhood cancer survivors, including the Children’s Oncology Group (COG),9 the Scottish Intercollegiate Guidelines Network (SIGN),10 the Late Effects Group of the United Kingdom Children’s Cancer and Leukaemia Group (UKCCLG),11 and the Dutch Childhood Oncology Group12 (Table 2). Subsequent collaborations among these groups and with the Pan-European Network for Care of Survivors After Childhood and Adolescent Cancer (PanCare)16 have yielded the International Late Effects of Childhood Cancer Guideline Harmonization Group (IGHG)17 initiative. Concern about increasingly recognized long-term treatment-related morbidity and the need to standardize follow-up care represent a common rationale for guideline development across all groups.
Table 2.
Overview of Clinical Guideline Initiatives
The goal of standardizing survivorship care is to facilitate opportunities for early detection and timely intervention to treat or prevent late effects. However, ineffective screening is wasteful of limited resources and may even lead to harm from overdiagnosis and psychological distress.18,19 With consideration of the limited feasibility of conducting trials to test late effects screening recommendations for childhood cancer survivors, guideline development for this population typically involves a hybrid approach and uses information from existing outcomes literature to characterize groups at high risk of treatment-related complications and expert consensus to formulate screening recommendations. Common to all initiatives is multidisciplinary collaboration that includes late effects experts in pediatric and radiation oncology, pediatric and medical subspecialties, and primary care; nurses; and patient advocates. In addition, inclusion of individuals with formal training in evidence-based methodology for guideline development has improved the rigor of literature assessment and informed a robust research agenda to address key knowledge deficits related to survivor care.
Although pediatric cancer survivorship guideline developers have used similar methods to formulate late effects screening recommendations, the scope/content, format, and methods used to update and disseminate information to clinicians and survivors and to integrate guideline care within health care systems have varied. The COG guidelines feature an extensive list of exposure-based recommendations for each potential agent or modality used in treatment protocols, some of which have limited published evidence to guide care but may provide signals for outcomes that require ongoing monitoring. In contrast, guidelines from UKCCLG, SIGN, and Dutch Childhood Oncology Group are organized by key organ/system topics that focus on more-common late effects.
Pediatric guideline development groups also vary in their procedures for guideline updates, which range from full review and updating of guideline content every 5 years by COG organ/system-based task forces,20 to review of targeted outcomes on the basis of relevant published literature by SIGN.10 Since 2010, all the major pediatric cancer survivorship guideline groups, as well as numerous individuals from institutions worldwide, have been working together to harmonize surveillance recommendations for highly prevalent late effects of childhood cancer. The IGHG collaboration performs systematic literature reviews, develops evidence-based summaries, evaluates the evidence by using methods established by the Cochrane Childhood Cancer Group, and formulates recommendations for each guideline topic17 (Fig 1). The IGHG has finalized and published guidelines related to surveillance for breast cancer,21 cardiomyopathy,22 premature ovarian insufficiency,23 and male gonadal toxicity,24 with a number of additional guidelines currently in development.
Fig 1.
International Guideline Harmonization Group (IGHG) process.
Guideline developers have used a variety of methods to disseminate recommendations to clinicians and survivors. Over the years, printed booklets and other guideline documents have largely been replaced by Web sites that provide options for downloading content. Cooperative group and professional society meetings have been important forums to increase awareness among care providers about long-term health risks related to childhood cancer and its treatment and the resources available to guide personalized risk-based care. Publication of systematic reviews with summaries of evidence that support guideline recommendations17,21-25 has been another means to disseminate knowledge about survivorship care to health care professionals. In countries with integrated national health care systems, ongoing dissemination initiatives involve the linking of survivorship care pathways with general practitioner information technology systems and collaboration with insurance providers.
An important barrier to the implementation of survivorship guidelines is the complexity of the recommendations, which are based on previous treatment. A Web-based survivorship care plan can support clinical decision making and help physicians and survivors to translate guideline recommendations to clinical care for individual survivors.26 Several groups have tackled this issue by developing Web-based resources to organize survivorship care plans. PanCare helped to develop a Web-based electronic survivorship passport tool that allows survivors and clinicians to enter diagnosis and treatment information and receive a treatment summary and individualized advice for late effects surveillance on the basis of international guidelines. An important objective of this tool is to empower survivors to seek the care they need.16 COG also has implemented an algorithm-driven Web-based tool, Passport for Care, that provides tailored late effects screening recommendations for individual survivors on the basis of their therapeutic exposures.26 These electronic platforms, which provide easy access to educational materials and screening recommendations, also may prompt and facilitate the uptake of guideline-based care for survivors who are no longer actively engaged in survivorship care.
