Abstract
Purpose:
The Centers for Medicare & Medicaid Services (CMS) identifies suboptimal management of treatment toxicities as a care gap and proposes the measurement of hospital performance on the basis of emergency department visits for 10 common symptoms. Current management strategies do not address symptom co-occurrence.
Methods:
We evaluated symptom co-occurrence in three patient cohorts that presented to a cancer hospital urgent care center in 2016. We examined both the CMS-identified symptoms and an expanded clinician-identified set defined as symptoms that could be safely managed in the outpatient setting if identified early and managed proactively. The cohorts included patients who presented with a CMS-defined symptom within 30 days of treatment, patients who presented within 30 days of treatment with a symptom from the expanded set, and patients who presented with a symptom from the expanded set within 30 days of treatment start. Symptom co-occurrence was measured by Jaccard index. A community detection algorithm was used to identify symptom clusters on the basis of a random walk process, and network visualizations were used to illustrate symptom dynamics.
Results:
There were 6,429 presentations in the CMS symptom-defined cohort. The network analysis identified two distinct symptom clusters centered around pain and fever. In the expanded symptom cohort, there were 5,731 visits and six symptom clusters centered around fever, emesis/nausea, fatigue, deep vein thrombosis, pain, and ascites. For patients who newly initiated treatment, there were 1,154 visits and four symptom clusters centered around fever, nausea/emesis, fatigue, and deep vein thrombosis.
Conclusion:
Uncontrolled symptoms are associated with unplanned acute care. Recognition of the complexity of symptom co-occurrence can drive improved management strategies.
INTRODUCTION
Patients who receive chemotherapy have, on average, one hospital admission and two emergency department visits per year, and 40% to 50% of these stem from symptoms related to their treatment.1 Acute hospitalizations account for 48% of total cancer expenditures.2 Considerable regional variation exists, which suggests that these hospitalizations may be avoidable.2 The Centers for Medicare & Medicaid Services (CMS) identified 10 conditions for hospital visits among patients who receive chemotherapy that are potentially preventable through appropriately managed outpatient care and communication plans. CMS plans to measure cancer hospitals’ performance on the basis of the frequency of emergency department visits and admissions for the following conditions: anemia, dehydration, diarrhea, emesis, nausea, neutropenia, fever, pain, pneumonia, and sepsis.3 This acute care reduces patients’ quality of life, delays their treatment, puts them at risk for nosocomial infections, augments their health care costs, and increases the burden on their family.3-5 These admissions also come at a high societal cost, with the 2010 average cost of a chemotherapy-related hospitalization at $22,000.1
Policymakers have recognized the need for better symptom management. Congress passed the 21st Century Cures Act in 2016 that authorizes $1.8 billion in funding for the Cancer Moonshot6 and identified improved symptom management as a priority area.7 In addition, the Medicare Access and CHIP Reauthorization Act of 2015 and the Oncology Care Model assess total cost of care and resource utilization. These costs are driven in part by unplanned acute care, which creates an additional impetus to improve symptom management.8,9 To address this need, organizations have developed evidence-based symptom management guidelines, including ASCO,10 the Oncology Nursing Society,11 and the National Comprehensive Cancer Network.12 The private market also has stepped in to develop clinical pathways and decision support for symptom triage and management.13-16
However, these pathways and guidelines typically are designed to address a symptom in isolation and fail to recognize the complexity of co-occurring symptoms. The presence of symptom clusters in oncology, defined as the co-occurrence of two or more symptoms, is well established through multiple patient-reported outcomes studies.17-20 However, a recent workshop on advancing symptom science through symptom cluster research sponsored by the National Institute for Nursing Research and the Office of Rare Disease Research, National Center for Advancing Translational Science, found that symptom cluster research is extremely limited and that a need exists for innovative analytic strategies to identify these clusters as well as targeted interventions to address them.21 In this vein, The National Cancer Institute introduced a funding mechanism for bridging the guideline implementation gap that calls for applications for clinical decision support systems that address co-occurrence.22
In this article, we introduce a new analytic methodology for symptom cluster identification that enables better cluster visualization. It has been established that physicians are uncomfortable with managing symptom clusters,23,24 and by better elucidating these clusters, we can improve management strategies. In addition, because the Medicare Access and CHIP Reauthorization Act compels a shift toward value-based care delivery, an understanding of potentially preventable symptom clusters and how they affect unplanned acute care is crucial. The connection between symptom clusters and resource utilization is not well understood. Thus, this analysis uses a novel methodology to identify and create network maps of potentially preventable symptom clusters for patients receiving active treatment who present to an urgent care center (UCC).
