Abstract
Objective:
Macronutrient regulation of hyperphagia and adiposity in PWS is poorly understood. We compared fasting and postprandial concentrations of hormones and metabolites in 8 PWS children (age 9–18 yr) fed, in random order, low carbohydrate, high fat (LC, 15%carb; 65%fat; 20% protein) and low fat, high carbohydrate (LF, 65%carb, 15%fat, 20% protein) diets matched for calories and protein.
Methods:
Participants were randomized to consume either the LC or LF diet during a first hospital admission and the second diet during a subsequent admission. Blood samples were obtained after overnight fasting and 1 hour after a mixed meal.
Results:
Relative to subjects consuming the LF diet, subjects consuming the LC diet had: lower post-prandial insulin concentrations (p=0.02); higher fasting and post-prandial GLP-1 concentrations (p<0.02); reduced ratio of fasting ghrelin to GLP-1 (p=0.0078); increased FFA and fatty acid oxidation, as assessed by concentrations of even-chain acylcarnitines (p<0.001); lower fasting TG and TG/HDL ratio (p<0.01); and higher concentrations of branch chain amino acids (p<0.01). There were no changes in glucose, GIP, PYY, or adiponectin. CRP, AST, and ALT were all higher (p<0.01) on the LC diet.
Conclusions:
Increases in GLP-1 with low carbohydrate feeding and reductions in the ratio of ghrelin to GLP-1 might limit food intake and improve glycemic control in PWS. Other potential benefits of carbohydrate restriction may include fat mobilization and oxidation and reductions in the TG/HDL ratio, a marker of insulin resistance. However increases in CRP, AST, and ALT necessitate longer-term studies of low carbohydrate efficacy and safety.
Keywords: GLP-1, PYY, ghrelin, insulin, branch chain amino acids, Prader-Willi Syndrome, low carbohydrate diet
Introduction
Prader-Willi syndrome (PWS) is the most common genetic obesity disorder, resulting from absence of expression of paternally-derived genes on chromosome 15q11.2-q131,2. Lack of satiety contributes to hyperphagia, which promotes increases in body fat mass1–3. In combination with reductions in lean body mass2,3, adiposity limits physical activity and exercise tolerance4 and predisposes to chronic co-morbidities including glucose intolerance, hypertension, hyperlipidemia, and sleep apnea2.
Attempts to control weight and prevent metabolic decompensation in PWS through dietary interventions have had variable and limited success. Energy restriction can decrease the rate of weight gain in selected patients5 but if severe may attenuate linear growth and reduce lean body mass6. One uncontrolled study7 found that PWS children consuming chronically a diet lower in carbohydrate (45% of calories) and higher in fat and protein (30% and 25% of calories, respectively) had lower body mass index (BMI) z-scores and lower body fat content than age-matched PWS children consuming a diet high in carbohydrate (~50–70% of daily calories) and low in fat and protein (10–23% and 10–15% of calories, respectively). These observations suggest that carbohydrate restriction might limit adiposity and weight gain in PWS. To that end, some parents have placed their PWS children on low carbohydrate diets in an attempt to limit fat deposition and reduce long-term metabolic risk. Yet there are no controlled studies comparing the effects of low carbohydrate and low fat diets on appetite-regulating hormones, insulin sensitivity, or glucose tolerance in PWS.
To assess the roles of dietary carbohydrate and fat in the control of appetite and metabolic function, we compared fasting and postprandial concentrations of various hormones and metabolites in 8 PWS children fed, sequentially and in randomized order, low carbohydrate, high fat (LC, 15%carb; 65%fat; 20% protein) and low fat, high carbohydrate diets (LF, 65%carb; 15%fat; 20% protein) diets matched for calories and protein content. We hypothesized that carbohydrate restriction would reduce the concentrations of the orexigenic hormone ghrelin, increase the concentrations of anorexigenic hormones including Glucagon-like Peptide 1 (GLP-1) and peptide YY (PYY), and increase insulin sensitivity as measured by fasting insulin and glucose concentrations, total and high molecular weight adiponectin, and the ratio of triglycerides (TG) to high density lipoprotein (HDL).
Methods
Study subjects:
9 children with PWS (3 males, 6 females) ranging in age from 5.1 to 17.7 years were recruited to the study. One child failed (for personal reasons) to complete the two dietary arms and was not included in the analysis of the results. The 8 children who completed the study ranged from 9.5–17.7 yr of age. BMI z scores of the 8 participants ranged from −0.6 to 2.7; only two of the children were considered obese (BMIz > 2.0). The child who failed to complete the study was only 5.1 yr of age.
Auxologic data and percent body fat of the 8 remaining children are shown in Table 1. All subjects had been taking GH continuously for at least 1 year prior to enrollment in the study (Table 1); no changes in GH therapy were made on any child during the course of the study. Two subjects were euthyroid on stable doses of thyroxine. No additional medications were introduced, discontinued, or modified during the study. Participants taking stimulant medication for ADHD/ADD were required to be on stable dosages for the 6 months preceding the study.
Table 1.
