Abstract
Purpose
Until recently, limited options existed for patients with advanced melanoma who experienced disease progression while receiving treatment with ipilimumab. Here, we report the coprimary overall survival (OS) end point of CheckMate 037, which has previously shown that nivolumab resulted in more patients achieving an objective response compared with chemotherapy regimens in ipilimumab-refractory patients with advanced melanoma.
Patients and Methods
Patients were stratified by programmed death-ligand 1 expression, BRAF status, and best prior cytotoxic T-lymphocyte antigen-4 therapy response, then randomly assigned 2:1 to nivolumab 3 mg/kg intravenously every 2 weeks or investigator’s choice chemotherapy (ICC; dacarbazine 1,000 mg/m2 every 3 weeks or carboplatin area under the curve 6 plus paclitaxel 175 mg/m2 every 3 weeks). Patients were treated until they experienced progression or unacceptable toxicity, with follow-up of approximately 2 years.
Results
Two hundred seventy-two patients were randomly assigned to nivolumab (99% treated) and 133 to ICC (77% treated). More nivolumab-treated patients had brain metastases (20% v 14%) and increased lactate dehydrogenase levels (52% v 38%) at baseline; 41% of patients treated with ICC versus 11% of patients treated with nivolumab received anti–programmed death 1 agents after randomly assigned therapy. Median OS was 16 months for nivolumab versus 14 months for ICC (hazard ratio, 0.95; 95.54% CI, 0.73 to 1.24); median progression-free survival was 3.1 months versus 3.7 months, respectively (hazard ratio, 1.0; 95.1% CI, 0.78 to 1.436). Overall response rate (27% v 10%) and median duration of response (32 months v 13 months) were notably higher for nivolumab versus ICC. Fewer grade 3 and 4 treatment-related adverse events were observed in patients on nivolumab (14% v 34%).
Conclusion
Nivolumab demonstrated higher, more durable responses but no difference in survival compared with ICC. OS should be interpreted with caution as it was likely impacted by an increased dropout rate before treatment, which led to crossover therapy in the ICC group, and by an increased proportion of patients in the nivolumab group with poor prognostic factors.
INTRODUCTION
There have been major advances in the treatment of advanced melanoma, with the development of agents that have changed clinical practice.1,2 Ipilimumab, an antibody to cytotoxic T-lymphocyte-associated-antigen-4 (CTLA-4), was the first therapy to demonstrate a survival improvement in metastatic melanoma in a phase III randomized clinical trial3,4; however, more than one half of patients do not derive benefit from ipilimumab.5 The combination of mitogen-activated protein kinase pathway inhibitors, including vemurafenib and cobimetinib, or dabrafenib and trametinib, is associated with a high response rate and increased survival compared with chemotherapy6,7; however, the use of BRAF inhibitors is restricted to approximately 50% of patients with melanoma who harbor BRAFV600 mutations, and most patients develop resistance to these inhibitors.6,8 Treatment options are needed when disease progression occurs with ipilimumab and BRAF inhibitor–based therapy. CheckMate 037 investigated treatments in patients with advanced melanoma who experienced progression on ipilimumab and a BRAF inhibitor (if BRAF mutated). At trial initiation, ipilimumab and vemurafenib were the only approved agents for the treatment of advanced melanoma that had demonstrated prolongation of overall survival (OS) in phase III registration studies,3,9 and no single chemotherapeutic agents were considered a standard of care for second-line therapy.
Nivolumab, a human IgG4 monoclonal antibody, inhibits the programmed death 1 (PD-1) immune checkpoint protein.10 Nivolumab and another PD-1 inhibitor, pembrolizumab, have shown increased efficacy compared with ipilimumab in metastatic melanoma11,12 and have now been approved for treatment. Combination nivolumab plus ipilimumab is also approved for metastatic melanoma and has demonstrated an unprecedented 2-year OS rate of 63.8%.13
Here, we report updated results of the phase III, randomized, open-label study, CheckMate 037, which previously demonstrated that nivolumab resulted in more patients achieving an objective response compared with chemotherapy in patients with metastatic melanoma who experienced progression after treatment with ipilimumab (plus a BRAF inhibitor, if BRAF-mutation positive).14 The coprimary end point of OS is presented here, as well as updated results for objective response rate (ORR), progression-free survival (PFS), and safety.