Several groups have aimed to disseminate guideline recommendations to childhood cancer survivors and their families through the development of patient educational materials. The COG guidelines are accompanied by health links that provide targeted lay health messages on 43 guideline-specific topics.27 Two additional European Union–funded projects, PanCare Childhood and Adolescent Survivor Care and Follow-Up Studies and PanCare quality-of-life studies, in collaboration with IGHG,17 present simple-language summaries for all these guidelines for use by survivors and nonspecialist clinicians as well as guidelines on fertility preservation, models of follow-up care, transition of care, and health promotion.28 Likewise, lay fact sheets about 24 late effects complement the UKCCLG guidelines.29
NEED FOR NOVEL METHODS TO REFINE SURVEILLANCE RECOMMENDATIONS
Current childhood cancer survivorship guidelines10,12,14,15,17 recommend screening for early detection of late complications in at-risk survivors to help to improve both survival and quality of life. Guideline recommendations are based on scientific knowledge focused on prevalence/incidence and risk factors for late complications, diagnostic test results, course of chronic health condition, and potential benefit of treatment.
A limitation of the current guideline recommendations is that knowledge is lacking about the benefit of the treatment of late effects in survivors. Recommendations for screening are based on scientific evidence from survivorship studies, on clinical trial results from the general population (whose risk profiles differ from that of childhood cancer survivors), and on expert consensus. The effectiveness of surveillance, however, is largely unknown. The feasibility of conducting clinical trials to optimize screening recommendations for survivors is limited because of requirements for large sample sizes, lengthy study periods, prohibitive costs, and ethical concerns. Other factors, such as genetic susceptibility, can contribute to individual risk or the effect of treatment. Guidelines include recommendations for groups of survivors, and translation to the individual survivor is not entirely straightforward. Moreover, survivors can have various types of chronic health conditions, and multiple morbidities can change the course of these conditions for an individual survivor. Therefore, several groups are exploring innovative methods, such as cumulative burden assessment and risk prediction and cost-effectiveness modeling, to refine surveillance recommendations.
Quantification of Cumulative Disease Burden
Among vulnerable populations, such as childhood cancer survivors, in whom multiple morbidities and recurrent disease are substantial drivers of disease burden, longitudinal studies are lacking with regard to the course of chronic health conditions. Use of common epidemiologic measures, such as incidence and prevalence of one health condition, has resulted in an underappreciation of the overall effect of chronic health conditions. Several burden scoring systems have been used to express multiple morbidities in survivors.30-33 Among these, the cumulative burden metric,31,32 an outcomes measure that is based on the mean cumulative count (MCC),33 offers a new approach to quantify disease burden over time, particularly when multiple/recurrent morbidities and competing risks are observed. The MCC allows investigators to estimate the mean number of all event occurrences of a condition of interest over a given period per survivor while taking into account censoring and competing risks. Interpreted and applied in the same manner, the cumulative burden metric extends the applications of the MCC by applying a clinical framework to analyze pathologies that evolve in a waxing and waning pattern over time and may or may not recur (eg, hypertension, hyperlipidemia).
An analysis of the association of anthracycline and cardiac radiation exposures with 21 different chronic cardiac conditions in a cohort of survivors of pediatric Hodgkin lymphoma demonstrated differences in inference between incidence and cumulative burden.31 Compared with using incidence alone, 50-year-old survivors and community controls seemed to have a similar disease burden. However, when cumulative burden was measured, 30-year-old survivors, on average, had nearly the same number of severe, life-threatening, or fatal chronic cardiac conditions as 50-year-old community controls. When the contributing chronic health conditions were assessed, the drivers and patterns of disease burden observed among 30-year-old survivors (myocardial infarction, valvular disease) and 50-year-old community controls (hypertension, hyperlipidemia) showed that survivors develop types of chronic cardiac conditions that are clinically considered more severe.
