Abstract
Purpose
Locoregional control for inflammatory breast cancers and chest wall recurrences is suboptimal, which has motivated interest in radiosensitization to intensify therapy. Preclinical studies have suggested a favorable therapeutic index when poly (ADP-ribose) polymerase inhibitors are used as radiosensitizers; clinical investigation is necessary to establish appropriate dosing and confirm safety.
Patients and Methods
We conducted a multi-institutional phase I study of veliparib and concurrent radiotherapy (RT) to the chest wall and regional lymph nodes in 30 patients with inflammatory or locally recurrent breast cancer after complete surgical resection. RT consisted of 50 Gy to the chest wall and regional lymph nodes plus a 10-Gy boost. A Bayesian time-to-event continual reassessment method escalated dose through four levels, with a 30% targeted rate of dose-limiting toxicity (DLT) measured during the 6 weeks of treatment plus 4 weeks of follow-up. DLTs were defined as confluent moist desquamation > 100 cm2, nonhematologic toxicity grade ≥ 3, toxicity that requires an RT dose delay > 1 week, absolute neutrophil count < 1,000/mm3, platelet count < 50,000/mm3, or hemoglobin < 8.0 g/dL if possibly, probably, or definitely related to study treatment.
Results
Five DLTs occurred: Four were moist desquamation (two each at 100 and 150 mg twice a day), and one was neutropenia (at 200 mg twice a day). The crude rate of any grade 3 toxicity (regardless of attribution) was 10% at year 1, 16.7% at year 2, and 46.7% at year 3. At year 3, six of 15 surviving patients had severe fibrosis in the treatment field.
Conclusion
Although severe acute toxicity did not exceed 30% even at the highest tested dose, nearly half of surviving patients demonstrated grade 3 adverse events at 3 years, which underscores the importance of long-term monitoring of toxicity in trials of radiosensitizing agents.
INTRODUCTION
Although locoregional control of breast cancer is excellent in many circumstances, outcomes remain unsatisfactory with conventional therapy in certain groups, including patients with locoregional recurrences after mastectomy and those diagnosed with inflammatory disease. Many chest wall recurrences are unresectable, which leaves radiotherapy as the sole modality of local therapy. Even when excision followed by radiotherapy is possible, such management can result in inadequate locoregional control, which compromises quality of life.1-4 In patients with inflammatory breast cancer, locoregional failure rates approach 20% despite aggressive multimodality treatment.
Radiation dose escalation can increase tumor control, but normal tissue tolerance limits this approach. Several groups have considered thermoradiotherapy5 or other approaches to radiosensitization,6,7 but a satisfactory technique suitable for widespread use has yet to be discovered.
Given the role of poly (ADP-ribose) polymerase (PARP) in DNA repair, the potential of PARP inhibitors as radiosensitizers is of interest. Radiosensitization by concurrent administration of a PARP inhibitor has been demonstrated in preclinical studies.8-15 One postulated mechanism is that radiation-induced single-strand breaks accumulate in cells in which PARP is inhibited, which leads to double-strand breaks at replication forks. Of note, because cancer cells are genetically unstable and often exhibit complex karyotypes that include large deletions, insertions, and unbalanced translocations, these cells are more susceptible than normal tissues to cytotoxicity induced by DNA-damaging agents.16,17 Deficiencies in mismatch repair and homologous recombination are prevalent in many malignancies. These deficiencies render cells more dependent on PARP for DNA repair and, hence, more sensitive to PARP inhibition.18 Higher expression of PARP in cancer cells compared with normal cells has been linked to the overall ability of cancer cells to sustain genotoxic stress.19-22 Consequently, PARP inhibitors have been proposed as radiosensitizers.
Veliparib (ABT-888) is a PARP inhibitor being investigated in Cancer Therapy Evaluation Program–sponsored trials prompted by promising preclinical findings. Specifically, in a panel of breast cancer and normal breast epithelial cell lines, veliparib preferentially radiosensitized breast cancer (v normal) cells with enhancement ratios of up to 2.3 independent of intrinsic breast cancer subtype or BRCA mutational status.23 Because the toxicity profile of a radiosensitizing agent is expected to depend on the body site treated and dose of radiotherapy delivered, specific research was needed to evaluate further the combination of PARP inhibition and radiotherapy in breast cancer. This motivated TBCRC 024, a multicenter phase I trial, to determine the maximum tolerated dose of veliparib in combination with chest wall and nodal radiotherapy and to make recommendations for phase II dosing.
