The yeast Saccharomyces cerevisiae is a commonly used microbial host for production of various biochemical compounds. From a physiological perspective, biosynthesis of these compounds competes with biomass formation in terms of carbon and/or energy equivalents. Fermentation processes functioning at extremely low or near-zero growth rates would prevent loss of feedstock to biomass production. Establishing S. cerevisiae cultures in which growth is restricted by the limited supply of a non-energy substrate therefore could have a wide range of industrial applications but remains largely unexplored. In this work we accomplished near-zero growth of S. cerevisiae through limited supply of a non-energy nutrient, namely, the nitrogen or phosphorus source, and carried out a quantitative physiological study of the cells under these conditions. The possibility to achieve near-zero-growth S. cerevisiae cultures through limited supply of a non-energy nutrient may offer interesting prospects to develop novel fermentation processes for high-yield production of bio-based chemicals.
KEYWORDS: carbon excess, near-zero growth, non-energy limitation, retentostat, yeast physiology
ABSTRACT
So far, the physiology of Saccharomyces cerevisiae at near-zero growth rates has been studied in retentostat cultures with a growth-limiting supply of the carbon and energy source. Despite its relevance in nature and industry, the near-zero growth physiology of S. cerevisiae under conditions where growth is limited by the supply of non-energy substrates remains largely unexplored. This study analyzes the physiology of S. cerevisiae in aerobic chemostat and retentostat cultures grown under either ammonium or phosphate limitation. To compensate for loss of extracellular nitrogen- or phosphorus-containing compounds, establishing near-zero growth rates (μ < 0.002 h−1) in these retentostats required addition of low concentrations of ammonium or phosphate to reservoir media. In chemostats as well as in retentostats, strongly reduced cellular contents of the growth-limiting element (nitrogen or phosphorus) and high accumulation levels of storage carbohydrates were observed. Even at near-zero growth rates, culture viability in non-energy-limited retentostats remained above 80% and ATP synthesis was still sufficient to maintain an adequate energy status and keep cells in a metabolically active state. Compared to similar glucose-limited retentostat cultures, the nitrogen- and phosphate-limited cultures showed aerobic fermentation and a partial uncoupling of catabolism and anabolism. The possibility to achieve stable, near-zero growth cultures of S. cerevisiae under nitrogen or phosphorus limitation offers interesting prospects for high-yield production of bio-based chemicals.
IMPORTANCE The yeast Saccharomyces cerevisiae is a commonly used microbial host for production of various biochemical compounds. From a physiological perspective, biosynthesis of these compounds competes with biomass formation in terms of carbon and/or energy equivalents. Fermentation processes functioning at extremely low or near-zero growth rates would prevent loss of feedstock to biomass production. Establishing S. cerevisiae cultures in which growth is restricted by the limited supply of a non-energy substrate therefore could have a wide range of industrial applications but remains largely unexplored. In this work we accomplished near-zero growth of S. cerevisiae through limited supply of a non-energy nutrient, namely, the nitrogen or phosphorus source, and carried out a quantitative physiological study of the cells under these conditions. The possibility to achieve near-zero-growth S. cerevisiae cultures through limited supply of a non-energy nutrient may offer interesting prospects to develop novel fermentation processes for high-yield production of bio-based chemicals.
INTRODUCTION
The yeast Saccharomyces cerevisiae is an established microbial host for production of a wide range of biochemical compounds (1, 2). Current aerobic processes for production of ATP-requiring (“anabolic”) products are typically biphasic, with separate growth and production phases. Complete uncoupling of growth and product formation could enable a further reduction of the loss of feedstock to biomass production. In theory, such a complete uncoupling can be achieved in continuous processes performed at very low or near-zero specific growth rates. In practice, however, its implementation requires processes and microorganisms that, over prolonged periods of time, ensure a high viability and a high-biomass-specific product formation rate (qp) in the absence of growth.
For laboratory studies, near-zero specific growth rates are usually achieved in retentostats (3). A retentostat is a modification of the chemostat, in which effluent removal occurs through an internal or external filter module that causes complete biomass retention. Retentostats enable studies on microbial physiology at near-zero growth rates that are technically difficult to achieve in conventional chemostats, while their use avoids complete starvation by maintaining a constant supply of essential nutrients.
When growth in retentostat cultures is limited by the energy substrate, biomass accumulates in the reactor until the biomass-specific substrate consumption rate (qs) equals the energy substrate requirement for cellular maintenance (ms). Aerobic and anaerobic glucose-limited retentostat cultures of S. cerevisiae were shown to retain a high viability, as well as an extremely high heat shock tolerance, over periods of several weeks (4–7). Consistent with a growth-rate-independent requirement of ATP for cellular maintenance (8), observed values of qs at near-zero growth rates (μ < 0.002 h−1) were in good agreement with estimates of ms derived from measurements in glucose-limited chemostat cultures grown at a range of specific growth rates (4, 6).
From an applied perspective, it seems illogical to apply severely energy-limited cultivation regimes for production of compounds whose synthesis from sugar requires a net input of ATP. In nature, S. cerevisiae seems to have primarily evolved for growth in sugar-rich environments where, instead of the energy substrate, the nitrogen source is growth limiting (9, 10). Also in industrial substrates for S. cerevisiae, such as wine must or brewing wort, sugar is typically present in abundance, while growth becomes limited by the nitrogen source (11). As an alternative to nitrogen-limited cultivation, growth under extreme phosphate limitation may offer interesting options to uncouple growth from product formation. For example, S. cerevisiae, a nonoleaginous yeast, has been reported to accumulate high levels of specific fatty acids when the availability of phosphate is restricted (12).
Studies in exponentially growing chemostat cultures have revealed an extensive reprogramming of the yeast transcriptome, proteome, and fluxome in response to nitrogen and phosphorus limitation (13–16). In addition, nitrogen- and phosphorus-limited growth resulted in lower contents of protein and phospholipids, respectively, in yeast biomass (17, 18). In contrast to the wealth of data on the effects of different nutrient limitation regimes in actively growing cultures, information on aerobic S. cerevisiae cultures grown at near-zero growth rates is scarce. In anaerobic cultures, nitrogen-limited cultivation with biomass recycling has been explored to maximize ethanol yields (19, 20). Brandberg and coauthors (21), who investigated the impact of severe nitrogen limitation on ethanol production by S. cerevisiae, used incomplete cell recycling under anaerobic and microaerobic conditions.
The goal of the present study is to design and implement retentostat regimes for aerobic, nitrogen- and phosphate-limited growth of S. cerevisiae at near-zero specific growth rates and to use the resulting cultures for an experimental exploration of its quantitative physiology under these scientifically interesting and industrially relevant conditions, e.g., for the production of ATP-requiring (anabolic) products. An experimental setup was implemented that allowed for a smooth transition from low-growth-rate chemostat cultures to near-zero-growth-rate retentostat cultures. Metabolic fluxes, biomass composition, and cellular robustness were analyzed and compared with previously obtained data from glucose-limited chemostat and retentostat cultures.
RESULTS
Design of carbon-excess retentostat regimes.
To study the physiology of S. cerevisiae at near-zero growth rates under non-energy-limited conditions, retentostat regimes were designed in which growth was prevented by a severely limited supply of ammonium or phosphate. To avoid starvation, any loss of nitrogen or phosphate from such cultures, either by cell lysis or by excretion of N- or P-containing compounds from viable cells, should be compensated for. As a first approximation of the rates of N and P release by S. cerevisiae at near-zero growth rates, concentrations of N- and P-containing compounds were quantified in the outflow of an aerobic, glucose-limited retentostat culture. From these measurements, biomass-specific release rates of 8.1 μmol N/(g biomass)/h and 5.2 μmol P/(g biomass)/h were calculated (see Table S1 in the supplemental material). These rates were used to estimate required supply rates of ammonium and phosphate in nongrowing retentostat cultures limited by either of these two nutrients. For a target biomass concentration in the retentostats of 5 g/liter at a dilution rate of 0.025 h−1, 0.1 g/liter (NH4)2SO4 was included in the medium feed of the ammonium-limited cultures, while 0.014 g/liter KH2PO4 was used for phosphate-limited retentostat cultivation.
Aerobic growth of S. cerevisiae at nonlimiting concentrations of glucose leads to aerobic alcoholic fermentation (22). Based on trial experiments, glucose concentrations in the influent of ammonium- and phosphate-limited retentostats were set at 120 g/liter and 60 g/liter, respectively. These concentrations of the growth-limiting nutrients resulted in residual glucose concentrations of ca. 15 g/liter. Ethanol concentrations did not exceed 20 g/liter, which is well below the value of 5% (vol/vol) that has been reported to cause stress responses (23).
Growth and viability in ammonium- and phosphate-limited retentostat cultures.
Retentostat cultures were started by redirecting the effluent of steady-state ammonium- or phosphate-limited chemostat cultures, grown at a dilution rate of 0.025 h−1, through a membrane filter unit placed inside the reactor (see Materials and Methods). Replicate ammonium-limited retentostats were operated for 220 h with full biomass retention, after which fouling caused the membrane filters to clog. Membrane fouling was not observed in the phosphate-limited retentostats, which were operated with full biomass retention until, after 400 h, the biomass concentration had reached a stable value.