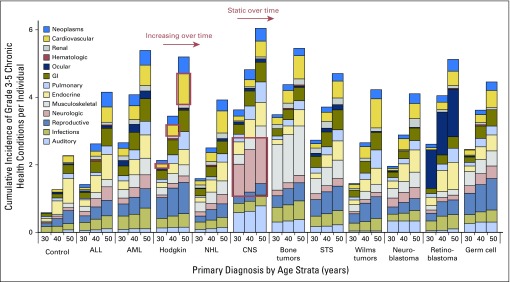
In a more comprehensive analysis, temporal trends in chronic illness among survivors across multiple organ systems and primary cancer subtypes were evaluated (Fig 2).32 In addition to substantial variation in burden across all groups, two global trends in morbidity were observed: Some conditions increase over time (eg, cardiac disease in survivors of Hodgkin lymphoma), and other conditions remain static (eg, neurologic adverse events among survivors of CNS tumors). From these data, a systematic re-evaluation of currently established risk strata within existing surveillance guidelines is now feasible and may better characterize subgroups of survivors at highest risk for recurrent illnesses who may benefit from more (or fewer) targeted screening interventions.
Fig 2.
Temporal patterns of severe, life-threatening, or fatal chronic health conditions across multiple organ systems and primary cancer subtypes. ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; NHL, non-Hodgkin lymphoma; STS, soft tissue sarcoma. Adapted with permission.35
When planning analyses and interpreting results that use the MCC and cumulative burden, investigators must carefully consider which chronic health conditions are included because every event is weighted equivalently in a single analysis. For example, if incorporated in the same study, myocardial infarction and hypertension events both would be counted equally. This assumption may make sense for descriptive studies but is not appropriate if the outcomes must consider the health-related quality-of-life burden that patients may experience.34
Risk Prediction Modeling to Personalize Follow-Up Care
Risk prediction modeling can translate scientific evidence into risk for an individual survivor. Incorporation of relevant clinical data into mathematical models allows for estimation of the risk of developing specific late effects, potentially in a time-specific manner according to age or time since the completion of cancer treatment. These models, when externally validated, may prove useful in the clinical setting by allowing clinicians to construct personalized risk profiles and screening recommendations from each survivor’s clinical data to predict his or her absolute risk of developing a particular late effect.35,36
For example, investigators from the Childhood Cancer Survivor Study, Emma Children’s Hospital, the National Wilms Tumor Study, and the St Jude Lifetime Cohort Study created models that use demographic and cancer treatment data to predict individual risk of heart failure among 5-year survivors.37 Three Childhood Cancer Survivor Study congestive heart failure risk scores were derived by incorporating sex, age at diagnosis, and exposure to anthracyclines and chest radiation into three prediction models that differed by level of detail on treatment exposures. Detailed exposure information, such as the protocol-specified radiation dose or the exact dose estimated by dosimetry, may be unavailable in certain settings. In these situations, the simple model that categorizes treatment exposures as yes or no can be used. With more-detailed exposure data, the standard model that incorporates clinical dose information or the heart-dose model that uses average radiation dose to the heart can be applied. The models have demonstrated reasonable discriminatory and predictive power on the basis of the area under the receiver operating characteristic curve and concordance statistics (range, 0.71 to 0.77 for ≥ 40 years of age), which were validated in two independent survivor cohorts. The model permitted identification of survivors at low-, moderate-, and high-risk for heart failure, which corresponds to cumulative incidence rates of heart failure at age 40 years of 0.5% (95% CI, 0.2% to 0.8%), 2.4% (95% CI, 1.8% to 3.0%), and 11.7% (95% CI, 8.8% to 14.5%), respectively. Of note, the more-complex standard and heart-dose models performed only modestly better than the simple model that featured readily available clinical information included in most survivor treatment summaries. These models can be used to risk adapt cardiomyopathy surveillance by identifying higher-risk individuals who may benefit from closer follow-up and intervention as well as to reduce overscreening in low-risk individuals.38 Similar risk-prediction models also recently have been developed to identify childhood cancer survivors at low, moderate, and high risk for ischemic heart disease and stroke.39 Additional personalized prediction modeling that incorporates recurrent and multiple outcomes using the cumulative burden metric, survivors’ germline genetic factors, and effect modifications of treatment exposures on the risk of specific late effects is under active development and aims to boost the clinical utility of prediction models through higher accuracy and precision.