PATIENTS AND METHODS
Study Population
Eligible patients were recruited from five institutions and had either locoregional recurrence of breast cancer after mastectomy that was subsequently excised or primary inflammatory breast cancer treated with mastectomy. To facilitate prescription of a single standard radiotherapy dose, patients were required to have undergone gross total excision of all locoregional disease. Adequate organ function, ability to swallow intact study drug, and an Eastern Cooperative Oncology Group performance status of 0 to 1 with at least a 6-month life expectancy also were required. Two weeks had to have elapsed since prior antineoplastic systemic therapy (except endocrine agents and bisphosphonates), with resolution of acute toxicity. Primary exclusion criteria were prior radiotherapy, breast reconstruction, concurrent treatment with antitumor agents other than hormonal therapy or bisphosphonates, gross residual locoregional tumor, surgery within 3 weeks of commencement of protocol therapy, pregnancy or breastfeeding, and serious comorbid illness.
Treatment
Treatment consisted of veliparib at one dose level (50, 100, 150, or 200 mg) taken orally twice a day throughout a 6-week course of radiotherapy. Doses were determined after consideration of phase 0 study findings. Intrasubject escalation of veliparib dose was not allowed.
Radiotherapy was administered using megavoltage linear accelerators once daily for 5 days/week. Targets were the entire chest wall and undissected axillary and supraclavicular nodes treated to 50 Gy in 2 Gy/fraction followed by a 10-Gy boost to the mastectomy scar. Internal mammary irradiation was discretionary, as was boost treatment to other sites (including the axilla). Radiotherapy dose was not allowed to exceed 60 Gy. Bolus (0.5 cm) to the chest wall was to be used every other treatment day for the first 50 Gy, beginning on the first day of treatment. The bolus could be discontinued or increased to daily application at the treating physician’s discretion.
End Points and Analytic Design
The trial was monitored using the time-to-event continual reassessment method,24,25 which assumes a model for the time to occurrence of toxicity as a function of dose and allows information from all patients enrolled in the trial to be used when a new patient is allocated to a dose level. Dose-limiting toxicity (DLT) was defined on the basis of toxicities possibly, probably, or definitely related to study drug (Table 1) that occurred during the acute observation period. The acute observation period, during which patients had weekly physician assessments, was the 6-week radiotherapy treatment period plus a 4-week follow-up period, including the clinical visit 10 weeks from radiotherapy initiation (a 70-day time period). The target rate for DLT was 30%.
Table 1.
Dose-Limiting Toxicity Definition
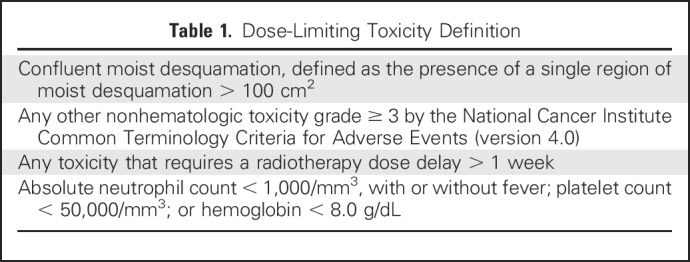
The primary study objective was to determine the maximum tolerated dose of veliparib that can be administered concurrently with standard radiotherapy to the chest wall and regional nodes. Secondary objectives were to describe the nature of toxicity that develops when veliparib is administered concurrently with chest wall and regional nodal radiotherapy. Under the continual reassessment method paradigm, the relationship between dose and toxicity is summarized by a single-parameter (α) logistic model that represents the assumed relationship prior to the collection of patient data. Information about the relationship between dose and toxicity can be summarized using the distribution of the parameter, updated according to the current data. Simulations suggested that a sample size of 30 evaluable patients would allow for identification of the correct dose level in the majority of trials.