METHODS
This study received a waiver of informed consent from the Memorial Sloan Kettering Cancer Center (MSKCC) institutional review board.
Data Source and Patients
We used data from the electronic health record (EHR) to identify patients who presented to MSKCC UCC in 2016 (23,341 unique visits). Staffed 24/7, the UCC is meant for urgent medical issues for MSKCC patients and is the central point of entry for unplanned hospital admissions.25,26 We filtered these presentations to patients who were receiving active treatment. We used the CMS definition of active treatment as having received an intravenous chemotherapy agent within 30 days of presentation but expanded this to include oral antineoplastic agents and immunotherapy.3,27 We excluded pediatric patients and patients with leukemia, consistent with the CMS quality measure.3,27 To determine the UCC presenting symptoms, we pulled from the EHR clinician-entered data fields for chief complaint and primary diagnoses. Consistent with prior literature, co-occurrence is defined as two or more symptoms that present in the same patient.21,23,28
We next identified the following three cohorts of patients to identify and map potentially preventable symptom clusters:
Cohort 1: patients who were receiving active treatment and presented to the UCC with one of the 10 CMS-defined potentially preventable symptoms.
Cohort 2: patients who were receiving active treatment but used a clinician-developed definition of a potentially preventable symptom. The CMS quality measure of potentially preventable acute care has received public feedback about the true preventability of these presentations and what conditions should be excluded from measurement.3 Not infrequently will an oncology patient present with both a preventable diagnosis, such as pain, and a nonpreventable diagnosis, such as a GI obstruction. In addition, with innovations in care delivery, such as mobile patient-reported outcomes and symptom care clinics, additional symptoms could be managed safely in the outpatient setting without an acute care visit. To have the greatest effect on patients, an understanding of the clustering of these additional symptoms is important. For the current analysis of cohorts 2 and 3, a potentially preventable symptom is defined as a symptom that could be managed safely in the outpatient setting if the clinical team identified said symptom early and managed it proactively. To create this list, an oncology nurse, a triage nurse practitioner, and a medical oncologist reviewed the 2015 list of urgent care chief complaints and primary diagnoses and identified, through consensus, those that met this definition (Appendix Table A1, online only). Patients were excluded from this analysis if they presented with a symptom that was determined to not meet this definition, even if they also presented with a potentially preventable symptom.
Cohort 3: patients who were receiving active treatment who presented to the UCC with a potentially preventable symptom but restricts the analysis to those who initiated treatment for the first time. We then examine UCC presentations within 30 days of the start of treatment. This time frame was chosen for several reasons: Symptom presence and severity is often at its worst in the first weeks after diagnosis and initial treatment,29 the Oncology Care Model uses as its index date the start of therapy,8 and we were able to evaluate the temporal component of symptom clusters.
Statistical Methods
In response to the call for new methods and approaches21 to symptom cluster analysis as well as the deficit described in clinician comfort in managing clusters,23 we created network symptom maps that provide a visual methodology for identifying the frequency of a symptom presentation as well as its co-occurrence with other symptoms. In the network visual, the node size is the frequency of symptom occurrences. We then used the Jaccard index to determine the strength of symptom co-occurrence, which in the network visual is the width of the edge (or connecting link) between symptoms. The Jaccard index is the prevalence of two co-occurring symptoms over their combined total presentations (both together and separately). Thus, the Jaccard index normalizes for relative size. For example, for all UCC presentations where patients presented for either fatigue or abdominal pain, a Jaccard index of 0.14 indicates that patients presented with both symptoms 14% of the time. Of note, a symptom is only visible if it has an edge, or association, with another symptom node. Consistent with the literature,28,30 we initially used a minimum co-occurrence count for all networks by keeping just the top N pairs, thus removing symptoms with low prevalence. We subsequently used a second filter on the basis of co-occurrence strength (Jaccard index) for all networks, similarly by keeping the top N pairs of those that remained and thus removing any with relatively high prevalence but low connection strength. For the CMS-identified potentially preventable symptoms, we filtered the top 20 by co-occurrence frequency, and of those, we retained the top 15 by Jaccard index. Because the number of symptoms included in the internally developed symptom list is greater in number, we used a higher top N by filtering for the top 50 pairings by co-occurrence frequency, and of those, we retained the top 30 by Jaccard index.