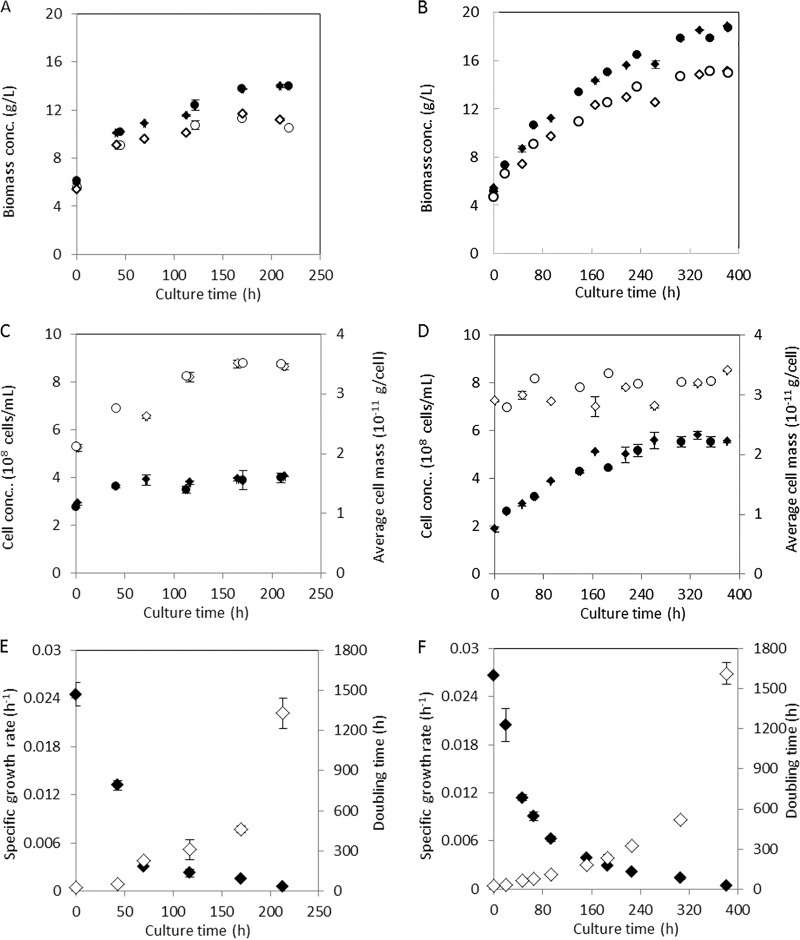
Irrespective of the nutrient limitation regime, the onset of retentostat cultivation led to a gradual increase of the biomass concentration (Fig. 1A and B). In ammonium-limited retentostats, the biomass concentration stabilized at ca. 14 g/liter after 150 h, while stabilization in the phosphate-limited cultures at ca. 18 g/liter occurred after 300 h. The increase in biomass concentration in the ammonium-limited retentostats mainly reflected an increase of the dry mass per cell, which was initially smaller than that in the phosphate-limited retentostats. Conversely, the biomass increase in phosphate-limited retentostats predominantly reflected an increase of the cell number (Fig. 1C and D).
FIG 1.
Biomass accumulation, cell counts, and specific growth rates in aerobic ammonium- and phosphate-limited retentostat cultures of S. cerevisiae CEN.PK113-7D. Data in panels A to D, obtained from independent duplicate cultures, are shown as circles and diamonds, and error bars indicate standard errors of analytical replicates on samples from the same culture. Data in panels E and F represent the averages and standard errors of measurements on duplicate retentostat cultures. (A and B) Total biomass (closed symbols) and viable biomass (open symbols) in ammonium-limited (A) and phosphate-limited (B) retentostat cultures. (C and D) Cell numbers (closed symbols) and average mass per cell (open symbols) in ammonium-limited (C) and phosphate-limited (D) retentostat cultures. (E and F) Specific growth rate (closed symbols) and doubling time (open symbols) in ammonium-limited (E) and phosphate-limited (F) retentostat cultures.
Culture viability was estimated by plate counts of CFU and by flow cytometry after CFDA/propidium iodide (PI) staining (Table S2). We observed a consistently lower viability in the CFU assays than in the CFDA/PI stains. A similar difference has previously been attributed to loss of viability of retentostat-grown cells during plating (4, 6). Based on PI staining, the viability of the ammonium- and phosphate-limited retentostat cultures toward the end of the experiments did not decrease below 80% and 90%, respectively (Fig. 1A and B and Table S2).
During retentostat cultivation, specific growth rates progressively decreased, reaching final values of 0.00056 ± 0.00010 h−1 and 0.00043 ± 0.00012 h−1 for the ammonium- and phosphate-limited cultures, respectively, corresponding to doubling times of 55 and 67 days (Fig. 1E and F). Based on these observations, death rates of 0.0018 ± 0.0001 h−1 and 0.0012 ± 0.0001 h−1 were calculated for prolonged ammonium- and phosphate-limited retentostat cultures, respectively, which were somewhat higher than the value of 0.00047 h−1 reported for carbon-limited aerobic retentostat cultures (6). The resulting gradual decrease of culture viability partially explained the difference between the observed biomass accumulation and the targeted values in the experimental design.
Quantitative physiology under extreme ammonium and phosphate limitation.
During retentostat cultivation, the biomass-specific consumption rates of glucose and oxygen and production rates of ethanol and CO2 asymptotically decreased over time and stabilized after approximately 100 h in the ammonium-limited cultures and after approximately 200 h in the phosphate-limited cultures (Fig. S1). At this stage, the specific growth rate of the cultures was lower than 0.002 h−1, growth stoichiometries became constant (Fig. 1E and F), and cells were assumed to be in a metabolic pseudo-steady state. Physiological parameters obtained from the preceding, slowly growing steady-state chemostat cultures (μ = 0.025 h−1) and from the pseudo-steady-state, near-zero-growth retentostat cultures (μ < 0.002 h−1) are summarized in Tables 1 and 2. As anticipated, the concentrations of the limiting nutrients (ammonium or phosphate) were below the detection limit, whereas glucose concentrations were between 10 and 20 g/liter in all cultures (Table 2). Carbon and degree-of-reduction balances yielded recoveries close to 100% (Table 2), indicating that no major metabolites had been overlooked in the analyses.
TABLE 1.
Biomass-specific net conversion rates of S. cerevisiae CEN.PK113-7D cultured in aerobic N- and P-limited slow-growth steady-state chemostats and near-zero-growth pseudo-steady-state retentostats
| Culture conditiona | Biomass-specific net conversion rateb |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| qglucose | qethanol | qbiomass | qglycerol | qsuccinate | qlactate | qacetate | qO2 | qCO2 | |
| N limited at SG | 14.0 ± 0.0 | 6.1 ± 0.2 | 0.96 ± 0.09 | 0.10 ± 0.01 | 0.40 ± 0.06 | 0.070 ± 0.001 | 0.017 ± 0.002 | 1.7 ± 0.0 | 5.5 ± 0.2 |
| P limited at SG | 16.6 ± 1.2 | 7.9 ± 0.3 | 0.99 ± 0.04 | 0.18 ± 0.00 | 0.15 ± 0.01 | 0.065 ± 0.001 | 0.21 ± 0.01 | 2.1 ± 0.0 | 6.6 ± 0.3 |
| N limited at NZG | 3.4 ± 0.1 | 1.5 ± 0.0 | —c | 0.18 ± 0.01 | 0.30 ± 0.01 | 0.048 ± 0.002 | 0.026 ± 0.001 | 0.71 ± 0.03 | 1.5 ± 0.0 |
| P limited at NZG | 3.1 ± 0.1 | 1.1 ± 0.1 | — | 0.14 ± 0.03 | 0.15 ± 0.00 | 0.000 ± 0.000 | 0.049 ± 0.005 | 0.94 ± 0.02 | 1.6 ± 0.1 |
Slow growth (SG), μ = 0.025 h−1; near-zero growth (NZG), μ < 0.002 h−1.
Data represent averages, with their standard errors, calculated from multiple measurements obtained from duplicate experiments. Biomass-specific rates are expressed in the unit of mCmol/gxv/h and were calculated per gram of viable biomass.
—, not calculated.
TABLE 2.
