Abstract
Cardiovascular disease (CVD), which includes cardiomyopathy/heart failure, coronary artery disease, stroke, pericardial disease, arrhythmias, and valvular and vascular dysfunction, is a major concern for long-term survivors of childhood cancer. There is clear evidence of increased risk of CVD largely attributable to treatment exposures at a young age, most notably anthracycline chemotherapy and chest-directed radiation therapy, and compounded by traditional cardiovascular risk factors accrued during decades after treatment exposure. Preclinical studies are limited; thus, it is a high priority to understand the pathophysiology of CVD as a result of anticancer treatments, taking into consideration the growing and developing heart. Recently developed personalized risk prediction models can provide decision support before initiation of anticancer therapy or facilitate implementation of screening strategies in at-risk survivors of cancer. Although consensus-based screening guidelines exist for the application of blood and imaging biomarkers of CVD, the most appropriate timing and frequency of these measures in survivors of childhood cancer are not yet fully elucidated. Longitudinal studies are needed to characterize the prognostic importance of subclinical markers of cardiovascular injury on long-term CVD risk. A number of prevention trials across the survivorship spectrum are under way, which include primary prevention (before or during cancer treatment), secondary prevention (after completion of treatment), and integrated approaches to manage modifiable cardiovascular risk factors. Ongoing multidisciplinary collaborations between the oncology, cardiology, primary care, and other subspecialty communities are essential to reduce therapeutic exposures and improve surveillance, prevention, and treatment of CVD in this high-risk population.
INTRODUCTION
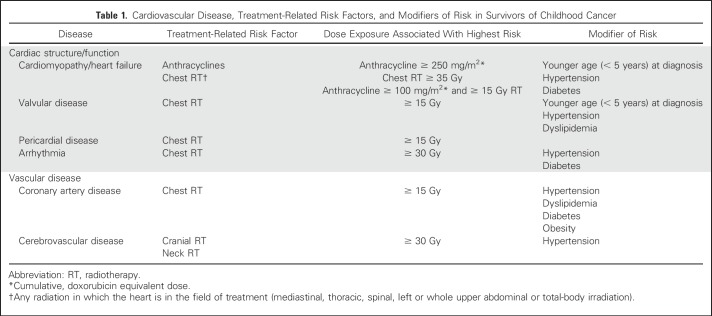
Cardiovascular disease (CVD), which includes cardiomyopathy/heart failure, coronary artery disease, stroke, pericardial disease, arrhythmias, and valvular and vascular dysfunction, is a major concern for long-term survivors of childhood cancer.1-5 These survivors are seven times more likely than the general population to die as a result of CVD, making CVD the leading cause of noncancer mortality in this population.6 This increased risk for development of CVD is largely attributed to cancer treatment exposures at a young age, most notably anthracycline (eg, doxorubicin, daunorubicin, epirubicin, idarubicin), mitoxantrone chemotherapy, and chest-directed radiation therapy (RT). It is estimated that as many as one in eight survivors of childhood cancer treated with anthracyclines and chest RT will experience a life-threatening cardiovascular event 30 years after treatment of childhood cancer.5 However, the cumulative burden of CVD, including both the quantity and severity of events, is inherently different when comparing survivors of individual cancers (eg, Hodgkin lymphoma) to both the general population7 and other disease groups.8 These differences are largely due to well-characterized clinical and treatment-related modifiers of risk related to cumulative dose exposure of cardiotoxic agents and modalities (Table 1).1-5 The large body of literature on CVD-related outcomes in survivors of childhood cancer has informed risk-based guidelines for early detection and treatment of CVD. The purpose of this review article is to highlight emerging paradigms in childhood cancer survivorship and CVD, with a focus on preclinical and clinical models of anthracycline-induced cardiotoxicity, the epidemiology of heart failure and coronary artery disease, cardiovascular sequelae associated with newer cancer therapies, and strategies for screening and prevention, taking into consideration lessons learned from oncology and cardiology populations.
Table 1.
Cardiovascular Disease, Treatment-Related Risk Factors, and Modifiers of Risk in Survivors of Childhood Cancer
PATHOPHYSIOLOGY OF TREATMENT-RELATED HEART FAILURE
Anthracyclines such as doxorubicin are frequently used to treat childhood cancer, and there is a well-characterized dose-dependent risk of cardioxicity and heart failure with these agents. However, the exact mechanism of anthracycline-induced cardiotoxicity has not been fully elucidated. Early studies pointed to cardiotoxicity through reduction–oxidation reaction cycling and the generation of reactive oxygen species.9 More recently, topoisomerase IIB (Top2) has been proposed to be a mediator of doxorubicin-mediated cardiac injury.10 Experimental evidence for this model is supported by cardioprotection from doxorubicin in mice with cardiomyocyte-specific deletion of Top2b, the gene encoding Top2.10 Other models suggest roles for impairment of mitochondrial biogenesis,11 mitochondrial iron accumulation,12 and transcription factors such as aryl hydrocarbon receptor13 and hypoxia-inducible factor14 in the pathogenesis of anthracycline-induced cardiotoxicity. These models are not necessarily mutually exclusive. For example, Top2 may mediate doxorubicin-induced myocardial injury by defective mitochondrial biogenesis and reactive oxygen species formation.10 Caution must be applied to generalize in vitro and mice studies to the toxicity observed in humans. For example, many of the early mice studies used high doses of doxorubicin leading to acute cardiotoxicity, which do not truly recapitulate the chronic development of cardiomyopathy in humans.9
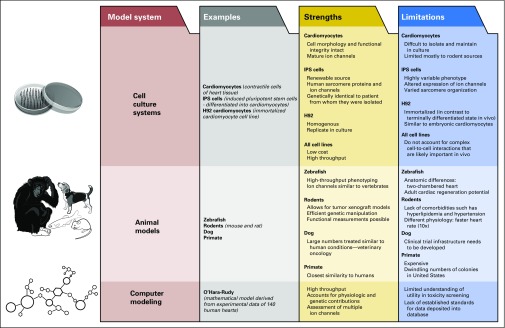
There is a paucity of preclinical studies that have evaluated chronic cardiotoxicity in the context of the growing and developing heart—an especially important consideration when extrapolating laboratory findings to survivors of childhood cancer. A recent study15 used mouse models to show how mitochondria from the hearts of young mice and humans are primed for apoptosis, predisposing them to undergo cell death in response to genotoxic damage as a result of chemotherapy or radiation exposure. Conversely, mitochondria from adult hearts were more resistant to pro-apoptotic signaling, leading to cellular resistance to doxorubicin-induced cardiomyopathy.15 These findings may explain, in part, the higher incidence of cardiotoxicity seen in children compared with adults16 and argue for the use of pediatric-specific preclinical models to help elucidate the pathophysiology of cardiotoxicity in children. This can be done using a variety of platforms, taking into consideration the advantages and limitations of each model (Fig 1).