PATIENTS AND METHODS
Eligibility Criteria
Patients were at least age 18 years with histologically confirmed, unresectable stage IIIC or IV metastatic melanoma and Eastern Cooperative Oncology Group performance status 0 or 1.14 Patients with BRAF wild-type metastatic melanoma must have experienced progression after treatment with anti–CTLA-4, and patients with BRAFV600 mutation must have experienced progression after treatment with anti–CTLA-4 and a BRAF inhibitor. Key exclusion criteria included active brain metastases; prior treatment with anti–PD-1, anti–programmed death ligand 1 (PD-L1), or anti–PD-L2; grade 4 toxicity or use of infliximab during previous ipilimumab treatment; and primary ocular melanoma.14
Study Design and Treatment
The study design and treatments have been previously described.14 In this randomized, controlled, open-label phase III trial, patients were randomly assigned 2:1 to nivolumab 3 mg/kg intravenously every 2 weeks or investigator’s choice chemotherapy (ICC), which consisted of dacarbazine 1,000 mg/m2 every 3 weeks or carboplatin area under the curve 6 plus paclitaxel 175 mg/m2 every 3 weeks. This was an open-label design study because the different toxicity profiles of the comparators made the study infeasible to blind. Patients were stratified by PD-L1 expression, BRAF status, and best response to prior CTLA-4 therapy, and were treated until progression or unacceptable toxicity. Patients who experienced clinical benefit and who tolerated nivolumab were allowed to continue beyond progression per investigator.
Coprimary end points were the proportion of patients who achieved an objective response per independent radiologic review committee and OS comparison of nivolumab versus ICC. Secondary end points included PFS comparison per independent radiologic review committee assessment, evaluation of PD-L1 expression as a predictive biomarker for ORR and OS, and evaluation of health-related quality of life as assessed by European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire C30 (EORTC QLQ-C30). Exploratory objectives included assessment of overall safety and tolerability as well as changes in health status by the European Quality of Life-5 Dimensions (EuroQoL EQ-5D).
The study protocol was approved by institutional review boards of the participating centers and performed in accordance with the Declaration of Helsinki and the International Conference on Harmonisation Guidelines for Good Clinical Practice. Written patient consent was obtained before the start of the study and a data monitoring committee was established for oversight.
Efficacy and Safety Assessments
Tumor response and progression were assessed by using Response Evaluation Criteria in Solid Tumors (v1.1; RECIST).15 Radiographic assessments were performed at baseline and week 9 after random assignment, every 6 weeks for the first year, and then every 12 weeks until disease progression, death, or study withdrawal. End point definitions are available in the Data Supplement. PD-L1 expression was measured via PD-L1 immunohistochemistry assay (Dako, Burlingame, CA) as previously described.16
Deaths, adverse events (AEs), serious AEs, AEs that led to discontinuation, and select AEs—with time to onset and resolution—are summarized for all treated patients. AEs were graded according to National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0.
Health-related quality of life was assessed at baseline, every cycle (ICC), or every other cycle (nivolumab) for the first 6 months, then every 6 weeks and at follow-up and survival visits; assessments were EORTC QLQ-C30 version 317 and EuroQoL EQ-5D summary index and visual analog scale.18
Statistical Analysis
Efficacy end points were based on the intent-to-treat population. Approximately 390 patients were to be randomly assigned with an α allocation of 0.1% and OS with an α allocation of 4.9%. At least 260 deaths were required to provide 90% power to detect a hazard ratio (HR) of 0.65 with an overall two-sided type I error of 4.9%; 263 deaths occurred by the database lock. Final OS α boundary was 0.0446 (or adjusted 95.54% CI) for OS and 0.049 for PFS when OS was statistically significant. Time-to-event distribution—PFS, time to response, duration of response—was estimated by using the Kaplan-Meier technique. Median and 95% CIs were estimated on the basis of the Brookmeyer and Crowley methodology. Rates at fixed time points were derived from the Kaplan-Meier estimate along with their corresponding transformed 95% CIs. CIs for binomial proportions were derived by using the Clopper-Pearson method.
RESULTS
Patients and Treatment
From December 21, 2012, to January 10, 2014, 631 patients were enrolled from 90 sites in 14 countries; 405 patients were randomly assigned, with 268 treated in the nivolumab group and 102 in the ICC group (Fig 1). A higher proportion of patients who were randomly assigned to ICC did not receive treatment compared with patients who were randomly assigned to nivolumab (23% v 2%; Fig 1 and Data Supplement). Patient demographics have been reported14 and were generally balanced, with the exception that a larger proportion of patients on nivolumab versus ICC had brain metastases (20% v 14%) and increased lactate dehydrogenase levels (52% v 38%; Table 1).
Fig 1.
Trial design. ICC, investigator’s choice chemotherapy.
Table 1.