Baseline subject characteristics: anthropometrics, body composition, blood pressure and medications.
| Sex | Age (yrs) | Weight (kg) | Height (cm) | BMI (kg/m2) | BMI z-score | BodPod % fat | WC (cm) | WC z-score | SBP (mmHg) | DBP (mmHg) | Medication exposure | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 10.7 | 91.5 | 148 | 41.8 | 2.7 | 53.6 | 114 | 2.3 | 135 | 69 | GH |
| 2 | M | 13.1 | 41.5 | 155.0 | 17.3 | −0.6 | 38.7 | 74 | 0.6 | 120 | 80 | GH |
| 3 | F | 14 | 58.5 | 168.5 | 20.6 | 0.3 | 34.4 | 85 | 1.2 | 95 | 59 | GH; Risperdal; Thyroxine |
| 4 | F | 10 | 49.0 | 142.0 | 24.3 | 1.8 | 54.8 | 84 | 1.8 | 129 | 61 | GH; Prozac |
| 5 | F | 9.5 | 34.1 | 132.6 | 19.4 | 1.0 | 31.7 | 64 | 0.4 | 127 | 73 | GH; Prevacid |
| 6 | F | 14.7 | 53.9 | 152.0 | 23.3 | 0.9 | 35.7 | 84 | 1.0 | No data | No data | GH; Thyroxine |
| 7 | F | 17.7 | 84.3 | 150.4 | 37.3 | 2.2 | 49.8 | 105 | 1.6 | 118 | 74 | GH; Lo-estrin; Singulair |
| 8 | F | 13.3 | 55.6 | 161.5 | 21.3 | 0.7 | 35.7 | 75 | 0.6 | 118 | 73 | GH |
M = Male; F = Female; BMI = body mass index; BodPod: Air Displacement Plethysmograph (ADP) determined body composition (fat vs. lean); WC = waist circumference; SBP = systolic blood pressure; DBP = diastolic blood pressure; GH = growth hormone. BMI percentile and z-scores calculated using EpiInfo (CDC, Atlanta, GA). Note: All subjects had been taking GH continuously for at least 1 year prior to enrollment in the study.
Study participants were admitted to the confined clinical research centers at Duke University Medical Center and the University of Alberta; participants and parents were not allowed additional food besides what was provided by the study. No changes in home dietary management were instituted between the two study visits.
Medical History Questionnaire.
Parents were asked to complete a medical history questionnaire. This questionnaire was used to obtain information regarding participants’ current and recent medications, including GH use and dose.
Anthropometric Methods.
Weight was measured to the nearest 0.1kg using the same calibrated scales. Height was measured to the nearest 0.1cm using wall-mounted stadiometers for children. BMI percentile and BMI standard deviation score were calculated using EpiInfo (CDC, Atlanta, GA). Waist circumference (WC) was recorded to the nearest 0.1cm with a non-stretch measuring tape between the bottom of the lower rib and the iliac crest. Waist standard deviation score was calculated using LMS tables for waist-circumference z-scores in children aged 5–19 years8. Body composition (total lean tissue mass, total body fat mass and percent body fat) was measured by air displacement plethysmography (BOD POD, Life Measurement Inc.).
Diet composition and administration.
Participants were randomized to consume either the low carbohydrate (LC) or low fat (LF) diet during a first hospital admission; 4 weeks later they were re-admitted to the research centers and administered the other diet. Each child received three meals and two snacks per day during each of the two 72-hour admissions. All food intake was overseen and monitored by research center nurses and staff. Participants consumed breakfast, lunch, and dinner meals within a mean duration of 25 mins; morning and afternoon snacks were consumed within an average of 15 mins duration.
The LC diet contained 15% carbohydrate, 65% fat, and 20% protein. The LF diet contained 65% carbohydrate, 15% fat, and 20% protein. The ratio of simple:complex carbohydrates, and polyunsaturated:saturated fatty acids was the same for all meals.
The LC and LF diets were matched for calorie as well as protein content.
The caloric content of the meals was determined by assessment of energy requirements. Energy requirements were estimated using Dietary Reference Intakes Estimated Energy Requirement (EER) age group and gender specific equations9,10 adjusted for the PWS population. PWS children have lower relative lean mass compared to children without PWS; thus energy requirements are lower11. To account for this difference, we calculated energy requirements using 80% of EER12. Physical activity coefficients used in the calculation were based on parent-reported usual activity level (sedentary, low active, active or very active). Participants remained weight stable during the intervention; there were no significant changes in weight between the first and second admissions (weight change 0.9 ± 0.8%, p = 0.46).
All participants were provided a daily multivitamin/mineral supplement formulated for children on each of the study visit days. Subjects were strongly encouraged to drink at least 6 glasses of permitted fluids daily. Participants were allowed 2 hours per day for physical activity; up to one hour was permitted for outdoor walks with parental supervision.
Measurement of hormone and metabolite concentrations.
Overnight fasting blood samples were obtained after administration of the diets for 72 hours. Post-prandial (60 min after supper) samples were obtained 60 hours after initiating the diet. We did not compare hormone and metabolite concentrations obtained during controlled administration of the LC and LF diets to baseline values obtained at the time of admission. This is because baseline values reflected participants’ uncontrolled diets prior to admission. Comparisons between values obtained under supervised and controlled conditions (during the dietary intervention) with those obtained under uncontrolled conditions (baseline) would be impossible to interpret.
Blood samples were collected on ice in tubes containing EDTA and aprotinin as previously described13. Plasma hormone and metabolite concentrations were measured in the Duke Metabolomics facility using methods described previously in detail14. Samples for alanine transaminase (ALT), aspartate aminotransferase (AST), and high-sensitivity C-reactive protein (CRP) were assayed on a Beckman DxC 600 clinical analyzer (Brea, CA).
Subjective Measurements of Hunger.
We used a questionnaire to attempt to assess pre-prandial hunger and post-prandial satiety in the subjects during the admission. Careful observation by hospital nurses and dieticians indicated that responses to questions were severely limited by lack of patient understanding and defects in communication and could not be considered reliable.
Statistical analysis.
Hormone and metabolite concentrations were natural log (ln) transformed to ensure normality. Statistical differences between hormone and metabolite concentrations obtained during consumption of the LC and LF diets were assessed using paired t tests. Correlations among the various hormones and metabolites were assessed by linear regression analysis using the statistical program R (Department of Statistics, Institute of Agriculture and Natural Resources and the College of Arts and Sciences, University of Nebraska-Lincoln). A p value < 0.05 was considered statistically significant.