The colored symptom clusters then were created by using a community detection algorithm called walktrap,31 which uses the concept of random walks.32 Conceptually, a random walk is an unsupervised process that involves a series of random steps.32 In the context of a network, the algorithm determines which nodes should cluster together by repeatedly completing a series of random finite steps from each node to its neighboring/connecting nodes, and then from the resulting paths, determine which nodes tend to reside within the same community, or cluster, on the basis of their calculated random walk probability. In applying this algorithm, use of a uniform and small number of steps in the random walk (which we left at the default step value t = 4) is recommended. We did not establish a priori the number of clusters; instead, we allowed the algorithm to determine the number of clusters, or k. The only element we specified in the algorithm was that it should use the Jaccard index as a weight. The Jaccard index thus influences the probability of the random walk as opposed to leaving all adjacent steps open to equal chance of being traversed by a random walk. For example, if a particular node has a connection strength with an adjacent node (Jaccard index, 0.2) that is higher than its connection strength with another adjacent node (Jaccard index, 0.1), the setting of the Jaccard index as a weight means that the probability the random walk will traverse to the former node is somewhat higher than the latter. This makes intuitive sense because the clusters should consider the relative connection strength of nodes when determining which symptoms should cluster together. With that said, we found that the specification of this parameter made little difference with respect to the final output largely because of the nature in which the walktrap algorithm works: Random walks tend to produce the same communities whether a weight is specified because they tend to traverse around densely connected nodes. Finally, we convey frequency by node size, co-occurrence strength by edge width, and clusters by color in the form of a network visualization.
We performed all analysis in R (www.r-project.org) and used the R package iGraph to run the walktrap algorithm. Cytoscape (www.cytoscape.org) was used to generate the network visuals.
RESULTS
Cohort 1
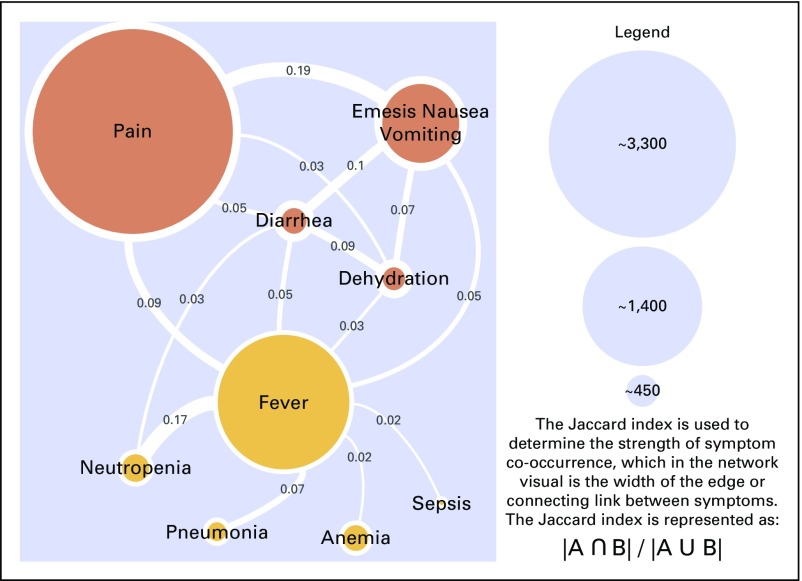
There were 9,424 UCC visits in 2016 among patients who were receiving active treatment of which 6,429 (68.2%) were for CMS-defined potentially preventable conditions. The median age for this cohort was 60 years, and 58.3% were women. Most patients were white (69.7%) followed by black (10.4%) and Asian (9%). The most common cancers were breast (13.4%), lung (10.4%), and colorectal (9.6%). As can be seen in the network visual (Fig 1), pain was the most frequent cause for UCC presentation (n = 3,368) followed by fever (n = 2,152) and nausea/emesis (n = 1,376). Of note, nausea and emesis were collapsed as one node to be consistent with how these symptoms are reported in the UCC EHR data fields. Pain shared a cluster with GI-related symptoms, including nausea/emesis and diarrhea, as well as with dehydration. Meanwhile, fever became a separate cluster with neutropenia, pneumonia, anemia, and sepsis and was the only symptom node that shared an edge with all other nodes. The Jaccard index was strongest for pain co-occurring with nausea/emesis (0.19) and for fever co-occurring with neutropenia (0.17).
