Abstract
Objectives
The National Lung Screening Trial (NLST) revealed that low-dose computed tomography (LDCT) screening could reduce lung cancer mortality in heavy smokers. Lung screening with LDCT was implemented in July 2019 as part of the National Cancer Screening Program in Korea for heavy smokers who meet NLST criteria [smokers aged 55–74 years with 30 pack-years (PY) or more, excluding former smokers with more than 15 years since smoking cessation]. This study evaluated NLST-eligible heavy smokers’ adherence to general medical checkup and cancer screening guidelines.
Methods
Using the Korea National Health and Nutrition Examination Survey (KNHANES) from 2010 to 2012, we compared adherence of Korean adults (55–74 years, n = 5,480) to general medical checkup and cancer (gastric, colorectal, breast, and cervical) screening guidelines according to self-reported smoking status. Smoking and PY data were available, but no data indicating when former smokers ceased smoking were available. Accordingly, smoking status was only classified as NLST (smokers with a history ≥ 30 PY) and non-NLST. Individuals who met NLST criteria were subdivided into current (NLST-current) and former smokers (NLST-former). Multivariable logistic regression was used to evaluate adherence to screening recommendations as a function of the study group (NLST-current, NLST-former, non-NLST) using possible covariates (sociodemographic factors, health-related behaviors, comorbidities, and self-reported health status).
Results
Weighted prevalence of NLST-current was 9.7%, of NLST-former was 9.6%, and of non-NLST was 80.7%. Overall screening rates were 70.7% (medical checkup), 59.1% (stomach cancer), 58.1% (colorectal cancer), 59.1% (breast cancer), and 48.9% (cervical cancer). Adherence to colorectal cancer screening and medical checkup was lower in NLST-current than non-NLST (AOR 0.59; 95% CI 0.44–0.78 for colorectal cancer; AOR 0.70; 95% CI 0.52–0.95 for medical checkup). Screening practices for other cancers were not different.
Conclusions
Current heavy smokers meeting NLST criteria were less likely to have colorectal cancer screening or general medical checkup. Understanding the screening practices of this target population might enable the development of more effective plans to implement lung screening and improve screening compliance for other cancers.
Introduction
Lung cancer is one of the most common malignancies and a leading cause of cancer death among both men and women [1]. Lung cancer frequently presents at advanced stages, and the prognosis is poor. Given that the stage of the tumor has the greatest impact on lung cancer prognosis, early diagnosis is important. In 2011, the National Lung Screening Trial (NLST) showed that low-dose computed tomography (LDCT) screening led to a 20.3% reduction in lung cancer mortality and a 6.7% decrease in all-cause mortality [2]. The NLST selected participants with a high risk of lung cancer based on age and cumulative tobacco smoke exposure. Participants were required to be 55 to 74 years old and heavy smokers with a history of 30 pack-years (PY) or more, except for ex-smokers with more than 15 years since smoking cessation [2]. Based on this evidence, the National Cancer Information Center has been recommending lung cancer screening for people who meet the NLST criteria since 2015 in Korea [3].
Cigarette smoking is the leading preventable cause of death [4, 5] and is known to cause cancer in various organs, including the lungs [6, 7], colon [8–10], breast [11, 12], stomach [13, 14], and uterus/cervix [15]. Moreover, smokers tend to have unhealthy lifestyle behaviors [16–18] and to comply less with cancer screening guidelines than never-smokers [19–21]. Therefore, although smoking cessation remains the most important cancer prevention method, subjects who smoke may potentially receive the greatest benefit from improved cancer screening, which could detect occult diseases at earlier stages.
In Korea, organized screening for stomach, colorectal, breast, and cervical cancer has been provided at no or minimal cost by the government as part of the National Cancer Screening Program (NCSP) since 2001 [5]. Since July 2019, lung cancer screening with LDCT has been included in the NCSP, and the target population is heavy smokers who meet the NLST criteria [22]. Understanding the screening practice pattern for this target population is important for the development of strategic plans to implement LDCT screening as well as to improve screening compliance for other cancers.
This study evaluated adherence to general medical checkup and cancer screening recommendations in heavy smokers who met NLST criteria.
Materials and methods
Study participants
We used data collected during the fifth Korea National Health and Nutrition Examination Survey (KNHANES V), which was conducted by the Korea Centers for Disease Control and Prevention from January 2010 to December 2012. The KNHANES is a cross-sectional, nationally representative survey conducted to determine the health and nutritional status of the civilian, noninstitutionalized Korean population.