Cost-Effectiveness Modeling in Defining Optimal Type and Frequency of Surveillance
Cost-effectiveness analysis offers a potential alternative to clinical trials by determining the optimal type and frequency of screening through mathematical modeling.40 With use of this approach, a model is constructed that reflects the disease processes on which a screening strategy is superimposed. The effectiveness of the screening modality (sensitivity/specificity), treatments available for screening-detected complications, the attendant survival rates and quality of life, and the costs of screening (including downstream health care costs) are incorporated into the model to calculate the quality-adjusted life-years (QALYs) and health care costs associated with screening. QALYs and costs are compared with those of the comparator screening strategy (including no screening/usual care) to estimate the relative effectiveness and determine whether screening provides good value for the health benefits gained.34
For example, a cost-effectiveness analysis was performed to evaluate the COG15 recommendation for lifetime echocardiographic screening for asymptomatic survivors who were treated with anthracyclines and chest radiation. In survivors with a high risk for heart disease,41 screening was shown to increase QALYs and deemed cost-effective compared with no screening. However, the analysis also showed that similar effectiveness could be achieved with less-frequent screening and at lower cost. In addition, subgroups of survivors who derived little benefit from screening were identified. Cost-effectiveness analysis thus can assist in expeditiously determining the potential effectiveness and cost-effectiveness of screening recommendations and can be used to identify potential refinements to current recommendations. Furthermore, cost-effectiveness modeling can be a valuable tool to assess the potential costs and burdens of various screening strategies for survivors and the health care system by providing valuable information to inform both clinical care and health policy. Nevertheless, with consideration of the limitations of available evidence to inform screening recommendations, decisions about screening should be shared between the clinician and survivor, should take into account the potential benefits and harms of screening (especially when evidence for benefit of screening is equivocal), and should respect the values and concerns of each individual survivor.
RESEARCH AGENDA
As more children and adolescents with cancer become long-term survivors, the field of survivorship care will continue to expand. Several countries have already developed guideline recommendations for late effects surveillance and have joined to harmonize these recommendations internationally to enhance long-term follow-up care and quality of life for these survivors. Nevertheless, ongoing efforts to quantify the cumulative disease burden among subpopulations of survivors and develop individual risk prediction profiles and cost-effectiveness models represent new approaches to refine and personalize screening recommendations. Risk profiling theoretically could be used to allocate the most intensive screenings to survivors at highest risk and maximize the potential benefits while minimizing screening (and its attendant harms) for survivors at lowest risk. The use of risk prediction modeling to guide the counseling of survivors about their individual risk for future complications could significantly enhance follow-up care and inform health-related decision making among survivors. Thus, a research agenda focused on evidence-based, harmonized delivery of personalized cost-effective care for survivors informed by risk prediction could enhance outcomes and quality of life for this vulnerable population and represents a worthy objective for future international survivorship collaborations.
Footnotes
Dr. Hudson is supported by St. Jude Children's Research Hospital Cancer Center support grant number 5P30CA021765-33, U01 CA195547, and the American Lebanese Syrian Associated Charities.
Drs. Skinner, Hjorth, and Kremer have received support from the 7th Framework Programme of the EU, PanCareSurfUp (257505).
AUTHOR CONTRIBUTIONS
Conception and design: Wendy Landier, Roderick Skinner, W. Hamish Wallace, Smita Bhatia, Leontien C. Kremer, Melissa M. Hudson
Collection and assembly of data: Wendy Landier, Roderick Skinner, Lars Hjorth, Renée L. Mulder, F. Lennie Wong, Yutaka Yasui, Nickhill Bhakta, Louis S. Constine, Leontien C. Kremer, Melissa M. Hudson
Data analysis and interpretation: Wendy Landier, Roderick Skinner, W. Hamish Wallace, Smita Bhatia, Lars Hjorth, Renée L. Mulder, F. Lennie Wong, Yutaka Yasui, Nickhill Bhakta, Louis S. Constine, Leontien C. Kremer, Melissa M. Hudson
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Surveillance for Late Effects in Childhood Cancer Survivors
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Wendy Landier
Research Funding: Merck Sharp & Dohme (Inst)
Roderick Skinner
Consulting or Advisory Role: Clinigen Group