Fig 1.
Cohort 1: Oncology patients who were receiving active treatment and presented to urgent care with a Centers for Medicare & Medicaid Services–defined potentially preventable symptom (6,429 urgent care visits).
Cohort 2
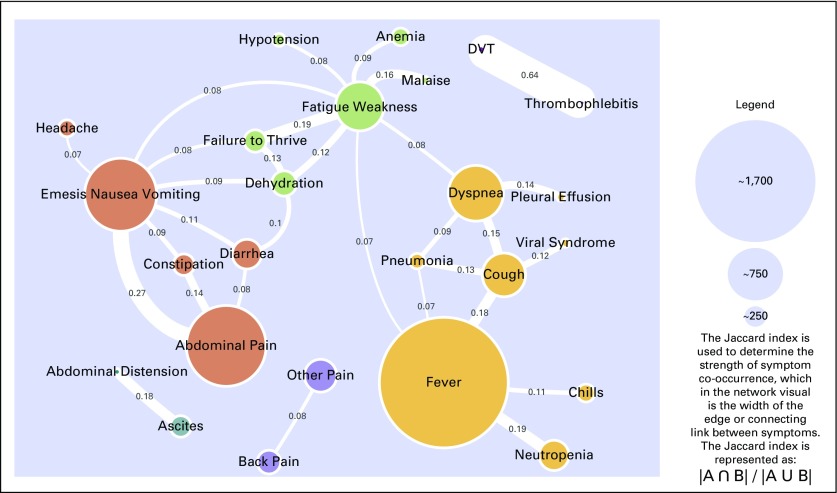
Cohort 2 reflects the clinically developed definitions of a potentially preventable (n = 106) and a not potentially preventable symptom (n = 93; Appendix Table A1). The introduction of not potentially preventable symptoms has a disqualifying effect on UCC visits. For example, if a patient presents with both nausea (preventable) and a stroke (not preventable), the patient would count toward the CMS criteria but would be disqualified per internally developed criteria. In addition, the CMS definition includes all pain presentations, whereas in cohort 2, pain was disaggregated by location. For this cohort, there were 9,424 UCC visits of which 5,731 (60.8%) were for potentially preventable symptoms. The median age was 60 years, and 59.2% were women with a similar demographic breakdown. The most common cancers were again breast (14.8%), lung (10.0%), and colorectal (8.2%) Fever was the most frequently presenting symptom (n = 1,714) followed by abdominal pain (n = 1,065) and nausea/emesis (n = 963). Although similar patterns of clustering were seen, given the broader symptom definition, new clusters emerged (Fig 2). Abdominal pain shared a cluster with GI symptoms, including nausea/emesis, diarrhea, and constipation, and fever continued to share a cluster with neutropenia and pneumonia but added symptoms, including cough, chills, and dyspnea. Four additional clusters emerged centered around fatigue/weakness, other pain, DVT, and ascites. Three of these clusters consisted of only two symptoms—ascites/abdominal distension, other pain/back pain, and DVT/thrombophlebitis—and were so closely associated with one another and not the other symptoms that they became their own clusters separate from the main symptom network. The Jaccard Index was strongest for DVT co-occurring with thrombophlebitis (0.64) and abdominal pain co-occurring with nausea/emesis (0.27).
Fig 2.
