Abstract
Plants present a delimited reservoir of biologically active compounds. Many plants synthesize several compounds of secondary metabolism, such as alkaloids, terpenoids, phenolics, steroids, etc. Such compounds are generally thought to be involved in plant–insect interactions. Phytoecdysteroids are a class of chemicals that plants synthesize; these compounds are analogues of molting hormones produced by insects. In this work, the effect of the 20-hydroxyecdysone, which is a molecule that belongs to the family of phytoecdysteroids, was tested on an insect pest, Tribolium castaneum (Herbst). Firstly, the effect of this molecule on post-embryonic development parameters was tested after ingestion at 300, 600, 900, and 1,200 ppm. Secondly, the effect of the 20-hydroxyecdysone was also tested on the biological parameters (proteins, alpha-amylase, detoxification enzymes). The results of the post-embryonic parameters test showed an important induction of larval mortality and a significant reduction of pupation and adult emergence rates. On the other hand, the test on the biological parameters showed that the 20-hydroxyecdysone caused a significant decrease in the levels of soluble proteins in treated larvae. In addition, the alpha-amylase activity was significantly inhibited by the ingestion of the phytoecdysteroid. And there was also a disruption of detoxification enzymes. The whole of the disturbances recorded in this work prove that phytoecdysteroids are thought to have potential value on T. castaneum control.
Keywords: insect pest, phytoecdysteroid, toxicity, biochemical parameter
Plants are continually exposed to damage caused by many agents including insects, (Talbot 2005). The life of those pests is ensured by the presence of green plants, which are considered as an important source of nutrition for heterotrophic living beings. Therefore, a better understanding of the plant–insect interaction is really necessary (Schoonhoven et al. 2005). Statistics show that the tithe of annually produced plant biomass is consumed by insects, which makes insects a huge threat to agricultural productivity (Stamp 1996, Coupe and Cahill 2003). Following the damage produced by these insect pests, plants induce resistance in response to these phytophagous (Stamp 1996, Dicke and Loon 2000). In fact, plant resistance can be induced by the biosynthesis of groups of secondary metabolites such as phenolics, terpenes, alkaloids, and steroids (Fraenkel 1959).
Phytoecdysteroids are natural steroids synthesized by plants. They have a similar chemical structure to ecdysteroids, which are molting hormones produced by insects. Phytoecdysteroids were found to have defensive potential against pests’s attacks (Dinan 2001, Schmelz et al. 2002). Blackford and Dinan (1997) studied the effect of ingested phytoecdysteroids against Cynthia cardui (Linnaeus, 1758) (Lepidoptera: Nymphalidae) larvae, and they found that phytoecdysteroids prolonged the larval and pupal periods, induced mortality, and provoked a disruption of the adult emergence. Tanaka et al. (1994) reported that phytoecdysteroids disrupted the physiological parameters of Bombyx mori (Linnaeus, 1758) (Lepidoptera: Bombycidae). However, certain species remain unsusceptible to the exogenous application of phytoecdysteroids; it is the case of Heliothis virescens (Fabricius, 1777) (Lepidoptera: Noctuidae) (Kubo et al. 1987) and Ostrinia nubilalis (Hübner, 1796) (Lepidoptera: Pyralidae) (Gelman et al. 1991).
Diverse researchers have showed that sterols affect negatively the biochemical and physiological parameters of herbivorous insects. However, the losses of stored grains caused by insect infestation is a serious problem. Therefore, testing the effect of phytoecdysteroids on insect pests of stored products should be focused on by more studies. Chaitanya et al. (2011) reported that the ingested phytoecdysteroids disrupt the physiological parameters of Corcyra cephalonica (Stainton, 1866) (Lepidoptera: Pyralidae). Rharrabe et al. (2009) showed that phytoecdysteroids provoke a disruption in the post-embryonic parameters of Plodia interpunctella (Hübner, 1813) (Lepidoptera: Pyralidae).
Among insect pests, Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae) is the major pest that causes quantitative and qualitative damage to post harvested products (Burkholder and Faustini 1991). In this study, the effect of 20-hydroxyecdysone, a molecule belonging to the family of phytoecdysteroids, was tested on the development, and alpha-amylase and detoxification enzyme activities of the stored products’ insect pest, T. castaneum. We chose 20-hydroxyecdysone in preference to any other molecule for this study because it is the most common and the main phytoecdysteroid contained in plants and also the major biologically active ecdysteroid of insects (Dinan 2001).
Materials and Methods
Insect Rearing
Tribolium castaneum was raised in the laboratory under standard conditions of temperature of 26 ± 2°C, relative humidity of 75 ± 5% and subjected to a diet consisting of wheat flour with yeast in a proportion of 5%. Five larval instars were displayed by T. castaneum under these conditions. Larvae of the fourth stage are left to fast for 20 h before submitting them to experimentation in order to induce them to take food.