Fig 1.
Preclinical models of cardiotoxicity. Robust and diverse preclinical models that include cell culture systems, animal models, and computer simulations have provided a better understanding of the molecular and cellular mechanisms that mediate cancer treatment–related cardiotoxicity. The strengths and limitations of these preclinical models are highlighted. Studies are under way to combine information obtained from clinical observational studies with preclinical models, facilitating the development of novel cardioprotective strategies.
CHARACTERIZATION OF CVD RISK
In the general population, calculators such as the Framingham Risk Score17 are routinely used to estimate CVD risk to guide therapeutic interventions (eg, aggressive management of cardiovascular risk factors [hypertension, dyslipidemia]) to lower the likelihood of developing cardiac events such as myocardial infarction or heart failure. However, risk estimates derived from the Framingham Risk Score do not account for key drivers of risk in survivors of cancer, such as anthracycline chemotherapy or chest RT. The need to understand survivor-specific risks has prompted the development of heart failure prediction models that take into account both clinical (age at diagnosis, sex) and treatment-related (anthracycline dose, chest RT) risk factors to reliably classify individuals into low, moderate, and high risk, (ie, the incidence of heart failure at 40 years is 0.5%, 2.4%, and 11.7%, respectively).18 This can then provide a framework on which to determine future screening strategies and interventions.
However, there is marked individual variability in the prevalence and severity of heart failure in survivors of childhood cancer that is not explained exclusively by risk factors such as cumulative anthracycline dose.19 Studies have used genome-wide association20-22 or candidate gene23-26 approaches to describe how key host genetic polymorphisms could result in differential risk for cardiotoxicity among survivors with otherwise similar clinical and treatment-related risk factors. Variations in genes involved in anthracycline transport and metabolism (eg, SLC28A3,23 UGT1A6,23,27 ABCC1,26 HFE,28,29 CBR325), cardiomyocyte injury and antioxidant defense (eg, RARG,20 CELF422,27, HAS321,27), and cardiac remodeling (eg, RARG,20 HAS321,27) have been independently associated with increased risk of anthracycline-induced cardiotoxicity. Validation studies are under way to confirm many of the unique (and at times discrepant) findings from these studies. Ultimately, the ability to accurately identify individuals with a genetic predisposition for cardiotoxicity would provide the opportunity to tailor cancer therapy at the time of diagnosis, keeping in mind health outcomes that extend beyond the immediate treatment period.
EMERGING THERAPIES AND THE POTENTIAL FOR FUTURE CVD RISK
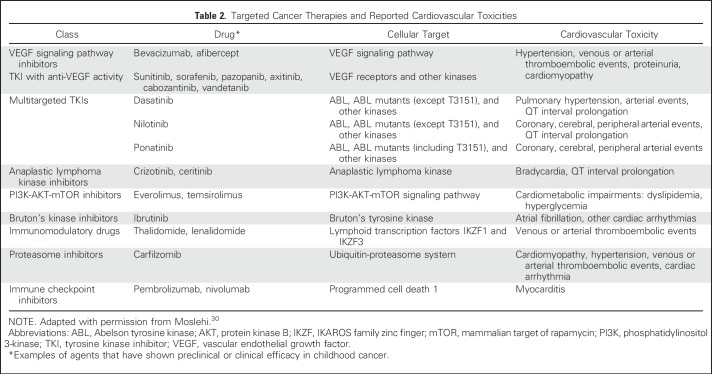
The introduction of targeted cancer therapies into adult oncology practice, while dramatically altering prognosis for certain cancers, has brought to light new cardiovascular sequelae (Table 2).30 Specifically, the recognition that abnormal activation of kinases plays a critical role in tumorigenesis led to introduction of small molecular tyrosine kinase inhibitors as a strategy for cancer treatment.30 However, each tyrosine kinase inhibitor can target more than one kinase, resulting in adverse effects. For example, although both dasatinib and imatinib inhibit the Abelson tyrosine kinase in Philadelphia chromosome (Ph)–positive chronic myelogenous leukemia (CML) and acute lymphoblastic leukemia (ALL), each kinase inhibitor targets a diverse array of additional kinase receptors, leading to a different toxicity profile for each drug.30 Dasatinib can lead to pulmonary hypertension and possibly arterial ischemic events, whereas imatinib is relatively well tolerated in adults with Ph-positive CML and ALL.31 Sorafenib, an FLT3 kinase inhibitor typically used in AML, attenuates vascular endothelial growth factor receptors, leading to hypertension, cardiomyopathy, ischemia, and vascular complications in up to approximately 25% of patients.32 Crizotinib, an anaplastic lymphoma kinase inhibitor used to treat a number of solid cancers, including neuroblastoma, can result in bradycardia.33 Finally, immune checkpoint inhibitors (eg, inhibitors of programmed cell death-1) have been shown to have unprecedented activity in hematologic malignancies such as Hodgkin lymphoma but in rare instances can cause fulminant myocarditis.34
Table 2.