RESULTS
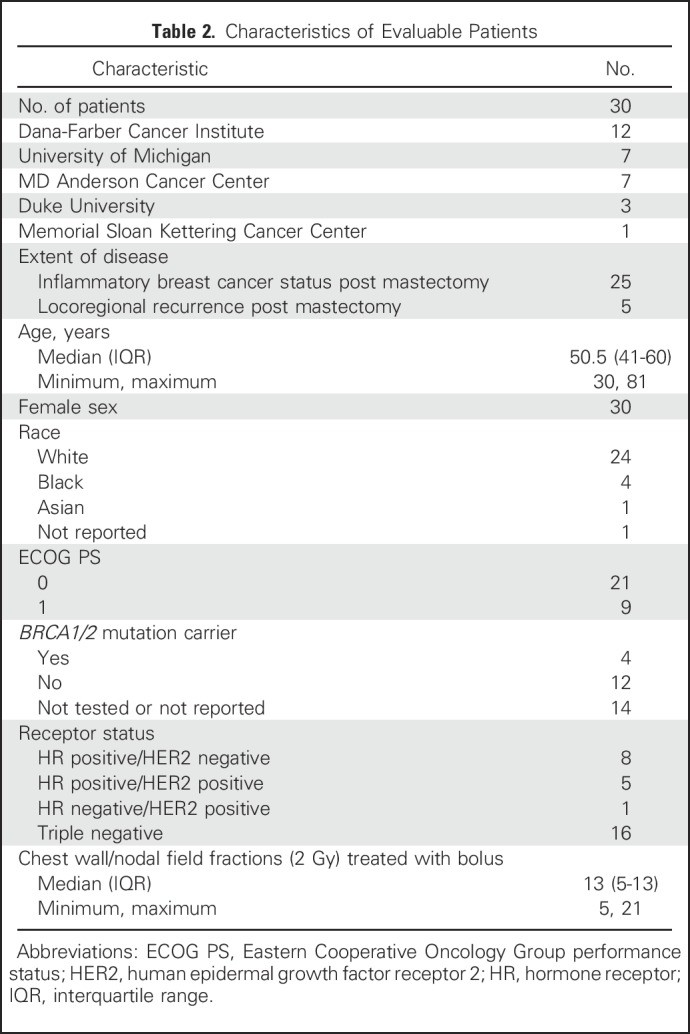
Between June 2012 and November 2014, 33 patients were enrolled, of whom 30 were evaluable with primary inflammatory (n = 25) or locally recurrent breast cancer (n = 5). The median number of fractions treated with bolus was 13 (interquartile range, 5 to 13). Characteristics of the evaluable sample are reported in Table 2. Of the 30 patients, 27 (90%) had treatment to the internal mammary region.
Table 2.
Characteristics of Evaluable Patients
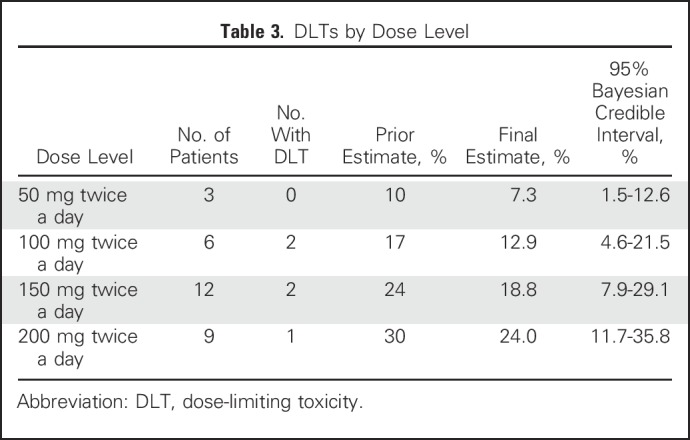
There were five DLTs in total (Table 3): two at 100 mg twice a day (moist desquamation), two at 150 mg twice a day (moist desquamation), and 1 at 200 mg twice a day (neutropenia). Of the five patients who experienced DLTs, four completed 60 Gy of radiotherapy (which required < 2 weeks of delay), and one completed 54 Gy (6 Gy less than the planned and standard dose of 60 Gy); toxicity resolved within 1 month of completion of therapy, similar to typical radiation dermatitis. The posterior probability of DLT did not exceed 30%, even at the highest dose level assessed (200 mg twice of day).
Table 3.
DLTs by Dose Level
Two serious adverse events developed within the first 2 years of follow-up. One patient experienced a grade 4 wound infection and late radiation dermatitis with ulceration, flap failure, induration, and severe telangiectasia 6 months after completion of study treatment. The second patient experienced a grade 3 brachial vein thrombosis in the setting of post-treatment lymphedema and fibrosis observed 1.5 years after treatment. Both patients had received 150 mg twice a day of veliparib.