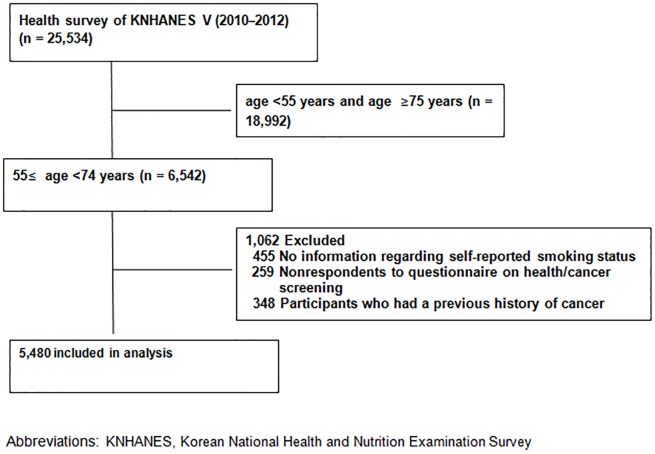
The KNHANES is composed of a health questionnaire, nutrition survey, and health examination; participants were chosen by proportional allocation-systematic sampling with multistage stratification (by age, sex, and region). All data used in this study were fully anonymized prior to assessment. All procedures and terms and conditions of the survey have been complied with and were performed in accordance with the Declaration of Helsinki 7th version, and informed consent was obtained from all participants. The dataset and questionnaire is provided with guidelines for calculating a health-related index through the KCDC online site (https://knhanes.cdc.go.kr/knhanes/eng/index.do). Of the 25,534 participants, the final study population included a total of 5,480 individuals aged 55 to 74 years (Fig 1).
Fig 1. Flow diagram for selection of the study population.
In the KNHANES, written informed consent was provided by every participant. The KNHANES survey was approved by the Institutional Review Board of the Korea Centers for Disease Control and Prevention (IRB No. 2010-02CON-21-C, 2011-02CON-06-C, 2012-01EXP-01-2C). Further ethical approval for the use of KNHANES data are not required because publicly available datasets were used in this study.
Smoking status
In the survey, all participants were asked if they had smoked at least 100 cigarettes in their lifetime. Those who responded “yes” were further asked if they currently smoke cigarettes, which enabled individuals to be categorized as current smokers or former smokers. PY information was available for both current and ex-smokers; however, the KNHANES V did not indicate when ex-smokers quit smoking. Therefore, individuals were categorized into two groups according to their self-reported smoking status: NLST (smokers with a history of ≥30 PY) and non-NLST (never-smokers and smokers with a history of <30 PY). Individuals who met the NLST criteria were subdivided into current (NLST-current) and former smokers (NLST-former).
Adherence to general medical checkups and cancer screening recommendations
Using the KNHANES for each cancer type, the participants were asked whether they had ever had a screening test, and if so, the length of time since the last test.
Adherence to general medical checkup guidelines was determined by asking participants if they had seen a doctor for a general medical checkup within the past two years. To define adherence to cancer screening guidelines, the NCSP [5] was used to determine age- and sex-appropriate cancer screening compliance; stomach cancer screening (endoscopy or upper gastrointestinal series) is recommended every 2 years in men and women 40 years of age or older; colorectal cancer screening (colonoscopy or barium enema) is recommended every 5 years in men and women 50 years of age or older; breast cancer screening (mammography) is recommended every 2 years in women 40 years of age or older; and cervical cancer screening (Papanicolaou (Pap) smear) is recommended every 2 years in women 30 years of age or older.
Independent variables
Independent variables that have been associated with screening practices included sociodemographic variables [age [23, 24], sex, marital status [23, 25, 26], education level [23, 24, 27], household income [25, 26, 28], insurance status [23, 24, 27], and body mass index (BMI)], health-related lifestyle factors (drinking alcohol [28] and exercise), and health status (history of chronic diseases such as hypertension, diabetes, dyslipidemia, and perceived health status) [23, 24, 29].
Sociodemographic variables included current age (age of the respondents was categorized into five-year groups; 55 to 59, 60 to 64, 65 to 69, and 70 to 74) and marital status (unmarried, separated, widowed, and divorced participants were allocated ‘‘no spouse” status). Household income level was divided into national quartile groups (lowest quartile, second to third quartile, and highest quartile). Education level was categorized as less than elementary, middle/high school, and college or higher. National insurance status (national health insurance or medical aid), private health insurance (no, yes), and weight status (BMI <25 kg/m2 or BMI ≥25 kg/m2) were also included [30].
