We observed that the S. antibioticus extracellular tyrosinase secretion level was even higher in its nonnatural translationally conjugated fusion protein form than in the natural complex of two separated polypeptides. The results of this study demonstrate that tyrosinase-expressing P. fluorescens can be a stable source of bacterial tyrosinase through exploiting the secretory machinery of P. fluorescens.
KEYWORDS: MelC2, Pseudomonas fluorescens, Streptomyces antibioticus, biofilms, fusion protein, membrane transport, protein secretion, twin-arginine translocation, tyrosinase
ABSTRACT
Tyrosinase is a monooxygenase that catalyzes both the hydroxylation of p-hydroxyphenyl moieties to o-catechols and the oxidation of o-catechols to o-quinones. Apart from its critical functionality in melanogenesis and the synthesis of various neurotransmitters, this enzyme is also used in a variety of biotechnological applications, most notably mediating covalent cross-linking between polymers containing p-hydroxyphenyl groups, forming a hydrogel. Tyrosinases from the genus Streptomyces are usually secreted as a complex with their caddie protein. In this study, we report an increased secretion efficiency observed when the Streptomyces antibioticus tyrosinase gene melC2 was introduced into Pseudomonas fluorescens along with its caddie protein gene melC1, which has the DNA sequence for the Tat (twin-arginine translocation) signal.
IMPORTANCE We observed that the S. antibioticus extracellular tyrosinase secretion level was even higher in its nonnatural translationally conjugated fusion protein form than in the natural complex of two separated polypeptides. The results of this study demonstrate that tyrosinase-expressing P. fluorescens can be a stable source of bacterial tyrosinase through exploiting the secretory machinery of P. fluorescens.
INTRODUCTION
Tyrosinase is a monooxygenase that has copper ions in its active site. It has a wide range of substrate selectivities, hydroxylating p-hydroxyphenyl moieties into 3,4-dihydroxyphenyl (o-catechol) moieties and oxidizing the latter into o-quinones (1, 2). Although their structures are not uniform, tyrosinases exist ubiquitously in various organisms, including prokaryotic and eukaryotic microorganisms, fungi, plants, insects, invertebrates, and mammals. In these biological systems, tyrosinase plays a key role in pigment formation (e.g., melanogenesis), neurotransmitter biosynthesis, the primary immune response (e.g., dopamine synthesis), and wound healing (3). Apart from its physiological importance, tyrosinase has also drawn attention in academic and industrial fields for its biotechnological applications, such as biomaterial adhesion, bioremediation, and industrial melanin production (1, 2, 4). Most notably, tyrosinase promotes covalent cross-linkages in polymers containing p-hydroxyphenyl groups by converting them to 3,4-dihydroxyphenyl groups and then to 3,4-quinones, which can spontaneously form covalent bonds with 3,4-dihydroxyphenyl groups under oxidative conditions (5). Interestingly, hydrogels, loosely cross-linked hydrophilic polymers, can be prepared via this reaction by cross-linking chitosan or chondroitin sulfate-tyramine, both of which contain p-hydroxyphenyl moieties (6, 7). In addition, tyrosinase is utilized in the production of l-3,4-dihydroxyphenylalanine (l-DOPA) (8), removal of phenols (9), phenolic biosensors (10), and cross-linking of enzymes (11). Bacterial tyrosinases are potent candidates for these biotechnological applications (1, 2). A vast number of fungi or bacteria were found to produce tyrosinase, including Agaricus bisporus (12), Streptomyces albus (13), Bacillus megaterium (14), Verrucomicrobium spinosum (15), and Rhizobium etli (16). Despite these diverse sources, the only commercially available tyrosinase is from the fungus Agaricus bisporus (12). Nevertheless, apart from commercial purposes, tyrosinase from the bacterial genus Streptomyces is the most thoroughly characterized (17–20). There have been many attempts to mass-produce tyrosinase by overexpressing bacterial tyrosinases in bacterial hosts. Most of these approaches involve isolating tyrosine-secreting microorganisms (21) or expressing tyrosine in Escherichia coli for purification following cell lysis (22). In this study, we aimed to produce tyrosinase from Streptomyces antibioticus in a bacterial expression host via secretion. Secretion-based production of proteins greatly reduces the effort for purification and eliminates inclusion body problems (23).
The Streptomyces tyrosinase operon is composed of two genes, melC1 and melC2 (20). MelC2 is the tyrosinase core enzyme, carrying the active site with cresolase and catecholase activities. However, MelC2 alone is inactive (in the apotyrosinase form), and it requires MelC1 for full activation. MelC1, the tyrosinase transactivator protein or “caddie protein,” is known to be responsible for copper insertion into the active site of MelC2 by forming a transient complex with MelC2 (20). Also, MelC1 is hypothesized to facilitate the secretion of MelC2 in wild-type S. antibioticus since it contains a characteristic Tat (twin-arginine translocation) signal peptide sequence (24, 25). MelC1 is postulated to carry MelC2 during secretion (26). After the completion of copper insertion and secretion, MelC1 dissociates from MelC2, and the copper-incorporated MelC2 holoenzyme exhibits tyrosinase activity (27).
For the expression of the tyrosinase, we chose Pseudomonas fluorescens ΔtliA ΔprtA (28), which had several advantages over other bacterial hosts: (i) it can be cultured in high-cell-density fermentation (29); (ii) it has the Tat system to secrete tyrosinase, eliminating the tedious steps of purifying Streptomyces tyrosinase from other intracellular proteins from lysed cells; and (iii) it can be employed for further applications utilizing the well-documented ATP-binding cassette (ABC) transporter system (for further details, see Discussion).
RESULTS
Construction of plasmids harboring recombinant genes and accidental identification of the MelC2h-MelC1 fusion protein.
We chose a widely used shuttle vector, pDSK519, as the expression vector in both E. coli and P. fluorescens. The melC2 tyrosinase gene with a His6 tag at the C terminus or N terminus was amplified via PCR using the primers listed in Table 1 and cloned into pDSK519, each generating pDSK-hMelC2 and pDSK-MelC2h (Fig. 1). Next, the melC1 caddie protein gene was amplified via PCR and inserted into pDSK-hMelC2 and pDSK-MelC2h to generate pDSK-hMelC2/C1 and pDSK-MelC2h/C1, respectively. We selected clones that exhibited the strongest tyrosinase activity based on the halo size during the E. coli colony isolation step. Interestingly, the clone with the strongest tyrosinase activity among the pDSK-MelC2h/C1 samples turned out to carry a deletion mutation where the stop codon of melC2h was removed. As a result, it produced a fusion protein of MelC2h and MelC1, for which MelC1 was translated in frame with MelC2h, separated by a short linkage peptide. We renamed this plasmid pDSK-MelC2h-C1, with a hyphen representing the fusion of the two proteins, as opposed to pDSK-MelC2h/C1, which properly had a stop codon at the end of melC2h and produced monomeric MelC2h as initially planned. When we performed a brief comparison of melanin synthesis rates in plate activity assays, pDSK-MelC2h-C1 synthesized more melanin than did pDSK-MelC2h/C1. In order to verify that this was not a result of any other plasmid mutations, we reconstructed pDSK-MelC2h-C1 with a dedicated primer (where the primer itself had no stop codon at the end of MelC2h), and we observed similar results (see Fig. S1 in the supplemental material). These results suggest that the MelC2h-MelC1 fusion protein potentially has better production and/or secretion efficiency than that of monomeric MelC2h expressed with MelC1. Therefore, we designed the following experiments to compare tyrosinase expression and secretion.
TABLE 1.