Post-Embryonic Development
20-Hydroxyecdysone (95% of purity) was dissolved in 5% methanol in distilled water. The phytoecdysteroid tested was mixed with wheat flour at concentration of 300, 600, 900, and 1,200 ppm. For control lot, methanol alone in distilled water was added to diet. The solvent was evaporated from the diet at 37°C in an oven for 48 h. Petri dishes were prepared in which starved larvae were put (with no food) to ensure that the effect was due to 20-hydroxyecdysone ingestion and not to a deterrence effect. Mortality, pupation, and adult emergence rate were observed every 2 d over 26 d. Mortality was identified by brown coloration with no noticeable movements. Note that insects of T. castaneum were maintained under standard conditions throughout the monitoring period. Ten larvae were added to each petri dish for all the experiments and five replicates (five petri dishes) were performed for treated, starved, and control larvae.
Biochemical Parameters
The biochemical study of some parameters such as the rate of proteins, and alpha-amylase and detoxification enzyme activities was established in relation to the toxic effects resulting from the ingestion of the 20-hydroxyecdysone.
Ten replicates were performed for treated, starved, and control larvae. For the biochemical parameters, larvae were treated for 7 d before starting the analysis.
Soluble Protein Rate
For protein measurements, three larvae were homogenized in 1 ml of Tris-HCl buffer (50 mM, pH 7) and soluble protein content was determined according to the method of (Bradford 1976).
Alpha-Amylase Activity
Measurement of alpha-amylase activity was performed according to (Valencia et al. 2000) with minor amendments. Three larvae were homogenized in acetate buffer (50 mM) with NaCl (10 M) and CaCl2 (20 M) at pH 5. Three larvae were incubated at 37°C for 15 min. Then, the iodine reagent was added, and the mixture centrifuged at 4,000 × g for 10 min. The alpha-amylase activity was expressed by the quantity of starch consumed per unit of time by larvae (the absorbance was read at 580 nm).
Detoxification Enzymes
Esterase activity was determined using 1-NA (1-naphthyl acetate). Three larvae were added to mixture (pH 6.5) and incubated 1 h at 30°C. The absorbance was read at 600 nm. Specific activity was calculated by using a standard curve of 1-naphthol according to (Gomori 1953).
Glutathione S-transferase (GST) activity was measured using 1 mM CDNB (1-chloro- 2,4-dinitrobenzene), 5 mM reduced glutathione, and 0.1 M Tris buffer, pH 7. The increment in absorbance at 340 nm was recorded during 5 min at 30°C to determine the nanomoles of substrate conjugated per minute per milligram of protein, using a molar extinction coefficient of 9.6/mM /cm (Habig et al. 1974).
P450 monooxygenases activity was measured according to Masters et al. (1965). Mixture of NADPH generating system at pH 7 and cytochrome c solution was incubated 1 h at 30°C. The reaction was stopped by methanol, then centrifuged at 12,000 rpm for 5 min. The absorbance was read at 550 nm to determine the nanomoles of cytochrome c reduced per minute per milligram of protein, using a molar extinction coefficient of 27.6/mM/cm.
Statistical Analysis
The results obtained were analyzed by the analysis of variance (ANOVA) using Statistica (1997) software to determine the statistical significance in the effect of 20-hydroxyecdysone on biological and physiological parameters of T. castaneum. Honest Significant Difference (HSD) testing was carried out using the Tukey test with untreated group as the control. A significant level of 0.05 was used for all statistical tests.
Results
Effects of 20-Hydroxyecdysone on Development Parameters
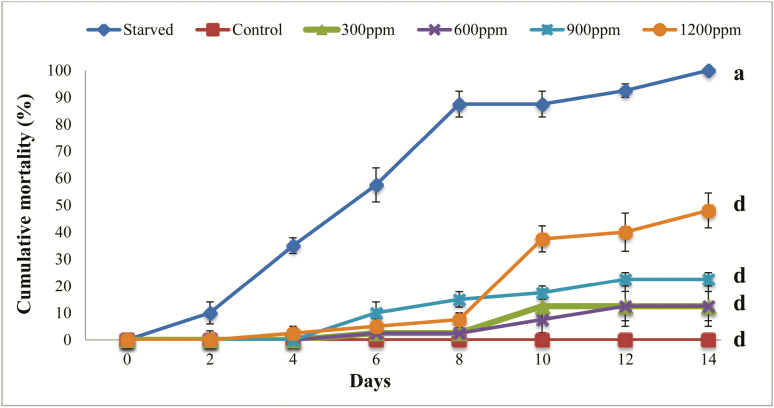
Ingestion of 20-hydroxyecdysone by the larvae of T. castaneum induced mortality from the 4th day of treatment for the highest dose (1,200 ppm) and increased to achieve its highest rate by the 14th day (Fig. 1). The percentage of inhibition compared to the control was (42%). For the other doses, mortality began from the sixth day and achieved its maximum either by the day 10 (for 300 ppm) or 12 (for 600 ppm and 900 ppm), whereas the mortality of starved larvae reached it maximum by day 14. It is noted that control larvae did not show mortality throughout the monitoring period. Statistical analysis showed that larval mortality effect was very highly significant for all treatment and for starved larvae as compared to the control (F = 51.2–90.1; df = 5–18; P < 0.001).