Targeted Cancer Therapies and Reported Cardiovascular Toxicities
EARLY SCREENING AND CVD DETECTION
In the general population, biologic markers are widely used to detect subclinical or overt CVD, identify risk for future disease, and guide treatment strategies.35 As an example, lipid markers (total cholesterol, LDL, or HDL) are accepted screening markers of CVD risk and measures of response to lipid-lowering therapy. In heart failure, N-terminal pro brain natriuretic peptide is a guideline-recommended measure of diagnosis and prognosis.36 Two-dimensional echocardiography is widely used to detect abnormalities in cardiac left ventricular ejection fraction (LVEF), diastolic function, and valvular disease. More recently, advanced imaging modalities, such as tissue characterization by cardiac magnetic resonance imaging (MRI)37,38 and echocardiography-derived myocardial deformation imaging (strain), have been used to detect subclinical cardiac dysfunction in populations at risk for heart failure (eg, after myocardial infarction).39
Although guidelines exist for the application of blood biomarkers, echocardiography, and cardiac MRI in the general population,36 the timing and frequency of these measures in at-risk survivors of childhood cancer has not been established. Research has suggested that elevated troponin and N-terminal pro brain natriuretic peptide levels during anthracycline exposure can be associated with subclinical cardiac dysfunction,40,41 but the association between these early findings and subsequent clinically significant disease (eg, symptomatic heart failure) is less clear. Studies evaluating myocardial strain imaging have demonstrated a high prevalence of abnormal longitudinal strain in childhood survivors exposed to anthracyclines and/or chest radiotherapy, despite the presence of preserved systolic function (LVEF).42 Longitudinal studies are needed to examine the prognostic value of abnormal strain in these survivors. Cardiac MRI has been used to improve functional characterization43 and to detect adverse LV remodeling, including fibrosis, in survivors of childhood cancer, but as with strain, the long-term prognostic utility of these abnormalities has yet to be determined in this population.38
Despite these limitations, for certain diseases, such as anthracycline-related cardiomyopathy, routine screening for asymptomatic cardiac systolic dysfunction (abnormal LVEF) may be cost effective, allowing for pharmacologic and lifestyle interventions to slow the progression to symptomatic disease.44,45 With this in mind, the International Late Effects of Childhood Cancer Guideline Harmonization Group provided a uniform guideline for cardiomyopathy screening (Fig 2).1 Routine surveillance is recommended for survivors at high risk for cardiomyopathy, defined as those with anthracycline exposure ≥ 250 mg/m2, or ≥ 35 Gy chest RT, or a combination of ≥ 100 mg/m2 of anthracyclines and ≥ 15 Gy chest RT. Although screening may be reasonable for survivors with lower-dose exposures, evidence directly comparing specific screening modalities or schedules is lacking. On the basis of expert consensus, echocardiography remains the recommended primary surveillance modality, is to begin no later than 2 years after exposure, and should be repeated a minimum of every 5 years thereafter. Cardiology consultation is strongly recommended for those with asymptomatic cardiomyopathy.1 Simulation models to estimate the efficacy of surveillance guidelines44,45 suggest that although routine surveillance reduces risks for heart failure and extends life expectancy, less-frequent assessments may be more cost effective for certain subsets of survivors who are at lower risk.
Fig 2.
Harmonized recommendations for cardiomyopathy surveillance for survivors of childhood cancer. Green represents a strong recommendation with a low degree of uncertainty (high quality evidence). Yellow (moderate-quality evidence) and orange (weak-quality evidence) represent moderate-level recommendations. Red represents a recommendation against a particular intervention, with harms outweighing benefits. ACE, angiotensin-converting enzyme; AHA, American Heart Association; ESC, European Society of Cardiology; LV, left ventricular. Reprinted with permission.1
Characterization of vascular disease in survivors of childhood cancer is also important, because accelerated atherosclerosis is a contributing factor to coronary and carotid artery disease after cancer therapy.3 Studies among survivors of Hodgkin lymphoma were the first to recognize the association between chest RT and future coronary artery disease.46 To date, investigations of vascular biomarkers and functional testing in survivors of cancer have been limited by heterogeneous populations and small numbers of survivors studied. Brouwer et al47 quantified a panel of blood biomarkers of arterial injury (plasminogen activator inhibitor, plasminogen activator inhibitor type-1), and imaging (intima-media thickness) among 277 survivors of childhood cancer (median age, 18 years; range, 5 to 31 years after treatment).47 Survivors of childhood cancer who received RT had increased intima-media thickness outside the radiation field and had higher blood biomarkers of arterial injury compared with sibling controls, suggesting subclinical vascular injury may be present decades after completion of cancer-directed therapy.47
Challenges impede the widespread implementation of these vascular screening modalities. Serum biomarkers have been variably applied, and the ideal biomarker(s) to detect or diagnose subclinical disease, or even determine prognosis, remains elusive. Lack of reproducibility, interlab and intrapatient variability, and challenges in the interpretation of these biomarkers in asymptomatic individuals have limited their use in routine clinical practice. Additional studies are needed to define the relationship between one-time measures of subclinical vascular disease and subsequent progression to clinically overt disease. Continued follow-up of survivors across their lifespan is necessary to test and develop evidence-based recommendations for screening and intervention.