Primers used in this study
| Primer | Sequencea | Feature(s) |
|---|---|---|
| SphI-His6-melC2 | GCATGCCcatcaccatcaccatcac/atgacagtccgcaaaaaccaa | SphI site, His6 tag, start codon, MelC2 sense primer |
| melC2-XbaI | TCTAGA/ttatacatcaaaggtgtaatgac | XbaI site, MelC2 antisense primer, stop codon |
| XbaI-RBS-melC1 | TCTAGAAGGAAACAGCT/atgccggaactcacccgtcg | XbaI site, RBS, start codon, MelC1 sense primer |
| melC1-KpnI | GGTACC/tcagttggaggggaagggga | KpnI site, MelC1 antisense primer, stop codon |
| SphI-melC2 | GCATGCC/atgacagtccgcaaaaaccaa | SphI site, start codon, MelC2 sense primer |
| melC2-His6-XbaIb | TCTAGA/ttagtgatggtgatggtgatgtacatcaaaggtgtaatgacgc | XbaI site, MelC2 antisense primer, His6 tag, stop codon |
| SphI-melC1 | GCATGCG/atgccggaactcacccgtcg | SphI site, start codon, MelC1 sense primer |
| melC1-PstI | CTGCAGgttggaggggaaggggag | PstI site, MelC1 antisense primer |
| PstI-melC2 | CTGCAG/atgacagtccgcaaaaaccaa | PstI site, start codon, MelC2 sense primer |
| melC2-XbaI-2 | TCTAGAtacatcaaaggtgtaatgacgc | XbaI site, MelC2 antisense primer |
| XbaI-melC1 | TCTAGA/atgccggaactcacccgtcg | XbaI site, start codon, MelC1 sense primer |
| F-TatA | TCCCCTCCAACTGAGaacttttacagaggaattgcag | In-fusion insertion into the KpnI/EcoRI site |
| TatC-B | GACGGCCAGTGAATTtcacggctgggtcgctgg | In-fusion insertion into the KpnI/EcoRI site |
The start and stop codons are in italics with a slash, and the RBS is underlined. The sequence for the His6 tag is shown in boldface type. Lowercase letters represent the coding region, and capital letters represent the restriction enzyme sites or sequences in the plasmid. GGG was added in front of the restriction enzyme sites for proper enzyme cleavage.
The sequence TCTAGA/ttagtgatggtgatggtgatgtacatcaaaggtgtaatgacgc has one base missing (the underlined “g”) and was amplified in the construction of pDSK-MelC2h-C1.
FIG 1.

Gene organization of the mel expression plasmids used in this experiment. Two genes, melC1 and melC2, were inserted into pDSK519 with various arrangements. In any case, the upstream gene was inserted in frame with the lacZα fragment, and the second gene (if any) was inserted with its own RBS. The slashes in the plasmid name indicate that the two genes were expressed as two separate polypeptides, whereas the hyphens denote that the genes were connected into a fused polypeptide. The plasmids for the fusion proteins, pDSK-MelC2h-C1, pDSK-MelC2h-C1, and pDSK-MelC1-C2h, were constructed with melC1 and melC2 placed in frame without a stop codon between the two genes in pDSK519. pDSK519 has a lac promoter, which shows constitutive expression in P. fluorescens because there is no lacI gene in the P. fluorescens genome or in the plasmid.
Expression patterns of His6-tagged MelC2 and MelC1 in P. fluorescens and E. coli.
After transforming E. coli and P. fluorescens with the plasmid constructs (making them express MelC2h, MelC2h/C1, and MelC2h-C1, respectively), we examined the expression and secretion of tyrosinase via Western blot analysis (Fig. 2A and B). In addition, we directly visualized the tyrosinase activity of these cells by streaking individual colonies on lysogeny broth (LB) agar plates supplemented with l-tyrosine and copper(II) ion and observing the formation of a dark melanin halo by tyrosinase (Fig. 2C and D). Finally, we centrifuged liquid cultures and tested the culture supernatant for the activity of tyrosinase via a solution-based l-DOPA assay (Fig. 2E and F). Based on the Western blot analyses, the expression patterns showed considerable differences depending on the cell host species and the presence of melC1. In general, tyrosinase was secreted with a higher efficiency in P. fluorescens than in E. coli, in terms of the supernatant-to-total signal strength ratio and the culture supernatant activity. In E. coli, the secretion of tyrosinase was minimal in all the variants MelC2h, MelC2h/C1, and MelC2h-C1. In contrast, the Western band intensity of tyrosinase in the supernatant, the degree of melanin pigment formation in the plate assay, and the supernatant activity in the l-DOPA assay were all significantly higher in the engineered P. fluorescens strain.
FIG 2.
Comparison of expression and secretion levels of melC2 and melC1 in E. coli and P. fluorescens. (A and B) The expression and secretion patterns of His6-tagged MelC2 and MelC1 were analyzed by Western blotting using an anti-His6 tag primary antibody in E. coli (A) and P. fluorescens (B). Both the cell pellet (labeled “C”) and the supernatant (labeled “S”) samples were analyzed in parallel. Cells of both species with different plasmids expressing tyrosinase were grown in LB for 1 and 3 days, respectively, each at the optimal growth temperature depending on the organism. Identifiable bands of His6-tagged MelC2 and MelC1 are indicated with arrows. The images were cropped from a single image of a single membrane, with all brightness and contrast adjustments performed identically. (C and D) E. coli (C) and P. fluorescens (D) cells with different plasmids expressing tyrosinase were streaked onto LB agar containing CuCl2 and l-tyrosine for a tyrosinase activity assay, and cells of both strains were grown at the optimal temperature. (E and F) The supernatants of E. coli (E) and P. fluorescens (F) cultures were collected by centrifugation and analyzed for tyrosinase activity via an l-DOPA assay by measuring the change in the A475. Student’s t test revealed that the difference between pDSK-MelC2h/C1 and pDSK-MelC2h-C1 was significant. The error bars represent the sample standard deviations. (A to F) C2h represents pDSK-MelC2h, C2h/C1 represents pDSK-MelC2h/C1, and C2h-C1 represents pDSK-MelC2h-C1. The original uncropped-image data are provided in Fig. S4 in the supplemental material.
In wild-type S. antibioticus, the source of the mel genes, the MelC1 caddy protein activates MelC2 by copper insertion and aids secretion mediated by the Tat pathway (26). In E. coli, pDSK-MelC2h resulted in no significant melanin pigment formation and very low culture supernatant tyrosinase activity. In contrast, brown pigments were formed around the colonies, and the l-DOPA assay showed greater activity for P. fluorescens cells harboring pDSK-MelC2h, indicating that they had tyrosinase activity (Fig. 2B and D). This means that MelC2 alone exhibited tyrosinase activity even without MelC1 in P. fluorescens. Nevertheless, the presence of MelC1 further enhanced the expression levels, secretion efficiency, and supernatant activity of tyrosinase in recombinant P. fluorescens. In addition, the production of melanin pigment around colonies and the l-DOPA assay activity escalated in pDSK-MelC2h/C1 and pDSK-MelC2h-C1 cells, compared to cells with pDSK-MelC2h. In summary, tyrosinase secretion and expression were stronger in P. fluorescens than in E. coli, and coexpressed MelC1 enhanced the secretion. However, there was a difference depending on the fusion of the proteins, which is detailed below.
By comparing cells expressing MelC2 and MelC1 as separate polypeptides (pDSK-MelC2h/C1) and cells expressing them as a fused single polypeptide (pDSK-MelC2h-C1), we observed increases in the secretion efficiency (Fig. 2B), melanin formation (Fig. 2D), and culture supernatant activity. In the l-DOPA assay, recombinant cells expressing the fusion protein had higher tyrosinase activity than recombinant cells expressing MelC2 and MelC1 as separate polypeptides in P. fluorescens (Fig. 2F). Nevertheless, we were not sure whether the higher supernatant activity of pDSK-MelC2h-C1 cells was a result of higher specific activity or a higher secretion level. Therefore, for verification, we set up the next experiment.
Purification and characterization of secreted tyrosinase.
P. fluorescens cells harboring pDSK-hMelC2/C1 or pDSK-MelC2h-C1 were cultured in LB medium in a flask in order to express and secrete tyrosinases into the supernatant. The supernatant of the flask liquid culture was collected by centrifugation and tested for tyrosinase activity (Fig. 3A). Subsequently, we directly purified the protein with affinity chromatography using nickel-nitrilotriacetic acid (Ni-NTA) agarose resin, which binds the His6 tag on MelC2. The activity of the purified tyrosinase was also measured, and the molar specific activity was calculated based on the molecular mass of each protein (Fig. 3B). Before the purification step, the culture supernatant of pDSK-hMelC2/C1 had 0.39 U/ml activity on average, while pDSK-MelC2h-C1 had 0.48 U/ml activity on average, showing that the culture of the fusion protein-producing cells had higher tyrosinase activity in the supernatant. The purified proteins had similar molar specific activities, 14.4 s−1 and 14.9 s−1, respectively, which signifies that the higher supernatant activity of the fusion protein-producing cell culture (Fig. 2F) is likely to be a result of higher molar concentrations of the active tyrosinase protein.