Fig. 1.
Effect of 20-hydroxyecdysone on mortality of Tribolium castaneum larvae. Each point represents the mean ± SE of five replicates. Means in the same column followed by different letters indicate that the difference between controls and treated or starved larvae is statistically significant as determined by the Tukey’s HSD test. Between the letters ‘a’ and ‘b’, the effect is significant (P < 0.05). Between the letters ‘a–c’, the effect is very significant (P < 0.01). Between the letters ‘a–d’, the effect is very highly significant (P < 0.001).
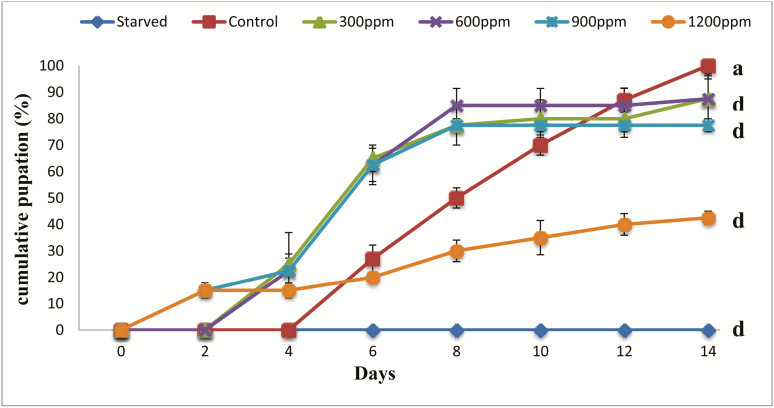
Results of pupation for control, treated, and starved larvae are grouped together in Fig. 2, Pupation began on the sixth day in control larvae and then increased rapidly throughout the monitoring period to reach its maximum value on day 14. In larvae treated with 20-hydroxyecdysone, precocious pupation appears 4 d earlier at 1,200 ppm and 900 ppm and 2 d earlier at 600 and 300 ppm as compared to control larvae. For starved larvae, we did not record any pupation throughout the monitoring period. Statistical analysis show that the treatment as well as starvation had a very highly significant effect on pupation (F = 8.9–25.6; df = 5–18; P < 0.001).
Fig. 2.
Effect of 20-hydroxyecdysone on pupation of Tribolium castaneum. Each point represents the mean ± SE of five replicates. Means in the same column followed by different letters indicate that the difference between controls and treated or starved larvae is statistically significant as determined by the Tukey’s HSD test. Between the letters ‘a’ and ‘b’, the effect is significant (P < 0.05). Between the letters ‘a–c’, the effect is very significant (P < 0.01). Between the letters ‘a–d’, the effect is very highly significant (P < 0.001).
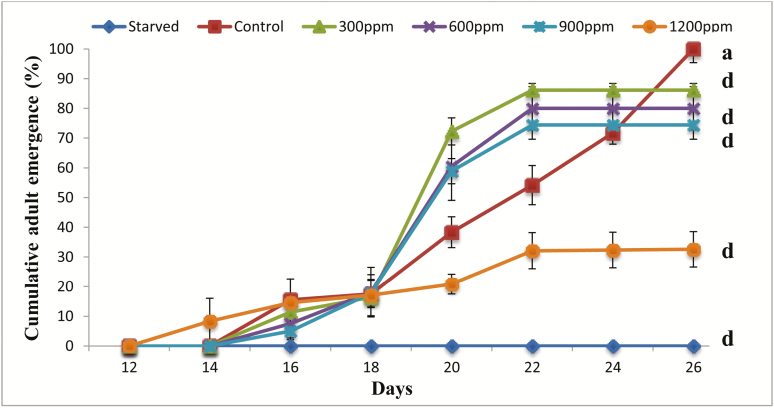
The emergence rate of adults is calculated from the number of pupae formed. Our results showed that this percentage was significantly affected by the presence of the 20-hydroxyecdysone in larval diet (Fig. 3). The adult emergence started from the 16th day for the control larvae and reached its highest rate on day 26. Similarly, for treated larvae with doses of 300, 600, and 900 ppm, it started on day 16 as well. However, for larvae treated with the dose of 1,200 ppm, the adult emergence began 2 d earlier. It is noted that the adult emergence rate increased throughout the monitoring period in larvae treated with all doses. Statistical analysis showed that larval mortality effect was very highly significant for all treatment and for starved larvae as compared with control (F = 19.8–77.4; df = 5–18; P < 0.001).
Fig. 3.