Baseline Characteristics of Patients
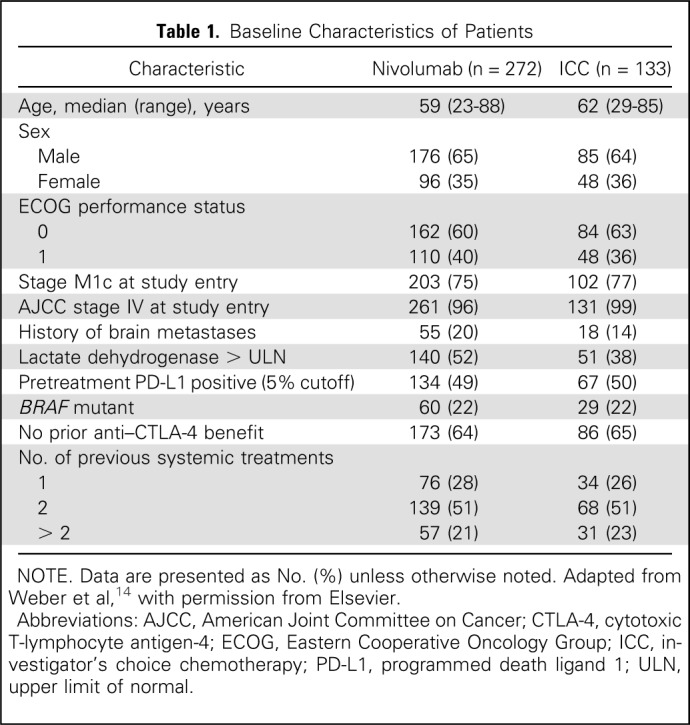
With a database lock of March 29, 2016, and follow-up of approximately 2 years, median duration of therapy was 4.7 months (95% CI, 3.3 to 6.0) for nivolumab and 2.0 months (95% CI, 1.6 to 2.8) for ICC. More patients received systemic therapy after randomly assigned therapy in the ICC arm (83 [62%] of 133) compared with the nivolumab arm (109 [40%] of 272; Data Supplement). Specifically, anti–PD-1 therapy was administered to 54 (41%) of 133 patients in the ICC group versus 29 (11%) of 272 patients in the nivolumab group, and ipilimumab to 14 (11%) and 13 (5%) patients in the ICC and nivolumab groups, respectively (Data Supplement). As case report forms for this study did not adequately capture all post–random assignment/poststudy therapy, the 41% of patients in the ICC arm who received anti–PD-1 therapy is likely underestimated.
Efficacy
In the randomly assigned population, median OS was 15.7 months (95% CI, 12.9 to 19.9) for the nivolumab group versus 14.4 months (95% CI, 11.7 to 18.2) for ICC (HR, 0.95; 95.54% CI, 0.73 to 1.24; Fig 2A). Survival rates at 1 year were 58.9% (95% CI, 52.8% to 64.5%) in the nivolumab group and 55.1% (95% CI, 46.1% to 63.3%) for ICC; 2-year rates were 38.7% (95% CI, 32.8% to 44.5%) and 33.9% (95% CI, 25.8% to 42.1%), respectively. Nivolumab had a higher rate of death compared with ICC in the first 3 months, which may be a result of group imbalance in poor prognostic factors. A multivariable analysis demonstrated that Eastern Cooperative Oncology Group performance status (HR, 0.64; 95% CI, 0.49 to 0.83; 0 v 1), brain metastases (HR, 0.61; 95% CI, 0.45 to 0.83; no v yes), and elevated lactate dehydrogenase (HR, 0.60; 95% CI, 0.46 to 0.78; ≤ upper limit of normal v > upper limit of normal) were all associated with shorter survival, and there were more patients in the nivolumab group for two of these three factors. No notable differences in OS were observed in prespecified subgroup analysis, although an HR of > 1.10 was observed for patients with BRAF mutation, those younger than 65 years, those with a history of brain metastases, and those with PD-L1 expression < 5% (Data Supplement).
Fig 2.
(A and B) Overall survival (OS) in all randomly assigned patients and OS censoring at the start of programmed death 1 (PD-1) or programmed death-ligand 1 (PD-L1) agent after assigned therapy in investigator’s choice chemotherapy (ICC). (A) Kaplan-Meier curves for OS in all randomly assigned patients. Median OS was 15.7 months (95% CI, 12.9 to 19.9) in the nivolumab (NIVO) group and 14.4 months (95% CI, 11.7 to 18.2) in the ICC group (hazard ratio for death, 0.95; 95.54% CI, 0.73 to 1.24; P = .716). (B) Kaplan-Meier curves for OS in all treated patients censoring at the start of PD-1 or PD-L1 agent after assigned therapy in ICC. Median OS was 16.4 months (95% CI, 12.9 to 20.3) in the NIVO group and 11.8 months (95% CI, 9.9 to 14.4) in the ICC group (hazard ratio for death, 0.81; 95.54% CI, 0.59 to 1.11).
Given the higher number of ICC patients who received subsequent systemic treatment, OS was investigated in a sensitivity analysis by censoring at the start of the PD-1/PD-L1 therapy that was received after assigned therapy in the ICC group. In contrast to the main OS analysis, this assessment was performed only in the treated patient population. With the recognition of possible selection bias in these patients, an OS difference was observed with a median OS of 16.4 months (95% CI, 12.9 to 20.3) for the nivolumab group and 11.8 months (95% CI, 9.9 to 14.4) for the ICC group (HR, 0.81; 95% CI, 0.59 to 1.1; Fig 2B).