STRATEGIES FOR CVD PREVENTION
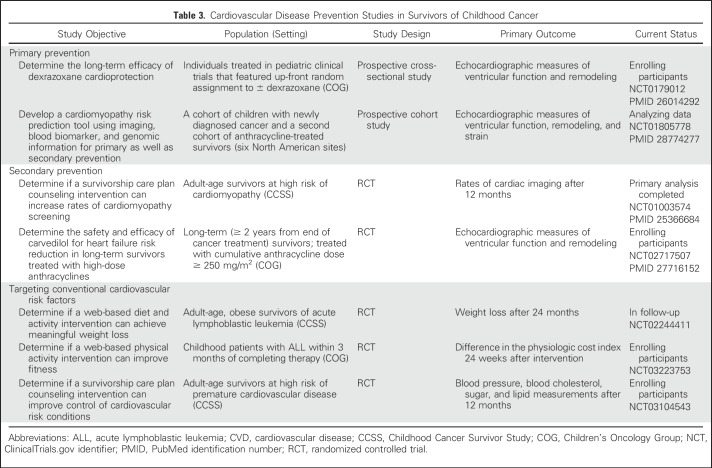
The existing large body of observational studies has facilitated the development of CVD prevention trials across the survivorship spectrum. Trials covering primary prevention (before or during cancer treatment), secondary prevention (after completion of treatment), as well as integrated approaches to improve CVD screening and the management of modifiable cardiovascular risk factors (Table 3).
Table 3.
Cardiovascular Disease Prevention Studies in Survivors of Childhood Cancer
Primary Prevention
In the oncology community, it is generally understood that anthracyclines and/or chest RT should only be used if they provide established antitumor efficacy over less cardiotoxic regimens.48 If anthracyclines cannot be avoided, several strategies to minimize cardiotoxicity have been proposed.
Less cardiotoxic analogs.
In adults, liposomal-encapsulated doxorubicin is favored over conventional doxorubicin, but pediatric randomized controlled trial (RCT) data are lacking.49 An RCT comparing the doxorubicin analog pirarubicin with daunorubicin in the treatment of childhood leukemia demonstrated equivalent survival and seemingly less subclinical cardiotoxicity.50 Anthracycline-loaded nanoparticles also seem promising,51 but studies have been limited to animal and in vitro models.
Longer anthracycline infusion durations.
Although an infusion duration of ≥ 6 hours has been shown to be cardioprotective in adults, RCTs in childhood leukemia have not identified a protective effect.52
Cardioprotective agents.
RCTs in children with different malignancies show some relatively short-term (< 10 years from treatment) cardioprotection with dexrazoxane, whereas concerns about interference of dexrazoxane with antitumor efficacy of anthracyclines and increased risk of secondary malignancies have not been substantiated.53-56 An ongoing Children’s Oncology Group (COG) study (ALTE11C2; ClinicalTrials.gov identifier: NCT0179012) is examining the long-term (> 10 years) efficacy of dexrazoxane in survivors treated in RCTs across a range of anthracycline exposures (100 to 360 mg/m2). Other agents, such as coenzyme Q1057 and amifostine,58 have not been shown to be effective in small pediatric RCTs; however, carvedilol may have potential efficacy.59 Strategies to minimize the risk of cardiotoxicity resulting from chest RT include limiting the radiation dose and volume, tailoring radiation fields to exclude as much of the heart as possible, deep-inspiration breath holding, or use of intensity-modulated RT or proton RT.16 Unfortunately, despite these efforts, cardiotoxicity can still occur, and secondary prevention is thus necessary.
Secondary Prevention
Strategies for intervening in survivors who have been exposed to cardiotoxic therapies have mostly been adapted from studies in adults with asymptomatic cardiac dysfunction resulting from causes other than anthracyclines. These include early screening and initiation of angiotensin-converting enzyme (ACE) inhibitors and β-blockers to prevent the progression of subclinical cardiac dysfunction to symptomatic heart failure.36 Several animal studies have shown that statins protect against anthracycline-induced cardiotoxicity,60,61 and there have been some retrospective62 and prospective63 human studies to suggest that these agents protect against deterioration of cardiac function in adults with cancer. The aldosterone antagonist spironolactone has also been shown to prevent cardiac deterioration in patients with breast cancer.64
In survivors of childhood cancer, timely dissemination of a survivorship care plan and counseling can result in a two-fold increase in cardiomyopathy screening rates.65 However, there are few studies to guide which pharmacologic agents, if any, should be used once cardiac dysfunction has been identified. A retrospective study of 18 survivors treated with anthracycline showed that although the ACE inhibitor enalapril improved LV structure and function in individuals with cardiac dysfunction,66 this was short lived. The only RCT in survivors of childhood cancer treated with anthracycline assessed the effectiveness of enalapril versus placebo in survivors with normal LVEF but at high risk for heart failure because of a history of cardiac dysfunction.67 The study failed to show an effect on its primary outcome (myocardial contractility index), but there was improvement in other cardiac measures (LV wall stress). Because β-blockers may be more likely to reverse the chronic cardiac remodeling than ACE inhibitors, an ongoing COG study (ALTE1621) is assessing the effect of carvedilol (v placebo) on prognostic markers of LV remodeling in approximately 250 survivors treated with high-dose (≥ 250 mg/m2) anthracyclines (ClinicalTrials.gov identifier: NCT0271750).