Travel, Accommodations, Expenses: Medac
W. Hamish Wallace
No relationship to disclose
Lars Hjorth
Stock or Other Ownership: BioInvent
Renée L. Mulder
No relationship to disclose
F. Lennie Wong
No relationship to disclose
Yutaka Yasui
No relationship to disclose
Nickhill Bhakta
No relationship to disclose
Louis S. Constine
Honoraria: UpToDate, Springer, Lippincott
Travel, Accommodations, Expenses: IBA Worldwide
Smita Bhatia
No relationship to disclose
Leontien C. Kremer
No relationship to disclose
Melissa M. Hudson
Consulting or Advisory Role: Coleman Supportive Oncology Initiative for Children with Cancer, Oncology Research Information Exchange Network, Pfizer, Princess Máxima Center
REFERENCES
- 1.Phillips SM, Padgett LS, Leisenring WM, et al. : Survivors of childhood cancer in the United States: Prevalence and burden of morbidity. Cancer Epidemiol Biomarkers Prev 24:653-663, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Trama A, Botta L, Foschi R, et al. : Survival of European adolescents and young adults diagnosed with cancer in 2000-07: Population-based data from EUROCARE-5. Lancet Oncol 17:896-906, 2016 [DOI] [PubMed] [Google Scholar]
- 3.Oeffinger KC, Mertens AC, Sklar CA, et al. : Chronic health conditions in adult survivors of childhood cancer. N Engl J Med 355:1572-1582, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Fidler MM, Reulen RC, Winter DL, et al. : Long term cause specific mortality among 34 489 five year survivors of childhood cancer in Great Britain: Population based cohort study. BMJ 354:i4351, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hudson MM, Ness KK, Gurney JG, et al. : Clinical ascertainment of health outcomes among adults treated for childhood cancer. JAMA 309:2371-2381, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robison LL, Hudson MM: Survivors of childhood and adolescent cancer: Life-long risks and responsibilities. Nat Rev Cancer 14:61-70, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Landier W, Armenian SH, Lee J, et al. : Yield of screening for long-term complications using the children’s oncology group long-term follow-up guidelines. J Clin Oncol 30:4401-4408, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edgar AB, Duffin K, Borthwick S, et al. : Can intensity of long-term follow-up for survivors of childhood and teenage cancer be determined by therapy-based risk stratification? BMJ Open 3:e002451, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Landier W, Bhatia S, Eshelman DA, et al. : Development of risk-based guidelines for pediatric cancer survivors: The Children’s Oncology Group long-term follow-up guidelines from the Children’s Oncology Group Late Effects Committee and nursing discipline. J Clin Oncol 22:4979-4990, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Wallace WH, Thompson L, Anderson RA: Long term follow-up of survivors of childhood cancer: Summary of updated SIGN guidance. BMJ 346:f1190, 2013 [DOI] [PubMed] [Google Scholar]
- 11.Wallace WH, Blacklay A, Eiser C, et al. : Developing strategies for long term follow up of survivors of childhood cancer. BMJ 323:271-274, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dutch Childhood Oncology Group : Guidelines for follow-up in survivors of childhood cancer 5 years after diagnosis, 2010. https://www.skion.nl/workspace/uploads/vertaling-richtlijn-LATER-versie-final-okt-2014_2.pdf
- 13.Scottish Intercollegiate Guidelines Network : Long-term follow up of survivors of childhood cancer, 2014. http://www.sign.ac.uk/guidelines/fulltext/132/index.html
- 14.United Kingdom Children’s Cancer Study Group Late Effects Group : Therapy based long term follow up practice statement, 2005. http://www.cclg.org.uk/write/mediauploads/member%20area/treatment%20guidelines/ltfu-full.pdf
- 15.Children’s Oncology Group : Long-term follow-up guidelines for survivors of childhood, adolescent, and young adult cancers, version 4.0, 2013. http://www.survivorshipguidelines.org [PubMed]
- 16.Hjorth L, Haupt R, Skinner R, et al. : Survivorship after childhood cancer: PanCare: A European Network to promote optimal long-term care. Eur J Cancer 51:1203-1211, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kremer LC, Mulder RL, Oeffinger KC, et al. : A worldwide collaboration to harmonize guidelines for the long-term follow-up of childhood and young adult cancer survivors: A report from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Pediatr Blood Cancer 60:543-549, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Germino JC, Elmore JG, Carlos RC, et al. : Imaging-based screening: Maximizing benefits and minimizing harms. Clin Imaging 40:339-343, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kotwal AA, Schumm P, Mohile SG, et al. : The influence of stress, depression, and anxiety on PSA screening rates in a nationally representative sample. Med Care 50:1037-1044, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.American Academy of Pediatrics Section on Hematology/Oncology Children’s Oncology Group : Long-term follow-up care for pediatric cancer survivors. Pediatrics 