FIG 3.

Characterization of purified MelC2 and the fusion protein MelC2h-MelC1. (A) Secreted tyrosinase activity in the unpurified supernatant of a large-volume culture of P. fluorescens harboring pDSK-hMelC2/C1 or pDSK-MelC2h-C1. Volumetric activity was measured via an l-DOPA assay by measuring the change in the A475. (B) After purification, the molar specific activities of purified tyrosinases from the culture supernatants of P. fluorescens cells harboring pDSK-hMelC2/C1 and cells harboring pDSK-MelC2h-C1 were compared. Tyrosinase was purified using an Ni-NTA agarose column. (C) The purified tyrosinase from P. fluorescens harboring pDSK-hMelC2/C1 or pDSK-MelC2h-C1 was loaded onto an SDS-PAGE gel. (D) The MelC2h-MelC1 fusion protein from pDSK-MelC2h-C1 was analyzed by SDS-PAGE after long-term 4°C storage or diluted-trypsin treatment. (E) MelC2h-MelC1, after extended storage or trypsin treatment, was analyzed by Western blotting with a His tag antibody. (F) Culture supernatants of MelC2h/C1 and MelC2h-C1 with or without brief trypsin treatment were analyzed via Western blotting. (A and B) Error bars represent the sample standard deviation of the three samples. Lanes (D to F): 1, MelC2h-C1 (47.8 kDa); 2, MelC2h-C1 after 6-month storage at 4°C; 3, MelC2h-C1 after brief trypsin treatment; 4, MelC2h/C1 (32.6 kDa); 5, MelC2h-C1 (47.8 kDa).
To verify the purity of the product, we analyzed the purified samples by SDS-PAGE and subsequent Coomassie brilliant blue gel staining (Fig. 3C). Gel electrophoresis revealed that MelC2 and MelC1 were indeed the major bands in all of the samples. Specifically, pDSK-hMelC2/C1 samples had the 32.6-kDa MelC2 band and the 14.5-kDa MelC1 band as their major bands (with minor bands that are expected to be multimers), whereas the pDSK-MelC2h-C1 sample had the fusion protein with a molecular weight of 47.8 kDa as its major band, accompanied by the oxidized form (which appears slightly below the major band).
There were a few interesting phenomena that we observed for the purified tyrosinase samples. First, the MelC1 caddie protein was not cleaved, even after secretion. MelC1 contains an N-terminal Tat secretion signal which is cleaved in wild-type S. antibioticus upon secretion, but MelC1 and the purified fusion protein MelC2h-MelC1 were not cleaved. We verified this by analyzing MelC1 and MelC2h-MelC1 bands by SDS-PAGE. The band was excised from the gel and analyzed by fast protein liquid chromatography (FPLC) and matrix-assisted laser desorption ionization–time of flight mass spectroscopy (MALDI-TOF MS), after it was treated with trypsin. The MALDI-TOF MS results matched the expected fragmentation patterns of the MelC2h-MelC1 fusion protein in its intact form (Fig. S2).
We also analyzed the MelC2h-MelC1 fusion protein stored for a month at 4°C, to examine effects of long-term storage and possible degradation patterns. The sample after prolonged storage exhibited 47.8-kDa, ∼36-kDa, and ∼12-kDa fragments (Fig. 3D, lane 2), possibly due to nonenzymatic spontaneous peptide bond cleavage. Among them, the 47.8-kDa and ∼36-kDa bands were also detected by Western blotting using an anti-His6 tag antibody (Fig. 3E, lane 2). This means that the 47.8-kDa band is the MelC2h-MelC1 fusion protein and that the ∼36-kDa band is MelC2h with the ∼3-kDa N-terminal part of MelC1, suggesting that the ∼12-kDa fragment is the remaining part of the cleaved MelC1 protein. These results show that the long-term storage of the fusion protein degrades the fusion protein in half, near the N-terminal region of MelC1. The degradation pattern was also very similar to the trypsin digestion pattern of the fusion protein (Fig. 3D and E, lane 3). Note that the original MelC2 protein was not cleaved by the same level of trypsin treatment that cleaved the MelC2h-MelC1 fusion protein (Fig. 3F). This further supports our hypothesis that the MelC2h-MelC1 fusion protein has a relatively exposed, less structured region between the MelC2h region and the MelC1 region.
Verification of the secretion mechanism in the heterologous expression host.
In wild-type S. antibioticus, the MelC2 apoenzyme does not have a signal sequence, but the Tat signal sequence in the caddie protein MelC1 is recognized, and the entire complex of MelC2 and MelC1 is transported together (26, 30). This is a fairly common feature among Tat substrates (31). We set up the following experiment to verify whether this Tat-dependent pathway is also responsible for the secretion of MelC2 tyrosinase that we observed in the heterologous expression host Pseudomonas fluorescens.
We identified a tat operon within the P. fluorescens genome (submitted to GenBank; see “Data availability” in Materials and Methods), and the three genes related to the bacterial Tat pathway (32, 33) were cloned. These genes are tatA, tatB, and tatC. We observed an increase of the culture supernatant tyrosinase activity when the tatABC operon of P. fluorescens was overexpressed by inserting tatABC into pDSK-MelC2h/C1 and pDSK-MelC2h-C1 (Fig. 4A and B). In addition, when the critical twin-arginine sequence of the Tat signal peptide of MelC1 was replaced by alanines, the culture supernatant activity was reduced (Fig. 4C and D).
FIG 4.
Evidence for the Tat dependence of tyrosinase secretion in P. fluorescens. (A and B) The tatABC operon was PCR amplified from P. fluorescens ΔtliA ΔprtA and inserted into pDSK-hMelC2/C1 (A) and pDSK-MelC2h-C1 (B). The tyrosinase activity in the culture supernatant was analyzed via an l-DOPA assay, measured by the change in the A475. (C and D) The twin-arginine residues of MelC1’s Tat signal peptide were replaced by two alanine residues (ΔSSTat) and expressed in P. fluorescens. The tyrosinase activities of pDSK-MelC2h/C1 (C) and pDSK-MelC2h-C1 (D) in the culture supernatants were examined by an l-DOPA assay. The secretion efficiency of the tyrosinase was reduced in the mutated proteins, in both the separated polypeptide version and the fusion protein version. The error bars represent the sample standard deviations.
Other fusion proteins of MelC2 and MelC1.
As mentioned above, the fusion protein of MelC2 and MelC1, MelC2h-MelC1, was produced by chance, leaving an important question as to whether or not this was the optimal form of the fusion. Therefore, we tried to verify whether a similar secretion-enhancing effect was observed when we changed the sequential arrangement of the fusion. We constructed two other plasmids that make translationally fused proteins: pDSK-hMelC2-C1(‡) and pDSK-MelC1-C2h(§). Along with the previously constructed fusion protein-expressing plasmid pDSK-MelC2h-C1(†) and the control plasmid pDSK-hMelC2/C1(*), these plasmids were tested for their secretion using methods identical to those demonstrated in Fig. 2 (Fig. 5). (The symbols *, †, ‡, and § are used to aid the reader in keeping track of the long plasmid names used in this section. These symbols are used in Fig. 4 as well.) In this experiment, pDSK-MelC2h-C1(†) (the fusion protein plasmid tested as described above) had the strongest Western signal in the supernatant and had the most prominent halo in the activity assay. pDSK-hMelC2/C1(*) (separated expression of MelC2 and MelC1) had a smaller halo and a weaker supernatant Western signal. Plasmid pDSK-hMelC2-C1(‡), despite being a fusion protein, had a halo size comparable to that of pDSK-hMelC2/C1(*) and a very weak supernatant band signal in the Western blot assay. Finally, pDSK-MelC1-C2h(§) had a very low expression level, and the size of the halo was the smallest. The His6 tag location possibly gave a slight difference in protein structure between pDSK-MelC2h-C1(†) and pDSK-hMelC2-C1(‡), affecting their secretory behaviors. We expect that the His6 tag between MelC2 and MelC1 in pDSK-MelC2h-C1(†) played a role as a linker between the two proteins, allowing secretory machineries to access the signal sequence of MelC1. It is also interesting that MelC1-MelC2h(§) had very low levels of expression and secretion. The N-terminal Tat signal sequence of MelC1 is present immediately after the N-terminal short LacZα fragment in this fusion protein, which means that the smaller LacZα fragment was less suited than the larger MelC2h fragment in MelC2h-MelC1 for expression and secretion.