Ethical Approval.
The study protocol was approved by the Duke University Medical Center Institutional Review Board (Pro00049226) and the University of Alberta Research Ethics Board (Pro00040087). Informed consent was provided by parents; subject assent was provided in cases in which the child was able to understand the purpose and procedures of the study.
Results
Hormonal effects of carbohydrate restriction (Table 2a and Figure 1)
Table 2a.
Effects of low carbohydrate (LC) vs low fat (LF) feeding on hormones, lipid concentrations and fatty acid metabolites.
| LC fasting | LF fasting | LC post-prandial | LF post-prandial | |
|---|---|---|---|---|
| Glucose, mmol/L | 4.5 ± 0.2 | 4.8 ± 0.2 | 4.9 ± 0.1 | 5.2 ± 0.3 |
| Insulin, pmol/L | 1.3 ± 0.4 | 1.3 ± 0.2 | 1.8 ± 0.4* | 5.8 ± 2.1 |
| GLP-1, pg/ml | 20.0 ± 3.6* | 12.3 ± 2.9 | 34.2 ± 5.0* | 22.4 ± 5.1 |
| Ghrelin, pg/ml | 690.8 ± 147.7* | 873.1 ± 149.9 | 656.1 ± 97.1 | 678.0 ± 215.1 |
| Ghrelin/GLP-1, pg/ml | 50.6 ± 17.1* | 103.4 ± 28.6 | 24.9 ± 3.6 | 47.4 ± 12.2 |
| GIP, pg/ml | 73.1 ± 16.4* | 48.0 ± 9.3 | 466.5 ± 65.7 | 481.8 ± 49.0 |
| PYY, pg/ml | 7.9 ± 5.4 | 40.9 ± 17.3 | 120.0 ± 22.5 | 108.8 ± 19.4 |
| FFA, mmol/L | 1.1 ± 0.2* | 0.6 ± 0.1 | 0.7 ± 0.1** | 0.2 ± 0.03 |
| FAO (even-chain acylcarnitines) | Increased*** | Increased*** | ||
| TG, mmol/L | 0.8 ± 0.1* | 1.4 ± 0.3 | 0.9 ± 0.2 | 1.3 ± 0.3 |
| HDL, mmol/L | 1.4 ± 0.1* | 1.3 ± 0.1 | 1.4 ± 0.1 | 1.3 ± 0.1 |
| TG/HDL, mmol/L | 1.4 ± 0.2* | 2.6 ± 0.7 | 1.6 ± 0.3 | 2.5 ± 0.8 |
| Total Adiponectin, ng/ml | 5424 ± 899 | 5185 ± 840 | 5490 ± 861 | 4641 ± 699 |
| HMWAdipo, ng/ml | 3509 ± 672 | 3533 ± 672 | 3560 ± 644 | 3818 ± 696 |
LC = Low carbohydrate - high fat diet (15%carb; 65%fat; 20% protein); LF = Low fat - high carbohydrate diet (65%carb; 15%fat; 20% protein); GLP-1 = Glucagon-like peptide 1; GIP = glucose-dependent insulinotropic polypeptide; PYY = Peptide YY; FAA = free fatty acids; FAO = fatty acid oxidation; TG = Triglycerides; HDL = high-density lipoproteins. HMWAdipo = high-molecular-weight adiponectin; data presented as mean + standard error (SE). Statistical analysis: paired t-tests; correlations were assessed by linear regression analysis (program R, Dept. of Statistics, Institute of Agriculture and Natural Resources and the College of Arts and Sciences, University of Nebraska-Lincoln).
A p value < 0.05 was considered statistically significant;
p<0.01;
p<0.001.
For International System of Units (SI), see AMA Manual of Style, 10th edition.
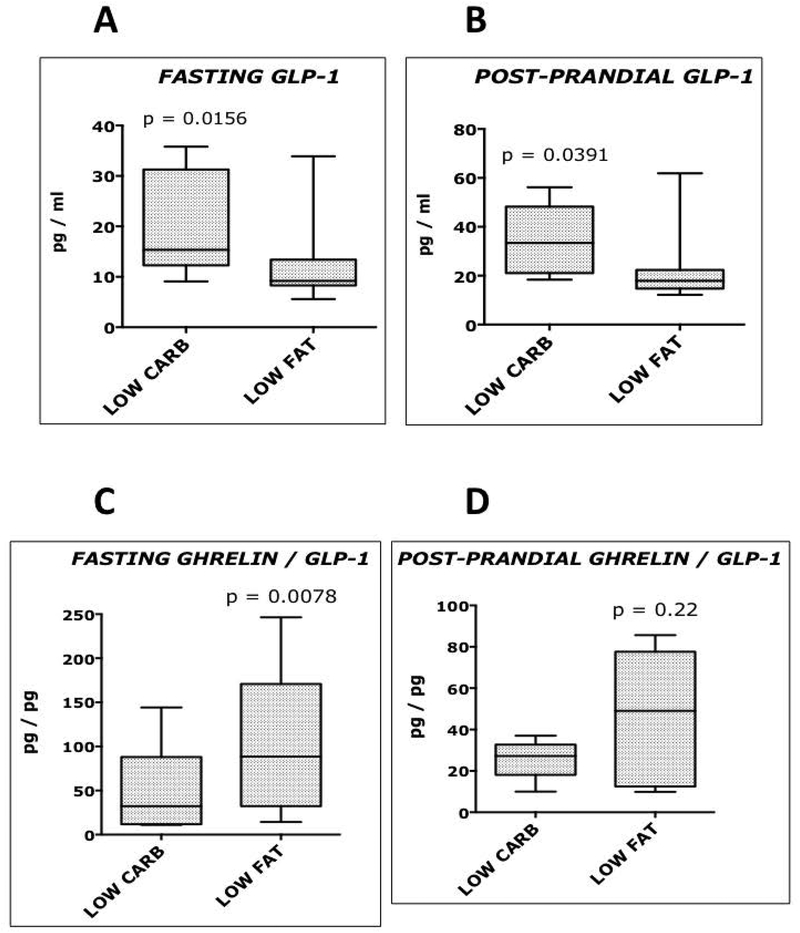
Figure 1.