For health-related lifestyle factors, alcohol consumption was assessed using the Alcohol Use Disorders Identification Test (AUDIT, in which scores of ≥12 are heavy drinkers and <12 are not heavy drinkers) [31]. Routine exercisers were defined as people who performed at least low-intensity physical activity, which was defined as walking or commuting for >30 minutes more than three times per week.
Comorbidities include self-reported physician’s diagnoses of hypertension, diabetes, and dyslipidemia, and perceived health status is categorized as good, normal, and poor.
Statistical analysis
Baseline characteristics are presented as percentages (± standard errors of percentages) for categorical variables and as estimated means (± standard errors of means) for continuous variables according to smoking status. Categorical variables and continuous variables were compared using the chi-square test and the Student’s t-test, respectively. The chi-square test was used to compare screening rates between subjects with different smoking statuses (NLST-current, NLST-former, non-NLST); P values < .016 were considered significant using Bonferroni’s method in case of multiple comparisons.
Using multivariable logistic regression, we calculated the odds ratio (OR) and 95% confidential interval (CI) for the probability of receiving screening as a function of the study group (NLST-current, NLST-former, non-NLST) and other covariates. Gradual modeling was used for adjusting potential covariates. Covariates in model 1 included sociodemographic factors, such as sex, age group, BMI, education level, marital status, insurance status, and private insurance; covariates in model 2 included sociodemographic factors and behavioral risk factors (alcohol consumption and exercise); and covariates in model 3 included sociodemographic factors, behavioral risk factors, and personal health status (comorbidities and perceived health status).
All analyses were adjusted for the complex survey design in the KNHANES using the complex sample analysis program in Predictive Analytics Software 18.0 (SPSS Inc., Chicago, Illinois, USA), and P values < .05 (two-sided) were considered significant.
Results
General characteristics of the study population
Characteristics of the study sample are shown in Table 1. Of the 5,480 participants (mean age, 63.1 ± 0.1 years; men, 48.0%), the weighted prevalence of NLST-current was 9.7% (unweighted n = 432), NLST-former was 9.6% (unweighted n = 489) and of non-NLST was 80.7% (unweighted n = 4,559).
Table 1. Characteristics of Korean adults (55–74 years) according to self-reported smoking status (n = 5,480).
| Characteristics | Non-NLST (unweighted n = 4,559) |
NLST-former (unweighted n = 489) |
NLST-current (unweighted n = 432) |
P value |
|---|---|---|---|---|
| Sex, male | 36.1 (0.7) | 98.0 (0.9) | 97.3 (1.1) | < .001* |
| Age (mean, years) | 63.2 (0.1) | 63.7 (0.3) | 61.6 (0.3) | < .001† |
| BMI (mean, years) | 24.3 (0.1) | 24.3 (0.2) | 23.3 (0.2) | < .001† |
| Obesity (BMI ≥25 kg/m2) | 38.7 (1.0) | 39.8 (2.7) | 25.7 (2.3) | .001* |
| Spouse, yes | 80.7 (0.7) | 90.8 (1.8) | 90.8 (1.7) | < .001* |
| Household income | .846* | |||
| Lowest quartile | 28.9 (1.0) | 28.2 (2.4) | 31.4 (2.6) | |
| 2nd and 3rd quartile | 51.2 (1.1) | 50.3 (2.7) | 49.4 (2.7) | |
| Highest quartile | 19.9 (0.9) | 21.4 (2.4) | 19.2 (2.4) | |
| Education | < .001* | |||
| Less than elementary school | 52.6 (1.1) | 42.3 (2.9) | 39.0 (3.0) | |
| Middle or high school | 37.9 (1.0) | 47.0 (2.8) | 54.1 (3.0) | |
| College and above | 9.5 (0.7) | 10.7 (1.6) | 6.9 (1.5) | |
| Insurance | .005* | |||
| National health insurance | 97.5 (0.3) | 94.6 (1.3) | 96.5 (1.0) | |
| Medical aid | 2.5 (0.3) | 5.4 (1.3) | 3.5 (1.0) | |
| Private Insurance, yes | 59.3 (1.0) | 56.9 (2.8) | 55.0 (3.2) | .316* |
| Heavy alcohol drinker | 15.2 (0.8) | 36.3 (2.9) | 49.3 (2.8) | < .001* |
| Routine exercise | 46.8 (1.0) | 49.9 (2.8) | 44.4 (3.2) | .390* |
| HTN | 41.0 (1.0) | 45.0 (2.7) | 29.5 (2.6) | < .001* |
| DM | 14.0 (0.6) | 19.0 (2.2) | 23.1 (2.5) | < .001* |
| Hyperlipidemia | 19.1 (0.8) | 16.9 (2.2) | 15.3 (2.3) | .217* |
| Perceived health status | .266* | |||
| Good to very good | 28.6 (0.9) | 28.6 (2.5) | 24.4 (2.5) | |
| Normal | 45.2 (1.0) | 49.1 (2.8) | 49.2 (2.8) | |
| Poor to very poor | 26.2 (0.8) | 22.3 (2.4) | 26.4 (2.6) |
Values are weighted means (standard errors of means) or weighted percentages (standard errors of percentages).