FIG 5.

Comparison of expression levels of fusion proteins of MelC2 and MelC1 depending on the arrangement of the genes. (A) P. fluorescens cells with different plasmids expressing tyrosinase were streaked onto LB agar plates containing CuCl2 and l-tyrosine and grown at 25°C for 3 days. (B) The expression patterns of MelC2 and its fusion proteins were analyzed via Western blotting. “*” represents plasmid pDSK-hMelC2/C1, “†” represents pDSK-MelC2h-C1, “‡” represents pDSK-hMelC2-C1, and “§” represents pDSK-MelC1-C2h. These symbols are used in the text as well. Interestingly, despite the fact that it has a Tat signal sequence at the N-terminal region of MelC1, MelC1-C2h(§) was not secreted.
Whole-cell-catalyzed hydrogel formation.
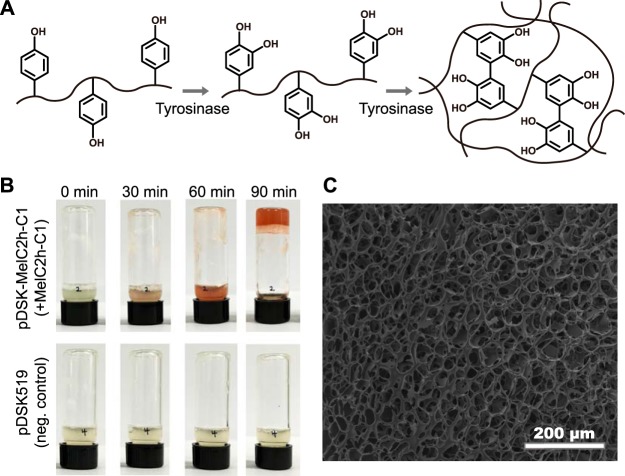
The amount of tyrosinase produced and secreted in this study was about 0.5 U/ml in P. fluorescens, using the pDSK519 vector. This corresponds to approximately 26 mg/liter tyrosinase (14.9 s−1 corresponds to 19.2 U/mg in the case of the fusion protein). The tyrosinase activity in this study is lower than those in previous studies by other investigators, 0.4 U/ml intracellular tyrosinase in E. coli (22), 86 mg/liter in E. coli (14), and 4.62 U/ml by S. antibioticus fermentation (21). However, we mainly focused on secretion-based production (without cell lysis) and in situ hydrogel formation. One of the unique applications of secretion-based tyrosine production is whole-cell catalysis. Utilizing the cross-linking activity of the secreted tyrosinase, bioengineered P. fluorescens can create a hydrogel (loosely cross-linked hydrophilic polymers capable of holding a large volume of water within their grid) from polymers with p-hydroxyphenyl groups. Furthermore, this involves the incorporation of the cells themselves, creating a stable matrix that prevents cell dispersion that may happen in an open environment, which enables even further applications in perhaps maritime and ecological fields. A tyrosinase-expressing recombinant P. fluorescens culture was used to prepare the microorganism-encapsulated hydrogel using the model polymer (Fig. 6). Microbial solutions containing recombinant P. fluorescens (A600 of 10) harboring the pDSK-MelC2h-C1 or pDSK519 control vector were mixed with an equal volume of an 8% (wt/vol) phenol-conjugated chitosan (CHI-PHE) polymer solution (Fig. 6B). The reaction mixture was observed every 30 min. After 90 min, the reaction solution solidified. The p-hydroxyphenyl groups conjugated on chitosan were converted into 3,4-dihydroxyphenyl (DOPA-like) groups by tyrosinase and then conjugated with each other to make a cross-linked system of hydrophilic polymers, a hydrogel. The cells were also incorporated into the hydrogel matrix via covalent bonds between the quinone groups generated on the polymer and amine (NH2–) or thiol (SH–) groups exposed on the bacterial cell surface, via reductive amination and reductive thiolation (Fig. 6A). The hydrogel structure was lyophilized and observed (Fig. 6C), showing that the whole-cell-catalyzed synthesis of the hydrogel was successful. The prepared hydrogel can be applied as a whole-cell catalyst utilizing the enzymatic activity of the encapsulated recombinant cells.
FIG 6.
Whole-cell-catalyzed hydrogel formation by MelC2h-MelC1 tyrosinase fusion protein-expressing cells. (A) Molecular mechanism of synthesis of the hydrogel. Tyrosinase converts tyrosine to DOPA, which can form a bond with neighboring DOPA to produce an interpolymer cross-link or make covalent bonds with thiol or amino groups on the surface of bacterial cells to produce a hydrogel of cells and polymers. (B) Investigation of hydrogel formation with tyrosinase-expressing P. fluorescens. A bacterial culture of P. fluorescens harboring pDSK-MelC2h-C1 (A600 = 10) was mixed with an 8% chitosan polymer, with p-hydroxyphenyl groups conjugated via a 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC) reaction. After 90 min, a hydrogel was formed and adhered to the bottom of the vial in the group where the fusion protein MelC2h-MelC1 was expressed but not in the control group (cells harboring the pDSK519 vector). (C) The hydrogel generated in panel B was lyophilized and observed using a scanning electron microscope. The white part is the network of dry cell matter interconnected with each other.
DISCUSSION
In this research, we aimed for heterologous expression and secretion-based production of bacterial tyrosinase mainly for whole-cell catalyst application. During the course of the study, we also discovered that the fusion between the tyrosinase apoenzyme gene melC2 and the caddie protein gene melC1 leads to an enhanced level of tyrosinase activity in the culture supernatant. Our analysis therefore mainly focuses on two aspects: the heterologous secretion-based production of tyrosinase and the characteristics of the tyrosinase fusion protein.
The first notable point was that even in heterologous expression hosts, the caddie protein MelC1 not only promoted the tyrosinase activity of recombinant MelC2 but also enhanced its secretion. The second point was that when the tyrosinase secretion levels of cells expressing MelC2 and MelC1 as separate polypeptides and cells expressing the MelC2h-MelC1 fusion protein were compared, the latter was higher.
In wild-type S. antibioticus, the tyrosinase MelC2 is secreted by the Tat pathway (25). Bacterial precursor proteins translocated via the Tat pathway have an unusually long signal peptide with a conserved twin-arginine sequence, S/T-R-R-x-ϕ-ϕ (where ϕ is a hydrophobic amino acid), that is essential for transport (34, 35) and cleaved after protein translocation (20, 36). According to previous reports, the signal sequence of the Tat pathway is dependent on the host organism, and the Tat signal of the Bacillus subtilis glucose-fructose oxidoreductase does not work in the heterologous expression host E. coli (37). However, from our results, where we found secreted tyrosinase in E. coli and P. fluorescens, we expect that MelC2 and MelC1 can also be translocated by E. coli and P. fluorescens Tat pathways, despite having a Tat signal sequence of foreign origin. This is further supported by our verification experiments.
As the results in Fig. 3 demonstrate, we verified that both the independent peptides MelC1 and MelC2 and the fusion protein MelC2h-MelC1 were secreted in an intact form without any cleavage of the Tat signal sequence. It seems that the signal peptide of S. antibioticus is not cleaved by P. fluorescens signal peptidase, consistent with previous reports stating that the signal peptidase of Gram-positive bacteria has a specificity different from that of Gram-negative bacteria (38, 39).
We expected a total defect of secretion when the two “invariable” arginine residues of the Tat signal were replaced by alanine residues, but the results of the Tat signal mutation experiments show that secretion was not completely blocked (Fig. 4C and D). However, this was consistent with more recent understandings of the Tat secretion pathway (40, 41).