Physiological parameters of S. cerevisiae CEN.PK113-7D cultured in aerobic N- and P-limited slow-growth steady-state chemostats and near-zero-growth pseudo-steady-state retentostatsa
| Culture condition | Viability (%) | RQb | Yx/sc | Yp/sd | Carbon recovery (%) | Reduction recovery (%) | Residual nutrient concn |
||
|---|---|---|---|---|---|---|---|---|---|
| Glucose (g/liter) | NH4+ (mM) | PO43− (mM) | |||||||
| N limited at SG | 93 ± 0 | 3.1 ± 0.0 | 0.059 ± 0.000 | 0.32 ± 0.12 | 95 ± 1 | 95 ± 1 | 16.5 ± 0.2 | BD | 18.4 ± 0.9 |
| P limited at SG | 91 ± 0 | 3.1 ± 0.1 | 0.052 ± 0.000 | 0.36 ± 0.00 | 99 ± 6 | 99 ± 3 | 18.2 ± 0.7 | 40.5 ± 2.0 | BD |
| N limited at NZG | 80 ± 0 | 2.1 ± 0.1 | — | 0.33 ± 0.02 | 101 ± 2 | 98 ± 3 | 15.0 ± 2.0 | BD | 18.2 ± 0.9 |
| P limited at NZG | 90 ± 0 | 1.7 ± 0.0 | — | 0.28 ± 0.03 | 98 ± 2 | 99 ± 1 | 10.3 ± 0.2 | 53.5 ± 2.7 | BD |
Data represent averages, with their standard errors, calculated from multiple measurements obtained from duplicate experiments. Slow growth (SG), μ = 0.025 h−1; near-zero growth (NZG), μ < 0.002 h−1; BD, below detection limit of assay; —, not calculated.
RQ, respiratory quotient (qCO2/qO2).
Yx/s, yield of biomass (g biomass/[g glucose consumed]).
Yp/s, yield of ethanol (g ethanol/[g glucose consumed]).
In the slow-growing (μ = 0.025 h−1) chemostat cultures, the biomass-specific rates of glucose and oxygen consumption, as well as ethanol and carbon dioxide production, were consistently higher in the phosphate-limited cultures than in the ammonium-limited cultures (Table 1). In line with these observations, the phosphate-limited cultures showed a lower biomass yield and higher ethanol yield on glucose. Respiratory quotients (RQ; ratio of CO2 production to O2 consumption rate) were identical for the two nutrient limitation regimes, indicating that the difference in biomass yield of the chemostat cultures was not caused by different contributions of respiratory and fermentative metabolism. Furthermore, the sum of the specific production rates of the four minor by-products (glycerol, succinate, lactate, and acetate), which accounted for less than 4% of the consumed glucose, were not significantly different for the two limitation regimes and also were not responsible for the observed difference in biomass yield.
In the pseudo-steady-state near-zero-growth retentostat cultures, the observed ethanol yields on glucose (Table 2) were 71% and 53% of the theoretical maximum (0.51 g ethanol/[g glucose]) for the ammonium- and phosphate-limited regimes, respectively. Consistent with this observation, significant oxygen consumption occurred in these cultures, and their RQ values were significantly lower than those of the preceding chemostat cultures. The difference was most pronounced for the phosphate-limited cultures. These observations indicate that near-zero growth achieved by phosphate limitation leads to a more respiratory metabolism than was observed in the preceding slowly growing, phosphate-limited chemostats. Formation of by-products accounted for 16% and 11% of the supplied glucose in the ammonium- and phosphate-limited near-zero-growth cultures, respectively. Glycerol and succinate were the main contributors, with succinate accounting for 9% of the consumed glucose in the ammonium-limited culture.
Biomass composition under extreme ammonium and phosphate limitation.
To analyze the impact of extreme ammonium and phosphate limitation on biomass composition, biomass samples from slow-growing, steady-state chemostat cultures and from near-zero-growth-rate pseudo-steady-state retentostat cultures were analyzed for their elemental and macromolecular compositions (Table 3). In the chemostat cultures as well as in the retentostat cultures, the content of the growth-limiting element in the biomass was strongly reduced relative to that of the culture grown under the other nutrient limitation (Table 3). This difference was even more pronounced in the retentostat cultures than in the preceding chemostat cultures. The nitrogen content of biomass from ammonium-limited retentostat cultures was ca. 2-fold lower than that of the corresponding phosphate-limited retentostats, while the phosphorus content of biomass from the phosphate-limited retentostats was 3.5-fold lower than that of biomass from the ammonium-limited retentostats. In both phosphate-limited chemostats and retentostats, a low phosphorus content was accompanied by a 2- to 3-fold higher sulfur content than that in the corresponding ammonium-limited cultures. The increased sulfur content in phosphate-limited cultures may be due to sulfate uptake by high-affinity phosphate transporters (14). Compared with glucose-limited chemostat cultures of the same S. cerevisiae strain at a similar dilution rate (D = 0.022 h−1) (Table 3), the biomass protein content and the total nitrogen content of cells grown in the ammonium-limited chemostat cultures were more than 60% and 50% lower, respectively. Similarly, in the phosphate-limited chemostat cultures, the phosphorus content of the biomass was ca. 50% lower.
TABLE 3.
Biomass elemental compositions of S. cerevisiae CEN.PK113-7D cultured in aerobic N- and P-limited slow-growth steady-state chemostats and near-zero-growth pseudo-steady-state retentostatsa
| Culture condition | μ (h−1) | fC (%)b | fH (%) | fN (%) | fO (%) | fP (%) | fS (%) | Sum (%) | C-molc wt (g) |
|---|---|---|---|---|---|---|---|---|---|
| N limited | 0.025 | 47.0 ± 0.0 | 7.2 ± 0.0 | 3.5 ± 0.1 | 39.5 ± 0.4 | 1.3 ± 0.0 | 0.16 ± 0.01 | 98 ± 0 | 25.6 ± 0.0 |
| P limited | 0.025 | 44.0 ± 0.2 | 7.0 ± 0.1 | 5.3 ± 0.0 | 36.9 ± 0.3 | 0.50 ± 0.01 | 0.39 ± 0.03 | 94 ± 0 | 24.3 ± 0.3 |
| N limited | <0.002 | 49.5 ± 0.4 | 7.6 ± 0.0 | 2.5 ± 0.0 | 39.5 ± 1.1 | 1.1 ± 0.0 | 0.11 ± 0.01 | 100 ± 1 | 27.3 ± 0.2 |
| P limited | <0.002 | 47.3 ± 0.1 | 7.2 ± 0.0 | 4.6 ± 0.1 | 37.4 ± 0.0 | 0.29 ± 0.00 | 0.27 ± 0.01 | 97 ± 0 | 25.4 ± 0.1 |
| C limited | 0.022 | 45.6 | 6.8 | 6.6 | 37.3 | 1.0 | 0.22 | 97 | 26.4 |
Data represent averages, with standard errors, of measurements from duplicate cultures and are compared with published values from aerobic glucose-limited (C-limited) chemostat culture of the same strain (49). Slow growth, μ = 0.025 h−1; near-zero growth, μ < 0.002 h−1.
Balanced elemental mass fractions f in %.
The amount of biomass (g) containing 1 mol of carbon.
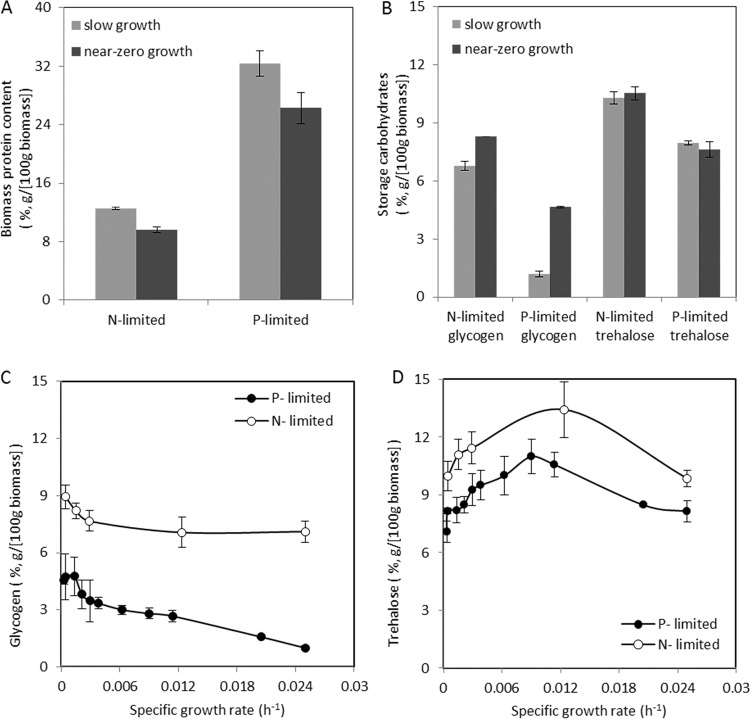
Consistent with their low nitrogen content, ammonium-limited chemostat and retentostat cultures showed a ca. 2.5-fold lower biomass protein content than the corresponding phosphate-limited cultures, with the lowest protein content (9.6%) measured in the ammonium-limited retentostats (Fig. 2A). Conversely, glycogen contents were higher (5.8-fold in chemostats and 1.8-fold in retentostats) in ammonium-limited cultures than in phosphate-limited cultures, while trehalose contents were only 30 to 40% higher in the ammonium-limited cultures (Fig. 2B). When analyzed throughout the retentostat experiments, glycogen contents in the ammonium-limited cultures remained consistently high, while they increased with declining specific growth rate in the phosphate-limited cultures (Fig. 2C). For both nutrient limitation regimes, the trehalose content reached a maximum at a specific growth rate of ca. 0.01 h−1 (Fig. 2D).