Both ORR and median duration of response in the updated results were notably higher for nivolumab versus ICC at 27% versus 10% and 32 months versus 13 months, respectively (Table 2). Results were similar to those observed in the previous analysis, although duration of response was now reached for the nivolumab group; however, time to response for nivolumab versus ICC is similar at 2.2 months versus 2.1 months currently and 2.1 months versus 3.5 months previously.14 In addition, nivolumab demonstrated more durable responses than did ICC; 69% of responses in the nivolumab group compared with 62% in the ICC group were ongoing at the end of the study period for individual patients (Fig 3). There was no improvement in PFS for nivolumab compared with ICC; median PFS was 3.1 months (95% CI, 2.3 to 3.5) for nivolumab versus 3.7 months (95% CI, 2.3 to 5.3) for ICC (HR, 1.0; 95.1% CI, 0.78 to 1.436; Fig 4), which was decreased compared with the earlier analysis that reported median PFS at 4.7 months (95% CI, 2.3 to 6.5) versus 4.2 months (95% CI, 2.1 to 6.3).14
Table 2.
Response to Treatment via IRRC Analysis

Fig 3.
Duration of response per independent radiologic review committee. Swimmer plots show time to first response and duration of response, as defined by RECIST v1.1, for responders who received nivolumab (NIVO) or investigator’s choice chemotherapy (ICC).
Fig 4.
Progression-free survival (PFS) by independent radiologic review committee (IRRC) assessment. Kaplan-Meier curves for PFS in all randomly assigned patients by IRRC assessment. Median PFS was 3.1 months (95% CI, 2.3 to 3.5) in the nivolumab (NIVO) group and 3.7 (95% CI, 2.3 to 5.3) in the investigator’s choice chemotherapy (ICC) group (hazard ratio for death or disease progression, 1.03; 95.1% CI, 0.78 to 1.436).
There were more patients in the nivolumab group who had quantifiable PD-L1 expression data (248 [91%] of 272 patients) compared with the ICC group (99 [74%] of 133 patients). In all PD-L1 expression subgroups tested, ORR was numerically higher for the nivolumab group relative to the ICC group (Data Supplement). In addition, a similar ORR was observed with ICC in all subgroups, whereas ORR increased with increasing PD-L1 expression in the nivolumab group. In a post hoc analysis that explored the association between response and the expression of PD-L1 across the continuum of expression (0% to 100%), no optimal threshold for PD-L1 expression was identified that could be used to select patients for nivolumab treatment.
Safety
Any-grade, treatment-related AEs occurred in 77% and 82% of patients in the nivolumab and ICC groups, respectively, including grade 3 and 4 AEs (14% and 34%, respectively; Table 3). The most common treatment-related AE in both groups was fatigue at 32% for nivolumab and 39% for ICC; AEs were similar to those reported previously.14 Any-grade, treatment-related AEs led to discontinuation in 5% of patients treated with nivolumab and 11% with ICC. Treatment-related AEs that led to discontinuation in two or more patients were increased ALT and pancreatitis (two patients each) in the nivolumab group and peripheral neuropathy (three patients), arthralgia (two patients), anemia (two patients), and thrombocytopenia (two patients) in the ICC group.
Table 3.
AEs
The most common treatment-related select AEs—AEs with a potential immunologic cause—in the nivolumab group were skin (38%) followed by GI (18%) and hepatic (11%; Data Supplement), as opposed to the previous analysis in which the third most common AE was endocrine (7.8%).14 Time to onset for patients with select AEs in the nivolumab group was shortest for hypersensitivity at 4.1 weeks, followed by skin at 6.1 weeks; a range of 46% to 86% of patients resolved, with the shortest time to resolution being 0.1 weeks for hypersensitivity and the longest being 29.1 weeks for endocrine AEs (Data Supplement). Although categorized as resolved, most patients with endocrine AEs continued to receive hormone therapy. Overall, 137 (51%) patients in the nivolumab group and 35 (34%) patients in the ICC group were managed with immune-modulating agents, the most common being topical corticosteroids (24% and 5%, respectively) and systemic corticosteroids (36% and 20%, respectively).
A total of 244 patients died during the study within 30 days of the last dose: 172 (64%) in the nivolumab group and 72 (71%) in the ICC group. Most deaths—165 of (96%) 172 and 69 of (96%) 72, respectively—were a result of disease progression.
Quality of Life
Quality of life in patients on nivolumab remained unchanged for all EORTC QLQ-C30 individual scales during the treatment course, with no score reaching minimal important difference (≥ 10 points). EORTC QLQ-C30 Global Health Status changes from baseline are shown in the Data Supplement. No clinically significant improvement was observed for either the EuroQoL EQ-5D utility index or the EuroQoL EQ-5D visual analog scale for nivolumab. At 12 weeks, the ICC group demonstrated a clinically significant decrease in the EuroQoL EQ-5D utility index.
DISCUSSION
Here, we report OS in nivolumab-treated patients with metastatic melanoma who experienced progression after treatment with ipilimumab (plus a BRAF inhibitor, if BRAF-mutation positive). Consistent with the initial report, the proportion of patients who achieved an objective response was higher for nivolumab than for ICC and responses were more durable.14 Responses were also consistent with those observed with pembrolizumab treatment in a similar patient population19; however, no survival or PFS difference was observed for nivolumab compared with ICC. The safety profile of nivolumab versus ICC was consistent with the original findings, with less toxicity observed for nivolumab compared with ICC.14 The majority of nivolumab treatment-related AEs were low grade and manageable using recommended treatment algorithms. Grade 3 and 4 treatment-related AEs were reported in 31% of ICC patients compared with 14% of nivolumab-treated patients.
