Abstract
Purpose
This is the first single-institution study of its size to characterize the treatment impact and to address the question of whether hemangioblastoma treatment with Gamma Knife Stereotactic Radiosurgery (GKRS) in both sporadic and VHL patients changes the characteristic saltatory hemangioblastoma growth pattern.
Methods
The authors reviewed a single-institution tumor registry to identify patients who had received GKRS for hemangioblastomas between January 1st, 1999, and December 31st, 2017.
Results
15 patients with 101 lesions met search criteria with a median age of first GKRS of 39.2 years (interquartile range [IQR] of 25.7–57.4 years), including 96 VHL and 5 sporadic lesions. The median time from GKRS to last follow-up was 5.4 years (IQR 2.3–11.5 years). 4 lesions (4%) and 3 patients (20%) experienced a local failure. The 1-year, 3-year, and 5-year freedom from new hemangioblastoma formation rates were 97%, 80%, and 46% respectively. Multivariate analysis revealed a reduction in tumor volume after GKRS. Several variables associated with a greater percent reduction in volume from GKRS to last follow-up: non-cystic status (p = .01), no prior craniotomy (p = .04), and follow-up time from GKRS (p < .0001).
Conclusions
GKRS is a successful long-term treatment option for hemangioblastomas changing the clinical course from saltatory growth to reduction in tumor volume. Non-cystic tumors and those without prior craniotomy were associated with a greater percent reduction in volume from GKRS at last follow-up.
Keywords: Gamma Knife, Hemangioblastoma, Oncology, Sporadic, Stereotactic radiosurgery, Von Hippel-Lindau
Introduction
CNS hemangioblastomas are World Health Organization Grade 1 tumors that may present either sporadically or in conjunction with von Hippel-Lindau disease (VHL) [1]. VHL is an autosomal dominant disorder with 95% penetrance by midlife and carries a substantial burden of CNS tumors; hemangioblastomas are often the first clinical manifestation and are the most common central nervous system (CNS) involvement [1]. VHL is caused by a germline mutation of a tumor suppressor gene at 3p25–26 on the short arm of chromosome 3 [1]. While sporadic and VHL-related hemangioblastomas share histological characteristics across tumors, the clinical course of these tumor populations may differ significantly [1, 2]. VHL patients, compared to sporadic patients, have lesions detected at younger ages, are more likely develop multiple tumors, and are more likely to develop lesions outside of the cerebellum [1, 3]. Both sporadic and VHL tumors may contain cystic components, a characteristic underlying many symptomatic lesions [2, 4]. In VHL, lesions are known to grow in a saltatory pattern with periods of quiescence punctuated by periods of growth [4, 5]. Previous series, due to lack of longitudinal volumetric analyses, have not demonstrated that stereotactic radiosurgery clearly alters the clinical course of this salutatory growth of hemangioblastomas [4, 5]. Thus, classic treatment for hemangioblastomas in VHL patients—a population with a higher likelihood for detection of CNS lesions ahead of symptom development—is still resection upon presentation of symptoms [4] as it is in sporadic lesions [2, 3].
Previous studies demonstrate positive patient outcomes using GKRS to treat hemangioblastomas [6–11], even advocating for treating lesions ahead of symptom development [8]. However disagreement remains regarding whether GKRS itself impacts the growth of the lesions, or if hemangioblastoma growth is too unpredictable to determine if lesions will need treatment ahead of symptom presentation [5]. In this study, we present a single institution retrospective series documenting the clinical outcomes and longitudinal imaging outcomes of treating hemangioblastomas with Gamma Knife Stereotactic Radiosurgery (GKRS) with our institutional philosophy of treating these tumors at first evidence of detectable growth as opposed to waiting for development of clinical symptoms. We present a lesion-by-lesion walk-through of the impact GKRS radiosurgery has on lesion progression to address existing doubt regarding how GKRS radiosurgery impacts the clinical course of a treated lesion (see Supplementary Content) alongside a traditional report of patient treatment outcome and demographic data.