We also expect that the fusion protein has its MelC2 and MelC1 “domains” folded relatively independent of each other, reserving the folding structure of MelC1, which is essential for Tat secretion. Long-term storage resulted in nonenzymatic spontaneous peptide bond cleavage, and this usually happens at bonds directly following aspartic acid or asparagine residues in a high-dielectric-constant environment (42, 43). The spontaneous peptide bond cleavage degraded only the region between MelC2 and MelC1 (Fig. 3E). Therefore, this region is expected to be highly solvent accessible. Also, brief trypsin digestion cleaved the fusion protein in half at approximately the same position despite the fusion protein carrying many lysine and arginine residues (Fig. 3E and F). These two results suggest that the region between the two domains is relatively flexible and solvent accessible, while within each domain, the fold is stable and prevents access of the protease.
As both the dimeric and the fused forms of MelC1 and MelC2 were secreted in P. fluorescens, we were able to speculate on the structure of the fusion protein and the mechanism of its secretion. We constructed the structure of each domain of the fusion protein by homology-based structure prediction based on the dimeric structure of Streptomyces castaneoglobisporus tyrosinase and its caddie protein (Fig. 7A). In this complex of wild-type MelC2 and MelC1, residue Tyr113 of MelC1 fits in the active-site crevice of MelC2 (see Fig. S3 in the supplemental material), just like the pin of a thumbtack (36). This interaction between MelC2 and MelC1 was proposed to be an important feature of tyrosinase activation by a previous study (27).
FIG 7.

Structure model of the MelC1/MelC2 dimer complex and schematic model of the fusion protein. (A) The modeling structure of the MelC1/MelC2 complex was predicted using SWISS-MODEL with Streptomyces castaneoglobisporus tyrosinase (20) as a template (PDB accession no. 3AWU), which has 81.6% sequence identity to MelC1/MelC2. MelC1 is in green, whereas MelC2 is in blue. The tyrosine (Tyr113) of MelC1 acting like the pin of a thumbtack is shown in red. (B) The MelC2h-MelC1 fusion protein was produced so that MelC1 was fused in frame with MelC2 containing a His6 tag between MelC2 and MelC1. The fusion protein consisted of the two proteins bridged by a few linkage peptides and the His6 tag. The unstructured linkage peptide is drawn in yellow. The N terminus and C terminus of the fusion protein are shown as N′ and C′. (C) Hypothetical interaction between two molecules of the MelC2h-MelC1 fusion protein. The MelC1 domain of one molecule of the fusion protein interacts with the MelC2 domain of another molecule, creating a complex so that a subsection of it (circled in cyan) resembles the naturally occurring MelC2/MelC1 complex (shown in panel A).
According to previous studies, the secretion of tyrosinase protein is mediated by the Tat pathway, and the Tat signal sequence in MelC1 facilitates the secretion of the entire complex (3). Moreover, the MelC1 domain must activate the MelC2 domain by interacting with it, meaning that the interaction between the MelC2 and MelC1 domains, similar to the structure in Fig. 7A, must form somehow in the fusion protein.
There could be two possibilities for the mechanism of secretion and activation of the MelC2h-MelC1 fusion protein (Fig. 7B). The first option involves an intramolecular interaction between the MelC2 domain and the MelC1 domain, where the MelC1 domain interacts with the MelC2 domain of the same molecule and activates it. The higher level of secretion can be explained by the increased chance of the MelC2 domain forming a complex with MelC1, as intramolecular interactions occur much more often than intermolecular reactions. In the second option, the MelC1 domain of one molecule activates the MelC2 domain of another molecule, by forming a “tandem dimer” (Fig. 7C). If this scenario is true, then the higher secretion efficiency may be a result of one cycle of secretion transporting two molecules of the fusion protein. There is also a chance that both mechanisms play a role in the secretion and activation of the MelC2h-MelC1 fusion protein. The exact mechanism remains to be elucidated.
A potential downstream application comes from the expression host P. fluorescens, which carries a well-documented ATP-binding cassette (ABC) transporter system capable of secreting various nonnative proteins after they are charge optimized (23, 44). These recombinant-protein-secreting P. fluorescens cells could be combined with tyrosinase-secreting P. fluorescens, prepared in this study, to make a biocatalytic hydrogel matrix that secretes enzymes (Fig. 6). The matrix-incorporated cells are resistant to dispersion, and this might prove useful when the whole-cell catalysts are needed in an open environment, such as marine oil spillage sites.
In conclusion, tyrosinase was successfully produced and secreted in the nonnatural expression host P. fluorescens. In addition, the secretion of tyrosinase could be enhanced by conjugating tyrosinase translationally with its caddie protein, enhancing the productivity of a tyrosinase-secreting culture. Furthermore, we characterized various aspects of the fusion protein, including the findings that there was no significant difference in the molar specific activities between the nonfused original protein and the fusion protein and that the folds of MelC2 and MelC1 were relatively independent in the fusion protein. Altogether, these observations give us insight into the heterologous secretion of bacterial tyrosinase as well as provide a method for increasing the secretion efficiency of tyrosinase, enabling high-efficiency secretion-based production of tyrosinase in a nonnative expression host.
MATERIALS AND METHODS
Bacterial strains, growth conditions, and transformation.
The S. antibioticus tyrosinase gene melC2 was synthesized based on the amino acid sequence in the NCBI database (GenBank accession no. M11582), and the codons were optimized for P. fluorescens. The caddie protein gene melC1 was cloned from S. antibioticus KCTC1140, from the Korean Collection for Type Cultures. The construction of all plasmids used in this research was performed with E. coli XL1-Blue, and plasmid expression was carried out in P. fluorescens ΔtliA ΔprtA (28). Recombinant plasmids were transformed into E. coli XL1-Blue using the standard heat shock method. For expression, the constructed plasmids were introduced into P. fluorescens ΔtliA ΔprtA via electroporation. Electroporation was performed under conditions of 2.5 kV, 125 Ω, and a capacitance of 50 μF using electrocompetent cells prepared according to a protocol described previously (44). After transformation, P. fluorescens ΔtliA ΔprtA was cultured in lysogeny broth (LB) medium containing 30 μg/ml kanamycin at 25°C with a 180-rpm shaking incubator.
Construction of recombinant plasmids.
In this study, seven plasmids for protein expression were constructed, pDSK-hMelC2, pDSK-hMelC2/C1, pDSK-MelC2h/C1, pDSK-MelC2h, and pDSK-MelC2h-C1 for mel expression and pDSK-MelC1-C2h and pDSK-hMelC2-C1 for the fusion protein. Here, slashes (as in pDSK-hMelC2/C1) indicate that two genes (in this case, hmelC2 and melC1) are expressed as separate polypeptides. On the other hand, the hyphen in pDSK-MelC2h-C1 indicates that MelC2h and MelC1 are fused into a single polypeptide. The tyrosinase genes melC1 and melC2 were amplified via PCR, using the genomic DNA of a Streptomyces antibioticus strain as the template, and inserted into the expression vector pDSK519 along with a hexahistidine tag (His6) sequence. The primers used in this study are listed in Table 1. For pDSK-hMelC2, the melC2 gene was amplified with the primer set SphI-His6-melC2 and melC2-XbaI to include the His6 tag sequence at the N terminus. The PCR product was excised with SphI and XbaI and inserted into pDSK519 in frame with a lacZα fragment. Similarly, for pDSK-hMelC2/C1, the melC1 gene along with its RBS (ribosome-binding site) were amplified with the primer set XbaI-RBS-melC1 and melC1-KpnI. The PCR product was digested with XbaI and KpnI and inserted into pDSK519, downstream of melC2. For pDSK-MelC2h, the melC2 gene was amplified with the primer set SphI-melC2 and melC2-His6-XbaI to include the His6 tag sequence at the C terminus. The PCR product was cut with SphI and XbaI and inserted in frame with the lacZα fragment into pDSK519. For pDSK-MelC2h-C1, the melC1 gene was also inserted into pDSK519 downstream of melC2 using the same method as the one described above for pDSK-hMelC2/C1. By PCR error, melC1 and its RBS were fused in frame with melC2. For pDSK-MeC1-C2h, melC1 amplified with primers SphI-melC1 and melC1-PstI and melC2 amplified with primers PstI-MelC2 and MelC2-His6-XbaI were inserted into pDSK519 with the two genes in frame and without a stop codon between the two genes. For pDSK-hMelC2-C1, melC2 amplified with primers SphI-His6-melC2 and melC2-XbaI-2 and melC1 amplified with primers XbaI-melC1 and melC1-KpnI were inserted into pDSK519 in a similar way. The constructed plasmids were verified by DNA sequencing and transformed into P. fluorescens ΔtliA ΔprtA via electroporation for their expression.