Effect of 20-hydroxyecdysone on adult emergence of Tribolium castaneum. Each point represents the mean ± SE of five replicates. Means in the same column followed by different letters indicate that the difference between controls and treated or starved larvae is statistically significant as determined by the Tukey’s HSD test. Between the letters ‘a’ and ‘b’, the effect is significant (P < 0.05). Between the letters ‘a–c’, the effect is very significant (P < 0.01). Between the letters ‘a–d’, the effect is very highly significant (P < 0.001).
Effects of 20-Hydroxyecdysone on Soluble Protein Rate
Protein content in control larvae decreases very significantly (P < 0.001) for starved and all treated larvae in a dose-dependent manner (Table 1). Protein content in starved larvae of T. castaneum is also significantly reduced (P < 0.001).
Table 1.
Effect of 20-hydroxyecdysone on protein and alpha-amylase activity of Tribolium castaneum larvae after 7 d of treatment
| Treatment | Protein (µg/larvae) |
α-Amylase (µg of starch consumed/larva) |
|---|---|---|
| Control | 233.15 ± 24a | 174.63 ± 1.7a |
| 300 ppm | 151.32 ± 15b | 161.25 ± 2.06b |
| 600 ppm | 149.13 ± 5b | 150.53 ± 2.5b |
| 900 ppm | 110.07 ± 20c | 145.15 ± 2.2c |
| 1,200 ppm | 95.7 ± 16c | 134.95 ± 1.9c |
| Starved | 48.34 ± 7.7d | 132.38 ± 2.6c |
Means in the same column followed by different letters indicate that the difference between controls and treated or starved larvae is statistically significant as determined by the Tukey’s HSD test. Between the letters ‘a’ and ‘b’, the effect is significant (P < 0.05). Between the letters ‘a–c’, the effect is very significant (P < 0.01). Between the letters ‘a–d’, the effect is very highly significant (P < 0.001).
Effects of 20-Hydroxyecdysone on Alpha-Amylase Activity
The results of the assay for alpha-amylase activity in control larvae, larvae treated with different doses of 20-hydroxyecdysone, and starved larvae are shown in (Table 1). The amount of starch consumed in the larvae treated with 20-hydroxyecdysone decreased, as the dose of the treatment increased, in a very high significant way (P < 0.001).
Effects of 20-Hydroxyecdysone on Detoxification Enzymes
The results of 20-hydroxyecdysone effect on detoxification enzymes of T. castaneum are regrouped in (Table 2). In treated larvae, esterase activity using 1-Na as substrate increased at low concentration (300 ppm) but decreased when using higher concentration (1,200 ppm) of 20-hydroxyecdysone.
Table 2.
Effect of 20-hydroxyecdysone on detoxification enzyme activities of Tribolium castaneum larvae after 7 d of treatment
| Specific activitya | |||
|---|---|---|---|
| EST (1-NA) | GST (CDNB) | P450 (cytochrome c) | |
| Control | 0.31 ± 0.04a | 10.2 ± 5.1a | 0.72 ± 0.12a |
| 300 ppm | 0.5 ± 0.05b | 43.1 ± 7.3d | 1.18 ± 0.13d |
| 600 ppm | 0.42 ± 0.02b | 74.2 ± 5.4d | 0.77 ± 0.1a |
| 900 ppm | 0.39 ± 0.07b | 228.9 ± 6.8d | 0.64 ± 0.12a |
| 1,200 ppm | 0.29 ± 0.05c | 337.4 ± 4.6d | 0.33 ± 0.06c |
| Starved | 0.37 ± 0.04a | 53.15 ± 7.2b | 0.49 ± 0.09b |
Means in the same column followed by different letters indicate that the difference between controls and treated or starved larvae is statistically significant as determined by the Tukey’s HSD test. Between the letters ‘a’ and ‘b’, the effect is significant (P < 0.05). Between the letters ‘a–c’, the effect is very significant (P < 0.01). Between the letters ‘a–d’, the effect is very highly significant (P < 0.001).
aSpecific activity: EST (1-NA): mmol of β-naphtol formed per minute per milligrams of proteins; GST (CDNB): nanomoles of substrate conjugated per minute per milligram of protein; P450 (cytochrome c): nanomoles of cytochrome c reduced per minute per milligram of protein.
GST using CDNB as substrate increased in treated larvae in a dose-dependent manner. Statistical analysis shows that 20-hydroxyecdysone had a very highly significant effect (P < 0.001) on GST activity for all doses.
P450 monooxygenases activity increased only at 300 ppm; this activity was inhibited significantly when larvae of T. castaneum were exposed to 1,200 ppm of 20-hydroxyecdysone. Starved larvae showed also a significant inhibition of this activity.