*Pearson’s chi-squared test.
†Student’s t-test
Abbreviations: BMI, body mass index; HTN, hypertension; DM, diabetes mellitus
NLST-current and NLST-former had a higher proportion of male subjects than non-NLST (proportion of male subjects: 97.3% NLST-current, 98.0% NLST-former, 36.1% non-NLST, p < .001). Household income, private insurance, routine exercise, presence of hyperlipidemia, and perceived health status were not significantly different between subjects with different smoking status.
General medical checkup and cancer screening practices
National health and cancer screening rates in NLST-current, NLST-former, and non-NLST are shown in Table 2. In Korean adults aged 55–74 years, the overall adherence to general medical checkup guidelines was 70.7%. The overall adherence to stomach cancer screening recommendations was 59.1%, colorectal cancer was 58.1%, breast cancer was 59.1%, and cervical cancer was 48.9%. In NLST-current, the adherence for general medical checkups, and for stomach, colorectal, breast, and cervical cancer screenings were 64.2%, 52.8%, 46.1%, 59.1%, and 48.9%, respectively. The screening rates for general medical checkups (P = .011; 62.4% in NLST-current vs. 71.6% in non-NLST) and colorectal cancer (P < .001; 46.1% in NLST-current vs. 59.1% in non-NLST) were significantly lower in NLST-current compared with non-NLST.
Table 2. Medical checkup and cancer screening rates among Korean adults aged 55–74 years according to self-reported smoking status.
| Non-NLST (unweighted n = 4,559) |
NLST-former (unweighted n = 489) |
NLST-current (unweighted n = 432) |
Total (unweighted n = 5,480) |
P value | |
|---|---|---|---|---|---|
| General medical checkup in last 2 yrs | 71.6 (0.9) | 70.2 (2.6) | 64.2 (3.1) | 70.7 (0.9) | .038b |
| Stomach cancer screening in last 2 yrs | 59.9 (1.0) | 59.3 (2.9) | 52.8 (3.1) | 59.1 (0.9) | .075 |
| Colorectal cancer screening in last 5 yrs | 59.1 (1.0) | 61.8 (2.8) | 46.1 (2.9) | 58.1 (1.0) | < .001c |
| Breast cancer screening in last 2 yrsa | 59.2 (1.1) | 74.4 (15.3) | 40.5 (21.2) | 59.1 (1.1) | .487 |
| Cervical cancer screening in last 2 yrsa | 48.9 (1.2) | 74.7(15.3) | 36.0 (21.7) | 48.9 (1.2) | .341 |
aFemales only (n = 3,164)
bP = .011 for NLST-current vs. non-NLST, P = .612 for NLST-former vs. non-NLST
cP < .001 for NLST-current vs. non-NLST, P = .362 for NLST-former vs. non-NLST
Crude and adjusted OR for medical checkups and cancer screenings according to smoking status are shown in Table 3. For general medical checkups, NLST-current had lower adherence than non-NLST in the unadjusted model (OR 0.71; 95% CI 0.55–0.93) and even after adjusting for covariates (AOR 0.70; 95% CI 0.52–0.95 for model 3). NLST-current had a lower adherence to colorectal cancer screening recommendations than non-NLST even after adjusting for sociodemographic factors, health-related behaviors, and comorbidities (AOR 0.59; 95% CI 0.44–0.78). However, NLST-former showed no significant difference in adherence to general medical checkups and colorectal cancer screening. Furthermore, adherence to other cancer (gastric, breast, and cervical cancer) screenings was not different according to the self-reported smoking status.