Plasmid construction for Tat verification experiments.
We amplified the P. fluorescens tatABC operon with primers F-TatA and TatC-B and integrated the PCR product into pDSK-hMelC2/C1 and pDSK-MelC2h-C1 with in-fusion PCR, utilizing the KpnI and EcoRI restriction sites. This integration site was downstream of the mel genes and was simultaneously controlled by the lac promoter of the pDSK519 vector. We sequenced the completed plasmids and tested their expression and secretion in E. coli. For additional verification, we made a Tat signal mutant of pDSK-hMelC2/C1 and pDSK-MelC2h-C1 by replacing the Thr-Arg-Arg tripeptide sequence of the MelC1 Tat signal sequence by Ala-Ala-Ala. The first few residues of the MelC1 Tat signal, Thr-Arg-Arg-Arg-Ala-Leu, are converted to Ala-Ala-Ala-Arg-Ala-Leu by this mutation. Mutagenesis was verified by DNA sequencing. The completed plasmids were tested for their expression and secretion in P. fluorescens.
Expression patterns of His6-tagged MelC2 and MelC1 in P. fluorescens and E. coli.
The expressions of tyrosinase in P. fluorescens ΔtliA ΔprtA and E. coli XL1-Blue were compared in both liquid and solid media to analyze the expression and secretion patterns. For analysis in liquid medium, E. coli XL1-Blue and P. fluorescens ΔtliA ΔprtA cells containing the constructed plasmids were cultured in 5 ml LB with 60 μg/ml kanamycin at their optimal growth temperatures of 37°C and 25°C, respectively, with shaking at 180 rpm. After 1 and 3 days of incubation, respectively, the cultures were harvested upon reaching an A600 of 3. The cell pellets and supernatant were separated via centrifugation. Laemmli sample buffer was added to the cell and supernatant, and the solution was loaded onto a 10% polyacrylamide gel. After electrophoresis, the proteins were transferred onto a nitrocellulose membrane (Amersham) and analyzed via Western blotting using anti-His6 antibodies. Protein signals were detected using the SuperSignal West Pico enhanced chemiluminescence substrate (Pierce) and a WesternBright Sirius Western blot detection kit (Advansta), and Western blot images were captured using the Azure c600 Western blot imaging system. For analysis with solid medium, E. coli XL1-Blue and P. fluorescens ΔtliA ΔprtA cells containing the constructed plasmids were streaked onto LB agar medium containing 30 μg/ml kanamycin, 0.1 mM CuCl2, and 2 mM l-tyrosine and incubated at 25°C. Expressed tyrosinase is expected to oxidize tyrosine present in the medium to form a dark-brown melanin pigment, which serves as a qualitative measure of tyrosinase expression.
Affinity chromatography of His6-tagged tyrosinase.
His6-tagged tyrosinase secreted by P. fluorescens ΔtliA ΔprtA was purified using His6 tag affinity chromatography. P. fluorescens ΔtliA ΔprtA containing pDSK-hMelC2/C1 or pDSK-MelC2h-C1 was cultured in 200 ml LB with 60 μg/ml kanamycin for 3 days. The culture supernatant was separated from the cell extract via centrifugation at 3,000 rpm for 1 h. A 15-ml open column filled with a 3-ml bed volume of Ni-NTA resin (Qiagen) was used for purification. The column-bound proteins were eluted with 10 rounds of 1 ml of 250 mM imidazole to yield 10 fractions of eluted protein. All purification steps, except the elution step, were done by using a Gilson Minipuls Evolution peristaltic pump with a 9-rpm running speed. Fractions containing the purified protein were concentrated using Amicon centrifugal filters with a molecular weight cutoff (MWCO) of 10 kDa. Consequently, the purified protein was further analyzed by an activity assay.
Analysis of tyrosinase activity via a solution-based l-DOPA assay.
Tyrosinase activity was observed using a microplate-based spectrophotometric assay as described previously by Lerch and Ettlinger (45). Dopachrome produced by mushroom tyrosinase was diluted to 0 to 8 mM dopachrome in 200 μl distilled water to plot a standard curve. The obtained formula was A475 = 0.127 × cdopachrome, where cdopachrome is the concentration of dopachrome in the solution in millimolars. The tyrosinase activity assay was performed at 25°C using a microplate with 8 mM l-DOPA as the substrate. A 33-μl tyrosinase solution (culture supernatant, purified culture supernatant, or diluted stock tyrosinase solution) was added to 165 μl of 8 mM l-DOPA and 2 μl of 1 mM CuCl2, the concentration of which was determined through multiple trials. Maximum tyrosinase activity was observed when the Cu2+ ion concentration was around 10 μM, and the activity rapidly decreased when the Cu2+ ion concentration was below 1 μM or above 500 μM. The change in the absorbance at 475 nm (A475) was measured 10 min using a VersaMax microplate reader (Molecular Devices). The high imidazole concentration (250 mM imidazole) used to elute tyrosinase from the Ni-NTA column may yield a background reaction even in the absence of tyrosinase, so we diluted the purified protein 10-fold, reducing the imidazole concentration to 25 mM. The activity was calculated from the initial rate of change of the absorbance at 475 nm, per unit volume of the unpurified culture supernatant (units per milliliter). The protein concentration was determined using a Bradford assay (Bio-Rad). A standard curve was obtained using different concentrations of bovine serum albumin (BSA) in 25 mM imidazole (since we diluted the tyrosinase samples with 250 mM imidazole 10-fold). It is also noteworthy that the protein concentration measured by the Bradford assay was lower by 2.03-fold than the protein concentration measured based on the absorbance at 280 nm, based on the extinction coefficient predicted by the ExPASy ProtParam Web server. The specific activity of the purified tyrosinase was calculated from the measured enzyme activity divided by the protein concentration. Analyses of the statistical significance of differences between two measured values were performed using paired-sample, single-tailed Student’s t test. The set P value threshold was 5%. We used paired analysis because we prepared the samples in batches, and the samples within a single batch shared the same growth conditions.
Identification of sequences using mass spectrometry.
Trypsin-digested peptides of the MelC1 monomer and the MelC2h-MelC1 fusion protein were analyzed using MALDI-tandem time of flight (TOF/TOF) MS and nano-electrospray ionization quadrupole time of flight (NanoESI-Q-TOF) MS at the Korea Basic Science Institute (Seoul, South Korea). To analyze the proteins, tryptic peptides were separated and fractionated on an Äkta micro-FPLC system (GE Healthcare). The mass spectra for each fraction were obtained using a MALDI-TOF/TOF 5800 instrument (AB Sciex) and identified using ProteinPilot 4.0.8085 software against a database of MelC1 monomer and complex protein sequences. The MelC2h-MelC1 protein was also analyzed and identified using a nano-Acquity ultraperformance liquid chromatography (UPLC) system (Waters), a Synapt ion mobility-mass spectrometry instrument (Waters), and PLGS 2.3 software (Waters).
Synthesis of phenol-conjugated chitosan.
The phenol moieties [more accurately, 3-(p-hydroxyphenyl)propionyl groups] were conjugated to a chitosan backbone using the 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC) reagent. First, chitosan extracted from shrimp (Sigma) was dissolved in a 0.1 M HCl solution, and the pH was gradually increased to pH 5.5 using 5 N NaOH for an optimal EDC coupling reaction. Next, 3-(p-hydroxyphenyl)propionic acid dissolved in distilled water and EDC dissolved in ethanol were slowly added to the chitosan solution. The reaction mixture was stirred vigorously at room temperature for 12 h, and the pH of the reaction mixture was monitored. After 12 h, the reaction mixture was dialyzed (MWCO of 3,500; SpectraPor, USA) against acidified distilled water for 24 h and in distilled water for another 4 h. The final product was lyophilized and kept in a moisture-free desiccator until further experiments were performed. The degree of substitution was determined via UV-visible (UV-vis) spectrophotometric analysis by the absorbance at 280 nm caused by the phenol group content. Standard solutions of 3-(p-hydroxyphenyl)propionic acid were used to generate a standard curve of the phenol concentrations, and the phenol content was quantified. UV-vis spectrophotometric analysis was used to calculate the degree of 3-(p-hydroxyphenyl)propionyl substitution on the chitosan backbone, which was revealed to be approximately 10%.