Fasting and postprandial Glucagon-like peptide 1 (GLP-1) and Ghrelin concentrations. Comparison of subjects consuming a low carbohydrate, high fat diet (15%carb; 65%fat; 20% protein), and the same subjects consuming a low fat, high carbohydrate diet (65%carb; 15%fat; 20% protein). A p value < 0.05 was considered statistically significant.
Compared with subjects consuming the low fat, high carbohydrate (LF) diet, the same subjects consuming the low carbohydrate, high fat (LC) diet had lower postprandial insulin concentrations (p=0.02), similar fasting and post-prandial glucose concentrations, higher fasting (p=0.0156) and postprandial (p=0.039) GLP-1 concentrations, and a reduced ratio of fasting ghrelin to GLP-1 (p=0.0078). In contrast, there were no differences in the concentrations of glucose-dependent insulinotropic polypeptide (GIP) or peptide YY (PYY) in the LC and LF groups.
Dietary effects on fatty acid and amino acid metabolites (Tables 2a and 2c, and Figures 2 and 3)
Table 2c.
Effects of low carbohydrate (LC) vs low fat (LF) feeding on plasma amino acids.
| LC fasting | LF fasting | LC post-prandial | LF post-prandial | |
|---|---|---|---|---|
| Alanine | 334.9 ± 23.7 | 345.7 ± 21.9 | 356.1 ± 25.8** | 289.9 ± 22.8 |
| Proline | 139.6 ± 9.6 | 148.5 ± 8.1 | 202.1 ± 11.2** | 329.1 ± 23.5 |
| Valine | 369.2 ± 20.7** | 251.9 ± 13.1 | 427.8 ± 22.1** | 341.6 ± 20.7 |
| Leucine/Isoleucine | 269.9 ± 19.7** | 177.6 ± 10.1 | 334.1 ± 17.6 ** | 260.7 ± 20.4 |
| Sum BCAA | 639.1 ± 40.2** | 429.6 ± 23.1 | 762.0 ± 38.6** | 602.3 ± 40.6 |
| Histidine | 72.3 ± 2.3** | 83.7 ± 1.9 | 87.0 ± 3.7 | 94.8 ± 4.6 |
| Phenylalanine | 60.7 ± 3.1* | 66.9 ± 3.2 | 78.5 ± 3.5** | 94.4 ± 4.6 |
| Tyrosine | 58.9 ± 3.7** | 68.1 ± 5.2 | 91.5 ± 5.6** | 122.2 ± 8.2 |
| Arginine | 97.3 ± 8.6 | 115.7 ± 4.7 | 104.8 ± 6.3** | 140.2 ± 6.1 |
| Citrulline | 23.8 ± 1.9 | 24.4 ± 2.1 | 24.2 ± 1.5** | 32.6 ± 2.8 |
BCAA =branched-chain amino acids (Leucine/Isoleucine, Valine). Data presented as mean + standard error (SE). Statistical analysis: paired t-tests; correlations were assessed by linear regression analysis (program R, Dept. of Statistics, Institute of Agriculture and Natural Resources and the College of Arts and Sciences, University of Nebraska-Lincoln).
A p value < 0.05 was considered statistically significant;
p<0.01
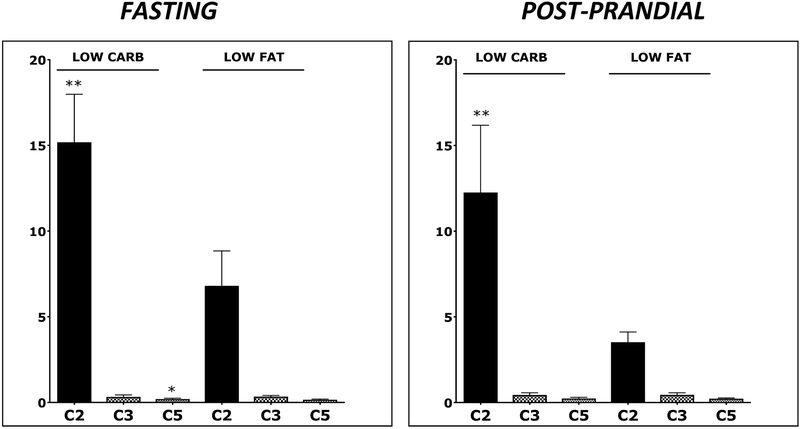
Figure 2.
Plasma concentrations of acetyl (C2), proprionyl (C3), and isovaleryl (C5) carnitines. C2 is a major product of fatty acid oxidation; C3 and C5 are products of the catabolism of the branch chain amino acids and methionine. Comparison of subjects consuming a low carbohydrate, high fat diet (15%carb; 65%fat; 20% protein), and the same subjects consuming a low fat, high carbohydrate diet (65%carb; 15%fat; 20% protein). *A p value < 0.05 was considered statistically significant; ** p<0.01.