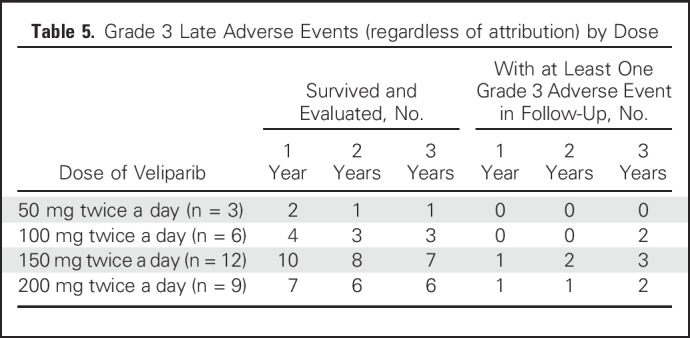
No deaths occurred within 30 days of completing treatment. Late events are listed in Table 4. Given the challenges of attribution after the acute treatment phase, all adverse events reported are included, regardless of attribution. At the 1-year follow-up visit, 20 patients were alive and evaluated for toxicity: Two had severe (grade 3) fibrosis in the radiation field (of whom one also had grade 3 skin induration and grade 3 fracture), and 11 patients had hyperpigmentation (all grade 1). At the 2-year follow-up visit, 18 patients were alive and evaluated for toxicity: Three exhibited severe fibrosis (one since the 1-year follow-up visit, and both patients who had grade 3 fibrosis at 1 year also having grade 3 skin induration at 2 years), and six had hyperpigmentation (five grade 1, one grade 2). At the 3-year follow-up visit, 15 patients were alive and evaluated for toxicity: Six exhibited grade 3 fibrosis, with two of these six also exhibiting grade 3 skin induration and two also exhibiting grade 3 lymphedema. Two of the six patients with grade 3 fibrosis had experienced an acute DLT, and four had not. In addition to the six patients with grade 3 fibrosis, one demonstrated grade 3 lymphedema without severe fibrosis. The crude rate of any grade 3 toxicity was 10% at year 1, 16.7% at year 2, and 46.7% at year 3. Grade 3 toxicity was observed at 3 years at all dose levels except 50 mg twice a day (Table 5).
Table 4.
Adverse Events Reported During Follow-Up, Regardless of Attribution
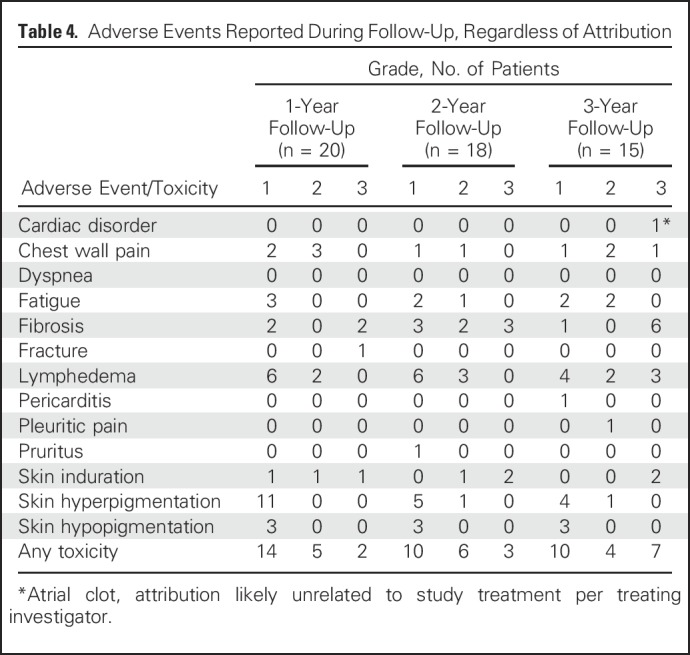
Table 5.