Methods
Patient population
The Institutional Review Board at our institution approved the following study. A search of the Department of Radiation Oncology Gamma Knife Tumor Registry was conducted to identify patients who received treatment for hemangioblastomas between January 1st, 1999, and December 31st, 2017. Patients were included if their lesions had at least one follow-up measurement. Patient history, demographic information, and long-term follow-up information were collected from individual electronic medical records.
At our institution, patients are offered treatment with GKRS for hemangioblastomas if the lesions: (1) exhibit documented growth, or (2) cause mild symptoms. For the subset of patients with VHL, many experienced evidence of recurrent growth of multiple lesions over time. Five of 10 VHL patients had prior craniotomies before GKRS. Two of these patients, patients 8 and 12 (Table 2), comprising 5 lesions were resection failures. For the subset of patients with sporadic lesions, all of those treated with GKRS experienced recurrence following resection with craniotomies. In both cases, further—likely numerous—craniotomies for resection were comparatively less attractive treatment options.
Table 2.
Individual characteristics in 15 patients
| Case | Sex | VHL | Num ber of GKRS |
Total number of lesions |
Lesions treated per GKRS |
Age at GKRS (years) |
Median prescription dose |
Failure | Freedom from local failure (months) |
Freedom from new hemangio blastoma forma tion (months) |
Time to last follow- up (months) |
Death | Death notes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | No | 1 | 1 | 1 | 57 | 21 | None | 86 | No | |||
| 2 | M | No | 1 | 1 | 1 | 47 | 20 | None | 95 | No | |||
| 3 | F | No | 1 | 1 | 1 | 61 | 12 | None | 70 | No | |||
| 4 | M | No | 1 | 1 | 1 | 57 | 18 | Local×1 | 4 | 4 | No | ||
| 5 | F | No | 1 | 1 | 1 | 57 | 15 | None | 6 | No | |||
| 6 | M | Yes | 3 | 20 | 9/6/5 | 38/44/49 | 16 | New×15 | 57/65 | 20/77/142 | Yes | Hemangioblastomatosis | |
| 7 | F | Yes | 2 | 12 | 8/4 | 39/49 | 18 | New×8 | 123 | 65/188 | No | ||
| 8 | F | Yes | 2 | 4 | 3/1 | 70/81 | 16 | New×3 | 136 | 12/149 | No | ||
| 9 | F | Yes | 2 | 11 | 5/6 | 38/43 | 16 | New×5 | 53 | 11/64 | No | ||
| 10 | M | Yes | 2 | 5 | ¼ | 15/19 | 20 | New×1 | 54 | 27/81 | No | ||
| 11 | F | Yes | 5 | 26 | 3/8/5/4/6 | 38/4¼2/49/50 | 20 | Local×1/ New×20 |
128 | 10/12/36/80 | 35/45/125/137/173 | No | |
| 12 | F | Yes | 1 | 1 | 1 | 75 | 15 | None | 26 | No | |||
| 13 | M | Yes | 1 | 3 | 3 | 25 | 18 | Local×2 | 6/55 | 54 | No | ||
| 14 | F | Yes | 2 | 10 | 7/3 | 25/28 | 16l | New×7 | 38 | 6/44 | No | ||
| 15 | F | Yes | 1 | 4 | 4 | 13 | 15 | None | 28 | No | |||
VHL von Hippel-Lindau disease, GKRS Gamma Knife Stereotactic Radiosurgery
Gamma Knife technique
Evaluation by a neurosurgeon and a radiation oncologist was performed prior to obtaining informed consent for GKRS. On the day of treatment, the patient received local anesthetic, and was subsequently fitted with a 4-pin Leksell stereotactic headframe (Elekta Instrument AB Stockholm, Stockholm, Sweden). The patient later underwent a high-resolution, stereotactic MRI with contrast enhancement. Prior to 2006, a 1.5 T scanner (General Electric, Milwaukee, WI) was used for treatment planning. For 2006 and beyond, a 3 T scanner was used (General Electric, Milwaukee, WI). All patients received two MRI sequences, a Tl-weighted 3D Fast Spoiled Gradient axial sequence with 1 mm thickness and no gap, and a second Tl-weighted axial sequence with 3 mm thickness and no gap. Treatment planning was performed using the Leksell GammaPlan system (Elekta Instrument AB Stockholm, Stockholm, Sweden). The Leksell model B or C Gamma Knife or Perfexion Unit (Elekta Instrument AB Stockholm, Stockholm, Sweden) is used to deliver the treatment. The patients were treated by 3 neurosurgeons and 5 radiation oncologists over an 18-year period. Dosing (Table 1) was done at the discretion of the practicing physicians and was generally based on the calculated volume of the tumor on the treatment planning MRI. In general, smaller tumors were more likely to be treated with a higher margin dose. Plans were generally prescribed to the 50% isodose line. The median tumor target volume was 28.0 mm3 at time of treatment (see Table 1). Between 1 and 9 lesions for a participant were treated in a single day; mean (SD) = 3.9 (2.5) lesions.