FIG 2.
Biomass protein and storage carbohydrate (glycogen and trehalose) contents in aerobic ammonium- and phosphate-limited (N- and P-limited) cultures of S. cerevisiae CEN.PK113-7D. Data represent the averages and standard errors from multiple measurements on duplicate cultures. (A and B) Biomass protein (A) and storage carbohydrates (glycogen and trehalose) (B). Samples were withdrawn from the steady-state, slow-growth (μ = 0.025 h−1) chemostat cultures and the pseudo-steady-state, near-zero-growth (μ < 0.002 h−1) retentostat cultures. (C and D) Glycogen (C) and trehalose (D) contents versus the specific growth rate in the prolonged retentostat cultures.
Metabolic flux analysis.
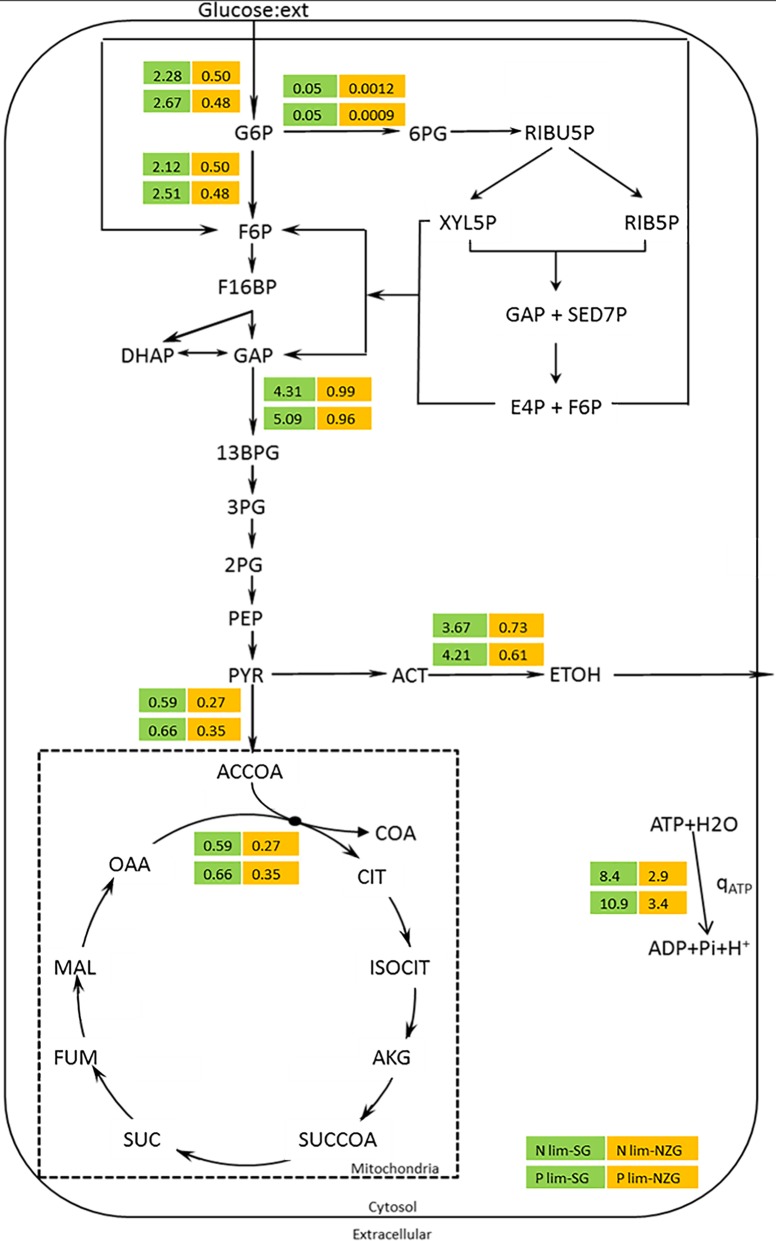
To further investigate the physiological differences between extreme ammonium and phosphate limitation, metabolic flux analysis was performed for both the slow-growing, steady-state chemostat cultures (μ = 0.025 h−1) and near-zero-growth, pseudo-steady-state retentostat cultures (μ < 0.002 h−1) (Fig. 3 and Table S3). At a specific growth rate of 0.025 h−1, fluxes through glycolysis, pyruvate decarboxylase, and alcohol dehydrogenase were significantly higher, while the tricarboxylic acid (TCA) cycle flux was slightly higher in the phosphate-limited cultures than in the ammonium-limited cultures. This observation indicated a higher contribution of catabolism in the phosphate-limited cultures. Assuming a P/O ratio of 1 (24), biomass-specific rates of ATP turnover were ca. 1.3-folder higher in the phosphate-limited chemostat cultures than in the corresponding ammonium-limited cultures (Fig. 3).
FIG 3.
Metabolic flux analysis in aerobic ammonium- and phosphate-limited (N- and P-limited) cultures of S. cerevisiae CEN.PK113-7D. Flux values present the steady-state, slow-growth (SG) (μ = 0.025 h−1) chemostat cultures (numbers on green background) and the pseudo-steady-state, near-zero-growth (NZG) (μ < 0.002 h−1) retentostat cultures (numbers on orange background). Data are expressed in millimoles of per gram of viable biomass per hour and represent the averages for duplicate cultures. Complete flux analysis values and standard errors are presented in Table S3 in the supplemental material. Glucose:ext, extracellular metabolite glucose.
In the retentostats, fluxes through the pentose-phosphate pathway (PPP) were extremely low, which is consistent with the strictly assimilatory role of this central metabolic pathway in S. cerevisiae (25). The glycolytic flux was nearly identical for the two nutrient limitations. Conversely, distributions of pyruvate over alcoholic fermentation and TCA cycle were different. Consistent with their lower RQ, phosphate-limited retentostat cultures channeled a higher fraction of the pyruvate into the TCA cycle than the ammonium-limited retentostat cultures. Estimated non-growth-associated ATP consumption was higher in the phosphate-limited retentostats (3.4 ± 0.2 mmol ATP/[g viable biomass]/h) than in the ammonium-limited retentostats (2.9 ± 0.1 mmol ATP/[g viable biomass]/h) (Fig. 3).
Energetics under extreme ammonium and phosphate limitation.
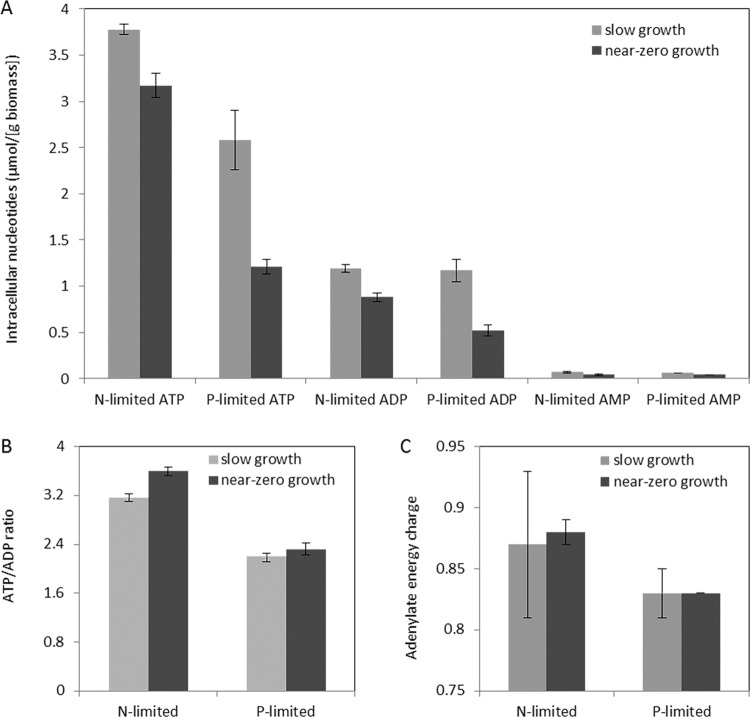
Nitrogen and phosphate limitation can both be characterized as non-energy-limited cultivation regimes. However, because phosphate plays a vital role in cellular energy metabolism and energy status, the intracellular nucleotide levels (ATP, ADP, and AMP) and corresponding adenylate energy charge and ATP/ADP ratios were quantified for both chemostat and retentostat conditions (Fig. 4). Intracellular levels of all three adenine nucleotides were consistently higher in the chemostats than in the retentostats. Comparing these two limitations, both in slow-growth and near-zero-growth cultures, intracellular ATP and AMP levels were consistently lower under phosphate limitation than under ammonium limitation. In addition, phosphate-limited near-zero-growth cultures also showed ca. 40% lower ADP levels than the corresponding ammonium-limited cultures, while ADP levels were identical in phosphate- and ammonium-limited, slow-growing chemostat cultures (Fig. 4A).