Grade 3 Late Adverse Events (regardless of attribution) by Dose
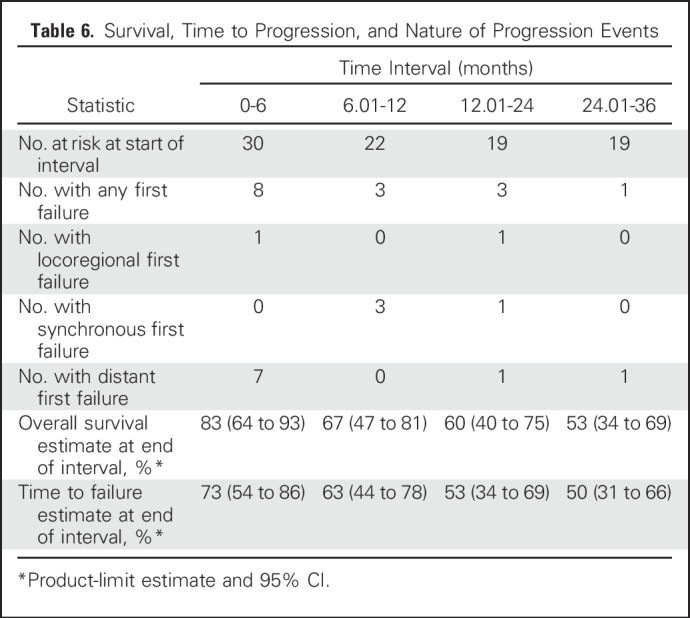
Of the 30 patients, 15 experienced disease control failures during the 3 years of follow-up, and 13 died (all after recurrence). Of the 15 patients who experienced disease control failures, the site of first failure was locoregional only in two, one of whom subsequently had a distant failure; both locoregional and distant in four; and distant only in nine (Table 6).
Table 6.
Survival, Time to Progression, and Nature of Progression Events
DISCUSSION
In this multicenter phase I trial, severe acute toxicity did not exceed the prespecified target of 30%, even at the highest tested dose of veliparib (200 mg twice a day), and we observed no grade 4 or 5 events. However, given observations of grade 3 late toxicity in nearly one half of all patients evaluated at 3 years, we recommend a phase II dose of 50 mg twice a day if veliparib is investigated further for radiosensitization in patients with breast cancer at high risk of locoregional recurrence and in need of treatment intensification. Although some of the late adverse events we observed might have occurred even in the absence of the investigational agent and with standard therapy, severe late toxicity is relatively uncommon with standard therapy alone, so we believe that a cautious approach is prudent. No severe adverse events were seen at 50 mg, although few patients received that dose in this trial. Future studies should include assessment of patient-reported quality of life and compare outcomes in patients treated with combination therapy with those among patients treated with radiotherapy alone.
This trial exemplifies many of the challenges discussed by National Cancer Institute working groups with regard to phase I studies of radiation modifiers.26 Phase I studies of potential radiosensitizing agents are challenging because the toxicity profile is expected to vary on the basis of the site being irradiated, which necessitates multiple trials to identify appropriate dosing particular to each site. This also is complicated by the ethical imperative to conduct such studies in subgroups of patients with a sufficiently dire prognosis to warrant consideration of participation in high-risk research, but most patients with metastatic cancer do not receive definitive doses of radiotherapy. In breast cancer, the vast majority of patients who receive definitive radiotherapy have excellent outcomes and are not candidates for a phase I study. Multicenter cooperation is necessary to recruit sufficient numbers of patients with uncommon presentations, such as inflammatory breast cancer, in which definitive radiation doses are used and locoregional control suboptimal.
The tendency for phase I studies to recruit patients with a dire prognosis is a challenge because this precludes the ability of such studies to provide useful illumination of activity or efficacy. It is important not to misinterpret the disease control findings from this study. Many patients treated in the current study experienced disease control failures, including six of 30 with locoregional failures either as an isolated first failure or simultaneously with a distant failure, but without a comparable control group, comment on efficacy from the current findings is impossible. The substantial rates of failure observed in this trial likely reflect a selection effect, whereby enrolling physicians appropriately offered the study to patients with the most aggressive disease in whom a study that investigates the safety of a regimen could be justified.