Discussion
In this study, it was found that the 20-hydroxyecdysone possesses larvicidal activity against T. castaneum. Obtained results demonstrated that when the phytoecdysteroid is added to the food of larvae, it causes negative effects that occur through significant larval mortality, and through inhibition of development. Similar effects were observed after ecdysone analogs had been ingested in Tribolium confusum (du Val, 1861) (Coleoptera: Tenebrionidae) larvae diet, they inhibited growth and development of this insect pest (Robbins et al. 1970). A developmental anomalies during metamorphosis were also shown after certain phytoecdysteroids had been incorporated into an artificial diet of larvae of the leek-moth Acrolepiopsis assectella (Zeller, 1839) (Lepidoptera: Hyponomeutoïdea) (Arnault and Sláma 1986). Rharrabe et al. (2010) found that ingested phytoecdysteroids increased mortality and provoked a disruption of development in P. interpunctella (Hübner) as well.
The obtained results in this study revealed that the mortality increased significantly after ingestion of 20-hydroxyecdysone in a dose-dependent manner, and the larval mortality was registered due to toxic effect of this phytoecdysteroid. Precocious pupation and adult emergence were registered, with a reduction of their percentages compared to controls. Similar effects were observed when phytoecdysteroids ingested to the diet of Leptinotarsa decemlineata (Say, 1824) (Coleoptera: Chrysomelidae) larvae (Zolotar’ et al. 2001), P. interpunctella (Hübner) (Rharrabe et al. 2009). Macedo et al. (2011) reported that the incorporation of Talisine from Talisia esculenta (A. St. Hil) Radlk (Sapindales: Sapindaceae) seeds into an artificial diet fed to Anticarsia gemmatalis (Hübner, 1818) (Lepidoptera: Noctuidae) affects larval growth, pupal weight, development and mortality, adult fertility, and longevity and produces malformations in pupae and adult insects. Boukouvala et al. (2016) demonstrated that the exposure to pyrrole derivatives causes insecticidal effect against T. confusum.
To fully clarify the effects of 20-hydroxyecdysone on T. castaneum, the results of ingested phytoecdysteroid on biochemical parameters were analyzed. These results revealed a significant drop in protein rate in larvae of T. castaneum. This drop could be due to a reduction of protein synthesis mainly in the fat body following the deterioration of the internal tissues of insects, or to a strong mobilization of proteins as a response to the nutritional stress caused by the exposure of the larvae to the phytoecdysteroid applied. Similar effects were observed after exposure of P. interpunctella larvae to 20-hydroxyecdysone, and it induces a decrease in protein content (Rharrabe et al. 2009). Such disruption was recorded in B. mori after ingesting phytoecdysteroids from Radix achyranthes (Blume) (Caryophyllales: Amaranthaceae) (Miao et al. 2004). Similar effects were also observed after the azadirachtin ingestion in Schistocerca gregaria (Forsskål, 1775) (Orthoptera: Acrididae) (Rao and Subrahmanyam 1986) and P. interpunctella (Rharrabe et al. 2008). Concerning digestive enzyme, the results clearly showed that the ingestion of 20-hydroxyecdysone causes an inhibition of alpha-amylase activity in a dose-dependent manner. This effect can be due to a direct inhibition of the activity of the enzymes themselves or indirectly to a perturbation of some neuropeptides (as Sulfakinin) that involved in alpha-amylase signaling and regulation (Zels et al. 2014). This indirect inhibition can also be due to a cytotoxic effect of 20-hydroxyecdysone on the epithelial cells synthesizing the digestive enzyme key, alpha-amylase. Similar studies had previously shown that some plant-based molecules inhibit the activity of alpha-amylase in T. castaneum (Jbilou et al. 2008), P. interpunctella (Bouayad et al. 2012), and Spodoptera eridania (Cramer, 1782) (Lepidoptera: Noctuidae) (Shannag et al. 2015). In fact, there are at least three alpha-amylases found in T. castaneum (Channale et al. 2016).
Insect pests can overcome the negative effects of plant defensive compounds by employing the action of detoxification enzymes (Robinson et al. 1987, Blackford et al. 1996, Rharrabe et al. 2007). Regarding the obtained results concerning detoxification enzymes, an inhibition of esterase and P450 monooxygenase activities with high doses of treatments was registered. This inhibition could be due to the severe toxicity of 20-hydroxyecdysone. Bilal et al. (2018) reported that activities of esterase decreased in Oxycarenus hyalinipennis (Costa, 1843) (Heteroptera: Lygaeidae) after treatment with bifenthrin. Moreover, the results also showed that GST activity increased significantly in a dose–response manner with all treatments in response to the ingestion of the phytoecdysteroid tested. This enzyme is mobilized for detoxification of the exogenous substance. Caballero et al. (2008) reported that the ingestion of terpenoids in the beet armyworm, Spodoptera exigua (Hübner, 1808) (Lepidoptera: Noctuidae) increased GST activity. Such results were found when plants extracts inducted GST activity in P. interpunctella (Bouayad et al. 2013), Spodoptera litura (Xu et al. 2015), and Bradysia odoriphaga (Yang & Zhang) (Diptera: Sciaridae) (Zhu et al. 2017). Tribolium castaneum is resistant to xenobiotic owing a potential detoxification genes suggesting major shifts in insecticide processing and immunity pathways (Oppert et al. 2018). Thus, the understanding of gene expression profiles in the insect gut can provide insight into a better understanding of the mechanisms involved in the regulation of genes coding for proteins involved in detoxification of xenobiotic (Shi et al. 2012, Kalsi and Palli 2017). Studies reported that the expression of gene responsible for the detoxification in T. castaneum was mediated after exposure to diflubenzuron (Merzendorfer et al. 2012) and deltamethrin (Kalsi and Palli 2015). Similar results were found in B. mori when detoxifying enzymes-related genes’ expression was upregulated compared to the control after exposure to phoxim (Cheng et al. 2018).