Cohort 2: Oncology patients who were receiving active treatment and presented to urgent care with a potentially preventable symptom (5,731 urgent care visits). A potentially preventable symptom is defined as that which could be managed safely in the outpatient setting if the clinical team identifies said symptom early and manages it proactively. DVT, deep vein thrombosis.
Cohort 3
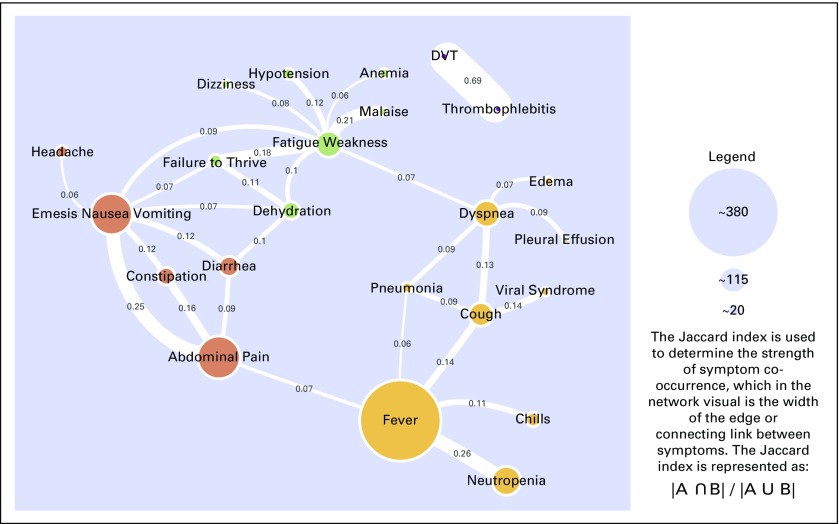
This cohort includes only patients who newly started antineoplastic therapy and presented with a potentially preventable symptom, defined as in cohort 2, within 30 days of treatment initiation. This cohort comprised 1,845 UCC visits of which 1,154 (62.5%) were for potentially preventable symptoms. The cohort median age was 60 years, and 56.3% were women. Most patients again were white (70.1%). The most common cancers now were lung (11.9%), breast (10.4%) and non-Hodgkin lymphoma (10.3%). The most prevalent symptoms again were fever (n = 382), abdominal pain (n = 195), and nausea/emesis (n = 188). The clusters were similar to cohort 2, with consistency centered around nausea/emesis, fever, fatigue/weakness, and DVT (Fig 3). However, the ascites and other pain clusters were not present, which perhaps reflected that these patients were newly diagnosed and that these symptoms had not yet emerged. The Jaccard index was strongest for DVT co-occurring with thrombophlebitis (0.69) and fever co-occurring with neutropenia (0.26).
Fig 3.
Cohort 3: Oncology patients who newly initiated antineoplastic therapy and presented to urgent care within 30 days of treatment start with a potentially preventable condition (1,154 urgent care visits). A potentially preventable symptom is defined as that which could be managed safely in the outpatient setting if the clinical team identifies said symptom early and manages it proactively. DVT, deep vein thrombosis.
DISCUSSION
This article answers a call to action in the literature for innovation21,33 with respect to symptom cluster research by harnessing a novel methodology to identify clusters and contextualizing those clusters in the current health care environment that focuses on value in patient care and resource utilization. In doing so, this article produces learnings with respect to symptom cluster research and the identification and management of potentially preventable symptoms in oncology patients who are receiving active treatment.
This article addresses several deficiencies in symptom cluster research. First, Miaskowski33 identified the need to determine how information in the EHR can be used to determine symptom clusters. By using EHR data fields for UCC primary diagnoses and chief complaints, we elicited symptom information and linked it to unplanned acute care. Unplanned acute care is of increasing importance to patients because it disrupts patients’ treatment trajectories as well as their lives outside the clinic. Use of the EHR also allowed for granular symptom data, such as a particular site of pain rather than just pain itself, which is absent from traditional sources of patient-reported symptom assessments. This disaggregation allows for the detection of more varied and nuanced associations between symptoms, such as the relationship between headache and nausea/emesis that could denote patients with brain metastases and a relationship that would not be elicited if pain complaints were aggregated. We also focused on a segment of the oncology population for whom a paucity of data exists with regard to symptom clusters34 and of particular importance to policymakers, those who are undergoing active treatment. In examining cohorts 2 and 3, a temporal consistency was found in the appearance of the fever, nausea/emesis, fatigue/weakness, and DVT clusters. However, new clusters emerged in cohort 2 for pain and ascites, which likely reflects progression of disease and is unlikely to be related to treatment toxicities; thus, this prompts a better understanding of symptoms as patients advance through their treatment.