In patients with advanced melanoma who have experienced progression on ipilimumab and a BRAF inhibitor (if BRAF mutated), both nivolumab and pembrolizumab have shown ORR benefit over ICC.14,19 Median pembrolizumab OS was reported at 13.4 months (95% CI, 11.0 to 16.4) for 2 mg/kg, which did not achieve a significant difference compared with chemotherapy.20 Median OS reported in the current study was higher for both nivolumab and ICC at 15.7 months and 14.4 months, respectively, and was not statistically different, similar to the pembrolizumab study.
The standard of care for melanoma in many countries has evolved since trial initiation, with anti–PD-1 as monotherapy and in combination with anti–CTLA-4 becoming first-line options, making first-line ipilimumab treatment obsolete. However, there are still cases in which treatment with ipilimumab is used for patients with advanced melanoma, particularly in areas of the world where anti–PD-1 therapy is not available as first-line therapy. In addition, because patients are living longer, second-line treatment after ipilimumab is important. Many patients treated with ipilimumab do not achieve a response, ultimately experience progression after treatment, or need to discontinue treatment because of immune-related toxicity.21 In addition, approximately 50% of patients who are treated with BRAF and MEK inhibitors will experience progression as a result of mitogen-activated protein kinase resistance within 12 months of therapy.22,23 These results, along with similar data with pembrolizumab, demonstrate that these patients can be treated effectively with anti–PD-1 therapy.
The current study showed that durable objective responses were achieved with nivolumab, but no survival difference. Several confounding factors likely impacted OS, which suggests that the results need to be interpreted with caution. A primary factor was the open-label design of the trial with crossover potential for patients to enter a PD-1/PD-L1 antibody trial or receive approved agents after experiencing progression in the ICC arm. Indeed, 41% of patients in the ICC group versus 11% in the nivolumab group received a subsequent anti–PD-1/PD-L1 agent. In patients who were treated, a numeric survival difference was observed between treatment groups with censoring at the start of anti–PD-1/PD-L1 treatment after assigned therapy in the ICC group; however, this was a sensitivity analysis and there is possible bias associated with these types of analyses. In addition, a high proportion of patients who were randomly assigned to ICC compared with those who were randomly assigned to nivolumab (23% v 1%) dropped out as soon as the random assignment occurred before receiving assigned chemotherapy treatments. Many of these patients went on to receive pembrolizumab in available phase I studies, which may have skewed the results.
Differences in patient population general health could also affect the survival curves. Two indicators of poor prognosis—brain metastases and elevated lactate dehydrogenase—were more frequent in the nivolumab group compared with the ICC group. In addition, systemic corticosteroids were used to manage immune-related AEs in 36% of patients in the nivolumab group. This may be attributed, in part, to the increased frequency of poor prognostic factors in this treatment group and may have had a detrimental effect on efficacy in nivolumab-treated patients.
ORR of 10% and median PFS of 3.7 months for the ICC group are similar to a previous study of patients with advanced melanoma who experienced progression on dacarbazine and whose ORR was 11% and PFS was 17.9 weeks after treatment with carboplatin plus paclitaxel.24 This consistency reinforces the impact of the increased ORR of 27% versus 10% for nivolumab compared with ICC in the current study.
In conclusion, although there were no survival differences between nivolumab and ICC treatments, nivolumab treatment after progression on ipilimumab with or without a BRAF inhibitor does provide a higher rate of response and more durable responses. Some situations may still exist that necessitate the use of ipilimumab as first-line therapy and nivolumab provides a safer option with a better maintained quality of life for patients who have experienced failure with prior systemic therapies compared with cytotoxic chemotherapy. The OS outcome may have been impacted by the increased dropout rate before treatment and increased systemic therapy received after assigned therapy in the ICC group, as well as an increased proportion of patients with poor prognostic factors in the nivolumab group. Despite the lack of survival advantage, nivolumab remains an effective option for PD-1 inhibitor–naive patients who experienced failure with ipilimumab and a BRAF inhibitor if BRAF mutated.
ACKNOWLEDGMENT
We thank the patients and investigators who participated in the CheckMate 037 trial. Professional medical writing and editorial assistance were provided by Melissa Kirk, PhD, CMPP, and Cara Hunsberger at StemScientific, an Ashfield Company, funded by Bristol-Myers Squibb. We also acknowledge Dako for the collaborative development of the PD-L1 immunohistochemistry 28-8 pharmDx assay.
Footnotes
Supported by Bristol-Myers Squibb. JL is supported by the Royal Marsden/Institute of Cancer Research NIHR Biomedical Research Centre for Cancer Research.
Presented at the 2016 Society for Melanoma Research Congress, Brisbane, Queensland, Australia, November 6-9, 2016.
Clinical trial information: NCT01721746.