Another challenge is that a radiosensitization approach may result in late effects not captured in the time window for DLT ascertainment. Not all patients with severe enough disease to justify inclusion in a phase I trial may survive long enough to allow for assessment of late events. Although the time-to-event continual reassessment method algorithm can expand the observation window to include subacute effects, it is not practical to delay dose escalation decisions in a phase I trial until a full year or longer has elapsed. Yet, certain effects of radiotherapy may not be apparent until late after treatment. As this trial demonstrates, in some circumstances the maximal acutely tolerated dose may result in unacceptable late effects that are not apparent during the course of the study. In the current setting, we believe that subsequent studies should evaluate activity with a dose of 50 mg twice a day, with careful long-term monitoring of toxicity. Particular caution is necessary if patients dissimilar to those in the current phase I population are included, such as those who receive breast conservation or breast reconstruction.
Since the initiation of this trial, findings were reported from a trial of radiotherapy in combination with veliparib for CNS metastases of non–small-cell lung cancer.27 That trial did not demonstrate differences in toxicity or disease control in patients randomly assigned to twice-a-day treatment with 50 mg veliparib, 200 mg veliparib, or placebo in combination with radiotherapy, which indicates that PARP inhibition does not change the therapeutic index of radiotherapy to the whole brain, at least not at the doses of radiotherapy used in the trial. This further underscores the importance of investigating both safety and efficacy of radiosensitizing agents in different settings. The brain trial used a lower total dose of radiotherapy (30 Gy) in higher doses per fraction (3 Gy). With larger doses per fraction, so much damage might be done that inhibition of PARP does not meaningfully affect the proportion of cells that survive after each fraction. Because smaller doses per fraction are necessary when treating to the high total doses needed for breast cancer, veliparib might lead to meaningful radiosensitization in that context.
Despite these negative data in lung cancer brain metastases, good reason remains to hypothesize that low-dose PARP inhibition would radiosensitize cancer cells more than normal tissues in the setting of breast cancer. Emerging data suggest that at least in cell lines, although heterogeneity of response exists within each lineage, breast cancers have much higher radiation resistance than lung cancers.28 Thus, the incremental benefit added by PARP inhibition in lung cancer brain metastases was likely blunted not only by the higher dose/fraction and the blood-brain barrier but also by relative intrinsic sensitivity compared with breast. Thus, the targeting of DNA repair in combination with radiotherapy as an approach toward radiosensitization continues to be an important avenue for ongoing investigation in breast cancer. Indeed, PARP inhibition in combination with radiotherapy soon will be the focus of a National Cancer Institute Breast Cancer Steering Committee–approved phase II trial led by SWOG in patients with inflammatory breast cancer.
In summary, questions of radiosensitization cannot be answered by extrapolation from other disease sites (where radiotherapy ose and fractionation and expected toxicities will differ, as may penetration of drug) or with other tumor types (which differ in their level of radioresistance and baseline dysregulation of DNA repair). Only by investigating such approaches specifically in patients with breast cancer are we able to rigorously evaluate its potential.
ACKNOWLEDGMENT
We thank Daniel Hayes, MD, and the TBCRC locoregional committee for contributions to the study design.
Footnotes
Supported by the Translational Breast Cancer Research Consortium (TBCRC), the Breast Cancer Research Foundation (both through its support of the TBCRC and through a grant to L.P.), the University of Michigan Comprehensive Cancer Center (through a grant from the Clinical Translational Resource Allocation Committee to R.J.), and the Michigan Institute for Clinical and Health Research through grant UL1TR000433. The TBCRC also receives support from the Avon Foundation, the Breast Cancer Research Foundation, and Susan G. Komen. This was an investigator-initiated study for which study drug and distribution were provided by AbbVie. AbbVie and the funding bodies played no role in the design or conduct of the trial, its interpretation, the writing of the manuscript, or the decision to submit for publication.
Clinical trial information: NCT01477489.