Table 1.
Patient and lesion characteristics
| Variable | Value |
|---|---|
| Number of patients | 15 |
| Number of lesions | 101 |
| Age at first GKRS (years) | |
| Mean (SD) | 44.1 (19.0) |
| Median (IQR) | 39.2 (31.7) |
| 25th–75th percentiles | 25.7–57.4 |
| Sex | |
| Female | 10 (67%) |
| Male | 5 (33%) |
| Number of lesions per patient | |
| Median (IQR) | 4 (10) |
| 25th–75th percentiles | 1–11 |
| Minimum–Maximum | 1–26 |
| Number of GKRSs | |
| Median (IQR) | 2.0 (1.0) |
| 25th–75th percentiles | 1–2 |
| Minimum-maximum | 1–5 |
| Patients with multiple GKRSs | |
| One GKRS | 7 (47%) |
| Multiple GKRS | 8 (53%) |
| Tumor volume at GKRS (mm3) | |
| Median (IQR) | 28.0 (151.9) |
| 25th–75th percentiles | 14.6–166.5 |
| Minimum–maximum | 2.0–9,200 |
| Prescription dose (Gy) | |
| Median (IQR) | 17.8 (3.9) |
| 25th–75th percentiles | 16.0–19.9 |
| Minimum–maximum | 12.0–21.0 |
| Location | |
| Infratentorial | 94 (93%) |
| Brainstem | 6 (6%) |
| Cerebellum | 85 (84%) |
| Cranial nerve | 1 (1%) |
| Foramen magnum | 2 (2%) |
| Supratentorial | 7 (7%) |
| Occipital lobe | 1 (1%) |
| Parasagittal | 1 (1%) |
| Temporal lobe | 4 (4%) |
| Thalamus | 1 (1%) |
| Local failure | |
| Lesions | 4 (4%) |
| Patients | 3 (20%) |
| Time to local failure (years) | |
| Minimum–maximum | 0.4–10.7 |
| New hemangioblastoma formation | |
| Lesions | 59 (58%) |
| Patients | 7 (47%) |
| Time to new hemangioblastoma formation (years) | |
| Kaplan–Meier median (95% CI) | 4.7 (4.4, 5.4) |
| Minimum–maximum | 0.8–10.2 |
| Time from GKRS to last follow-up (years) | |
| Median (IQR) | 5.4 (9.1) |
| 25th–75th percentiles | 2.3–11.5 |
| Minimum–maximum | 0.3–15.6 |
| Number of patient deaths | 1 (7%) |
GKRS Gamma Knife Stereotactic Radiosurgery, SD standard deviation, IQR interquartile range, CI confidence interval
Follow-up
3–6 months after the GKRS treatment date, an initial follow-up clinic visit with an MRI is completed, and further imaging generally occurred annually thereafter. For the 15 patients analyzed, the shortest follow-up was 4 months. Local treatment failure was defined on a per-lesion basis by mass growth or cystic growth necessitating resection. A local failure was determined to be a 25% increase in the volume over the nadir volume as calculated by contouring the tumors on post-treatment MRI. New hemangioblastoma formation is classified as the presence or growth of a new, non-overlapping hemangioblastoma lesion newly necessitating treatment, whereas prior scans indicated absence of a lesion or absence of lesion growth.