Table 3. Unadjusted and adjusted odds ratio (95% confidence interval) for medical checkup and cancer screening practices according to self-reported smoking status.
| Unadjusted OR (95% CI) |
Model 1 AOR (95% CI) |
Model 2 AOR (95% CI) |
Model 3 AOR (95% CI) |
|
|---|---|---|---|---|
| General medical checkup in last 2 yrs | ||||
| Non-NLST | Reference | Reference | Reference | Reference |
| NLST-former | 0.94 (0.72–1.21) | 0.85 (0.63–1.15) | 0.86 (0.63–1.17) | 0.84 (0.61–1.15) |
| NLST-current | 0.71 (0.55–0.93) | 0.67 (0.50–0.90) | 0.69 (0.51–0.93) | 0.70 (0.52–0.95) |
| Stomach cancer screening in last 2 yrs | ||||
| Non-NLST | Reference | Reference | Reference | Reference |
| NLST-former | 0.94 (0.77–1.25) | 0.94 (0.71–1.25) | 0.99 (0.73–1.32) | 0.97 (0.72–1.31) |
| NLST-current | 0.75 (0.59–0.96) | 0.74 (0.57–0.989) | 0.76 (0.57–1.01) | 0.77 (0.57–1.03) |
| Colorectal cancer screening in last 5 yrs | ||||
| Non-NLST | Reference | Reference | Reference | Reference |
| NLST-former | 1.12 (0.88–1.42) | 1.10 (0.83–1.46) | 1.11 (0.83–1.49) | 1.09 (0.81–1.46) |
| NLST-current | 0.59 (0.47–0.75) | 0.59 (0.45–0.77) | 0.59(0.45–0.79) | 0.59 (0.44–0.78) |
| Breast cancer screening in last 2 yrsa | ||||
| Non-NLST | Reference | Reference | Reference | Reference |
| NLST-former | 2.01 (0.41–9.84) | 4.95 (0.87–28.23) | 2.17 (0.25–18.93) | 2.35 (0.29–19.36) |
| NLST-current | 0.47 (0.08–2.65) | 0.51 (0.12–2.14) | 0.48 (0.11–2.04) | 0.49 (0.12–2.05) |
| Cervical cancer screening in last 2 yrsa | ||||
| Non-NLST | Reference | Reference | Reference | Reference |
| NLST-former | 3.04 (0.62–14.96) | 7.59 (1.19–48.27) | 3.26 (0.36–29.28) | 3.87 (0.42–35.24) |
| NLST-current | 0.59 (0.09–3.76) | 0.59 (0.13–2.74) | 0.62 (0.13–2.99) | 0.68 (0.15–3.20) |
aFemales only (n = 3,164)
Abbreviations: (A)OR, (adjusted) odds ratio, CI, confidence interval.
Model 1 was adjusted for sociodemographic factors such as sex, age group, obesity, education level, marital status, income level, insurance status, and private insurance. Model 2 was adjusted for sociodemographic factors and behavioral risk factors (alcohol consumption and exercise). Model 3 was adjusted for sociodemographic factors, behavioral risk factors, and personal health status (history of chronic disease and perceived health status).
Discussion
Lung cancer is one of the most common malignancies and a leading cause of cancer deaths in men and women [1]. NLST, the randomized controlled trial, showed a reduction in lung cancer mortality (20.3%) and in all-cause mortality (6.7%) with LDCT screening in comparison with subjects who underwent chest radiography [2]. NLST defined participants with a high risk of lung cancer based on age (55–74 years) and cumulative tobacco smoke exposure (heavy smokers with a history of 30 pack-years (PY) or more, except for ex-smokers with more than 15 years since smoking cessation) [2]. Based on this evidence, the United States Preventive Service Task Force published recommendations encouraging annual lung screenings for individuals at high risk for lung cancer in March 2014. In Korea, lung cancer screening has commonly been conducted as a part of opportunistic screening programs, based on physician recommendations and individual preferences. Recently, the Korean government implemented lung screening with LDCT for heavy smokers who met NLST criteria as part of NSCP.
Although it is known that smokers tend to have unhealthy behaviors [16–18] and are less compliance with screening guidelines than non-smokers [19–21], no study has examined adherence to medical checkup guidelines and national cancer screening recommendations specific to heavy smokers in Korea, who are the target population for lung screening. In the present study, NLST-current had lower adherence to general medical checkup guidelines and colorectal cancer screening recommendations compared with non-NLST, even after adjusting for covariates. NLST-former was no different from non-NLST in the adherence to general medical checkup and colorectal cancer screening. These findings are similar to a recent study [32] that showed large differences in health care seeking practices between current and former smokers. Current smokers are less likely to screen for breast and colorectal cancer compared to never and former smokers [32]. Further research is needed to identify barriers to screening among current smokers, with the goal of increasing acceptance and uptake of cancer screening among this population at a high risk for cancer. Current heavy smokers’ low adherence to general medical checkup and colorectal cancer screening guidelines suggests that healthcare workers should also encourage these screenings in addition to lung screening.