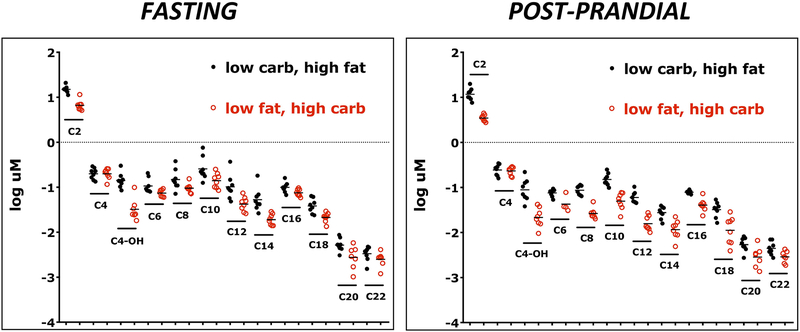
Figure 3.
Plasma concentrations of even chain acylcarnitines, which are products of fatty acid oxidation. Concentrations were log transformed to enable direct comparisons on the same figure. Comparison of subjects consuming a low carbohydrate, high fat diet (15%carb; 65%fat; 20% protein), and the same subjects consuming a low fat, high carbohydrate diet (65%carb; 15%fat; 20% protein). In all cases except C4/Ci4 and post-prandial C20 (p=0.0547), the concentrations of even chain acylcarnitines were significantly higher (p<0.05-p<0.0001) during low carbohydrate, high fat feeding than during low fat, high carbohydrate feeding
The concentrations of free fatty acids (FFA, p<0.01) and even-chain acylcarnitines (p<0.001) were higher during LC feeding, suggesting enhanced lipolysis and fatty acid oxidation. The only exception was C4/Ci4 (butyryl carnitine or isobutyrl carnitine), which is derived primarily from gut microbial fermentation of carbohydrates, fatty acids, and the branch chain amino acid valine15,16.
In contrast to the even chain acylcarnitines, the concentrations of propionyl carnitine (C3) were comparable in children fed the low carbohydrate and low fat diets (Figure 2). Fasting concentrations of isovaleryl carnitine (C5) were slightly but significantly higher during the low carbohydrate feeding (p=0.039).
C3 and C5 are products of the catabolism of methionine and the branch chain amino acids, which were higher (p<0.01) during low carbohydrate than during low fat feeding (Table 2c). In contrast, concentrations of the amino acids His, Phe, Tyr, Arg and Cit were lower (p<0.01) under fasting and/or post-prandial conditions.
Markers of insulin sensitivity and inflammation (Table 2a)
Markers of insulin resistance in obese children and adults include: high concentrations of the branch chain (leucine, isoleucine, valine) and aromatic (phenylalanine and tyrosine) amino acids17,18; low concentrations of total and high molecular weight adiponectin; fasting hyperinsulinemia; and an increase in the ratio of fasting triglycerides to HDL18,19. Children fed low carbohydrate diets had high concentrations of the branch chain amino acids but low concentrations of phenylalanine and tyrosine and a lower ratio of fasting TG to HDL. Adiponectin and fasting insulin and glucose concentrations were comparable during low carbohydrate and low fat feeding. On the other hand, markers of inflammation (CRP, 10.9 vs 4.2 ug/ml, p<0.01), and liver fat deposition (AST, 25.9 vs 17.8 U/L, p<0.001, and ALT, 18.3 vs 9.9 U/L, p<0.01) were higher during consumption of the LC diet (Tables 2a and 2b).
Table 2b.
Effects of low carbohydrate (LC) vs low fat (LF) feeding on CRP, AST, and ALT
| LC fasting | LF fasting | LC post-prandial | LF post-prandial | Fasting and post-prandial combined | ||
|---|---|---|---|---|---|---|
| LC | LF | |||||
| CRP, ug/ml | 10.5 ± 5.0 | 4.1 ± 2.5 | 11.3 ± 5.7 | 4.3 ± 2.8 | 10.9 ± 3.7** | 4.2 ± 1.8 |
| AST, U/L | 26.1 ± 2.3** | 18.4 ± 1.5 | 25.6 ± 2.4** | 17.1 ± 1.5 | 25.9 ± 1.6*** | 17.8 ± 1.0 |
| ALT, U/L | 19.3 ± 4.3 | 10.6 ± 1.7 | 17.3 ± 3.7 | 9.3 ± 2.1 | 18.3 ± 2.8** | 9.9 ± 1.3 |
LC = Low carbohydrate - high fat diet (15%carb; 65%fat; 20% protein); LF = Low fat - high carbohydrate diet (65%carb; 15%fat; 20% protein); CRP = C-reactive protein; ALT = alanine transaminase; AST = aspartate aminotransferase. Data presented as mean + standard error (SE). Statistical analysis: paired t-tests; correlations were assessed by linear regression analysis (program R, Dept. of Statistics, Institute of Agriculture and Natural Resources and the College of Arts and Sciences, University of Nebraska-Lincoln).
A p value < 0.05 was considered statistically significant;
p<0.01;
p<0.001.
For International System of Units (SI), see AMA Manual of Style, 10th edition.