All the results obtained in this work indicate that 20-hydroxyecdysone when ingested produced a disruption on larval growth, development, and an alteration of physiological parameters of T. castaneum. Therefore, new perspectives can be envisaged by starting a study of the insecticidal activity of the 20-hydroxyecdysone on T. castaneum, testifying the bioinsecticidal potential of phytoecdysteroids in control of insect pests of stored products, such as T. castaneum that invade homes, stores, and warehouses.
References Cited
- Arnault C., and Sláma K.. . 1986. Dietary effects of phytoecdysones in the leek-moth, Acrolepiopsis assectella Zell. (Lepidoptera: Acrolepiidae). J. Chem. Ecol. 12: 1979–1986. [DOI] [PubMed] [Google Scholar]
- Bilal M., Freed S., Ashraf M. Z., and Rehan A.. . 2018. Resistance and detoxification enzyme activities to bifenthrin in Oxycarenus hyalinipennis (Hemiptera: Lygaeidae). Crop Protect. 111: 17–22. [Google Scholar]
- Blackford M. J., and Dinan L.. . 1997. The effects of ingested 20-hydroxyecdysone on the larvae of Aglais urticae, Inachis io, Cynthia cardui (Lepidoptera: Nymphalidae) and Tyria jacobaeae (Lepidoptera: Arctiidae). J. Insect Physiol. 43: 315–327. [DOI] [PubMed] [Google Scholar]
- Blackford M., Clarke B., and Dinan L.. . 1996. Tolerance of the Egyptian cotton leafworm Spodoptera littoralis (Lepidoptera: Noctuidae) to ingested phytoecdysteroids. J. Insect Physiol. 42: 931–936. [Google Scholar]
- Bouayad N., Rharrabe K., Lamhamdi M., Nourouti N. G., and Sayah F.. . 2012. Dietary effects of harmine, a β-carboline alkaloid, on development, energy reserves and α-amylase activity of Plodia interpunctella Hübner (Lepidoptera: Pyralidae). Saudi J. Biol. Sci. 19: 73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouayad N., Rharrabe K., Ghailani N. N., Jbilou R., Castañera P., and Ortego F.. . 2013. Insecticidal effects of Moroccan plant extracts on development, energy reserves and enzymatic activities of Plodia interpunctella. Span. J. Agric. Res. 11: 189. [Google Scholar]
- Boukouvala M. C., Kavallieratos N. G., Athanassiou C. G., and Hadjiarapoglou L. P.. . 2016. Insecticidal effect of two novel pyrrole derivatives against two major stored product insect species. Crop Protect. 84: 1–7. [Google Scholar]
- Bradford M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72: 248–254. [DOI] [PubMed] [Google Scholar]
- Burkholder W. E., and Faustini D. L.. 1991. Biological methods of survey and control, pp. 361–372. InJ. R. Gorham (ed.), Ecology and Management of Food Industry Pests. Food and Drug Administration Technical Bulletin, AOAC Press. [Google Scholar]
- Caballero C., López-Olguín J., Ruiz M., Ortego F., and Castañera P.. . 2008. Antifeedant activity and effects of terpenoids on detoxication enzymes of the beet armyworm, Spodoptera exigua (Hübner). Span. J. Agric. Res. 6: 177. [Google Scholar]
- Chaitanya R. K., Sridevi P., Senthilkumaran B., and Gupta A. D.. . 2011. 20-Hydroxyecdysone regulation of H-fibroin gene in the stored grain pest Corcyra cephalonica, during the last instar larval development. Steroids 76: 125–134. [DOI] [PubMed] [Google Scholar]
- Channale S. M., Bhide A. J., Yadav Y., Kashyap G., Pawar P. K., Maheshwari V. L., Ramasamy S., and Giri A. P.. . 2016. Characterization of two coleopteran α-amylases and molecular insights into their differential inhibition by synthetic α-amylase inhibitor, acarbose. Insect Biochem. Mol. Biol. 74: 1–11. [DOI] [PubMed] [Google Scholar]
- Cheng X., Hu J., Li J., Chen J., Wang H., Mao T., Xue B., and Li B.. . 2018. The silk gland damage and the transcriptional response to detoxifying enzymes-related genes of Bombyx mori under phoxim exposure. Chemosphere 209: 964–971. [DOI] [PubMed] [Google Scholar]
- Coupe M. D., and Cahill J. F.. . 2003. Effects of insects on primary production in temperate herbaceous communities: a meta-analysis. Ecol. Entomol. 28: 511–521. [Google Scholar]