This study has several limitations. First, defining smokers based on self-reporting is regarded as reliable in population-based surveys in Western populations [33]. However, several studies reported an underestimation of the true number of smokers in Asian populations [34]. Furthermore, smoking rates in women are reported as very low in East Asian countries, and under-reporting of hidden female smokers is an emerging issue. Furthermore, the KNHANES V did not include valid information regarding when former smokers quit smoking. Therefore, a slight disjoint of group definitions according to smoking status in the present study (NLST-current, NLST-former, non-NLST) could not be avoided. NLST-current and NLST-former are not identical in the target population for lung screening (i.e., NLST-current are the target population for lung screening but NLST-former are not if they quit smoking for more than 15 years), and non-NLST are not identical to never-smokers (non-NLST include smokers less than 30 PY, as well as never-smokers). Second, response bias could have been introduced when participants were asked questions about lifestyle habits, history of recent medical checkups, and cancer screening practices. This survey explored whether participants were screened in past years, and there is a potential for recall bias. Third, expecting LDCT adherence has limitations even with a full understanding of the adherence patterns associated with medical check-ups and other cancer screening practices. The LDCT examination has distinct characteristics from other screening methods. It is a less invasive and painful procedure compared to endoscopy, colonoscopy, or Pap smears. However, some participants might avoid lung screening due to the possible harms of LDCT examination (i.e., potential for radiation-induced carcinogenesis, high false-positivity rates, and overdiagnosis issue) [35].
In conclusion, current heavy smokers who meet NLST criteria were less likely to be screened for a general medical checkup and colorectal cancer; however, no difference was observed in adherence to general medical checkups and other cancer screenings. Understanding the screening practice patterns for this target population that is subject to lung screening recommendations might enable the development of more effective plans to implement lung screening as well as to improve screening compliance for other cancers.
Data Availability
Data were obtained from the KNHANES (the Korea National Health and Nutrition Surveys). The authors specifically used the 2010-12 KNHANES dataset. These data can be downloaded from the KNHANES webpage (URL: https://knhanes.cdc.go.kr/knhanes/sub03/sub03_02_02.do) under the names HN10_ALL.SAV, HN11_ALL.SAV. and HN12_ALL.SAV.
Funding Statement
This study was supported by a grant from the National R&D Program for Cancer Control, Ministry of Health & Welfare, Republic of Korea (grant no. HA17C0045). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA: a cancer journal for clinicians. 2016;66(1):7–30. Epub 2016/01/09. 10.3322/caac.21332 . [DOI] [PubMed] [Google Scholar]
- 2.Kramer BS, Berg CD, Aberle DR, Prorok PC. Lung cancer screening with low-dose helical CT: results from the National Lung Screening Trial (NLST). J Med Screen. 2011;18(3):109–11. 10.1258/jms.2011.011055 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jang SH, Sheen S, Kim HY, Yim HW, Park BY, Kim JW, et al. The Korean guideline for lung cancer screening. J Korean Med Assoc. 2015;58:291–301. [Google Scholar]
- 4.WHO urges more countries to require large, graphic health warnings on tobacco packaging: the WHO report on the global tobacco epidemic, 2011 examines anti-tobacco mass-media campaigns. Cent Eur J Public Health. 2011;19(3):133, 51. . [PubMed] [Google Scholar]
- 5.Yoo KY. Cancer control activities in the Republic of Korea. Jpn J Clin Oncol. 2008;38(5):327–33. 10.1093/jjco/hyn026 . [DOI] [PubMed] [Google Scholar]