This research also adds a new element to our definition of potentially preventable symptoms: the concept of the disqualifying symptom. By focusing exclusively on presenting symptoms that were clinically determined to be manageable in the outpatient setting, we add nuance to the CMS work on potentially preventable presentations. These clusters allow us to further our thinking of interventions, especially as it relates to the idea of a sentinel symptom.14,21,24,25,33,34 The concept of the sentinel symptom is that of a triggering or driving symptom of a cluster. The network visualization approach is a powerful tool for visualizing all symptom relationships in a comprehensive and decipherable manner, which allows for the potential of better understanding sentinel symptoms.
This work had several limitations. By focusing on a heterogenous population, we were unable to determine clusters that might be specific to a certain malignancy or therapy. In addition, by limiting the analysis to symptoms that resulted in a UCC presentation, we miss the relationship with psychological symptoms, such as anxiety and depression, that affect symptom presentation but might not result in a UCC visit or be documented by acute care physicians. However, fatigue/weakness, malaise, and failure to thrive do appear in our clusters, and although not psychological symptoms, they reflect the physical and psychological toll the underlying malignancy and treatments have on patients. Finally, the definition of a potentially preventable symptom is subjective and might not be generalizable to other organizations, but it served as a starting point for this research.
Future work should focus on using the EHR and advancements in big data and machine learning to enable earlier detection for patients at risk for one of these clusters to enable timely intervention before UCC presentation. In addition, research around electronic clinical decision support of symptom clusters, including a focus on the sentinel symptom, is needed to untangle the complexities of symptom management.
ACKNOWLEDGMENT
Supported by an ASCO Health Policy Fellowship (to B.D.). B.D. and K.N. are co-first authors.
Appendix
Table A1.
The 2015 Urgent Care Center Primary Diagnoses Categorized as Preventable or Not Preventable
AUTHOR CONTRIBUTIONS
Conception and design: Bobby Daly, Kevin Nicholas, Dmitriy Gorenshteyn, Stefania Sokolowski, Lynn Adams, Lauren L. Katzen, Yeneat O. Chiu, Rori Salvaggio, Abigail Baldwin-Medsker, Kimberly Chow, Judith Nelson, Mikel Ross, Alice Zervoudakis, Wendy Perchick, Diane L. Reidy, Brett A. Simon
Financial support: Isaac Wagner
Administrative support: Lauren L. Katzen, Han Xiao, Wendy Perchick, Isaac Wagner
Provision of study materials or patients: Bobby Daly, Lynn Adams
Collection and assembly of data: Bobby Daly, Kevin Nicholas, Stefania Sokolowski, Lior Gazit, Lynn Adams, Mikel Ross, Wendy Perchick, Diane L. Reidy, Isaac Wagner
Data analysis and interpretation: Bobby Daly, Kevin Nicholas, Dmitriy Gorenshteyn, Stefania Sokolowski, Lior Gazit, Lynn Adams, Jennie Matays, Han Xiao, Rori Salvaggio, Kenneth K. Ng, Wendy Perchick, Diane L. Reidy, Brett A. Simon, Isaac Wagner
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Misery Loves Company: Presenting Symptom Clusters to Urgent Care by Patients Receiving Antineoplastic Therapy
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jop/site/ifc/journal-policies.html.