P. fluorescens encapsulated hydrogel experiment.
For the hydrogel experiment, CHI-PHE with an ∼10% degree of phenol conjugation was dissolved in distilled water to prepare an 8% (wt/vol) solution. Next, an equal amount of a P. fluorescens centrifugation-concentrated cell culture solution (A600 of 10) was mixed with the CHI-PHE solution. We examined the mixture every 10 min by inverting the vial to see if the solution had solidified.
Structural analysis of the MelC proteins.
SWISS-MODEL structural homology modeling (https://swissmodel.expasy.org/) was used to study the structure of the MelC1 and MelC2 complex and the MelC2h-MelC1 fusion protein (46). The model structure was predicted using the Streptomyces castaneoglobisporus tyrosinase holoenzyme (36) as a template (PDB accession no. 3AWU), which has 81.6% sequence identity to MelC1/MelC2. The representation of the three-dimensional (3D) protein models was prepared using PyMOL 1.8. All protein sequences used for the analysis are provided in Text S1 in the supplemental material.
Data availability.
The complete genome of Pseudomonas fluorescens strain SIK_W1 was submitted to GenBank under accession number NZ_CP031450.
Supplementary Material
ACKNOWLEDGMENTS
Our study was supported by the Global Frontier Project of the Intelligent Synthetic Biology Center, by midcareer scientist grant 310 (grant no. NRF-2014R1A2A1A01002855 to H.L.) from the National Research Foundation (NRF) of Korea, and by the R&E Program of the Korea Science Academy of Korea Advanced Institute of Science and Technology funded by the Ministry of Science, ICT, and Future Planning (J.H.A.).
We declare that we have no conflicts of interest with the contents of this article.
J.H.A. conceived and coordinated the study. J.R. and K.H.N. designed and performed the activity assay experiments in Fig. 3 and the digestion pattern analysis. J.P.P. performed the hydrogel experiments. J.H.C. designed and performed the mass spectroscopy and fragment analyses. J.P. and H.B. designed and performed the rest of the activity assays and all of the Western blot experiments. J.R. wrote the first submitted version of the manuscript, while H.B. prepared the later versions of the manuscript. H.B. suggested the present model of fusion protein secretion. H.L. conceived the hydrogel experiments, introduced the concept of a whole-cell catalyst, and provided intellectual concepts related to tyrosinase activity and spontaneous cleavage. All authors analyzed the results and approved the final version of the manuscript.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AEM.01350-19.
REFERENCES
- 1.Fairhead M, Thony-Meyer L. 2012. Bacterial tyrosinases: old enzymes with new relevance to biotechnology. N Biotechnol 29:183–191. doi: 10.1016/j.nbt.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 2.Faccio G, Kruus K, Saloheimo M, Thöny-Meyer L. 2012. Bacterial tyrosinases and their applications. Process Biochem 47:1749–1760. doi: 10.1016/j.procbio.2012.08.018. [DOI] [Google Scholar]
- 3.Claus H, Decker H. 2006. Bacterial tyrosinases. Syst Appl Microbiol 29:3–14. doi: 10.1016/j.syapm.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 4.Ercili-Cura D, Huppertz T, Kelly AL. 2015. Enzymatic modification of dairy product texture, p 71–97. In Chen J, Rosenthal A (ed), Modifying food texture. Woodhead Publishing, Cambridge, United Kingdom. doi: 10.1016/B978-1-78242-333-1.00004-8. [DOI] [Google Scholar]
- 5.Lee BP, Dalsin JL, Messersmith PB. 2002. Synthesis and gelation of DOPA-modified poly(ethylene glycol) hydrogels. Biomacromolecules 3:1038–1047. doi: 10.1021/bm025546n. [DOI] [PubMed] [Google Scholar]
- 6.Chen T, Embree HD, Brown EM, Taylor MM, Payne GF. 2003. Enzyme-catalyzed gel formation of gelatin and chitosan: potential for in situ applications. Biomaterials 24:2831–2841. doi: 10.1016/S0142-9612(03)00096-6. [DOI] [PubMed] [Google Scholar]
- 7.Jin R, Lou B, Lin C. 2013. Tyrosinase-mediated in situ forming hydrogels from biodegradable chondroitin sulfate-tyramine conjugates. Polym Int 62:353–361. doi: 10.1002/pi.4306. [DOI] [Google Scholar]
- 8.Seetharam G, Saville BA. 2002. l-DOPA production from tyrosinase immobilized on zeolite. Enzyme Microb Technol 31:747–753. doi: 10.1016/S0141-0229(02)00182-5. [DOI] [Google Scholar]
- 9.Battaini G, Monzani E, Casella L, Lonardi E, Tepper AW, Canters GW, Bubacco L. 2002. Tyrosinase-catalyzed oxidation of fluorophenols. J Biol Chem 277:44606–44612. doi: 10.1074/jbc.M207829200. [DOI] [PubMed] [Google Scholar]
- 10.Streffer K, Vijgenboom E, Tepper AWJW, Makower A, Scheller FW, Canters GW, Wollenberger U. 2001. Determination of phenolic compounds using recombinant tyrosinase from Streptomyces antibioticus. Anal Chim Acta 427:201–210. doi: 10.1016/S0003-2670(00)01040-0. [DOI] [Google Scholar]
- 11.Fairhead M, Thony-Meyer L. 2010. Cross-linking and immobilisation of different proteins with recombinant Verrucomicrobium spinosum tyrosinase. J Biotechnol 150:546–551. doi: 10.1016/j.jbiotec.2010.10.068. [DOI] [PubMed] [Google Scholar]
- 12.Wichers HJ, Recourt K, Hendriks M, Ebbelaar CE, Biancone G, Hoeberichts FA, Mooibroek H, Soler-Rivas C. 2003. Cloning, expression and characterisation of two tyrosinase cDNAs from Agaricus bisporus. Appl Microbiol Biotechnol 61:336–341. doi: 10.1007/s00253-002-1194-2. [DOI] [PubMed] [Google Scholar]
- 13.Dolashki A, Gushterova A, Voelter W, Tchorbanov B. 2009. Purification and characterization of tyrosinases from Streptomyces albus. Z Naturforsch C 64:724–732. doi: 10.1515/znc-2009-9-1019. [DOI] [PubMed] [Google Scholar]
- 14.Shuster V, Fishman A. 2009. Isolation, cloning and characterization of a tyrosinase with improved activity in organic solvents from Bacillus megaterium. J Mol Microbiol Biotechnol 17:188–200. doi: 10.1159/000233506. [DOI] [PubMed] [Google Scholar]
- 15.Fairhead M, Thony-Meyer L. 2010. Role of the C-terminal extension in a bacterial tyrosinase. FEBS J 277:2083–2095. doi: 10.1111/j.1742-4658.2010.07621.x. [DOI] [PubMed] [Google Scholar]
- 16.Lagunas-Muñoz VH, Cabrera-Valladares N, Bolívar F, Gosset G, Martínez A. 2006. Optimum melanin production using recombinant Escherichia coli. J Appl Microbiol 101:1002–1008. doi: 10.1111/j.1365-2672.2006.03013.x. [DOI] [PubMed] [Google Scholar]
- 17.Tseng HC, Lin CK, Hsu BJ, Leu WM, Lee YH, Chiou SJ, Hu NT, Chen CW. 1990. The melanin operon of Streptomyces antibioticus: expression and use as a marker in gram-negative bacteria. Gene 86:123–128. doi: 10.1016/0378-1119(90)90124-a. [DOI] [PubMed] [Google Scholar]
- 18.Popa C, Bahrim G. 2011. Streptomyces tyrosinase: production and practical applications. Innov Rom Food Biotechnol 8:1–7. [Google Scholar]
- 19.Katz E, Thompson CJ, Hopwood DA. 1983. Cloning and expression of the tyrosinase gene from Streptomyces antibioticus in Streptomyces lividans. J Gen Microbiol 129:2703–2714. doi: 10.1099/00221287-129-9-2703. [DOI] [PubMed] [Google Scholar]
