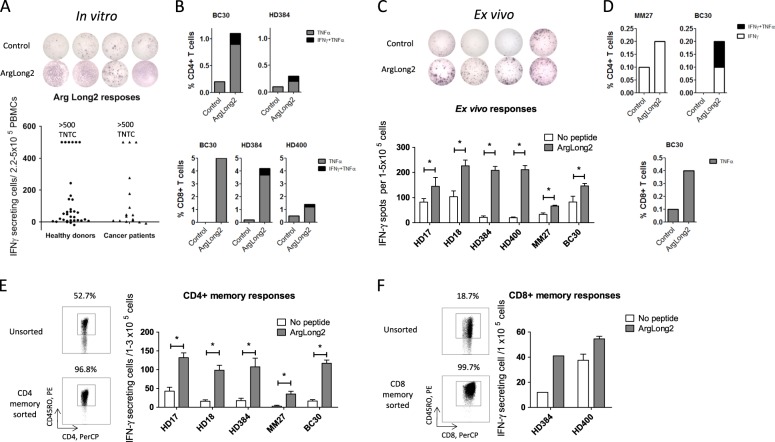
Increased arginase-1 (Arg-1) activity is one of the common mechanisms of immune inhibition in patients with cancer. Arg-1 is an enzyme catalyzing the first step in L-arginine metabolism and is expressed by various immunosuppressive myeloid cells as well as by cancer cells. Arg-1-associated deprivation of l-arginine downregulates the expression of the T cell receptor ζ chain and decreases cytokine production and proliferation of activated T cells.1,2
Recently, we described the existence of proinflammatory effector T cells that recognize Arg-1 and are found in both healthy donors and cancer patients.3 Importantly, however, the natural frequency, functional role and occurrence of these effector T cells is not yet well understood. Here, we examined the natural immunity towards Arg-1 in the periphery. We confirmed that frequent immune responses towards Arg-1 could be detected in healthy individuals (n = 33) as well as in cancer patients (n = 16; 8 melanoma, 6 multiple myeloma, 1 breast cancer, and 1 renal cell carcinoma) by ELISPOT after one stimulation with an Arg-1-derived peptide (ArgLong2, 38-mer, positions 169–206 in Arg-1) in vitro, with strong responses against Arg-1 detected in 22 of 33 healthy donors and 8 of 16 cancer patients (Fig. 1a). Furthermore, PBMCs from healthy donors and cancer patients showed both CD4+ and CD8+T cell responses against Arg-1 (Fig. 1b) in intracellular staining one week after in vitro stimulation with ArgLong2 and low-dose IL-2. Importantly, significant strong responses against Arg-1 were also detectable in the PBMCs of cancer patients and healthy donors ex vivo (Fig. 1c), and both CD4+ and CD8+ responses against Arg-1 were also seen in ex vivo intracellular staining of PBMCs from the same responders (Fig. 1d). These findings are especially noteworthy since (i) with very few exceptions, it has not been possible to detect tumor-associated antigen-specific T cells either by tetramer staining or by ELISPOT in PBMCs directly ex vivo4 and (ii) strong immune responses in healthy individuals suggest that Arg-1-specific T cells are a natural part of the immune system and may be important for immune homeostasis.5 To test this hypothesis, we isolated CD4+ and CD8+ memory T cells from PBMCs from healthy donors and cancer patients using magnetic bead sorting. We found statistically significant CD4+ memory responses against the ArgLong2 peptide in samples from two cancer patients and three healthy donors (Fig. 1e). Clear CD8+ memory responses were detected in samples from healthy donors (Fig. 1f). Our data show that Arg-1-specific self-reactive T cells are a naturally occurring part of the memory T cell repertoire of the human immune system. The suppressive effects of cells expressing high levels of Arg-1 are likely to hinder Arg-1-specific T cells themselves. Thus, in immune regulatory networks, Arg-1-specific T cells suppress the function of Arg-1+ regulatory immune cells and vice versa. This suppression is the likely reason why we do not see autoimmunity in healthy donors despite the very strong ex vivo Arg-1-specific T cell responses (Fig. 1c–f).
Fig. 1.