- Dicke M., and Loon J. J.. . 2000. Multitrophic effects of herbivore-induced plant volatiles in an evolutionary context. Entomol. Experiment. Appl. 97: 237–249. [Google Scholar]
- Dinan L. 2001. Phytoecdysteroids: biological aspects. Phytochemistry 57: 325–339. [DOI] [PubMed] [Google Scholar]
- Fraenkel G. S. 1959. The raison d’être of secondary plant substances. Science 129: 1466–1470. [DOI] [PubMed] [Google Scholar]
- Gelman D. B., Demilo A. B., Thyagaraja B. S., Kelly T. J., Masler E. P., Bell R. A., and Borkovec A. B.. . 1991. 3-Oxoecdysteroid 3b-reductase in various organs of the European corn borer, Ostrinia nubilalis (Hubner). Arch Insect Biochem. Physiol. 17: 93–106. [Google Scholar]
- Gomori G. 1953. Human esterases. J. Lab. Clin. Med. 42: 445–453. [PubMed] [Google Scholar]
- Habig W. H., Pabst M. J., and Jakoby W. B.. . 1974. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 249: 7130–7139. [PubMed] [Google Scholar]
- Jbilou R., Amri H., Bouayad N., Ghailani N., Ennabili A., and Sayah F.. . 2008. Insecticidal effects of extracts of seven plant species on larval development, alpha-amylase activity and offspring production of Tribolium castaneum (Herbst) (Insecta: Coleoptera: Tenebrionidae). Bioresour. Technol. 99: 959–964. [DOI] [PubMed] [Google Scholar]
- Kalsi M., and Palli S. R.. . 2015. Transcription factors, CncC and Maf, regulate expression of CYP6BQ genes responsible for deltamethrin resistance in Tribolium castaneum. Insect Biochem. Mol. Biol. 65: 47–56. [DOI] [PubMed] [Google Scholar]
- Kalsi M., and Palli S. R.. . 2017. Cap n collar transcription factor regulates multiple genes coding for proteins involved in insecticide detoxification in the red flour beetle, Tribolium castaneum. Insect Biochem. Mol. Biol. 90: 43–52. [DOI] [PubMed] [Google Scholar]
- Kubo I., Komatsu S., Asaka Y., and de Boer G.. . 1987. Isolation and identification of apolar metabolites of ingested 20-hydroxyecdysone in frass of Heliothis virescens larvae. J. Chem. Ecol. 13: 785–794. [DOI] [PubMed] [Google Scholar]
- Macedo M. L. R., Freire M. das G. M., Kubo C. E. G., and Parra J. R. P.. . 2011. Bioinsecticidal activity of Talisia esculenta reserve protein on growth and serine digestive enzymes during larval development of Anticarsia gemmatalis. Comp. Biochem. Physiol. C: Toxicol. Pharmacol. 153: 24–33. [DOI] [PubMed] [Google Scholar]
- Masters B. S. S., Kamin H., Gibson Q. H., and Williams C. H.. . 1965. Studies on the mechanism of microsomal triphosphopyridine nucleotide-cytochrome c reductase. J. Biol. Chem. 240: 921–931. [PubMed] [Google Scholar]
- Merzendorfer H., Kim H. S., Chaudhari S. S., Kumari M., Specht C. A., Butcher S., Brown S. J., Manak J. R., Beeman R. W., Kramer K. J., . et al. 2012. Genomic and proteomic studies on the effects of the insect growth regulator diflubenzuron in the model beetle species Tribolium castaneum. Insect Biochem. Mol. Biol. 42: 264–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao Y.-G., Shi L.-G., and Nair K. S.. . 2004. Ecdysteroid as a mediator in the regulation of silk protein synthesis and its influence on silkworm (Bom., Lepidoptera) genome. J. Appl. Entomol. 128: 348–353. [Google Scholar]
- Oppert B., Perkin L., Martynov A. G., and Elpidina E. N.. . 2018. Cross-species comparison of the gut: differential gene expression sheds light on biological differences in closely related tenebrionids. J. Insect Physiol. 106: 114–124. [DOI] [PubMed] [Google Scholar]
- Rao P. J., and Subrahmanyam B.. . 1986. Azadirachtin induced changes in development, food utilization and haemolymph constituents of Schistocerca gregaria Forskal. J. Appl. Entomol. 102: 217–224. [Google Scholar]
- Rharrabe K., Alla S., Maria A., Sayah F., and Lafont R.. . 2007. Diversity of detoxification pathways of ingested ecdysteroids among phytophagous insects. Arch. Insect Biochem. Physiol. 65: 65–73. [DOI] [PubMed] [Google Scholar]