Time to death was recorded in one instance and was calculated from the time of Gamma Knife treatment. Information on patient death was gathered through individual electronic medical records, the Social Security Death Index, and accessible local obituaries. Cause of death was determined to be neurological if progressive neurological deterioration occurred in the setting of unchanging systemic symptoms or simultaneously progressive neurologic and systemic deterioration as has previously been described [12].
Statistical methods
Descriptive characteristics are used to describe the patients and lesions of our sample. Frequencies and percentages are used for categorical measures and depending upon the distribution of the continuous measures, either means with standard deviations or medians and interquartile range (IQR) are used. Follow-up time was calculated from the date of GKRS to the most recent follow-up. Local failure was defined as the lesion requiring surgery and new hemangioblastoma formation was defined as the need for GKRS on subsequent tumors within the subject. Both local failure and new hemangioblastoma formation were censored at the last follow-up. Kaplan–Meier estimation was used to describe freedom from local failure and freedom from new hemangioblastoma formation. Percent change in volume was the primary outcome for this analysis and is defined as the percent change in the volume of the lesion from GKRS to each follow-up. Time to death was measured from the date of GKRS until death or censored at last follow-up. No post-surgical lesion measurements were used in the modeling of percent change, leaving 99 lesions in 15 subjects with at least one follow-up measurement for analysis. To meet the assumptions of the linear model, outliers in the percent change variable were windsorized to the 95th percentile and then percent change was log-transformed. Repeated measures mixed linear models were used to evaluate baseline patient and lesion characteristics on the log transformed percent change in lesion size following GKRS. Potential covariates were initially chosen based on scientific reasoning. Potential covariates were included in a base model along with GKRS volume and follow-up time and selected for the full model if the p-value was < .2. Potential covariates included cystic (yes/no), VHL (yes/no), sex (female/male), prior craniotomy (yes/no), prior GKRS treatment (yes/no), and location (infratentorial/supratentorial). VHL and prior craniotomy both met the criteria for inclusion in the full model, but only prior craniotomy was used since the two variables were collinear. All analyses were done using SAS v9.4 (SAS Institute Inc. Cary, NC).
Results
Summary statistics
Detailed follow-up data was available for 15 patients and a total of 101 lesions out of a total of 102 lesions treated. This included patients with VHL and sporadic cases. One lesion was excluded due to lack of scan quality covering this lesion, however this same patient had 26 other lesions included in this study. One patient died, and death was neurological in this case.
Table 1 summarizes descriptive statistics across the study group. The median age at first GRKS was 39 years. There was a mean of 4 lesions treated per patient (interquartile range 10). Eight patients (53%) received multiple GKRS treatments. The median tumor volume was 28.0 mm3 (interquartile range of 152 mm3). The median prescription dose was 17.8 Gy (interquartile range of 3.9 Gy). Ninety-three percent of the treated lesions were infratentorial. The median time from GKRS treatment to last follow-up was 5.4 years.
Table 2 summarizes individual treatment-based, tumor-based, and demographic-based data separated by patient including the number of GKRS treatments, total number of lesions, number of lesions treated per GKRS treatment, median prescription dose, failure status, freedom from new hemangioblastoma formation, time to last follow-up, and death with cause if relevant.
Five additional patients were noted in the initial query, but were lost to follow-up and not included; of these, 3 patients died with only one patient dying of a confirmed neurological death.
By search definition, all patients and lesions noted were treated with GKRS during the defined study period.