AUTHOR CONTRIBUTIONS
Conception and design: David Minor, Sandra D'Angelo, Jeffrey Weber
Administrative support: David Minor
Provision of study materials or patients: David Minor, Bart Neyns, Wilson H. Miller Jr, Bartosz Chmielowski, Paul Lorigan, Kenneth Grossmann, Jessica C. Hassel, Jeffrey Sosman, Nikhil Khushalani, Dirk Schadendorf
Collection and assembly of data: James Larkin, David Minor, Sandra D'Angelo, Bart Neyns, Wilson H. Miller Jr, Ralf Gutzmer, Gerald Linette, Bartosz Chmielowski, Paul Lorigan, Kenneth Grossmann, Jessica C. Hassel, Mario Sznol, Adil Daud, Dirk Schadendorf, Christoph Hoeller, Dana Walker, Christine Horak, Jeffrey Weber
Data analysis and interpretation: James Larkin, David Minor, Sandra D'Angelo, Michael Smylie, Wilson H. Miller Jr, Gerald Linette, Bartosz Chmielowski, Christopher D. Lao, Paul Lorigan, Kenneth Grossmann, Jessica C. Hassel, Mario Sznol, Jeffrey Sosman, Nikhil Khushalani, Dirk Schadendorf, Dana Walker, George Kong, Christine Horak, Jeffrey Weber
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Overall Survival in Patients With Advanced Melanoma Who Received Nivolumab Versus Investigator’s Choice Chemotherapy in CheckMate 037: A Randomized, Controlled, Open-Label Phase III Trial
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/site/ifc.
James Larkin
Research Funding: Pfizer, Bristol-Myers Squibb, MSD, Novartis
Travel, Accommodations, Expenses: Bristol-Myers Squibb, MSD, Pfizer, Eisai, GlaxoSmithKline, Roche
David Minor
Stock or Other Ownership: Bristol-Myers Squibb (I)
Consulting or Advisory Role: Bristol-Myers Squibb, Genentech, Merck, Pfizer
Speakers' Bureau: Bristol-Myers Squibb, Merck
Research Funding: Prometheus Laboratories
Sandra D'Angelo
Consulting or Advisory Role: EMD Serono, Nektar
Bart Neyns
Honoraria: Bristol-Myers Squibb, MSD, Roche, Amgen, Novartis
Speakers' Bureau: Novartis
Research Funding: Pfizer (Inst), Novartis (Inst)
Travel, Accommodations, Expenses: Bristol-Myers Squibb, MSD, Roche, Amgen, Novartis
Michael Smylie
Honoraria: Bristol-Myers Squibb, Novartis, Merck, Roche
Consulting or Advisory Role: Bristol-Myers Squibb, Merck
Wilson H. Miller Jr
Stock or Other Ownership: Bristol-Myers Squibb
Honoraria: Bristol-Myers Squibb, Merck, Roche, Novartis, GlaxoSmithKline
Consulting or Advisory Role: Bristol-Myers Squibb, Merck, Roche, Novartis
Research Funding: Argos (Inst), AstraZeneca (Inst), Bayer, Bristol-Myers Squibb (Inst), GlaxoSmithKline (Inst), MedImmune (Inst), Merck (Inst), Novartis (Inst), Roche (Inst)
Ralf Gutzmer
Honoraria: Bristol-Myers Squibb, Roche, GlaxoSmithKline, Novartis, MSD, Merck Serono, Amgen, Almirall, Boehinger Ingelheim, Pierre Fabre
Consulting or Advisory Role: Bristol-Myers Squibb, Roche, MSD, Novartis, Almirall, LEO, Amgen, Pfizer, Merck Serono, Pierre Fabre
Research Funding: Pfizer, Johnson & Johnson
Travel, Accommodations, Expenses: Bristol-Myers Squibb, Roche
Gerald Linette
No relationship to disclose
Bartosz Chmielowski
Consulting or Advisory Role: Amgen, Bristol-Myers Squibb, Merck, Genentech, Eisai, Immunocore
Speakers' Bureau: Genentech, Janssen Oncology
Travel, Accommodations, Expenses: Genentech, Bristol-Myers Squibb, Janssen Pharmaceuticals, Merck
Christopher D. Lao
Travel, Accommodations, Expenses: Bristol-Myers Squibb
Paul Lorigan
Consulting or Advisory Role: Merck Sharp & Dohme, Bristol-Myers Squibb, Novartis, Amgen, GlaxoSmithKline
Speakers' Bureau: Merck Sharp & Dohme, Novartis, Bristol-Myers Squibb, Roche
Travel, Accommodations, Expenses: Merck Sharp & Dohme, Bristol-Myers Squibb
Kenneth Grossmann
Consulting or Advisory Role: Genentech, Castle Biosciences, Bristol-Myers Squibb (I)
Jessica C. Hassel
Honoraria: Bristol-Myers Squibb, MSD, Roche, GlaxoSmithKline, Novartis, Amgen
Consulting or Advisory Role: MSD, Amgen
Research Funding: Bristol-Myers Squibb (Inst)
Travel, Accommodations, Expenses: Bristol-Myers Squibb, MSD, Amgen, GlaxoSmithKline
Mario Sznol
Stock or Other Ownership: Amphivena, Intensity Therapeutics, Adaptive Biotechnologies