FIG 4.
Intracellular adenosine phosphate concentrations (A), ATP/ADP ratio (B), and energy charge (C) in aerobic ammonium- and phosphate-limited (N- and P-limited) cultures of S. cerevisiae CEN.PK113-7D. Data represent the averages and standard errors of multiple measurements from duplicate cultures. Samples were withdrawn from the steady-state, slow-growth (μ = 0.025 h−1) chemostat cultures and the pseudo-steady-state, near-zero-growth (μ < 0.002 h−1) retentostat cultures.
Neither the ATP/ADP ratios nor the energy charge in the retentostat cultures differed from those in the corresponding slow-growing chemostat cultures (Fig. 4B and C). However, ATP/ADP ratios in the phosphate-limited cultures were 30 to 35% lower than those in the corresponding ammonium-limited cultures. A similar, less pronounced difference was observed for the adenylate energy charge. These results show that phosphate limitation indeed significantly affected cellular energy status.
DISCUSSION
Prolonged near-zero growth of S. cerevisiae under non-energy-limited conditions.
Retentostat cultivation of heterotrophic microorganisms typically involves a constant, growth-limiting supply rate of the carbon and energy substrate (3). The amount of viable biomass in such energy-limited retentostats asymptotically increases to a constant value, while the specific growth rate asymptotically approaches zero. In the resulting pseudo-steady states, biomass-specific substrate supply rates closely match cellular maintenance energy requirements (3). The retentostat regimes explored in this study, in which growth was restricted by supply of the nitrogen or phosphorus source, represented a fundamentally different scenario. While biomass also asymptotically increased to a constant value, the corresponding constant biomass-specific ammonium or phosphate consumption rates were not related to maintenance energy metabolism. Instead, they represented release of nitrogen- or phosphorus-containing compounds, which were removed via the cell-free effluent.
Excretion of nitrogen- or phosphorus-containing compounds by severely ammonium- or phosphate-limited yeast cultures appears counterintuitive. Instead, release of these compounds probably occurs by cell death and/or lysis. S. cerevisiae can express a range of specific and nonspecific amino acid permeases (26), while di- and tripeptides can be imported by Prt2p (27). Presence of amino acids in culture supernatants therefore is likely to reflect the kinetics of such transporters rather than a complete inability for amino acid reconsumption by viable cells. Consistent with this hypothesis, extracellular concentrations of amino acids in the ammonium-limited retentostats were lower than the Km values of the corresponding high-affinity S. cerevisiae amino acid permeases (see Table S4 in the supplemental material).
Biomass concentrations in the ammonium- and phosphate-limited retentostats reached values that were approximately 3-fold higher than the target value of 5 g/liter on which design of growth media and operating conditions were based. This difference could only partially be attributed to accumulation of nonviable biomass. In addition, strongly reduced contents of the growth-limiting element in the retentostat-grown biomass could explain this large discrepancy to a large extent.
As previously reported for glucose-limited cultures (21), ammonium- and phosphate-limited cultivation of S. cerevisiae at low- to near-zero growth rates led to increased intracellular levels of glycogen and trehalose. This observation confirms that glycogen and trehalose accumulation is a universal physiological response of S. cerevisiae under near-zero-growth conditions. In faster-growing chemostat cultures, nitrogen limitation also has been shown to lead to higher storage carbohydrate levels than other nutrient limitation regimes (28). Intracellular reserves of glycogen and trehalose enable survival during carbon and energy source starvation and can fuel cell cycle progression under carbon and energy source limitation (29). Additionally, upregulation of genes involved in synthesis, metabolism, and degradation of trehalose has been implicated in the extreme heat shock tolerance of glucose-limited retentostat cultures of S. cerevisiae (6, 30).
Energy metabolism of S. cerevisiae under extreme ammonium and phosphate limitation.
Despite strongly reduced phosphate content and low intracellular levels of adenosine nucleotides, the adenylate energy charge of 0.83 of the phosphate-limited chemostat and retentostat cultures was within the normal physiological range of 0.7 to 0.95 (31). The adenylate energy charge of 0.88 for the corresponding ammonium-limited cultures also indicated that cells were able to maintain their energy status under extreme nutrient restriction.
Consistent with the well-known tendency of S. cerevisiae to exhibit aerobic alcoholic fermentation when exposed to excess glucose (22), respiratory quotients (RQs) of all ammonium- and phosphate-limited cultures were above 1. RQ values were lowest at near-zero growth rates (Table S5), indicating that the contribution of fermentative metabolism decreased with decreasing specific growth rate. Even though S. cerevisiae has a low P/O ratio, respiratory catabolism of glucose yields much more ATP than fermentation (32). However, its maximum rate of fermentative ATP generation is approximately 2-fold higher than its maximum rate of respiratory ATP generation (33). These observations underlie a rate/yield trade-off hypothesis, according to which ATP can either be produced fast (but with a low efficiency) or efficiently (but at a lower maximum rate) (34). The shift toward a more respiratory metabolism in the near-zero-growth-rate retentostat cultures is entirely in line with this hypothesis.
Non-growth-associated rates of ATP turnover in the aerobic, non-energy-limited cultures were significantly higher than maintenance energy requirements estimated from aerobic and anaerobic energy-limited retentostat studies with the same S. cerevisiae strain (Fig. S3). While a similar uncoupling of anabolic energy demand and catabolic energy conservation has been reported for nitrogen-limited chemostat cultures, the underlying mechanism has not been elucidated (16, 21, 35–38). Quantification of the in vivo cytosolic concentrations of ammonium and ammonia recently showed that, in ammonium-limited chemostat cultures of S. cerevisiae grown at pH 5, cytosolic ammonia concentrations exceeded extracellular concentrations (39). Diffusion of ammonia from the cells, combined with reuptake of ammonium cation by the high-affinity uniporter Mep2 (40) and expulsion of its associated proton by the plasma membrane H+-ATPase Pma1, could lead to a futile cycle.
Extreme phosphate-limited growth of S. cerevisiae induces expression of PHO84, which encodes a high-affinity phosphate/proton symporter and vacuolar synthesis of inorganic polyphosphate (41). By acting as a phosphorus sink, polyphosphate sustains phosphate uptake at low extracellular concentrations (41, 42). Its synthesis in yeast requires activity of the vacuolar H+-ATPase (V-ATPase) to maintain a proton-motive force across the vacuolar membrane (41). Although high-affinity phosphate import and subsequent vacuolar polyphosphate synthesis must have resulted in increased ATP requirements, these are negligible compared to the observed non-growth-associated ATP requirements in the phosphate-limited retentostat cultures. Unless very significant turnover of the polyphosphate pool has occurred, these additional ATP requirements are likely to have been caused by other, yet-unknown processes.
Possible application of severe ammonium or phosphate limitation for industrial processes.
Metabolic engineering of S. cerevisiae has enabled the production of a wide range of compounds whose biosynthesis from sugars requires a net input of ATP (43). The specific rate of formation of such anabolic products is determined by the capacities and regulation of the enzymes of the product pathway and connected primary metabolic pathways, as well as by the continuous (re)generation of cofactors such as NAD(P)H, coenzyme A, and ATP. To optimize yields of such products, allocation of sugar to growth should be minimized. At the same time, ATP availability should not limit product formation rates. Theoretically, these goals can be reconciled by near-zero-growth-rate cultivation under non-energy-limited conditions. This study shows that, under ammonium limitation as well as under phosphate limitation, glucose-sufficient, near-zero-growth retentostat cultures of a laboratory strain of S. cerevisiae are able to maintain a normal energy charge and showed only a modest loss of culture viability. The extremely low protein content of biomass grown in the nitrogen-limited retentostats is likely to represent a disadvantage for high-level expression of heterologous product pathways. Moreover, nitrogen limitation is intrinsically poorly suited for production of proteins and other nitrogen-containing compounds. Extreme phosphate limitation did not affect biomass protein levels. However, relative to glucose-limited retentostats, both the ammonium- and phosphate-limited cultures showed increased rates of non-growth-associated ATP dissipation. This increase is undesirable in industrial contexts, as the resulting increased rate of sugar dissimilation would go at the expense of the product yield. Therefore, future research should aim at identifying the causes of non-growth-associated ATP dissipation and on their elimination, either by alternative nutrient limitation regimes, by strain engineering, or by alternative approaches to restrict cell division.
MATERIALS AND METHODS
Yeast strain and media.
The prototrophic, haploid yeast strain Saccharomyces cerevisiae CENPK 113-7D was used in this study (44). Working stocks were obtained by cultivation in YPD medium (10 g/liter Bacto yeast extract, 20 g/liter Bacto peptone, and 20 g/liter d-glucose). After addition of 30% (vol/vol) glycerol, culture aliquots were stored in sterilized Eppendorf tubes at –80°C.