Correlates of plasma GLP-1 and ghrelin (Figure 4)
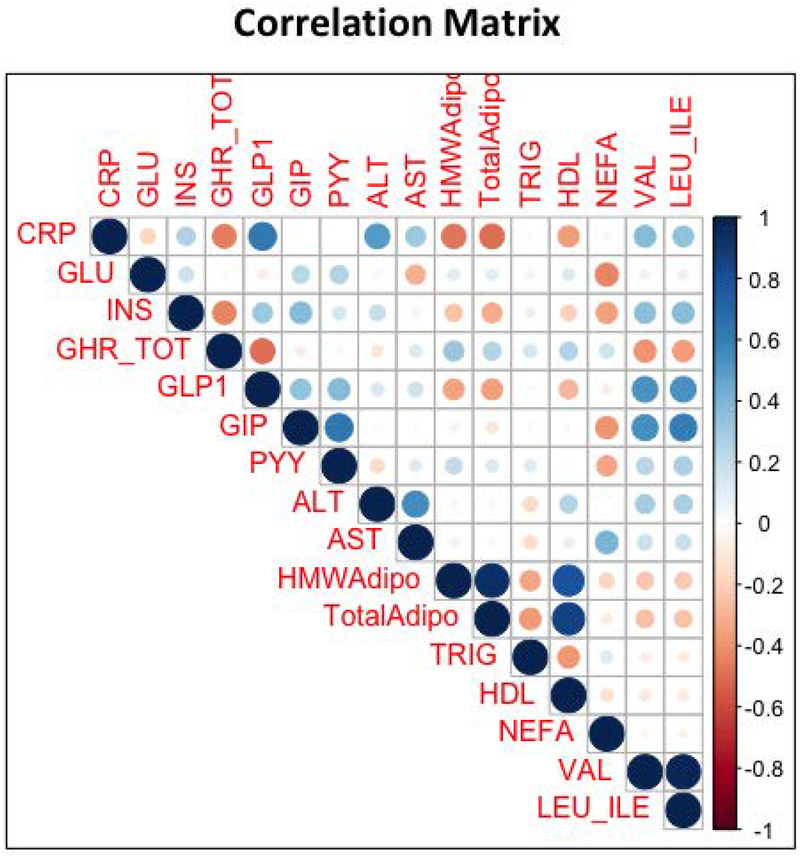
Figure 4.
Matrix showing the correlation coefficients between participants’ plasma concentrations of the following sets of variables (as measured in the present study): CRP = C-reactive protein; GLU = glucose; INS = insulin; GHR_TOT = total ghrelin; GLP-1 = Glucagon-like peptide 1; GIP = glucose-dependent insulinotropic peptide; PYY = Peptide YY; ALT = alanine transaminase; AST = aspartate aminotransferase; HMWAdipo = high-molecular-weight adiponectin; TotalAdipo = total adiponectin; TRIG = triglycerides; HDL = high-density lipoproteins; NEFA = non-esterified fatty acids; VAL = valine; LEU_ILE = leucine_isoleucine.
The strongest correlates of plasma GLP-1 across groups were the concentrations of the branch chain amino acids (R2 = 0.28, P<0.001) and CRP (R2= 0.39, P<0.001). GLP-1 also correlated positively with GIP (R2 = 0.12, P<0.01) and PYY (R2 = 0.15, P<0.01) and negatively with ghrelin (R2 = 0.25, p<0.001), and adiponectin (R2 = 0.13, p<0.01).
Total ghrelin correlated negatively with insulin (R2 = 0.23, p<0.001), GLP-1 (R2 = 0.22, p<0.001), and the branch chain amino acids (R2 = 0.13, p<0.01)
Discussion
Newborns with PWS have hypotonia and failure to thrive and often require tube feedings for several weeks to months to maintain weight gain and linear growth. This is followed by a period of hyperphagia and progressive increases in body fat content. The hyperphagia and adiposity in PWS children are associated with hyperleptinemia, hyperghrelinemia, and a blunted peptide YY response to high-fat (58%) feeding2,20,21. Likewise, the rise in PYY was attenuated in PWS adults following a high calorie chocolate snack containing 61% carbohydrate and 31% fat22. Fasting insulin concentrations are lower in PWS than in non-syndromic obesity and insulin sensitivity, as assessed by HOMA-IR, adiponectin, and the ratio of TG to HDL, is higher13.
Poorly understood are the roles of dietary macronutrients in the control of appetite, weight gain, and metabolic function in PWS. Weight gain in PWS is associated with preference for simple sugars23–25; together with limited evidence from an uncontrolled study cited previously7, this suggests that high carbohydrate intake might promote or sustain hyperphagia and fat deposition. We postulated that carbohydrate restriction might reduce orexigenic and increase anorexigenic drive and improve metabolic function as assessed by measures of insulin sensitivity.
The study we conducted is unique in comparing the effects of low carbohydrate, high fat and low fat, high carbohydrate diets rigidly matched for protein and calorie content. Each patient served as his or her own control, permitting direct comparisons independent of differences in age, sex, pubertal status, baseline BMI z-scores, or medication exposure. There were no variations in medications during the study, and no changes in pubertal status would have occurred during the short interval (1 month) between dietary arms. Moreover, the children remained in isocaloric balance as determined by absence of significant weight change between hospital admissions. To minimize any impact of age and pubertal status, we calculated the caloric content of the diets using estimates of energy requirement (EER)26 adjusted for the PWS population.
PWS is a rare condition (prevalence ~1/25,000); thus the number of children available for our study was limited. Nevertheless, we obtained statistically significant results that likely have important clinical implications. We found that carbohydrate restriction increased fasting and post-prandial GLP-1 concentrations and reduced the ratio of fasting ghrelin to GLP-1. In contrast, there were no effects of carbohydrate restriction on plasma GIP or PYY. Dietary fat stimulates an increase in GLP-1 secretion, with a potency equal to or greater than that of dietary carbohydrate or protein27–29. Conversely, dietary nutrients suppress ghrelin secretion, with carbohydrate being more potent, at least in obese children, than fat20. Given that GLP-1 reduces and ghrelin promotes food intake in rodents and humans30,31, the increases in GLP-1 and reductions in the ratio of ghrelin to GLP-1 in PWS fed a LC diet might serve to limit food intake and weight gain. A test of this hypothesis would require a long-term clinical trial.