Targeting Conventional Cardiovascular Risk Factors
Multiple studies in survivors of childhood and adult cancer show that conventional cardiovascular conditions like hypertension, diabetes, and dyslipidemia are more prevalent68,69 and tend to occur at younger ages compared with sibling controls or the general population,70-72 increasing the likelihood of under-diagnosis and under-treatment. Survivors exposed to anthracycline who have hypertension or diabetes are at an especially high risk of developing CVD later in life.69 Lifestyle factors are also important. In survivors of Hodgkin lymphoma, vigorous exercise has been associated with a lower risk of CVD in a dose-dependent manner, independent of clinical and treatment-related risk factors,73 and should be encouraged in all survivors of cancer per established guidelines.1,16 Greater adherence to healthier lifestyle patterns (eg, healthier [Mediterranean] diet) has also been associated with reduced CVD risk in adult survivors of cancer.68 At present, there are several clinical trials that have leveraged the information obtained from these observational studies to reduce under-diagnosis and under-treatment of modifiable cardiovascular risk factors (eg, ClinicalTrials.gov identifier: NCT03104543) and to improve adherence to recommended lifestyle practices (eg, ClinicalTrials.gov identifiers: NCT02244411 and NCT03223753).
SUMMARY AND FUTURE DIRECTIONS
The evolution of childhood cancer treatment during the past four decades (eg, reduction in cumulative anthracycline dose, elimination of or reduction in dose and/or volume of radiation to the heart) has brought with it a need to understand how temporal changes in both cancer treatment and screening for late effects have altered health outcomes in long-term survivors. Armstrong et al74 from the Childhood Cancer Survivor Study (CCSS) recently reported a 50% reduction in the cumulative incidence of CVD-related late mortality (death > 5 years from diagnosis) between survivors of childhood cancer treated in the 1970s and those treated in the 1990s. Studies are under way to examine whether there has been a comparable decrease in the incidence of other CVD-related outcomes (eg, nonmortality). This information is necessary for the development of comprehensive (clinical, treatment, genetic) risk prediction models relevant to survivors treated with contemporary approaches.
The introduction of newer targeted agents has, in some instances, facilitated the decrease in treatment intensity for childhood cancer (eg, Ph-positive [like] ALL or CML). As such, there is a need for long-term follow-up studies in children exposed to these agents, keeping in mind the relatively small number of patients treated to date and the long latency of CVD in survivors of childhood cancer. Clinical trials networks such as the COG or large cohort studies such as the CCSS could provide the necessary infrastructure to examine short-term (COG) and long-term cardiovascular (COG, CCSS) sequelae in children treated with newer targeted therapies.
The growing number of survivors of childhood cancer at risk for CVD makes it imperative to continue our efforts to investigate the pathophysiology of cardiac and vascular injury resulting from established or newer cancer treatments. Longitudinal studies are needed to better characterize the prognostic effect of subclinical markers of cardiovascular injury, facilitating much-needed interventions to halt or reverse the trajectory of chronic CVD. These efforts will undoubtedly be strengthened by ongoing multidisciplinary collaborations between the oncology, cardiology, primary care, and other subspecialty communities.1,16,75
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Collection and assembly of data: All authors
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Cardiovascular Disease in Survivors of Childhood Cancer: Insights Into Epidemiology, Pathophysiology, and Prevention
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Saro H. Armenian
No relationship to disclose
Gregory T. Armstrong
No relationship to disclose
Gregory Anue
Employment: Mednax (I)
Stock or Other Ownership: Mednax (I), Bristol-Myers Squibb
Other Relationship: Azaya Therapeutics
Eric J. Chow
No relationship to disclose
Matthew J. Ehrhadt
No relationship to disclose
Bonnie Ky
Consulting or Advisory Role: Roche, Bristol-Myers Squibb
Research Funding: Pfizer, Roche
Patents, Royalties, Other Intellectual Property: Have a patent on the use of neuregulin-1b as a biomarker in heart failure
Javid Moslehi
Consulting or Advisory Role: Novartis, Pfizer, Bristol-Myers Squibb, Takeda, Pharmacyclics, Regeneron, Daiichi Sankyo
Research Funding: Pfizer, Bristol-Myers Squibb
Daniel A. Mulrooney
No relationship to disclose
Paul C. Nathan
No relationship to disclose
Thomas D. Ryan
No relationship to disclose
Helena J. van der Pal
No relationship to disclose
Elvira C. van Dalen
No relationship to disclose
Leontien C.M. Kremer
No relationship to disclose
REFERENCES
- 1.Armenian SH, Hudson MM, Mulder RL, et al. : Recommendations for cardiomyopathy surveillance for survivors of childhood cancer: A report from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Oncol 16:e123-e136, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mulrooney DA, Armstrong GT, Huang S, et al. : Cardiac outcomes in adult survivors of childhood cancer exposed to cardiotoxic therapy: A cross-sectional study. Ann Intern Med 164:93-101, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lipshultz SE, Adams MJ, Colan SD, et al. : Long-term cardiovascular toxicity in children, adolescents, and young adults who receive cancer therapy: Pathophysiology, course, monitoring, management, prevention, and research directions: A scientific statement from the American Heart Association. Circulation 128:1927-1995, 2013. [Erratum: Circulation 128:e394, 2013] [DOI] [PubMed] [Google Scholar]