Consulting or Advisory Role: Bristol-Myers Squibb, Genentech, Amgen, AstraZeneca, MedImmune, Symphogen, Merus, Immune Design, Kyowa Hakko Kirin, Lion Biotechnologies, Nektar, Novartis, Eli Lilly, Pfizer, Janssen Oncology, Vaccinex, Merck Sharp & Dohme, Biodesix, Alexion Pharmaceuticals, Adaptimmune, Lycera, Theravance, Modulate, Omniox, Seattle Genetics, Inovio Pharmaceuticals, Agonox, Ignyta
Other Relationship: Haymarket Media, Research to Practice, TRM Oncology, Physician Education Resource, Imedex, AcademicCME, DAVAOncology, Clinical Care Options, Vindico, Prime Oncology
Adil Daud
Stock or Other Ownership: Oncosec
Honoraria: EMD Serono, Inovio Pharmaceuticals
Consulting or Advisory Role: Oncosec, Merck, GlaxoSmithKline
Research Funding: Merck (Inst), Schering Plough (Inst), GlaxoSmithKline (Inst), Pfizer (Inst), Genentech (Inst), Oncosec (Inst)
Patents, Royalties, Other Intellectual Property: Patent relating to test for immunotherapy
Jeffrey Sosman
Honoraria: Array, Genentech, Merck, Novartis
Consulting or Advisory Role: Array, Genentech, Merck, Novartis
Nikhil Khushalani
Stock or Other Ownership: Bellicum Pharmaceuticals
Consulting or Advisory Role: Bristol-Myers Squibb, Castle Biosciences, AstraZeneca, EMD Serono, Genentech
Research Funding: Bristol-Myers Squibb (Inst), Merck (Inst), Novartis (Inst), National Comprehensive Cancer Network (Inst), Pfizer (Inst), Threshold Pharmaceuticals (Inst), Eisai (Inst), TRACON Pharma (Inst), GlaxoSmithKline (Inst)
Dirk Schadendorf
Honoraria: Genentech, Novartis, Amgen, Bristol-Myers Squibb, Merck Sharp & Dohme, Boehringer Ingelheim, Sysmex, Immunocore, Grünenthal Group, Merck Serono
Consulting or Advisory Role: Genentech, Novartis, Bristol-Myers Squibb, Merck Sharp & Dohme, Merck Serono, Sysmex, Amgen, Grünenthal Group, Immunocore
Speakers' Bureau: Roche, Bristol-Myers Squibb, Merck Sharp & Dohme, Novartis, Amgen
Research Funding: Roche (Inst), Bristol-Myers Squibb (Inst), Merck Sharp & Dohme (Inst), Amgen (Inst), Novartis (Inst)
Travel, Accommodations, Expenses: Genentech, Bristol-Myers Squibb, Amgen, Merck, Merck Serono, Novartis
Christoph Hoeller
Honoraria: Amgen, Bristol-Myer Squibb, MSD, Novartis, Roche
Consulting or Advisory Role: Amgen, AstraZeneca, Bristol-Myer Squibb, MSD, Novartis, Roche
Research Funding: Roche
Dana Walker
Employment: Bristol-Myers Squibb
Stock or Other Ownership: Antares Pharmaceuticals (I)
George Kong
Employment: Bristol-Myers Squibb
Stock or Other Ownership: Bristol-Myers Squibb
Christine Horak
Employment: Bristol-Myers Squibb
Stock or Other Ownership: Bristol-Myers Squibb
Jeffrey Weber
Stock or Other Ownership: Altor BioScience, Celldex, CytomX Therapeutics
Honoraria: Bristol-Myers Squibb, Merck, Genentech, AbbVie, AstraZeneca, Daiichi Sankyo, GlaxoSmithKline, Eisai, Altor BioScience, Lion Biotechnologies, Amgen, Roche, Ichor Medical Systems, Celldex, cCam Biotherapeutics, Pieris Pharmaceuticals, CytomX Therapeutics, Nektar, Novartis, Medivation
Consulting or Advisory Role: Celldex, Ichor Medical Systems, cCam Biotherapeutics, Lion Biotechnologies, Pieris Pharmaceuticals, Altor BioScience, Bristol-Myers Squibb, Merck, Genentech, Roche, Amgen, AstraZeneca, GlaxoSmithKline, Daiichi Sankyo, AbbVie, Eisai, CytomX Therapeutics, Nektar, Novartis, Medivation
Research Funding: Bristol-Myers Squibb (Inst), Merck (Inst), GlaxoSmithKline (Inst), Genentech (Inst), Astellas Pharma (Inst), Incyte (Inst), Roche (Inst), Novartis (Inst)
Patents, Royalties, Other Intellectual Property: Named on a patent submitted by Moffitt Cancer Center for an IPILIMUMAB biomarker
named on a patent from Biodesix for a PD-1 antibody biomarker
Travel, Accommodations, Expenses: Bristol-Myers Squibb, GlaxoSmithKline, Daiichi Sankyo, Pieris Pharmaceuticals, cCam Biotherapeutics, Lion Biotechnologies, Roche, Celldex, Amgen, Merck, AstraZeneca, Genentech, Novartis
REFERENCES
- 1.McArthur GA, Ribas A: Targeting oncogenic drivers and the immune system in melanoma. J Clin Oncol 31:499-506, 2013 [DOI] [PubMed] [Google Scholar]
- 2.Menzies AM, Long GV: Recent advances in melanoma systemic therapy. BRAF inhibitors, CTLA4 antibodies and beyond. Eur J Cancer 49:3229-3241, 2013 [DOI] [PubMed] [Google Scholar]