Ammonium- and phosphate-limited (N- and P-limited) preculture and batch culture media were prepared as described by Boer et al. (16). For N-limited batch cultivation, the medium contained the following components: 1.0 g of (NH4)2SO4, 5.3 g of K2SO4, 3.0 g of KH2PO4, 0.5 g of MgSO4·7H2O, and 59 g of glucose per liter. For P-limited batch cultivation, the medium contained 5.0 g of (NH4)2SO4, 1.9 g of K2SO4, 0.12 g of KH2PO4, 0.5 g of MgSO4·7H2O, and 59 g of glucose per liter. In addition, 1 ml/liter trace element solution, 1 ml/liter vitamin solution, and 0.2 g/liter Pluronic 6100 PE antifoaming agent (BASF, Ludwigshafen, Germany) were added. Trace element and vitamin solutions were prepared as described by Verduyn et al. (45). The compositions of media for N- and P-limited chemostat cultivation were as described above, except that the glucose concentration was increased to 120 g/liter. For N-limited retentostat cultivation, the (NH4)2SO4 concentration in the medium feed was decreased to 0.1 g/liter and the glucose concentration was 60 g/liter. To maintain the same sulfur concentration, the K2SO4 concentration was increased to 6.46 g/liter, and the concentrations of the other compounds were the same as those in the chemostat medium. For P-limited retentostat cultivation, the KH2PO4 concentration was lowered to 0.014 g/liter and the glucose concentration was 60 g/liter.
Bioreactor setup.
Bench-scale, turbine-stirred 7-liter bioreactors (Applikon, Delft, The Netherlands) equipped with a single six-bladed Rushton turbine impeller, with a diameter of 85 mm, were used in this study. The working volume was controlled at 5 liters by placing the bioreactor on an electronic balance (Mettler Toledo, Columbus, OH, USA). During continuous cultivation, effluent was removed with a peristaltic pump to an effluent vessel, which was placed on an electronic balance for measurement of the dilution rate (D = 0.025 h−1). The culture temperature was maintained at 30 ± 0.1°C and the stirrer speed at 500 rpm. Aerobic conditions were maintained by sparging 0.5-vvm compressed air, controlled by a mass flow controller (Brooks 5850 TR; Hatfield, PA, USA). The dissolved oxygen (DO) concentration was measured on-line with a DO sensor (Mettler-Toledo GmbH, Greinfensee, Switzerland) and remained above 30% of air saturation in all experiments. Culture pH was controlled at 5.00 ± 0.05 by automated addition of either 2 M KOH or 2 M H2SO4, using a Biostat Bplus controller (Sartorius BBI Systems, Melsungen, Germany). Exhaust gas was cooled to 4°C by an in-line condenser and dried by a Nafion dryer (Permapure, Toms River, NJ, USA) before entering a combined paramagnetic/infrared NGA 2000 off-gas analyzer (Rosemount Analytical, Anaheim, CA, USA) for analysis of O2 and CO2 concentrations. Off-gas data were acquired with MFCS/win 3.0 software (Sartorius BBI Systems, Melsungen, Germany).
Precultures and batch, chemostat, and retentostat cultures.
Precultures, grown in 500-ml shake flasks containing 200 ml medium, were inoculated with 2 ml of stock culture and grown at 30°C and at 200 rpm for 8 h in a B Braun Certomat BS-1 incubator (Sartorius, Melsungen, Germany). Bioreactor batch cultures were started by transferring 400 ml of preculture to a bioreactor containing 4.6 liters of medium. After approximately 24 h of batch cultivation, a sharp decrease of the CO2 concentration in the off-gas and a corresponding increase of the dissolved oxygen concentration indicated that ammonium or phosphate was depleted. The bioreactors were then switched to chemostat cultivation mode and operated at a dilution rate of 0.025 h−1. Steady state was assumed to be achieved after 5 volume changes, in which stable (less than 3% difference over 2 volume changes) off-gas CO2 and O2 concentrations, culture dry weight, and cell counts were observed. At that stage, bioreactors were switched from chemostat to retentostat mode by redirecting the culture effluent through a filtration probe assembly (Applikon, Delft, The Netherlands). Each probe was fitted with a 0.22-μm tubular microfiltration polypropylene membrane (TRACE Analytics, Brunswick, Germany). Because of the limited flow rate capacity of each filter, four filtration probes were installed in each bioreactor. Before mounting on the filtration probe and autoclaving, membranes were hydrophilized overnight in 70% (vol/vol) isopropanol.
To avoid a sudden decrease of substrate concentrations during the switch from chemostat to retentostat mode, a gradual transition from chemostat to retentostat medium was accomplished by using two feed pumps. The resulting time-dependent concentrations of glucose and of the growth-limiting nutrient [(NH4)2SO4 or KH2PO4] in the medium are described by equation 1:
| (1) |
In this equation, τ is the time constant for the transition, which was set to a value of 16.67 h. Cs,ch, Cs,re, Fin,ch, and Fin,re, correspond to the nutrient concentrations in the chemostat and retentostat media and the feed rates from the corresponding medium reservoirs, respectively. Profiles of the resulting concentrations of the limited nutrient and of glucose in the retentostat feed media during the transition are provided in Fig. S3 in the supplemental material. The actual medium feed rates during the chemostat and retentostat phases for each experiment were calculated from the weight increase of the effluent vessels and the addition rates of base.
Biomass and viability assays.
Culture dry weight assays were carried out through a filtration, washing, and drying procedure as described previously (46). Total cell counts were quantified with a Z2 Coulter counter (50-μm aperture; Beckman, Fullerton, CA). Cell viabilities were determined through a FungaLight yeast CFDA AM/propidium iodide vitality kit (a cellular membrane integrity indicator) by flow cytometry and colony-forming unit counts (6).
Quantification of (by-)products and residual substrates.
Cell-free effluent samples were harvested from a sample port connected to the retentostat filters, immediately frozen in liquid nitrogen, and stored at –80°C until analysis. Effluent concentrations of glucose, ethanol, and by-products (glycerol, lactate, acetate, and succinate) were quantified with high-performance liquid chromatography (HPLC) using a Bio-Rad HPX-87H 300 column (7.8 mm). The column was eluted with phosphoric acid (1.5 mM, 0.6 ml/min). The detection was performed with a refractometer (Walters 2414) and a UV detector (210 nm; Walters 484). Concentrations of ammonium and phosphate were quantified with an ammonium cuvette test (0.02 to 2.5 mg/liter NH4+) and a phosphate trace cuvette test (0.03 to 1.5 mg/liter PO43−), respectively (Hach Lange GmbH, Düsseldorf, Germany).
Balance and rate calculations.
Biomass-specific glucose and oxygen consumption rates and biomass-specific production rates of ethanol, carbon dioxide, and by-products were calculated based on primary measurements of substrate/product concentration and flow rates in gas and liquid phases. Data reconciliation was performed as described previously (47). The consistencies of the obtained rates were evaluated by calculation of carbon and degree of reduction recoveries. Ethanol evaporation via the off-gas of the reactor was quantified as described previously (48) and was taken into account in calculation of ethanol production rates.
Assuming that only the viable biomass can replicate and lyse, the specific growth rates (μ) and death rates (kd) in retentostat cultures were calculated using equations 2 and 3, respectively:
| (2) |
| (3) |
Analysis of biomass composition.
Around 250 mg of lyophilized biomass was used to determine the elemental (C, H, N, O, P, and S) composition through complete combustion and subsequent gas analysis (carbon dioxide, water vapor, and nitrogen mass fractions), gas chromatography (oxygen), and inductively coupled plasma mass spectrometry (ICP-MS) (phosphorus and sulfur) (Energy Research Centre, Petten, The Netherlands). Biomass protein was quantified with the Biuret method as described previously (49). The trehalose content of the biomass was directly quantified by gas chromatography-tandem mass spectrometry (GC-MS/MS) (50) in intracellular metabolite samples prepared as described below. Glycogen content was quantified through an enzymatic hydrolysis method (6).
Quantification of intracellular metabolites.
A rapid sampling device connected to the bioreactor was used to rapidly withdraw broth samples for intracellular metabolite measurements (51). Approximately 1.2 g broth was taken and instantaneously quenched in precooled pure methanol (–40°C), followed by a washing procedure with 80% (vol/vol) aqueous methanol solution precooled to −40°C. Metabolite extraction was performed with 75% (vol/vol) ethanol (95°C, 3 min), followed by rapid vacuum evaporation until dryness. A detailed protocol has been described previously (47). Metabolite concentrations were quantified by isotope dilution mass spectrometry (liquid chromatography-IDMS/MS and GC-IDMS) using U-13C-labeled yeast cell extract as an internal standard (52). Metabolites from glycolysis, TCA cycle, and pentose-phosphate pathway as well as amino acids were quantified according to published protocols (53–55). Intracellular adenine nucleotide contents (ATP, ADP, and AMP) were measured according to reference 55. The adenylate energy charge (AEC) was calculated with equation 4:
| (4) |
Metabolic flux analysis.
Intracellular flux distributions during steady-state chemostat and pseudo-steady-state retentostat cultivation were calculated using a slightly modified version of a previously published stoichiometric model (56), in which the biomass composition was adapted according to the measurements of the biomass elemental compositions. The input variables used for the flux analysis are summarized in Table S3.