- 6.Yu YW, Wang CP, Han YF, Niu JJ, Zhang YZ, Fang Y. [Meta-analysis on related risk factors regarding lung cancer in non-smoking Chinese women]. Zhonghua Liu Xing Bing Xue Za Zhi. 2016;37(2):268–72. 10.3760/cma.j.issn.0254-6450.2016.02.024 . [DOI] [PubMed] [Google Scholar]
- 7.Lee PN, Forey BA, Coombs KJ. Systematic review with meta-analysis of the epidemiological evidence in the 1900s relating smoking to lung cancer. BMC Cancer. 2012;12:385 10.1186/1471-2407-12-385 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Botteri E, Iodice S, Bagnardi V, Raimondi S, Lowenfels AB, Maisonneuve P. Smoking and colorectal cancer: a meta-analysis. JAMA. 2008;300(23):2765–78. 10.1001/jama.2008.839 . [DOI] [PubMed] [Google Scholar]
- 9.Vineis P, Alavanja M, Buffler P, Fontham E, Franceschi S, Gao YT, et al. Tobacco and cancer: recent epidemiological evidence. J Natl Cancer Inst. 2004;96(2):99–106. 10.1093/jnci/djh014 . [DOI] [PubMed] [Google Scholar]
- 10.Sasco AJ, Secretan MB, Straif K. Tobacco smoking and cancer: a brief review of recent epidemiological evidence. Lung Cancer. 2004;45 Suppl 2:S3–9. 10.1016/j.lungcan.2004.07.998 . [DOI] [PubMed] [Google Scholar]
- 11.Gao CM, Ding JH, Li SP, Liu YT, Qian Y, Chang J, et al. Active and passive smoking, and alcohol drinking and breast cancer risk in chinese women. Asian Pac J Cancer Prev. 2013;14(2):993–6. 10.7314/apjcp.2013.14.2.993 . [DOI] [PubMed] [Google Scholar]
- 12.Gaudet MM, Gapstur SM, Sun J, Diver WR, Hannan LM, Thun MJ. Active smoking and breast cancer risk: original cohort data and meta-analysis. J Natl Cancer Inst. 2013;105(8):515–25. 10.1093/jnci/djt023 . [DOI] [PubMed] [Google Scholar]
- 13.Shikata K, Doi Y, Yonemoto K, Arima H, Ninomiya T, Kubo M, et al. Population-based prospective study of the combined influence of cigarette smoking and Helicobacter pylori infection on gastric cancer incidence: the Hisayama Study. Am J Epidemiol. 2008;168(12):1409–15. 10.1093/aje/kwn276 . [DOI] [PubMed] [Google Scholar]
- 14.Ladeiras-Lopes R, Pereira AK, Nogueira A, Pinheiro-Torres T, Pinto I, Santos-Pereira R, et al. Smoking and gastric cancer: systematic review and meta-analysis of cohort studies. Cancer Causes Control. 2008;19(7):689–701. 10.1007/s10552-008-9132-y . [DOI] [PubMed] [Google Scholar]
- 15.International Collaboration of Epidemiological Studies of Cervical C. Comparison of risk factors for invasive squamous cell carcinoma and adenocarcinoma of the cervix: collaborative reanalysis of individual data on 8,097 women with squamous cell carcinoma and 1,374 women with adenocarcinoma from 12 epidemiological studies. Int J Cancer. 2007;120(4):885–91. 10.1002/ijc.22357 . [DOI] [PubMed] [Google Scholar]
- 16.Berrigan D, Dodd K, Troiano RP, Krebs-Smith SM, Barbash RB. Patterns of health behavior in U.S. adults. Prev Med. 2003;36(5):615–23. 10.1016/s0091-7435(02)00067-1 . [DOI] [PubMed] [Google Scholar]
- 17.Ruidavets JB, Bataille V, Dallongeville J, Simon C, Bingham A, Amouyel P, et al. Alcohol intake and diet in France, the prominent role of lifestyle. Eur Heart J. 2004;25(13):1153–62. 10.1016/j.ehj.2003.12.022 . [DOI] [PubMed] [Google Scholar]
- 18.de Vries H, van ’t Riet J, Spigt M, Metsemakers J, van den Akker M, Vermunt JK, et al. Clusters of lifestyle behaviors: results from the Dutch SMILE study. Prev Med. 2008;46(3):203–8. 10.1016/j.ypmed.2007.08.005 . [DOI] [PubMed] [Google Scholar]
- 19.Shapiro JA, Seeff LC, Nadel MR. Colorectal cancer-screening tests and associated health behaviors. Am J Prev Med. 2001;21(2):132–7. . [DOI] [PubMed] [Google Scholar]
- 20.Carlos RC, Fendrick AM, Patterson SK, Bernstein SJ. Associations in breast and colon cancer screening behavior in women. Acad Radiol. 2005;12(4):451–8. 10.1016/j.acra.2004.12.024 . [DOI] [PubMed] [Google Scholar]