- 4.Bowers DC, McNeil DE, Liu Y, et al. : Stroke as a late treatment effect of Hodgkin’s disease: A report from the Childhood Cancer Survivor Study. J Clin Oncol 23:6508-6515, 2005 [DOI] [PubMed] [Google Scholar]
- 5.van der Pal HJ, van Dalen EC, van Delden E, et al. : High risk of symptomatic cardiac events in childhood cancer survivors. J Clin Oncol 30:1429-1437, 2012 [DOI] [PubMed] [Google Scholar]
- 6.Armstrong GT, Liu Q, Yasui Y, et al. : Late mortality among 5-year survivors of childhood cancer: A summary from the Childhood Cancer Survivor Study. J Clin Oncol 27:2328-2338, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhakta N, Liu Q, Yeo F, et al. : Cumulative burden of cardiovascular morbidity in paediatric, adolescent, and young adult survivors of Hodgkin’s lymphoma: An analysis from the St Jude Lifetime Cohort Study. Lancet Oncol 17:1325-1334, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhakta N, Liu Q, Ness KK, et al. : The cumulative burden of surviving childhood cancer: An initial report from the St Jude Lifetime Cohort Study (SJLIFE). Lancet 390:2569-2582, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gianni L, Herman EH, Lipshultz SE, et al. : Anthracycline cardiotoxicity: From bench to bedside. J Clin Oncol 26:3777-3784, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang S, Liu X, Bawa-Khalfe T, et al. : Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat Med 18:1639-1642, 2012 [DOI] [PubMed] [Google Scholar]
- 11.Suliman HB, Carraway MS, Ali AS, et al. : The CO/HO system reverses inhibition of mitochondrial biogenesis and prevents murine doxorubicin cardiomyopathy. J Clin Invest 117:3730-3741, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ichikawa Y, Ghanefar M, Bayeva M, et al. : Cardiotoxicity of doxorubicin is mediated through mitochondrial iron accumulation. J Clin Invest 124:617-630, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Volkova M, Palmeri M, Russell KS, et al. : Activation of the aryl hydrocarbon receptor by doxorubicin mediates cytoprotective effects in the heart. Cardiovasc Res 90:305-314, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leite de Oliveira R, Deschoemaeker S, Henze AT, et al. : Gene-targeting of Phd2 improves tumor response to chemotherapy and prevents side-toxicity. Cancer Cell 22:263-277, 2012 [DOI] [PubMed] [Google Scholar]
- 15.Sarosiek KA, Fraser C, Muthalagu N, et al. : Developmental regulation of mitochondrial apoptosis by c-Myc governs age- and tissue-specific sensitivity to cancer therapeutics. Cancer Cell 31:142-156, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Armenian SH, Lacchetti C, Barac A, et al. : Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 35:893-911, 2017 [DOI] [PubMed] [Google Scholar]
- 17.Wilson PW, D’Agostino RB, Levy D, et al. : Prediction of coronary heart disease using risk factor categories. Circulation 97:1837-1847, 1998 [DOI] [PubMed] [Google Scholar]
- 18.Chow EJ, Chen Y, Kremer LC, et al. : Individual prediction of heart failure among childhood cancer survivors. J Clin Oncol 33:394-402, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Armenian SH, Bhatia S: Chronic health conditions in childhood cancer survivors: Is it all treatment-related--or do genetics play a role? J Gen Intern Med 24:S395-S400, 2009. (suppl 2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aminkeng F, Bhavsar AP, Visscher H, et al. : A coding variant in RARG confers susceptibility to anthracycline-induced cardiotoxicity in childhood cancer. Nat Genet 47:1079-1084, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang X, Liu W, Sun CL, et al. : Hyaluronan synthase 3 variant and anthracycline-related cardiomyopathy: A report from the children’s oncology group. J Clin Oncol 32:647-653, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang X, Sun CL, Quiñones-Lombraña A, et al. : CELF4 variant and anthracycline-related cardiomyopathy: A Children’s Oncology Group genome-wide association study. J Clin Oncol 34:863-870, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Visscher H, Ross CJ, Rassekh SR, et al. : Validation of variants in SLC28A3 and UGT1A6 as genetic markers predictive of anthracycline-induced cardiotoxicity in children. Pediatr Blood Cancer 60:1375-1381, 2013 [DOI] [PubMed] [Google Scholar]
- 24.Blanco JG, Leisenring WM, Gonzalez-Covarrubias VM, et al. : Genetic polymorphisms in the carbonyl reductase 3 gene CBR3 and the NAD(P)H:quinone oxidoreductase 1 gene NQO1 in patients who developed anthracycline-related congestive heart failure after childhood cancer. Cancer 112:2789-2795, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Blanco JG, Sun CL, Landier W, et al. : Anthracycline-related cardiomyopathy after childhood cancer: Role of polymorphisms in carbonyl reductase genes--a report from the Children’s Oncology Group. J Clin Oncol 30:1415-1421, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Semsei AF, Erdelyi DJ, Ungvari I, et al. : ABCC1 polymorphisms in anthracycline-induced cardiotoxicity in childhood acute lymphoblastic leukaemia. Cell Biol Int 36:79-86, 2012 [DOI] [PubMed] [Google Scholar]