- 3.Hodi FS, O’Day SJ, McDermott DF, et al. : Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 363:711-723, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robert C, Thomas L, Bondarenko I, et al. : Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med 364:2517-2526, 2011 [DOI] [PubMed] [Google Scholar]
- 5.Callahan MK, Postow MA, Wolchok JD: Immunomodulatory therapy for melanoma: Ipilimumab and beyond. Clin Dermatol 31:191-199, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wood K, Luke JJ: Optimal use of BRAF targeting therapy in the immunotherapy era. Curr Oncol Rep 18:67, 2016 [DOI] [PubMed] [Google Scholar]
- 7.Spain L, Julve M, Larkin J: Combination dabrafenib and trametinib in the management of advanced melanoma with BRAFV600 mutations. Expert Opin Pharmacother 17:1031-1038, 2016 [DOI] [PubMed] [Google Scholar]
- 8.Ascierto PA, Kirkwood JM, Grob JJ, et al. : The role of BRAF V600 mutation in melanoma. J Transl Med 10:85, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chapman PB, Hauschild A, Robert C, et al. : Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med 364:2507-2516, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang C, Thudium KB, Han M, et al. : In vitro characterization of the anti-PD-1 antibody nivolumab, BMS-936558, and in vivo toxicology in non-human primates. Cancer Immunol Res 2:846-856, 2014 [DOI] [PubMed] [Google Scholar]
- 11.Robert C, Schachter J, Long GV, et al. : Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med 372:2521-2532, 2015 [DOI] [PubMed] [Google Scholar]
- 12.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. : Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 373:23-34, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hodi FS, Chesney J, Pavlick AC, et al. : Combined nivolumab and ipilimumab versus ipilimumab alone in patients with advanced melanoma: 2-year overall survival outcomes in a multicentre, randomised, controlled, phase 2 trial. Lancet Oncol 17:1558-1568, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weber JS, D’Angelo SP, Minor D, et al. : Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): A randomised, controlled, open-label, phase 3 trial. Lancet Oncol 16:375-384, 2015 [DOI] [PubMed] [Google Scholar]
- 15.Eisenhauer EA, Therasse P, Bogaerts J, et al. : New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 45:228-247, 2009 [DOI] [PubMed] [Google Scholar]
- 16.Wolchok JD, Kluger H, Callahan MK, et al. : Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med 369:122-133, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aaronson NK, Ahmedzai S, Bergman B, et al. : The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 85:365-376, 1993 [DOI] [PubMed] [Google Scholar]
- 18.EuroQol Group : EuroQol—A new facility for the measurement of health-related quality of life. Health Policy 16:199-208, 1990 [DOI] [PubMed] [Google Scholar]
- 19.Ribas A, Puzanov I, Dummer R, et al. : Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): A randomised, controlled, phase 2 trial. Lancet Oncol 16:908-918, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamid O, Puzanov I, Dummer R, et al: Final overall survival for KEYNOTE-002: Pembrolizumab (pembro) versus investigator-choice chemotherapy (chemo) for ipilimumab (ipi)-refractory melanoma. Presented at the ESMO 2016 Congress, Copenhagen, Denmark, October 7-11, 2016 [Google Scholar]
- 21.Restifo NP, Smyth MJ, Snyder A: Acquired resistance to immunotherapy and future challenges. Nat Rev Cancer 16:121-126, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shi H, Hugo W, Kong X, et al. : Acquired resistance and clonal evolution in melanoma during BRAF inhibitor therapy. Cancer Discov 4:80-93, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rizos H, Menzies AM, Pupo GM, et al. : BRAF inhibitor resistance mechanisms in metastatic melanoma: spectrum and clinical impact. Clin Cancer Res 20:1965-1977, 2014 [DOI] [PubMed] [Google Scholar]
- 24.Hauschild A, Agarwala SS, Trefzer U, et al. : Results of a phase III, randomized, placebo-controlled study of sorafenib in combination with carboplatin and paclitaxel as second-line treatment in patients with unresectable stage III or stage IV melanoma. J Clin Oncol 27:2823-2830, 2009 [DOI] [PubMed] [Google Scholar]