Responses against Arg-1 are found in CD4+ and CD8+ memory T cells in cancer patients and healthy donors. a – IFNγ ELISPOT responses against the ArgLong2 peptide in 33 healthy donors and 16 cancer patients. b, d – In vitro (b) and ex vivo (d) IFNγ and TNFα responses against ArgLong2 peptide in CD4+ (Top) and CD8+ (Bottom) T cells in intracellular staining of PBMCs from healthy donors (HD) and cancer patients. c – Ex vivo responses against ArgLong2 peptide in four healthy donors and two cancer patients in an IFNγ ELISPOT. e, f – CD4+ memory (e – Left) and CD8+ memory (f – Left) T cell staining in PBMCs and sorted memory cells. Ex vivo IFNγ ELISPOT of sorted CD4+ memory (e – Right) and CD8+ memory (f – Right) T cells (CD45RO+) from PBMCs of healthy donors and cancer patients. ELISPOT assays performed in triplicate or duplicate, average number of IFNγ-producing cells in peptide-stimulated and control wells + SEM. BC, breast cancer; and MM, malignant melanoma. Statistical analysis was performed using distribution-free resampling, using RStudio, and GraphPad v 5.01. TNTC, too numerous to count (≥500 spots)
The strong, natural proinflammatory immune response towards Arg-1 highlights the potential of Arg-1 epitopes in cancer immunotherapy. Many Arg-1-expressing immunosuppressive myeloid cells are not terminally differentiated and may be reverted under the proinflammatory stimulus of activated Arg-1-specific CD4+T cells or depleted by Arg-1-specific CD8+T cells, contrary to other current clinical strategies targeting tumor-associated macrophages.6 The 38 amino acid ArgLong2 peptide used in this study is efficient at stimulating Arg-1-specific CD4+ and CD8+T cell responses, which may both be vital for rebalancing the microenvironment. Furthermore, the ArgLong2 peptide could increase the effect of T cell-enhancing drugs such as checkpoint blockers in comparison to single approach therapies or current cancer vaccines that only aim to induce cancer-specific CD8+cytotoxic T cells.
Although it is known that self-reactive T cells with high affinity for a target/HLA complex undergo clonal deletion in the thymus, self-antigen-specific T cells are present in the blood at frequencies similar to those of T cells specific to non-self-antigens.7 Other autoreactive T cells, such as IDO- and PD-L1-specific proinflammatory T cells, have been described8,9 and in a similar manner, can recognize immune regulatory cells that express these proteins.5,10 Thus, the combination of epitopes from different target antigens of self-reactive T cells may be important in the development of new approaches for treating cancer by modulating the immune system through direct elimination of regulatory immune cells and boosting of local immune activation through effector cytokines.
Contributor Information
Evelina Martinenaite, Email: evelina.martinenaite@regionh.dk.
Mads Hald Andersen, Phone: +4538682602, Email: mads.hald.andersen@regionh.dk.
References
- 1.Singhal S, et al. Human tumor-associated monocytes/macrophages and their regulation of T cell responses in early-stage lung cancer. Sci. Transl. Med. 2019;11:11–479. doi: 10.1126/scitranslmed.aat1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vanpouille-Box C, Formenti SC. Dual transforming growth factor-beta and programmed death-1 blockade: a strategy for immune-excluded tumors? Trends Immunol. 2018;39:435–437. doi: 10.1016/j.it.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martinenaite E, et al. Frequent spontaneous adaptive immune responses towards arginase. Oncoimmunology. 2017;7:e1404215. doi: 10.1080/2162402X.2017.1404215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keilholz U, et al. Immunologic monitoring of cancer vaccine therapy: results of a workshop sponsored by the Society for Biological Therapy. J. Immunother. 2002;25:97–138. doi: 10.1097/00002371-200203000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Andersen MH. The balance players of the adaptive immune system. Cancer Res. 2018;78:1379–1382. doi: 10.1158/0008-5472.CAN-17-3607. [DOI] [PubMed] [Google Scholar]
- 6.Cassetta, L. & Pollard, J. W. Targeting macrophages: therapeutic approaches in cancer. Nat. Rev. Drug Discov. 17, 887–904 (2018). [DOI] [PubMed]
- 7.Yu W, et al. Clonal deletion prunes but does not eliminate self-specific alphabeta CD8(+) T lymphocytes. Immunity. 2015;19;42:929–941. doi: 10.1016/j.immuni.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sorensen RB, et al. Indoleamine 2,3-dioxygenase specific, cytotoxic T cells as immune regulators. Blood. 2011;117:2200–2210. doi: 10.1182/blood-2010-06-288498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Munir S, et al. HLA-restricted cytotoxic T cells that are specific for the immune checkpoint ligand PD-L1 occur with high frequency in cancer patients. Cancer Res. 2013;73:1674–1776. doi: 10.1158/0008-5472.CAN-12-3507. [DOI] [PubMed] [Google Scholar]
- 10.Andersen MH. Immune regulation by self-recognition: novel possibilities for anticancer immunotherapy. J. Natl. Cancer Inst. 2015;107:154. doi: 10.1093/jnci/djv154. [DOI] [PMC free article] [PubMed] [Google Scholar]