- 27.Leger KJ, Cushing-Haugen K, Hansen JA, et al. : Clinical and genetic determinants of cardiomyopathy risk among hematopoietic cell transplantation survivors. Biol Blood Marrow Transplant 22:1094-1101, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Armenian SH, Ding Y, Mills G, et al. : Genetic susceptibility to anthracycline-related congestive heart failure in survivors of haematopoietic cell transplantation. Br J Haematol 163:205-213, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lipshultz SE, Lipsitz SR, Kutok JL, et al. : Impact of hemochromatosis gene mutations on cardiac status in doxorubicin-treated survivors of childhood high-risk leukemia. Cancer 119:3555-3562, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moslehi JJ: Cardiovascular toxic effects of targeted cancer therapies. N Engl J Med 375:1457-1467, 2016 [DOI] [PubMed] [Google Scholar]
- 31.Moslehi JJ, Deininger M: Tyrosine kinase inhibitor-associated cardiovascular toxicity in chronic myeloid leukemia. J Clin Oncol 33:4210-4218, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li W, Croce K, Steensma DP, et al. : Vascular and metabolic implications of novel targeted cancer therapies: Focus on kinase inhibitors. J Am Coll Cardiol 66:1160-1178, 2015 [DOI] [PubMed] [Google Scholar]
- 33.Tartarone A, Gallucci G, Lazzari C, et al. : Crizotinib-induced cardiotoxicity: The importance of a proactive monitoring and management. Future Oncol 11:2043-2048, 2015 [DOI] [PubMed] [Google Scholar]
- 34.Johnson DB, Balko JM, Compton ML, et al. : Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med 375:1749-1755, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hlatky MA, Greenland P, Arnett DK, et al. : Criteria for evaluation of novel markers of cardiovascular risk: A scientific statement from the American Heart Association. Circulation 119:2408-2416, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yancy CW, Jessup M, Bozkurt B, et al. : 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Card Fail 23:628-651, 2017 [DOI] [PubMed] [Google Scholar]
- 37.Hundley WG, Bluemke D, Bogaert JG, et al. : Society for Cardiovascular Magnetic Resonance guidelines for reporting cardiovascular magnetic resonance examinations. J Cardiovasc Magn Reson 11:5, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kongbundansuk S, Hundley WG: Noninvasive imaging of cardiovascular injury related to the treatment of cancer. JACC Cardiovasc Imaging 7:824-838, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Plana JC, Galderisi M, Barac A, et al. : Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: A report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 15:1063-1093, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lipshultz SE, Miller TL, Scully RE, et al. : Changes in cardiac biomarkers during doxorubicin treatment of pediatric patients with high-risk acute lymphoblastic leukemia: Associations with long-term echocardiographic outcomes. J Clin Oncol 30:1042-1049, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lipshultz SE, Rifai N, Sallan SE, et al. : Predictive value of cardiac troponin T in pediatric patients at risk for myocardial injury. Circulation 96:2641-2648, 1997 [DOI] [PubMed] [Google Scholar]
- 42.Armstrong GT, Joshi VM, Ness KK, et al. : Comprehensive echocardiographic detection of treatment-related cardiac dysfunction in adult survivors of childhood cancer: Results from the St Jude Lifetime Cohort Study. J Am Coll Cardiol 65:2511-2522, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Armstrong GT, Plana JC, Zhang N, et al. : Screening adult survivors of childhood cancer for cardiomyopathy: Comparison of echocardiography and cardiac magnetic resonance imaging. J Clin Oncol 30:2876-2884, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wong FL, Bhatia S, Landier W, et al. : Cost-effectiveness of the Children's Oncology Group long-term follow-up screening guidelines for childhood cancer survivors at risk for treatment-related heart failure. Ann Intern Med 160:672-683, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yeh JM, Nohria A, Diller L: Routine echocardiography screening for asymptomatic left ventricular dysfunction in childhood cancer survivors: A model-based estimation of the clinical and economic effects. Ann Intern Med 160:661-671, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hancock SL, Donaldson SS, Hoppe RT: Cardiac disease following treatment of Hodgkin’s disease in children and adolescents. J Clin Oncol 11:1208-1215, 1993 [DOI] [PubMed] [Google Scholar]
- 47.Brouwer CA, Postma A, Hooimeijer HL, et al. : Endothelial damage in long-term survivors of childhood cancer. J Clin Oncol 31:3906-3913, 2013 [DOI] [PubMed] [Google Scholar]
- 48. doi: 10.1002/14651858.CD006647.pub4. van Dalen EC, Raphaël MF, Caron HN, et al: Treatment including anthracyclines versus treatment not including anthracyclines for childhood cancer. Cochrane Database Syst Rev 9:CD006647, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. doi: 10.1002/14651858.CD005006.pub2. van Dalen EC, Michiels EM, Caron HN, et al: Different anthracycline derivates for reducing cardiotoxicity in cancer patients. Cochrane Database Syst Rev 12:CD005006, 2010. [DOI] [PubMed] [Google Scholar]
- 50.Hori H, Kudoh T, Nishimura S, et al. : Acute and late toxicities of pirarubicin in the treatment of childhood acute lymphoblastic leukemia: Results from a clinical trial by the Japan Association of Childhood Leukemia Study. Int J Clin Oncol 22:387-396, 2017 [DOI] [PubMed] [Google Scholar]
- 51.Fojtu M, Gumulec J, Stracina T, et al. : Reduction of doxorubicin-induced cardiotoxicity using nanocarriers: A review. Curr Drug Metab 18:237-263, 2017 [DOI] [PubMed] [Google Scholar]