The concentrations of FFA and even-chain acylcarnitines were far higher during LC feeding than during LF feeding. These findings suggest that the LC diet may promote white adipose mobilization and/or triglyceride hydrolysis and increase fatty acid oxidation. Interestingly acetate, the end-product of fatty acid oxidation, stimulates colonic production of GLP-1 and suppresses food intake through induction of hypothalamic expression of pro-opiomelanocortin (POMC) and suppression of Agouti-related peptide (AgRP)32. Thus, carbohydrate restriction could in theory limit food intake through distinct but overlapping mechanisms. Food intake was rigidly controlled in our study by caregivers and research staff; attempts to assess subjective hunger and satiety in our patient cohort were unsuccessful, as patient intellectual and communication deficits made their responses to the questionnaire unreliable.
Carbohydrate restriction reduced postprandial insulin concentrations, fasting TG, and the ratio of TG to HDL, all of which suggest heightened insulin sensitivity17–19. On the other hand, total and high molecular weight adiponectin, a marker of insulin sensitivity13,17–19, was no different in LC-fed than in LF-fed subjects. Interestingly, carbohydrate restriction increased plasma concentrations of the branch chain amino acids, which are associated with insulin resistance17,18,33. Additionally, concentrations of CRP, AST, and ALT were higher during LC feeding than LF feeding. Increases in CRP and ALT suggest heightened inflammation and liver fat deposition, warranting concern and underlining the need for additional studies of the relative benefits and safety of long-term carbohydrate restriction in PWS.
In addition to the size of our cohort, a possible limitation of our study is the lack of assessment of the gut microbiome. This might be of interest, given that dietary modulation of gut microbiota was associated with weight loss in an uncontrolled investigation of children with simple obesity and PWS34. In addition, as noted previously, we did not compare hormone and metabolite concentrations obtained during controlled administration of the LC and LF diets to baseline values obtained at the time of admission. This was because baseline values reflected participants’ uncontrolled diets prior to admission. It is impossible to interpret comparisons between values obtained under supervised and controlled conditions (during the dietary intervention) with those obtained under uncontrolled conditions (baseline).
Anecdotal reports indicate that some families are adopting low carbohydrate diets for their children with PWS because of assumed or perceived benefits of carbohydrate restriction on hyperphagia and cognition. However in our cohort, low carbohydrate feeding increased markers of inflammation (c-reactive protein) and hepatic steatosis (AST/ALT), and salutary effects of low carbohydrate diets on appetite, weight gain, and cognitive function in PWS have not yet been demonstrated. More detailed studies of the safety and long-term effects of carbohydrate restriction on weight loss and metabolic function in Prader Willi patients would be useful in providing clinical guidance.
Acknowledgements:
Supported by a grant (to AH and MF) from the Foundation for Prader Willi Research (0016121) and by a NICHD T32 grant (5T32HD043029–15) awarded to the Duke Department of Pediatrics. We are grateful to Michelle Mackenzie, PhD for help with recruitment of study subjects and implementation of the intervention and to Cris Slentz, PhD for assistance with measurements of body fat content using the Bod Pod.
Footnotes
DISCLOSURE STATEMENT: The authors have nothing to disclose.
References
- 1.Butler MG. Prader-Willi Syndrome: Obesity due to Genomic Imprinting. Curr Genomics. 2011;12(3):204–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Irizarry KA, Miller M, Freemark M, Haqq AM. Prader Willi Syndrome: Genetics, Metabolomics, Hormonal Function, and New Approaches to Therapy. Adv Pediatr. 2016;63(1):47–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Orsso CE, Mackenzie M, Alberga AS, et al. The use of magnetic resonance imaging to characterize abnormal body composition phenotypes in youth with Prader-Willi syndrome. Metabolism. 2017;69:67–75. [DOI] [PubMed] [Google Scholar]
- 4.Rubin DA, Clark SJ, Ng J, Castner DM, Haqq AM, Judelson DA. Hormonal and metabolic responses to endurance exercise in children with Prader-Willi syndrome and non-syndromic obesity. Metabolism. 2015;64(3):391–395. [DOI] [PubMed] [Google Scholar]
- 5.Bonfig W, Dokoupil K, Schmidt H. A special, strict, fat-reduced, and carbohydrate-modified diet leads to marked weight reduction even in overweight adolescents with Prader-Willi syndrome (PWS). ScientificWorldJournal. 2009;9:934–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmidt H, Schwarz HP, Enders A. Dietary intervention in the first four years prevents abnormal weight gain but negatively affects height development in Prader-Willi syndrome. Acta Paediatr. 2001;90(4):468–469. [PubMed] [Google Scholar]
- 7.Miller JL, Lynn CH, Shuster J, Driscoll DJ. A reduced-energy intake, well-balanced diet improves weight control in children with Prader-Willi syndrome. J Hum Nutr Diet. 2013;26(1):2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharma AK, Metzger DL, Daymont C, Hadjiyannakis S, Rodd CJ. LMS tables for waist-circumference and waist-height ratio Z-scores in children aged 5–19 y in NHANES III: association with cardio-metabolic risks. Pediatr Res. 2015;78(6):723–729. [DOI] [PubMed] [Google Scholar]
- 9.Medicine Io. Dietary Reference Intakes: The Essential Guide to Nutrient Requirements. Washington, DC: The National Academies Press; 2006. [Google Scholar]