123:906-915, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mulder RL, Kremer LC, Hudson MM, et al. : Recommendations for breast cancer surveillance for female survivors of childhood, adolescent, and young adult cancer given chest radiation: A report from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Oncol 14:e621-e629, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Armenian SH, Hudson MM, Mulder RL, et al. : Recommendations for cardiomyopathy surveillance for survivors of childhood cancer: A report from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Oncol 16:e123-e136, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Dorp W, Mulder RL, Kremer LC, et al. : Recommendations for premature ovarian insufficiency surveillance for female survivors of childhood, adolescent, and young adult cancer: A report from the International Late Effects of Childhood Cancer Guideline Harmonization Group in collaboration with the PanCareSurFup Consortium. J Clin Oncol 34:3440-3450, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Skinner R, Mulder RL, Kremer LC, et al. : Recommendations for gonadotoxicity surveillance in male childhood, adolescent, and young adult cancer survivors: A report from the International Late Effects of Childhood Cancer Guideline Harmonization Group in collaboration with the PanCareSurFup Consortium. Lancet Oncol 18:e75-e90, 2017 [DOI] [PubMed] [Google Scholar]
- 25.Bowers DC, Nathan PC, Constine L, et al. : Subsequent neoplasms of the CNS among survivors of childhood cancer: A systematic review. Lancet Oncol 14:e321-e328, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poplack DG, Fordis M, Landier W, et al. : Childhood cancer survivor care: Development of the Passport for Care. Nat Rev Clin Oncol 11:740-750, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eshelman D, Landier W, Sweeney T, et al. : Facilitating care for childhood cancer survivors: Integrating Children’s Oncology Group long-term follow-up guidelines and health links in clinical practice. J Pediatr Oncol Nurs 21:271-280, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Mulder RL, van der Pal HJH, Levitt GA, et al. : Transition guidelines: An important step in the future care for childhood cancer survivors. A comprehensive definition as groundwork. Eur J Cancer 54:64-68, 2016 [DOI] [PubMed] [Google Scholar]
- 29.Children’s Cancer and Leukemia Group : Aftercure (late effects factsheets). http://www.cclg.org.uk/Aftercurefactsheets
- 30.Geenen MM, Cardous-Ubbink MC, Kremer LC, et al. : Medical assessment of adverse health outcomes in long-term survivors of childhood cancer. JAMA 297:2705-2715, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Bhakta N, Liu Q, Yeo F, et al. : Cumulative burden of cardiovascular morbidity in paediatric, adolescent, and young adult survivors of Hodgkin’s lymphoma: An analysis from the St Jude Lifetime Cohort Study. Lancet Oncol 17:1325-1334, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bhakta N, Liu Q, Ness KK, et al. : The cumulative burden of surviving childhood cancer: An initial report from the St Jude Lifetime Cohort Study (SJLIFE). Lancet 390:2569-2582, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dong H, Robison LL, Leisenring WM, et al. : Estimating the burden of recurrent events in the presence of competing risks: The method of mean cumulative count. Am J Epidemiol 181:532-540, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brodin NP, Vogelius IR, Maraldo MV, et al. : Life years lost--comparing potentially fatal late complications after radiotherapy for pediatric medulloblastoma on a common scale. Cancer 118:5432-5440, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Salz T, Baxi SS, Raghunathan N, et al. : Are we ready to predict late effects? A systematic review of clinically useful prediction models. Eur J Cancer 51:758-766, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brodin NP, Maraldo MV, Aznar MC, et al. : Interactive decision-support tool for risk-based radiation therapy plan comparison for Hodgkin lymphoma. Int J Radiat Oncol Biol Phys 88:433-445, 2014 [DOI] [PubMed] [Google Scholar]
- 37.Chow EJ, Chen Y, Kremer LC, et al. : Individual prediction of heart failure among childhood cancer survivors. J Clin Oncol 33:394-402, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.St Jude Children’s Research Hospital : CCSS Cardiovascular Risk Calculator. https://ccss.stjude.org/cvcalc
- 39.Chow EJ, Chen Y, Hudson MM, et al. : Prediction of ischemic heart disease and stroke in survivors of childhood cancer. J Clin Oncol 36:44-52, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sonnenberg FA, Beck JR: Markov models in medical decision making: A practical guide. Med Decis Making 13:322-338, 1993 [DOI] [PubMed] [Google Scholar]
- 41.Wong FL, Bhatia S, Landier W, et al. : Cost-effectiveness of the Children’s Oncology Group long-term follow-up screening guidelines for childhood cancer survivors at risk for treatment-related heart failure. Ann Intern Med 160:672-683, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]