AUTHOR CONTRIBUTIONS
Conception and design: Reshma Jagsi, Kent A. Griffith
Financial support: Reshma Jagsi
Administrative support: Reshma Jagsi
Provision of study materials or patients: Reshma Jagsi
Collection and assembly of data: Reshma Jagsi, Jennifer R. Bellon, Wendy A. Woodward, Janet K. Horton, Alice Ho, Corey Speers, Beth Overmoyer, Lori Pierce
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Concurrent Veliparib With Chest Wall and Nodal Radiotherapy in Patients With Inflammatory or Locoregionally Recurrent Breast Cancer: The TBCRC 024 Phase I Multicenter Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
Reshma Jagsi
Employment: University of Michigan
Consulting or Advisory Role: Amgen
Research Funding: AbbVie (Inst)
Kent A. Griffith
No relationship to disclose
Jennifer R. Bellon
Honoraria: UpToDate
Wendy A. Woodward
No relationship to disclose
Janet K. Horton
Honoraria: Oakstone, International Journal of Radiation Oncology Biology Physics, Qfix
Research Funding: Varian Medical Systems
Alice Ho
Employment: Cedars-Sinai Medical Center
Honoraria: Amgen
Consulting or Advisory Role: Amgen
Research Funding: Merck (Inst)
Travel, Accommodations, Expenses: Amgen
Felix Y. Feng
Stock or Other Ownership: PFS Genomics
Honoraria: Clovis Oncology
Consulting or Advisory Role: Medivation, Astellas Pharma, Janssen Pharmaceuticals, Sanofi, Dendreon, Ferring Pharmaceuticals, EMD Serono
Patents, Royalties, Other Intellectual Property: Patent on an approach for identifying determinants of radiosensitivity in cancer titled “Compositions and Methods for the Analysis of Radio Sensitivity” (patent No. EP 3047037 A4)
Corey Speers
Patents, Royalties, Other Intellectual Property: University of Michigan Health System
Beth Overmoyer
Research Funding: Eisai (Inst), GTx (Inst), Genetech (Inst), Incyte (Inst)
Travel, Accommodations, Expenses: Novartis
Michael Sabel
Patents, Royalties, Other Intellectual Property: Breast Cancer Ally and Melanoma Ally mobile technologies
Anne F. Schott
Research Funding: Dompé Farmaceutici (Inst), Pfizer (Inst), Novartis (Inst)
Patents, Royalties, Other Intellectual Property: Inventor of Systems and Methods for Tissue Imaging patent No. 8,185,186 (submitted on April 2008) for use of diffusion magnetic resonance imaging technology to quantitate response to neoadjuvant breast cancer therapy
Lori Pierce
Stock or Other Ownership: PFS Genomics
Patents, Royalties, Other Intellectual Property: UpToDate, PFS Genomics
REFERENCES
- 1.Bedwinek JM, Fineberg B, Lee J, et al. : Analysis of failures following local treatment of isolated local-regional recurrence of breast cancer. Int J Radiat Oncol Biol Phys 7:581-585, 1981 [DOI] [PubMed] [Google Scholar]
- 2.Aberizk WJ, Silver B, Henderson IC, et al. : The use of radiotherapy for treatment of isolated locoregional recurrence of breast carcinoma after mastectomy. Cancer 58:1214-1218, 1986 [DOI] [PubMed] [Google Scholar]
- 3.Schwaibold F, Fowble BL, Solin LJ, et al. : The results of radiation therapy for isolated local regional recurrence after mastectomy. Int J Radiat Oncol Biol Phys 21:299-310, 1991 [DOI] [PubMed] [Google Scholar]
- 4.Halverson KJ, Perez CA, Kuske RR, et al. : Isolated local-regional recurrence of breast cancer following mastectomy: Radiotherapeutic management. Int J Radiat Oncol Biol Phys 19:851-858, 1990 [DOI] [PubMed] [Google Scholar]
- 5.Welz S, Hehr T, Lamprecht U, et al. : Thermoradiotherapy of the chest wall in locally advanced or recurrent breast cancer with marginal resection. Int J Hyperthermia 21:159-167, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Taylor ME: Breast cancer: Chest wall recurrences. Curr Treat Options Oncol 3:175-177, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Suh WW, Schott AF, Hayman JA, et al. : A phase I dose escalation trial of gemcitabine with radiotherapy for breast cancer in the treatment of unresectable chest wall recurrences. Breast J 10:204-210, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Powell C, Mikropoulos C, Kaye SB, et al. : Pre-clinical and clinical evaluation of PARP inhibitors as tumour-specific radiosensitisers. Cancer Treat Rev 36:566-575, 2010 [DOI] [PubMed] [Google Scholar]