Supplementary Material
ACKNOWLEDGMENTS
This research was financed by the Netherlands Be-Basic research program (Be-Basic project FS10-04, uncoupling of microbial growth and product formation).
We thank Cor Ras, Patricia van Dam, Silvia Marine, and Johan Knoll for analytical support.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.01161-19.
REFERENCES
- 1.Nielsen J, Keasling JD. 2016. Engineering cellular metabolism. Cell 164:1185–1197. doi: 10.1016/j.cell.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 2.Jansen ML, van Gulik WM. 2014. Towards large scale fermentative production of succinic acid. Curr Opin Biotechnol 30:190–197. doi: 10.1016/j.copbio.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 3.Ercan O, Bisschops MM, Overkamp W, Jorgensen TR, Ram AF, Smid EJ, Pronk JT, Kuipers OP, Daran-Lapujade P, Kleerebezem M. 2015. Physiological and transcriptional responses of different industrial microbes at near-zero specific growth rates. Appl Environ Microbiol 81:5662–5670. doi: 10.1128/AEM.00944-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boender LG, de Hulster EA, van Maris AJ, Daran-Lapujade PA, Pronk JT. 2009. Quantitative physiology of Saccharomyces cerevisiae at near-zero specific growth rates. Appl Environ Microbiol 75:5607–5614. doi: 10.1128/AEM.00429-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boender LG, van Maris AJ, de Hulster EA, Almering MJ, van der Klei IJ, Veenhuis M, de Winde JH, Pronk JT, Daran-Lapujade P. 2011. Cellular responses of Saccharomyces cerevisiae at near-zero growth rates: transcriptome analysis of anaerobic retentostat cultures. FEMS Yeast Res 11:603–620. doi: 10.1111/j.1567-1364.2011.00750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vos T, Hakkaart XD, de Hulster EA, van Maris AJ, Pronk JT, Daran-Lapujade P. 2016. Maintenance-energy requirements and robustness of Saccharomyces cerevisiae at aerobic near-zero specific growth rates. Microb Cell Fact 15:111. doi: 10.1186/s12934-016-0501-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bisschops MM, Zwartjens P, Keuter SG, Pronk JT, Daran-Lapujade P. 2014. To divide or not to divide: a key role of Rim15 in calorie-restricted yeast cultures. Biochim Biophys Acta 1843:1020–1030. doi: 10.1016/j.bbamcr.2014.01.026. [DOI] [PubMed] [Google Scholar]
- 8.Pitt SJ. 1982. Maintenance energy: a general model for energy-limited and energy-sufficient growth. Arch Microbiol 133:300–302. [DOI] [PubMed] [Google Scholar]
- 9.Brice C, Cubillos FA, Dequin S, Camarasa C, Martinez C. 2018. Adaptability of the Saccharomyces cerevisiae yeasts to wine fermentation conditions relies on their strong ability to consume nitrogen. PLoS One 13:e0192383. doi: 10.1371/journal.pone.0192383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ibstedt S, Stenberg S, Bages S, Gjuvsland AB, Salinas F, Kourtchenko O, Samy JK, Blomberg A, Omholt SW, Liti G, Beltran G, Warringer J. 2015. Concerted evolution of life stage performances signals recent selection on yeast nitrogen use. Mol Biol Evol 32:153–161. doi: 10.1093/molbev/msu285. [DOI] [PubMed] [Google Scholar]
- 11.Taillandier P, Ramon Portugal F, Fuster A, Strehaiano P. 2007. Effect of ammonium concentration on alcoholic fermentation kinetics by wine yeasts for high sugar content. Food Microbiol 24:95–100. doi: 10.1016/j.fm.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 12.Kolouchová I, Maťátková O, Sigler K, Masák J, Řezanka T. 2016. Lipid accumulation by oleaginous and non-oleaginous yeast strains in nitrogen and phosphate limitation. Folia Microbiol (Praha) 61:431–438. doi: 10.1007/s12223-016-0454-y. [DOI] [PubMed] [Google Scholar]
- 13.Gutteridge A, Pir P, Castrillo JI, Charles PD, Lilley KS, Oliver SG. 2010. Nutrient control of eukaryote cell growth a systems: biology study in yeast. BMC Biol 8:68. doi: 10.1186/1741-7007-8-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tai SL, Boer VM, Daran-Lapujade P, Walsh MC, de Winde JH, Daran JM, Pronk JT. 2005. Two-dimensional transcriptome analysis in chemostat cultures. Combinatorial effects of oxygen availability and macronutrient limitation in Saccharomyces cerevisiae. J Biol Chem 280:437–447. doi: 10.1074/jbc.M410573200. [DOI] [PubMed] [Google Scholar]
- 15.Boer VM, Crutchfield CA, Bradley PH, Botstein D, Rabinowitz JD. 2010. Growth-limiting intracellular metabolites in yeast growing under diverse nutrient limitations. Mol Biol Cell 21:198–211. doi: 10.1091/mbc.e09-07-0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boer VM, de Winde JH, Pronk JT, Piper MD. 2003. The genome-wide transcriptional responses of Saccharomyces cerevisiae grown on glucose in aerobic chemostat cultures limited for carbon, nitrogen, phosphorus, or sulfur. J Biol Chem 278:3265–3274. doi: 10.1074/jbc.M209759200. [DOI] [PubMed] [Google Scholar]
- 17.Ramsay A, Douglas L. 1979. Effects of phosphate limitation of growth on the cell-wall and lipid composition of Saccharomyces cerevisiae. J Gen Microbiol 110:185–191. doi: 10.1099/00221287-110-1-185. [DOI] [PubMed] [Google Scholar]
- 18.Acquisti C, Kumar S, Elser JJ. 2009. Signatures of nitrogen limitation in the elemental composition of the proteins involved in the metabolic apparatus. Proc Biol Sci 276:2605–2610. doi: 10.1098/rspb.2008.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wada M, Kato J, Chibata I. 1981. Continuous production of ethanol in high concentration using immobilized growing yeast cells. Eur J Appl Microbiol Biotechnol 11:67–71. doi: 10.1007/BF00518045. [DOI] [Google Scholar]
- 20.Taniguchi M, Wakamiya K, Tsuchiya M, Matsuno R, Kamikubo T. 1983. Continuous ethanol production by cell-holding culture of yeasts. Eur J Appl Microbiol Biotechnol 18:201–206. doi: 10.1007/BF00501509. [DOI] [Google Scholar]
- 21.Brandberg T, Gustafsson L, Franzén CJ. 2007. The impact of severe nitrogen limitation and microaerobic conditions on extended continuous cultivations of Saccharomyces cerevisiae with cell recirculation. Enzyme Microb Technol 40:585–593. doi: 10.1016/j.enzmictec.2006.05.032. [DOI] [Google Scholar]
- 22.De Deken RH. 1966. The crabtree effect: a regulatory system in yeast. J Gen Microbiol 44:149–156. doi: 10.1099/00221287-44-2-149. [DOI] [PubMed] [Google Scholar]
- 23.Piper PW. 1995. The heat shock and ethanol stress responses of yeast exhibit extensive similarity and functional overlap. FEMS Microbiol Lett 134:121–127. doi: 10.1016/0378-1097(95)00431-9. [DOI] [PubMed] [Google Scholar]
- 24.Verduyn C, Postma E, Scheffers W, van Dijken J. 1990. Energetics of Saccharomyces cerevisiae in anaerobic glucose-limited chemostat cultures. J Gen Microbiol 136:405–412. doi: 10.1099/00221287-136-3-405. [DOI] [PubMed] [Google Scholar]
- 25.Steel CC, Grbin PR, Nichol AW. 2001. The pentose phosphate pathway in the yeasts Saccharomyces cerevisiae and Kloeckera apiculata, an exercise in comparative metabolism for food and wine science students. Biochem Mol Biol Educ 29:245–249. doi: 10.1016/S1470-8175(01)00097-2. [DOI] [Google Scholar]
- 26.Gournas C, Prévost M, Krammer E-M, André B. 2016. Function and regulation of fungal amino acid transporters: insights from predicted structure, p 69–106. In Ramos J, Sychrová H, Kschischo M (ed), Yeast membrane transport. Advances in experimental medicine and biology, vol 892 Springer, Cham, Switzerland. doi: 10.1007/978-3-319-25304-6_4. [DOI] [PubMed] [Google Scholar]
- 27.Melnykov AV. 2016. New mechanisms that regulate Saccharomyces cerevisiae short peptide transporter achieve balanced intracellular amino acid concentrations. Yeast 33:21–31. doi: 10.1002/yea.3137. [DOI] [PubMed] [Google Scholar]