- 21.Rakowski W, Meissner H, Vernon SW, Breen N, Rimer B, Clark MA. Correlates of repeat and recent mammography for women ages 45 to 75 in the 2002 to 2003 Health Information National Trends Survey (HINTS 2003). Cancer Epidemiol Biomarkers Prev. 2006;15(11):2093–101. 10.1158/1055-9965.EPI-06-0301 . [DOI] [PubMed] [Google Scholar]
- 22.Lung cancer screening for high-risk smokers between 55 and 74 years old. Goyang: The Ministry of Health and Welfare; 2019 [updated 30 July]. http://www.mohw.go.kr/eng/index.jsp
- 23.Kwak MS, Park EC, Bang JY, Sung NY, Lee JY, Choi KS. [Factors associated with cancer screening participation, Korea]. J Prev Med Public Health. 2005;38(4):473–81. . [PubMed] [Google Scholar]
- 24.Lee K, Lim HT, Park SM. Factors associated with use of breast cancer screening services by women aged >or = 40 years in Korea: the third Korea National Health and Nutrition Examination Survey 2005 (KNHANES III). BMC Cancer. 2010;10:144 10.1186/1471-2407-10-144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duffy CM, Clark MA, Allsworth JE. Health maintenance and screening in breast cancer survivors in the United States. Cancer Detect Prev. 2006;30(1):52–7.. [DOI] [PubMed] [Google Scholar]
- 26.Fukuda Y, Nakamura K, Takano T, Nakao H, Imai H. Socioeconomic status and cancer screening in Japanese males: Large inequlaity in middle-aged and urban residents. Environ Health Prev Med. 2007;12(2):90–6. 10.1007/BF02898155 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sheen V, Tucker MA, Abramson DH, Seddon JM, Kleinerman RA. Cancer screening practices of adult survivors of retinoblastoma at risk of second cancers. Cancer. 2008;113(2):434–41. 10.1002/cncr.23564 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kwon YM, Lim HT, Lee K, Cho BL, Park MS, Son KY, et al. Factors associated with use of gastric cancer screening services in Korea. World J Gastroenterol. 2009;15(29):3653–9. 10.3748/wjg.15.3653 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wardle J, Miles A, Atkin W. Gender differences in utilization of colorectal cancer screening. J Med Screen. 2005;12(1):20–7. 10.1258/0969141053279158 . [DOI] [PubMed] [Google Scholar]
- 30.Consultation WHOE. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363(9403):157–63. 10.1016/S0140-6736(03)15268-3 . [DOI] [PubMed] [Google Scholar]
- 31.Osaki Y, Ino A, Matsushita S, Higuchi S, Kondo Y, Kinjo A. Reliability and validity of the alcohol use disorders identification test—consumption in screening for adults with alcohol use disorders and risky drinking in Japan. Asian Pac J Cancer Prev. 2014;15(16):6571–4. 10.7314/apjcp.2014.15.16.6571 [DOI] [PubMed] [Google Scholar]
- 32.Sanford NN, Sher DJ, Butler S, Xu X, Ahn C, D’Amico AV, et al. Cancer Screening Patterns Among Current, Former, and Never Smokers in the United States, 2010–2015. JAMA Netw Open. 2019;2(5):e193759 10.1001/jamanetworkopen.2019.3759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wong SL, Shields M, Leatherdale S, Malaison E, Hammond D. Assessment of validity of self-reported smoking status. Health Rep. 2012;23(1):47–53. . [PubMed] [Google Scholar]
- 34.Jung-Choi KH, Khang YH, Cho HJ. Hidden female smokers in Asia: a comparison of self-reported with cotinine-verified smoking prevalence rates in representative national data from an Asian population. Tob Control. 2012;21(6):536–42. 10.1136/tobaccocontrol-2011-050012 . [DOI] [PubMed] [Google Scholar]
- 35.Bach PB, Mirkin JN, Oliver TK, Azzoli CG, Berry DA, Brawley OW, et al. Benefits and harms of CT screening for lung cancer: a systematic review. JAMA. 2012;307(22):2418–29. 10.1001/jama.2012.5521 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data were obtained from the KNHANES (the Korea National Health and Nutrition Surveys). The authors specifically used the 2010-12 KNHANES dataset. These data can be downloaded from the KNHANES webpage (URL: https://knhanes.cdc.go.kr/knhanes/sub03/sub03_02_02.do) under the names HN10_ALL.SAV, HN11_ALL.SAV. and HN12_ALL.SAV.