- Rharrabe K., Amri H., Bouayad N., and Sayah F.. . 2008. Effects of azadirachtin on post-embryonic development, energy reserves and α-amylase activity of Plodia interpunctella Hübner (Lepidoptera: Pyralidae). J. Stored Product. Res. 44: 290–294. [Google Scholar]
- Rharrabe K., Bouayad N., and Sayah F.. . 2009. Effects of ingested 20-hydroxyecdysone on development and midgut epithelial cells of Plodia interpunctella (Lepidoptera, Pyralidae). Pestic. Biochem. Physiol. 93: 112–119. [Google Scholar]
- Rharrabe K., Sayan F., and Lafont R.. . 2010. Dietary effects of four phytoecdysteroids on growth and development of the Indian meal moth, Plodia interpunctella. J. Insect Sci. 10: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins W. E., Kaplanis J. N., Thompson M. J., Shortino T. J., and Joyner S. C.. . 1970. Ecdysones and synthetic analogs: molting hormone activity and inhibitive effects on insect growth, metamorphosis and reproduction. Steroids 16: 105–125. [DOI] [PubMed] [Google Scholar]
- Robinson P. D., Morgan E. D., Wilson I. D., and Lafont R.. . 1987. The metabolism of ingested and injected [3H] ecdysone by final instar larvae of Heliothis armigera. Physiol. Entomol. 12: 321–330. [Google Scholar]
- Schmelz E. A., Grebenok R. J., Ohnmeiss T. E., and Bowers W. S.. . 2002. Interactions between Spinacia oleracea and Bradysia impatiens: a role for phytoecdysteroids. Arch. Insect Biochem. Physiol. 51: 204–221. [DOI] [PubMed] [Google Scholar]
- Schoonhoven L. M., van Loon J. J. A., and Dicke M.. . 2005. Insect-plant biology. 2nd ed. ed. Oxford University Press, Oxford, NY. [Google Scholar]
- Shannag H. K., Capinera J. L., and Freihat N. M.. . 2015. Effects of neem-based insecticides on consumption and utilization of food in larvae of Spodoptera eridania (Lepidoptera: Noctuidae). J. Insect Sci. 15: 152. [Google Scholar]
- Shi H., Pei L., Gu S., Zhu S., Wang Y., Zhang Y., and Li B.. . 2012. Glutathione S-transferase (GST) genes in the red flour beetle, Tribolium castaneum, and comparative analysis with five additional insects. Genomics 100: 327–335. [DOI] [PubMed] [Google Scholar]
- Stamp N. E. 1996. Developing a theory of plant-insect herbivore interactions: are we there yet? Bull. Ecol. Soc. Am. 77: 51–61. [Google Scholar]
- Statistica. 1997. Statistica statsoft Inc. release 5.1., Tulsa, OK. [Google Scholar]
- Talbot N. J. 2005. Emerging themes in plant–pathogen interactions. InBlackwell (ed.), Plant–pathogen interactions, Vol. 11. Annual Plant Reviews book series, Oxford, UK. [Google Scholar]
- Tanaka Y., Asaoka K., and Takeda S.. . 1994. Different feeding and gustatory responses to ecdysone and 20-hydroxyecdysone by larvae of the silkworm, Bombyx mori. J. Chem. Ecol. 20: 125–133. [DOI] [PubMed] [Google Scholar]
- Valencia A., Bustillo A. E., Ossa G. E., and Chrispeels M. J.. . 2000. Alpha-amylases of the coffee berry borer (Hypothenemus hampei) and their inhibition by two plant amylase inhibitors. Insect Biochem. Mol. Biol. 30: 207–213. [DOI] [PubMed] [Google Scholar]
- Xu Z. B., Zou X. P., Zhang N., Feng Q. L., and Zheng S. C.. . 2015. Detoxification of insecticides, allechemicals and heavy metals by glutathione S-transferase SlGSTE1 in the gut of Spodoptera litura: SlGSTE1 detoxification in Spodoptera litura. Insect Sci. 22: 503–511. [DOI] [PubMed] [Google Scholar]
- Zels S., Verlinden H., Dillen S., Vleugels R., Nachman R. J., and Vanden Broeck J.. . 2014. Signaling properties and pharmacological analysis of two sulfakinin receptors from the red flour beetle, Tribolium castaneum. PLoS ONE 9: e94502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu G., Luo Y., Xue M., Zhao H., Sun X., and Wang X.. . 2017. Effects of feeding on different host plants and diets on Bradysia Odoriphaga population parameters and tolerance to heat and insecticides. J. Econ. Entomol. 110: 2371–2380. [DOI] [PubMed] [Google Scholar]
- Zolotar’ R. M., Bykhovets A. I., and Kovganko N. V.. . 2001. Effect of certain phytoecdysteroids on larvae of Colorado beetle Leptinotarsa decemlineata. Chem. Nat. Compound. 37: 537–539. [Google Scholar]