Bobby Daly
Employment: Quadrant Holdings Corporation
Leadership: Quadrant Holdings
Stock and Other Ownership Interests: Quadrant Holdings (I), CVS Health, Johnson & Johnson, McKesson, Walgreens Boots Alliance, Lilly (I), Walgreens Boots Alliance (I)
Kevin Nicholas
No relationship to disclose
Dmitriy Gorenshteyn
No relationship to disclose
Stefania Sokolowski
No relationship to disclose
Lior Gazit
No relationship to disclose
Lynn Adams
No relationship to disclose
Jennie Matays
No relationship to disclose
Lauren L. Katzen
No relationship to disclose
Yeneat O. Chiu
No relationship to disclose
Han Xiao
No relationship to disclose
Rori Salvaggio
No relationship to disclose
Abigail Baldwin-Medsker
No relationship to disclose
Kimberly Chow
No relationship to disclose
Judith Nelson
No relationship to disclose
Mikel Ross
Honoria: Paxman
Kenneth K. Ng
No relationship to disclose
Alice Zervoudakis
No relationship to disclose
Wendy Perchick
No relationship to disclose
Diane L. Reidy
Honoraria: Novartis
Consulting or Advisory Role: Ipsen, Pfizer, Novartis
Research Funding: Novartis
Brett A. Simon
Patents, Royalties, Other Intellectual Property: patent application US2015/0290418, patent application US13/118109
Isaac Wagner
Consulting or Advisory Role: Nan Fung Life Sciences
REFERENCES
- 1.Kolodziej M, Hoverman JR, Garey JS, et al. : Benchmarks for value in cancer care: An analysis of a large commercial population. J Oncol Pract 7:301-306, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brooks GA, Li L, Uno H, et al. : Acute hospital care is the chief driver of regional spending variation in Medicare patients with advanced cancer. Health Aff (Millwood) 33:1793-1800, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Department of Health and Human Services. Centers for Medicare & Medicaid Services : Medicare Program; Hospital Inpatient Prospective Payment Systems for Acute Care Hospitals and the LongTerm Care Hospital Prospective Payment System and Policy Changes and Fiscal Year 2017 Rates; Quality Reporting Requirements for Specific Providers; Graduate Medical Education; Hospital Notification Procedures Applicable to Beneficiaries Receiving Observation Services; Technical Changes Relating to Costs to Organizations and Medicare Cost Reports; Finalization of Interim Final Rules With Comment Period on LTCH PPS Payments for Severe Wounds, Modifications of Limitations on Redesignation by the Medicare Geographic Classification Review Board, and Extensions of Payments to MDHs and Low-Volume Hospitals, 2016. https://www.gpo.gov/fdsys/pkg/FR-2016-08-22/pdf/2016-18476.pdf [PubMed]
- 4.Hassett MJ, O’Malley AJ, Pakes JR, et al. : Frequency and cost of chemotherapy-related serious adverse effects in a population sample of women with breast cancer. J Natl Cancer Inst 98:1108-1117, 2006 [DOI] [PubMed] [Google Scholar]
- 5.McKenzie H, Hayes L, White K, et al. : Chemotherapy outpatients’ unplanned presentations to hospital: A retrospective study. Support Care Cancer 19:963-969, 2011 [DOI] [PubMed] [Google Scholar]
- 6.National Cancer Institute : Cancer Moonshot. https://www.cancer.gov/research/key-initiatives/moonshot-cancer-initiative
- 7.National Cancer Institute : Cancer Moonshot–Funding Opportunities Resources. https://www.cancer.gov/research/key-initiatives/moonshot-cancer-initiative/funding/upcoming#impact
- 8.Centers for Medicare & Medicaid Services : Oncology Care Model, 2015. https://www.cms.gov/Newsroom/MediaReleaseDatabase/Fact-sheets/2015-Fact-sheets-items/2015-02-12.html
- 9.Polite BN: Oncology care model enhanced services: Long-term goals. Presented at ASCO Qual Care Symp Orlando, FL, March 3-4, 2017 [Google Scholar]
- 10.American Society of Clinical Oncology : Supportive care and treatment related issues, 2018. https://www.asco.org/practice-guidelines/quality-guidelines/guidelines/supportive-care-and-treatment-related-issues
- 11.Oncology Nursing Society : PEP Rating System Overview. https://www.ons.org/practice-resources/pep.