- 52. doi: 10.1002/14651858.CD005008.pub2. van Dalen EC, van der Pal HJ, Caron HN, et al: Different dosage schedules for reducing cardiotoxicity in cancer patients receiving anthracycline chemotherapy. Cochrane Database Syst Rev 4:CD005008, 2009. [DOI] [PubMed] [Google Scholar]
- 53.Shaikh F, Dupuis LL, Alexander S, et al. : Cardioprotection and second malignant neoplasms associated with dexrazoxane in children receiving anthracycline chemotherapy: A systematic review and meta-analysis. J Natl Cancer Inst 10.1093/jnci/djv357 [DOI] [PubMed] [Google Scholar]
- 54.Chow EJ, Asselin BL, Schwartz CL, et al. : Late mortality after dexrazoxane treatment: A report from the Children’s Oncology Group. J Clin Oncol 33:2639-2645, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kremer LC, van Dalen EC: Dexrazoxane in children with cancer: From evidence to practice. J Clin Oncol 33:2594-2596, 2015 [DOI] [PubMed] [Google Scholar]
- 56.Tebbi CK, London WB, Friedman D, et al. : Dexrazoxane-associated risk for acute myeloid leukemia/myelodysplastic syndrome and other secondary malignancies in pediatric Hodgkin’s disease. J Clin Oncol 25:493-500, 2007 [DOI] [PubMed] [Google Scholar]
- 57.Iarussi D, Auricchio U, Agretto A, et al. : Protective effect of coenzyme Q10 on anthracyclines cardiotoxicity: Control study in children with acute lymphoblastic leukemia and non-Hodgkin lymphoma. Mol Aspects Med 15:s207-s212, 1994. (suppl) [DOI] [PubMed] [Google Scholar]
- 58.Gallegos-Castorena S, Martínez-Avalos A, Mohar-Betancourt A, et al. : Toxicity prevention with amifostine in pediatric osteosarcoma patients treated with cisplatin and doxorubicin. Pediatr Hematol Oncol 24:403-408, 2007 [DOI] [PubMed] [Google Scholar]
- 59.El-Shitany NA, Tolba OA, El-Shanshory MR, et al. : Protective effect of carvedilol on adriamycin-induced left ventricular dysfunction in children with acute lymphoblastic leukemia. J Card Fail 18:607-613, 2012 [DOI] [PubMed] [Google Scholar]
- 60.Sharma H, Pathan RA, Kumar V, et al. : Anti-apoptotic potential of rosuvastatin pretreatment in murine model of cardiomyopathy. Int J Cardiol 150:193-200, 2011 [DOI] [PubMed] [Google Scholar]
- 61.Ramanjaneyulu SV, Trivedi PP, Kushwaha S, et al. : Protective role of atorvastatin against doxorubicin-induced cardiotoxicity and testicular toxicity in mice. J Physiol Biochem 69:513-525, 2013 [DOI] [PubMed] [Google Scholar]
- 62.Seicean S, Seicean A, Plana JC, et al. : Effect of statin therapy on the risk for incident heart failure in patients with breast cancer receiving anthracycline chemotherapy: An observational clinical cohort study. J Am Coll Cardiol 60:2384-2390, 2012 [DOI] [PubMed] [Google Scholar]
- 63.Acar Z, Kale A, Turgut M, et al. : Efficiency of atorvastatin in the protection of anthracycline-induced cardiomyopathy. J Am Coll Cardiol 58:988-989, 2011 [DOI] [PubMed] [Google Scholar]
- 64.Akpek M, Ozdogru I, Sahin O, et al. : Protective effects of spironolactone against anthracycline-induced cardiomyopathy. Eur J Heart Fail 17:81-89, 2015 [DOI] [PubMed] [Google Scholar]
- 65.Hudson MM, Leisenring W, Stratton KK, et al. : Increasing cardiomyopathy screening in at-risk adult survivors of pediatric malignancies: A randomized controlled trial. J Clin Oncol 32:3974-3981, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lipshultz SE, Lipsitz SR, Sallan SE, et al. : Long-term enalapril therapy for left ventricular dysfunction in doxorubicin-treated survivors of childhood cancer. J Clin Oncol 20:4517-4522, 2002 [DOI] [PubMed] [Google Scholar]
- 67.Silber JH, Cnaan A, Clark BJ, et al. : Enalapril to prevent cardiac function decline in long-term survivors of pediatric cancer exposed to anthracyclines. J Clin Oncol 22:820-828, 2004 [DOI] [PubMed] [Google Scholar]
- 68.Chow EJ, Baker KS, Lee SJ, et al. : Influence of conventional cardiovascular risk factors and lifestyle characteristics on cardiovascular disease after hematopoietic cell transplantation. J Clin Oncol 32:191-198, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Armstrong GT, Oeffinger KC, Chen Y, et al. : Modifiable risk factors and major cardiac events among adult survivors of childhood cancer. J Clin Oncol 31:3673-3680, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Oeffinger KC, Adams-Huet B, Victor RG, et al. : Insulin resistance and risk factors for cardiovascular disease in young adult survivors of childhood acute lymphoblastic leukemia. J Clin Oncol 27:3698-3704, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.van Waas M, Neggers SJ, Pieters R, et al. : Components of the metabolic syndrome in 500 adult long-term survivors of childhood cancer. Ann Oncol 21:1121-1126, 2010 [DOI] [PubMed] [Google Scholar]
- 72.Steinberger J, Sinaiko AR, Kelly AS, et al. : Cardiovascular risk and insulin resistance in childhood cancer survivors. J Pediatr 160:494-499, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jones LW, Liu Q, Armstrong GT, et al. : Exercise and risk of major cardiovascular events in adult survivors of childhood hodgkin lymphoma: A report from the Childhood Cancer Survivor Study. J Clin Oncol 32:3643-3650, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Armstrong GT, Chen Y, Yasui Y, et al. : Reduction in late mortality among 5-year survivors of childhood cancer. N Engl J Med 374:833-842, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lenihan DJ, Hartlage G, DeCara J, et al. : Cardio-oncology training: A proposal from the International Cardioncology Society and Canadian Cardiac Oncology Network for a New Multidisciplinary Specialty. J Card Fail 22:465-471, 2016 [DOI] [PubMed] [Google Scholar]