- 9.Russo AL, Kwon HC, Burgan WE, et al. : In vitro and in vivo radiosensitization of glioblastoma cells by the poly (ADP-ribose) polymerase inhibitor E7016. Clin Cancer Res 15:607-612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dungey FA, Caldecott KW, Chalmers AJ: Enhanced radiosensitization of human glioma cells by combining inhibition of poly(ADP-ribose) polymerase with inhibition of heat shock protein 90. Mol Cancer Ther 8:2243-2254, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dungey FA, Löser DA, Chalmers AJ: Replication-dependent radiosensitization of human glioma cells by inhibition of poly(ADP-Ribose) polymerase: Mechanisms and therapeutic potential. Int J Radiat Oncol Biol Phys 72:1188-1197, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Donawho CK, Luo Y, Luo Y, et al. : ABT-888, an orally active poly(ADP-ribose) polymerase inhibitor that potentiates DNA-damaging agents in preclinical tumor models. Clin Cancer Res 13:2728-2737, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Liu SK, Coackley C, Krause M, et al. : A novel poly(ADP-ribose) polymerase inhibitor, ABT-888, radiosensitizes malignant human cell lines under hypoxia. Radiother Oncol 88:258-268, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Veuger SJ, Curtin NJ, Richardson CJ, et al. : Radiosensitization and DNA repair inhibition by the combined use of novel inhibitors of DNA-dependent protein kinase and poly(ADP-ribose) polymerase-1. Cancer Res 63:6008-6015, 2003 [PubMed] [Google Scholar]
- 15.Albert JM, Cao C, Kim KW, et al. : Inhibition of poly(ADP-ribose) polymerase enhances cell death and improves tumor growth delay in irradiated lung cancer models. Clin Cancer Res 13:3033-3042, 2007 [DOI] [PubMed] [Google Scholar]
- 16.DePinho RA, Polyak K: Cancer chromosomes in crisis. Nat Genet 36:932-934, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Sharpless NE, DePinho RA: Telomeres, stem cells, senescence, and cancer. J Clin Invest 113:160-168, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Curtin NJ, Wang LZ, Yiakouvaki A, et al. : Novel poly(ADP-ribose) polymerase-1 inhibitor, AG14361, restores sensitivity to temozolomide in mismatch repair-deficient cells. Clin Cancer Res 10:881-889, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Wielckens K, Garbrecht M, Kittler M, et al. : ADP-ribosylation of nuclear proteins in normal lymphocytes and in low-grade malignant non-Hodgkin lymphoma cells. Eur J Biochem 104:279-287, 1980 [DOI] [PubMed] [Google Scholar]
- 20.Hirai K, Ueda K, Hayaishi O: Aberration of poly(adenosine diphosphate-ribose) metabolism in human colon adenomatous polyps and cancers. Cancer Res 43:3441-3446, 1983 [PubMed] [Google Scholar]
- 21.Tomoda T, Kurashige T, Moriki T, et al. : Enhanced expression of poly(ADP-ribose) synthetase gene in malignant lymphoma. Am J Hematol 37:223-227, 1991 [DOI] [PubMed] [Google Scholar]
- 22.Shiobara M, Miyazaki M, Ito H, et al. : Enhanced polyadenosine diphosphate-ribosylation in cirrhotic liver and carcinoma tissues in patients with hepatocellular carcinoma. J Gastroenterol Hepatol 16:338-344, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Feng FY, Speers C, Liu M, et al. : Targeted radiosensitization with PARP1 inhibition: Optimization of therapy and identification of biomarkers of response in breast cancer. Breast Cancer Res Treat 147:81-94, 2014 [DOI] [PubMed] [Google Scholar]
- 24.Cheung YK, Chappell R: Sequential designs for phase I clinical trials with late-onset toxicities. Biometrics 56:1177-1182, 2000 [DOI] [PubMed] [Google Scholar]
- 25.O’Quigley J, Pepe M, Fisher L: Continual reassessment method: A practical design for phase 1 clinical trials in cancer. Biometrics 46:33-48, 1990 [PubMed] [Google Scholar]
- 26.Colevas AD, Brown JM, Hahn S, et al. : Development of investigational radiation modifiers. J Natl Cancer Inst 95:646-651, 2003 [DOI] [PubMed] [Google Scholar]
- 27.Chabot P, Hsia TC, Ryu JS, et al. : Veliparib in combination with whole-brain radiation therapy for patients with brain metastases from non-small cell lung cancer: Results of a randomized, global, placebo-controlled study. J Neurooncol 131:105-115, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yard BD, Adams DJ, Chie EK, et al. : A genetic basis for the variation in the vulnerability of cancer to DNA damage. Nat Commun 7:11428, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]