- 12.National Comprehensive Cancer Network : NCCN guidelines for supportive care. https://www.nccn.org/professionals/physician_gls/default.aspx#supportive
- 13.Innovative Oncology Business Solutions : http://www.innovativeobs.com
- 14.Via Oncology : https://viaoncology.com/wp-content/media/via_triage_one_page_collateral.pdf
- 15.Navigating Cancer : https://www.navigatingcancer.com
- 16.Daly B, Zon RT, Page RD, et al. : Oncology clinical pathways: Charting the Landscape of pathway providers. J Oncol Pract 10.1200/JOP.17.00033 [epub ahead of print on February 7, 2018] [DOI] [PubMed] [Google Scholar]
- 17.Skerman HM, Yates PM, Battistutta D: Cancer-related symptom clusters for symptom management in outpatients after commencing adjuvant chemotherapy, at 6 months, and 12 months. Support Care Cancer 20:95-105, 2012 [DOI] [PubMed] [Google Scholar]
- 18.Rha SY, Lee J: Symptom clusters during palliative chemotherapy and their influence on functioning and quality of life. Support Care Cancer 25:1519-1527, 2017. [Erratum: Support Care Cancer 25:2671-2672, 2017] [DOI] [PubMed] [Google Scholar]
- 19.Molassiotis A, Wengström Y, Kearney N: Symptom cluster patterns during the first year after diagnosis with cancer. J Pain Symptom Manage 39:847-858, 2010 [DOI] [PubMed] [Google Scholar]
- 20.Dong ST, Butow PN, Tong A, et al. : Patients’ experiences and perspectives of multiple concurrent symptoms in advanced cancer: A semi-structured interview study. Support Care Cancer 24:1373-1386, 2016 [DOI] [PubMed] [Google Scholar]
- 21.Miaskowski C, Barsevick A, Berger A, et al. : Advancing symptom science through symptom cluster research: Expert panel proceedings and recommendations. J Natl Cancer Inst 109:djw253, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National Cancer Institute : NIH/NCI 377 - Bridging the guideline implementation gap: Clinical decision-support to improve cancer symptom management, 2017. https://sbir.cancer.gov/funding/contracts/377
- 23.Dong ST, Butow PN, Agar M, et al. : Clinicians’ perspectives on managing symptom clusters in advanced cancer: A semistructured interview study. J Pain Symptom Manage 51:706-717.e5, 2016 [DOI] [PubMed] [Google Scholar]
- 24.Williams LA: Clinical management of symptom clusters. Semin Oncol Nurs 23:113-120, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Memorial Sloan Kettering Cancer Center : The Urgent Care Unit at MSK. https://www.mskcc.org/cancer-care/patient-education/urgent-care-center
- 26.Lipitz-Snyderman A, Klotz A, Atoria CL, et al. : Impact of observation status on hospital use for patients with cancer. J Oncol Pract 11:73-77, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Centers for Medicare & Medicaid Services : Admissions and emergency department visits for patients receiving outpatient chemotherapy measure technical report, 2016. https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/HospitalQualityInits/Measure-Methodology.html
- 28.Kirkova J, Aktas A, Walsh D, et al. : Cancer symptom clusters: Clinical and research methodology. J Palliat Med 14:1149-1166, 2011 [DOI] [PubMed] [Google Scholar]
- 29.Bubis LD, Davis L, Mahar A, et al. : Symptom burden in the first year after cancer diagnosis: An analysis of patient-reported outcomes. J Clin Oncol 36:1103-1111, 2018 [DOI] [PubMed] [Google Scholar]
- 30.Zhong X, Lim EA, Hershman DL, et al. : Identifying severe adverse event clusters using the National Cancer Institute’s Common Terminology Criteria for Adverse Events. J Oncol Pract 12:e270-e280, e245-e246, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pons P, Latapy M. Computing Communities in Large Networks Using Random Walks. Berlin, Germany, Springer, 2005 [Google Scholar]
- 32.Zhang H, Raitoharju J, Kiranyaz S, et al. : Limited random walk algorithm for big graph data clustering. J Big Data 3:26, 2016 [Google Scholar]
- 33.Miaskowski C: Future directions in symptom cluster research. Semin Oncol Nurs 32:405-415, 2016 [DOI] [PubMed] [Google Scholar]
- 34.Esper P: Symptom clusters in individuals living with advanced cancer. Semin Oncol Nurs 26:168-174, 2010 [DOI] [PubMed] [Google Scholar]