- 20.Chen LY, Leu WM, Wang KT, Lee YH. 1992. Copper transfer and activation of the Streptomyces apotyrosinase are mediated through a complex formation between apotyrosinase and its trans-activator MelC1. J Biol Chem 267:20100–20107. [PubMed] [Google Scholar]
- 21.Sambasiva Rao KR, Tripathy NK, Mahalaxmi Y, Prakasham RS. 2012. Laccase- and peroxidase-free tyrosinase production by isolated microbial strain. J Microbiol Biotechnol 22:207–214. doi: 10.4014/jmb.1106.06031. [DOI] [PubMed] [Google Scholar]
- 22.Ren Q, Henes B, Fairhead M, Thony-Meyer L. 2013. High level production of tyrosinase in recombinant Escherichia coli. BMC Biotechnol 13:18. doi: 10.1186/1472-6750-13-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Byun H, Park J, Kim SC, Ahn JH. 2017. A lower isoelectric point increases signal sequence-mediated secretion of recombinant proteins through a bacterial ABC transporter. J Biol Chem 292:19782–19791. doi: 10.1074/jbc.M117.786749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chater KF, Biro S, Lee KJ, Palmer T, Schrempf H. 2010. The complex extracellular biology of Streptomyces. FEMS Microbiol Rev 34:171–198. doi: 10.1111/j.1574-6976.2009.00206.x. [DOI] [PubMed] [Google Scholar]
- 25.Schaerlaekens K, Schierova M, Lammertyn E, Geukens N, Anne J, Van Mellaert L. 2001. Twin-arginine translocation pathway in Streptomyces lividans. J Bacteriol 183:6727–6732. doi: 10.1128/JB.183.23.6727-6732.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leu WM, Chen LY, Liaw LL, Lee YH. 1992. Secretion of the Streptomyces tyrosinase is mediated through its trans-activator protein, MelC1. J Biol Chem 267:20108–20113. [PubMed] [Google Scholar]
- 27.Matoba Y, Kihara S, Muraki Y, Bando N, Yoshitsu H, Kuroda T, Sakaguchi M, Kayama K, Tai H, Hirota S, Ogura T, Sugiyama M. 2017. Activation mechanism of the Streptomyces tyrosinase assisted by the caddie protein. Biochemistry 56:5593–5603. doi: 10.1021/acs.biochem.7b00635. [DOI] [PubMed] [Google Scholar]
- 28.Son M, Moon Y, Oh MJ, Han SB, Park KH, Kim JG, Ahn JH. 2012. Lipase and protease double-deletion mutant of Pseudomonas fluorescens suitable for extracellular protein production. Appl Environ Microbiol 78:8454–8462. doi: 10.1128/AEM.02476-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chew LC, Ramseier TM, Retallack DM, Schneider JC, Squires CH, Talbot HW. 2005. Pseudomonas fluorescens, p 45–62. In Gellissen G. (ed), Production of recombinant proteins: novel microbial and eukaryotic expression system. Wiley-VCH, Weinheim, Germany. [Google Scholar]
- 30.Palmer T, Berks BC. 2012. The twin-arginine translocation (Tat) protein export pathway. Nat Rev Microbiol 10:483–496. doi: 10.1038/nrmicro2814. [DOI] [PubMed] [Google Scholar]
- 31.Berks BC, Palmer T, Sargent F. 2005. Protein targeting by the bacterial twin-arginine translocation (Tat) pathway. Curr Opin Microbiol 8:174–181. doi: 10.1016/j.mib.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 32.Bogsch EG, Sargent F, Stanley NR, Berks BC, Robinson C, Palmer T. 1998. An essential component of a novel bacterial protein export system with homologues in plastids and mitochondria. J Biol Chem 273:18003–18006. doi: 10.1074/jbc.273.29.18003. [DOI] [PubMed] [Google Scholar]
- 33.Sargent F, Stanley NR, Berks BC, Palmer T. 1999. Sec-independent protein translocation in Escherichia coli. A distinct and pivotal role for the TatB protein. J Biol Chem 274:36073–36082. doi: 10.1074/jbc.274.51.36073. [DOI] [PubMed] [Google Scholar]
- 34.Berks BC. 1996. A common export pathway for proteins binding complex redox cofactors? Mol Microbiol 22:393–404. doi: 10.1046/j.1365-2958.1996.00114.x. [DOI] [PubMed] [Google Scholar]
- 35.Niviere V, Wong SL, Voordouw G. 1992. Site-directed mutagenesis of the hydrogenase signal peptide consensus box prevents export of a beta-lactamase fusion protein. J Gen Microbiol 138:2173–2183. doi: 10.1099/00221287-138-10-2173. [DOI] [PubMed] [Google Scholar]
- 36.Matoba Y, Kumagai T, Yamamoto A, Yoshitsu H, Sugiyama M. 2006. Crystallographic evidence that the dinuclear copper center of tyrosinase is flexible during catalysis. J Biol Chem 281:8981–8990. doi: 10.1074/jbc.M509785200. [DOI] [PubMed] [Google Scholar]
- 37.Blaudeck N, Sprenger GA, Freudl R, Wiegert T. 2001. Specificity of signal peptide recognition in Tat-dependent bacterial protein translocation. J Bacteriol 183:604–610. doi: 10.1128/JB.183.2.604-610.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Dijl JM, Smith H, Bron S, Venema G. 1988. Synthesis and processing of Escherichia coli TEM-beta-lactamase and Bacillus licheniformis alpha-amylase in E. coli: the role of signal peptidase I. Mol Gen Genet 214:55–61. doi: 10.1007/BF00340179. [DOI] [PubMed] [Google Scholar]
- 39.van Roosmalen ML, Geukens N, Jongbloed JDH, Tjalsma H, Dubois J-YF, Bron S, van Dijl JM, Anné J. 2004. Type I signal peptidases of Gram-positive bacteria. Biochim Biophys Acta 1694:279–297. doi: 10.1016/j.bbamcr.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 40.Huang Q, Palmer T. 2017. Signal peptide hydrophobicity modulates interaction with the twin-arginine translocase. mBio 8:e00909-17. doi: 10.1128/mBio.00909-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hinsley AP, Stanley NR, Palmer T, Berks BC. 2001. A naturally occurring bacterial Tat signal peptide lacking one of the ‘invariant’ arginine residues of the consensus targeting motif. FEBS Lett 497:45–49. doi: 10.1016/s0014-5793(01)02428-0. [DOI] [PubMed] [Google Scholar]
- 42.Stephenson RC, Clarke S. 1989. Succinimide formation from aspartyl and asparaginyl peptides as a model for the spontaneous degradation of proteins. J Biol Chem 264:6164–6170. [PubMed] [Google Scholar]
- 43.Brennan TV, Clarke S. 1993. Spontaneous degradation of polypeptides at aspartyl and asparaginyl residues: effects of the solvent dielectric. Protein Sci 2:331–338. doi: 10.1002/pro.5560020305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ryu J, Lee U, Park J, Yoo DH, Ahn JH. 2015. A vector system for ABC transporter-mediated secretion and purification of recombinant proteins in Pseudomonas species. Appl Environ Microbiol 81:1744–1753. doi: 10.1128/AEM.03514-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lerch K, Ettlinger L. 1972. Purification and characterization of a tyrosinase from Streptomyces glaucescens. Eur J Biochem 31:427–437. doi: 10.1111/j.1432-1033.1972.tb02549.x. [DOI] [PubMed] [Google Scholar]
- 46.Arnold K, Bordoli L, Kopp J, Schwede T. 2006. The SWISS-MODEL workspace: a Web-based environment for protein structure homology modelling. Bioinformatics 22:195–201. doi: 10.1093/bioinformatics/bti770. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The complete genome of Pseudomonas fluorescens strain SIK_W1 was submitted to GenBank under accession number NZ_CP031450.