- 28.Hazelwood LA, Walsh MC, Luttik MA, Daran-Lapujade P, Pronk JT, Daran JM. 2009. Identity of the growth-limiting nutrient strongly affects storage carbohydrate accumulation in anaerobic chemostat cultures of Saccharomyces cerevisiae. Appl Environ Microbiol 75:6876–6885. doi: 10.1128/AEM.01464-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Silljé HHW, Paalman JWG, Schure EGT, Olsthoorn SQB, Verkleij AJ, Boonstra J, Verrips CT. 1999. Function of trehalose and glycogen in cell cycle progression and cell viability in Saccharomyces cerevisiae. J Bacteriol 181:396–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Petitjean M, Teste MA, Francois JM, Parrou JL. 2015. Yeast tolerance to various stresses relies on the trehalose-6P synthase (Tps1) protein, not on trehalose. J Biol Chem 290:16177–16190. doi: 10.1074/jbc.M115.653899. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 31.De la Fuente IM, Cortés JM, Valero E, Desroches M, Rodrigues S, Malaina I, Martínez L. 2014. On the dynamics of the adenylate energy system: homeorhesis vs homeostasis. PLoS One 9:e108676. doi: 10.1371/journal.pone.0108676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Gulik WM, Heijnen JJ. 1995. A metabolic network stoichiometry analysis of microbial growth and product formation. Biotechnol Bioeng 48:681–698. doi: 10.1002/bit.260480617. [DOI] [PubMed] [Google Scholar]
- 33.Sonnleitner B, Käppeli O. 1986. Growth of Saccharomyces cerevisiae is controlled by its limited respiratory capacity: formulation and verification of a hypothesis. Biotechnol Bioeng 28:927–937. doi: 10.1002/bit.260280620. [DOI] [PubMed] [Google Scholar]
- 34.Pfeiffer T, Schuster S, Bonhoeffer S. 2001. Cooperation and competition in the evolution of ATP-producing pathways. Science 292:504–507. doi: 10.1126/science.1058079. [DOI] [PubMed] [Google Scholar]
- 35.Larsson C, Nilsson A, Blomberg A, Gustafsson L. 1997. Glycolytic flux is conditionally correlated with ATP concentration in Saccharomyces cerevisiae: a chemostat study under carbon- or nitrogen-limiting conditions. J Bacteriol 179:7243–7250. doi: 10.1128/jb.179.23.7243-7250.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Varela C, Pizarro F, Agosin E. 2004. Biomass content governs fermentation rate in nitrogen-deficient wine musts. Appl Environ Microbiol 70:3392–3400. doi: 10.1128/AEM.70.6.3392-3400.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Larsson C, Stockar U, Marison I, Gustafsson L. 1993. Growth and metabolism of Saccharomyces cerevisiae in chemostat cultures under carbon-, nitrogen-, or carbon- and nitrogen-limiting conditions. J Bacteriol 175:4809–4816. doi: 10.1128/jb.175.15.4809-4816.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lidén G, Persson A, Gustafsson L, Niklasson C. 1995. Energetics and product formation by Saccharomyces cerevisiae grown in anaerobic chemostats under nitrogen limitation. Appl Microbiol Biotechnol 43:1034–1038. doi: 10.1007/BF00166921. [DOI] [PubMed] [Google Scholar]
- 39.Cueto-Rojas HF, Milne N, van Helmond W, Pieterse MM, van Maris AJA, Daran JM, Wahl SA. 2017. Membrane potential independent transport of NH3 in the absence of ammonium permeases in Saccharomyces cerevisiae. BMC Syst Biol 11:49. doi: 10.1186/s12918-016-0381-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marini A, Soussi-Boudekou S, Vissers S, Andre B. 1997. A family of ammonium transporters in Saccharomyces cerevisiae. Mol Cell Biol 17:4282–4293. doi: 10.1128/mcb.17.8.4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ogawa N, DeRisi J, Brown PO. 2000. New components of a system for phosphate accumulation and polyphosphate metabolism in Saccharomyces cerevisiae revealed by genomic expression analysis. Mol Biol Cell 11:4309–4321. doi: 10.1091/mbc.11.12.4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gerasimaitė R, Mayer A. 2016. Enzymes of yeast polyphosphate metabolism: structure, enzymology and biological roles. Biochem Soc Trans 44:234–239. doi: 10.1042/BST20150213. [DOI] [PubMed] [Google Scholar]
- 43.Borodina I, Nielsen J. 2014. Advances in metabolic engineering of yeast Saccharomyces cerevisiae for production of chemicals. Biotechnol J 9:609–620. doi: 10.1002/biot.201300445. [DOI] [PubMed] [Google Scholar]
- 44.Nijkamp JF, van den Broek M, Datema E, de Kok S, Bosman L, Luttik MA, Daran-Lapujade P, Vongsangnak W, Nielsen J, Heijne WH, Klaassen P, Paddon CJ, Platt D, Kötter P, van Ham RC, Reinders MJ, Pronk JT, de Ridder D, Daran J-M. 2012. De novo sequencing, assembly and analysis of the genome of the laboratory strain Saccharomyces cerevisiae CEN.PK113-7D, a model for modern industrial biotechnology. Microb Cell Fact 11:36. doi: 10.1186/1475-2859-11-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Verduyn C, Postma E, Scheffers WA, Van Dijken J. 1992. Effect of benzoic acid on metabolic fluxes in yeasts: a continuous culture study on the regulation of respiration and alcoholic fermentation. Yeast 8:501–517. doi: 10.1002/yea.320080703. [DOI] [PubMed] [Google Scholar]
- 46.Postma E, Verduyn C, Scheffers WA, Van Dijken J. 1989. Enzymic analysis of the crabtree effect in glucose-limited chemostat cultures of Saccharomyces cerevisiae. Appl Environ Microbiol 55:468–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lameiras F, Heijnen JJ, van Gulik WM. 2015. Development of tools for quantitative intracellular metabolomics of Aspergillus niger chemostat cultures. Metabolomics 11:1253–1264. doi: 10.1007/s11306-015-0781-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cueto-Rojas HF, Maleki Seifar R, Ten Pierick A, Heijnen SJ, Wahl A. 2016. Accurate measurement of the in vivo ammonium concentration in Saccharomyces cerevisiae. Metabolites 6:12. doi: 10.3390/metabo6020012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lange HC, Heijnen JJ. 2001. Statistical reconciliation of the elemental and molecular biomass composition of Saccharomyces cerevisiae. Biotechnol Bioeng 75:334–344. doi: 10.1002/bit.10054. [DOI] [PubMed] [Google Scholar]
- 50.Niedenführ S, ten Pierick A, van Dam PTN, Suarez-Mendez CA, Nöh K, Wahl SA. 2016. Natural isotope correction of MS/MS measurements for metabolomics and 13C fluxomics. Biotechnol Bioeng 113:1137–1147. doi: 10.1002/bit.25859. [DOI] [PubMed] [Google Scholar]
- 51.Lange HC, Eman M, van Zuijlen G, Visser D, van Dam JC, Frank J, Teixeira de Mattos M, Heijnen JJ. 2001. Improved rapid sampling for in vivo kinetics of intracellular metabolites in Saccharomyces cerevisiae. Biotechnol Bioeng 75:406–415. doi: 10.1002/bit.10048. [DOI] [PubMed] [Google Scholar]
- 52.Wu L, Mashego MR, van Dam JC, Proell AM, Vinke JL, Ras C, van Winden WA, van Gulik WM, Heijnen JJ. 2005. Quantitative analysis of the microbial metabolome by isotope dilution mass spectrometry using uniformly 13C-labeled cell extracts as internal standards. Anal Biochem 336:164–171. doi: 10.1016/j.ab.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 53.van Dam JC, Eman MR, Frank J, Lange HC, van Dedem GWK, Heijnen SJ. 2002. Analysis of glycolytic intermediates in Saccharomyces cerevisiae using anion exchange chromatography and electrospray ionization with tandem mass spectrometric detection. Anal Chim Acta 460:209–218. doi: 10.1016/S0003-2670(02)00240-4. [DOI] [Google Scholar]
- 54.Cipollina C, ten Pierick A, Canelas AB, Seifar RM, van Maris AJ, van Dam JC, Heijnen JJ. 2009. A comprehensive method for the quantification of the non-oxidative pentose phosphate pathway intermediates in Saccharomyces cerevisiae by GC-IDMS. J Chromatogr B Analyt Technol Biomed Life Sci 877:3231–3236. doi: 10.1016/j.jchromb.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 55.Seifar RM, Ras C, van Dam JC, van Gulik WM, Heijnen JJ, van Winden WA. 2009. Simultaneous quantification of free nucleotides in complex biological samples using ion pair reversed phase liquid chromatography isotope dilution tandem mass spectrometry. Anal Biochem 388:213–219. doi: 10.1016/j.ab.2009.02.025. [DOI] [PubMed] [Google Scholar]
- 56.Daran-Lapujade P, Jansen ML, Daran JM, van Gulik W, de Winde JH, Pronk JT. 2004. Role of transcriptional regulation in controlling fluxes in central carbon metabolism of Saccharomyces cerevisiae. A chemostat culture study. J Biol Chem 279:9125–9138. doi: 10.1074/jbc.M309578200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.