- 10.Institute of Medicine Committee to Review Dietary Reference Intakes for Vitamin D, Calcium. The National Academies Collection: Reports funded by National Institutes of Health In: Ross AC, Taylor CL, Yaktine AL, Del Valle HB, eds. Dietary Reference Intakes for Calcium and Vitamin D. Washington (DC): National Academies Press (US)National Academy of Sciences; 2011. [PubMed] [Google Scholar]
- 11.Alsaif M, Elliot SA, MacKenzie ML, Prado CM, Field CJ, Haqq AM. Energy Metabolism Profile in Individuals with Prader-Willi Syndrome and Implications for Clinical Management: A Systematic Review. Advances in nutrition (Bethesda, Md). 2017;8(6):905–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Butler MG, Theodoro MF, Bittel DC, Donnelly JE. Energy expenditure and physical activity in Prader-Willi syndrome: comparison with obese subjects. American journal of medical genetics Part A. 2007;143a(5):449–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haqq AM, Muehlbauer MJ, Newgard CB, Grambow S, Freemark M. The metabolic phenotype of Prader-Willi syndrome (PWS) in childhood: heightened insulin sensitivity relative to body mass index. J Clin Endocrinol Metab. 2011;96(1):E225–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haqq AM, Lien LF, Boan J, et al. The Study of the Effects of Diet on Metabolism and Nutrition (STEDMAN) weight loss project: Rationale and design. Contemp Clin Trials. 2005;26(6):616–625. [DOI] [PubMed] [Google Scholar]
- 15.Srinivas SR, Prasad PD, Umapathy NS, Ganapathy V, Shekhawat PS. Transport of butyryl-L-carnitine, a potential prodrug, via the carnitine transporter OCTN2 and the amino acid transporter ATB(0,+). Am J Physiol Gastrointest Liver Physiol. 2007;293(5):G1046–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Louis P, Flint HJ. Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol Lett. 2009;294(1):1–8. [DOI] [PubMed] [Google Scholar]
- 17.Newgard CB, An J, Bain JR, et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009;9(4):311–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Newbern D, Gumus Balikcioglu P, Balikcioglu M, et al. Sex differences in biomarkers associated with insulin resistance in obese adolescents: metabolomic profiling and principal components analysis. J Clin Endocrinol Metab. 2014;99(12):4730–4739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iwani NAKZ, Jalaludin MY, Zin RMWM, et al. Triglyceride to HDL-C Ratio is Associated with Insulin Resistance in Overweight and Obese Children. Sci Rep. 2017;7:40055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gumus Balikcioglu P, Balikcioglu M, Muehlbauer MJ, et al. Macronutrient Regulation of Ghrelin and Peptide YY in Pediatric Obesity and Prader-Willi Syndrome. J Clin Endocrinol Metab. 2015;100(10):3822–3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Irizarry KA, Bain J, Butler MG, et al. Metabolic profiling in Prader-Willi syndrome and nonsyndromic obesity: sex differences and the role of growth hormone. Clin Endocrinol (Oxf). 2015;83(6):797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rigamonti AE, Bini S, Piscitelli F, et al. Hedonic eating in Prader-Willi syndrome is associated with blunted PYY secretion. Food Nutr Res. 2017;61(1):1297553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martinez Michel L, Haqq AM, Wismer WV. A review of chemosensory perceptions, food preferences and food-related behaviours in subjects with Prader-Willi Syndrome. Appetite. 2016;99:17–24. [DOI] [PubMed] [Google Scholar]
- 24.Hinton EC, Holland AJ, Gellatly MSN, Soni S, Owen AM. An investigation into food preferences and the neural basis of food-related incentive motivation in Prader-Willi syndrome. J Intellect Disabil Res. 2006;50(Pt 9):633–642. [DOI] [PubMed] [Google Scholar]
- 25.Caldwell ML, Taylor RL. A clinical note on food preference of individuals with Prader-Willi syndrome: the need for empirical research. J Ment Defic Res. 1983;27 (Pt 1):45–49. [DOI] [PubMed] [Google Scholar]
- 26.Schofield WN. Predicting basal metabolic rate, new standards and review of previous work. Hum Nutr Clin Nutr. 1985;39 Suppl 1:5–41. [PubMed] [Google Scholar]
- 27.Gibbons C, Caudwell P, Finlayson G, et al. Comparison of postprandial profiles of ghrelin, active GLP-1, and total PYY to meals varying in fat and carbohydrate and their association with hunger and the phases of satiety. J Clin Endocrinol Metab. 2013;98(5):E847–855. [DOI] [PubMed] [Google Scholar]
- 28.Lindgren O, Carr RD, Deacon CF, et al. Incretin hormone and insulin responses to oral versus intravenous lipid administration in humans. J Clin Endocrinol Metab. 2011;96(8):2519–2524. [DOI] [PubMed] [Google Scholar]
- 29.Runchey SS, Valsta LM, Schwarz Y, et al. Effect of low- and high-glycemic load on circulating incretins in a randomized clinical trial. Metabolism. 2013;62(2):188–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dailey MJ, Moran TH. Glucagon-like peptide 1 and appetite. Trends Endocrinol Metab. 2013;24(2):85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wren AM, Seal LJ, Cohen MA, et al. Ghrelin enhances appetite and increases food intake in humans. J Clin Endocrinol Metab. 2001;86(12):5992. [DOI] [PubMed] [Google Scholar]
- 32.Frost G, Sleeth ML, Sahuri-Arisoylu M, et al. The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat Commun. 2014;5:3611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bloomgarden Z. Diabetes and branched-chain amino acids: What is the link? J Diabetes. 2018;10(5):350–352. [DOI] [PubMed] [Google Scholar]
- 34.Zhang C, Yin A, Li H, et al. Dietary Modulation of Gut Microbiota Contributes to Alleviation of Both Genetic and Simple Obesity in Children. EBioMedicine. 2015;2(8):968–984. [DOI] [PMC free article] [PubMed] [Google Scholar]