Clinical course of treated lesions
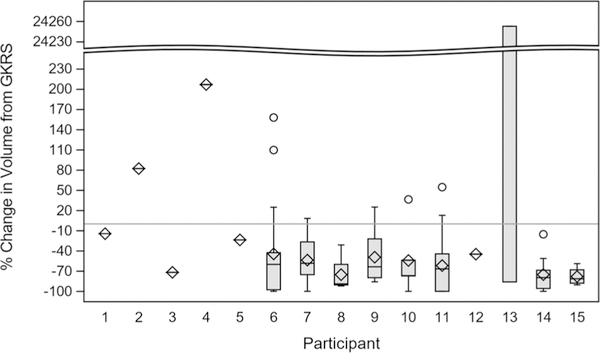
Figure 1 shows the distribution of percent (%) change in volume from GKRS to last follow-up across each patient. The sporadic patients are presented ahead of the VHL patients (see Supplemental Digital Content for per-lesion graphs of clinical course). The overall mean percent change in size was an increase of 192.3%, while the overall median change in size was a decrease of 66.7%.
Fig. 1.
Percent change in volume from GKRS to last follow-up separated by patient. Percent change was calculated for each participant. For each visit a total volume was calculated, then the percent change (with the GKRS visit as the reference) was calculated for each visit. The thick part of each histogram represents the bulk of the distribution of volume change for lesions within each patient, and the thin bars above and below represent the minimum and maximum percent change
Patterns of failure
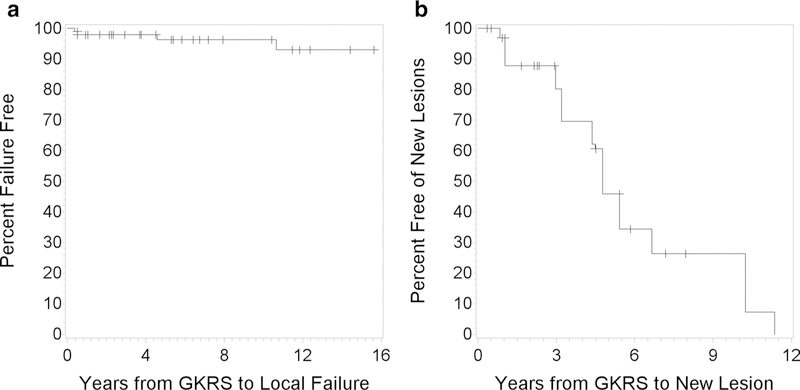
Figure 2a shows the Kaplan–Meier curve for freedom from local failure for the included lesions. Local failure occurred in 4 (4%) of 101 treated lesions across 3 (20%) of patients following GKRS with a freedom from local failure ranging from 0.4 to 10.7 years (see Table 1). All 4 tumor recurrences were treated with resection. The indication for resection was tumor enlargement in 2 of the lesions, and enlargement of a cyst in the other 2 cases. Three of the four treatment failures that were treated with resection exhibited mass effect and caused symptoms prior to resection. In the fourth case, surgery was performed as a means of preventing symptoms after continued tumor enlargement. All local failures occurred in infratentorial lesions that demonstrated significant relative growth (6.8–14 cc, 1 cc at baseline with growth that led to mass effect and ventriculomegaly, 0.2–4.1 cc, and 0.05–0.08 cc). No CTCAE grade 3–5 toxicities were present in the lesions across our study.
Fig. 2.
a Kaplan–Meier curve for freedom from local failure on a per-lesion basis. b Kaplan–Meier curve for freedom from new hemangioblastoma formation on a per-patient basis
Figure 2b shows the Kaplan–Meier curve for freedom from new hemangioblastoma formation for the included lesions. New hemangioblastomas occurred in 7 (47%) of patients, represented by 59 (58%) of the lesions included in this study with a median freedom from new hemangioblastoma formation of 5.4 years and an IQR of 9.1 years (see Table 1). At 1, 3, and 5 years following GKRS treatment there were 97%, 80%, and 46% patients free of new hemangioblastoma formation, respectively.
Predictors of post-treatment volume reduction
Multivariate analysis produced several predictive variables of a greater percent (%) decrease in volume following GKRS treatment, including increase in follow-up time (p < .0001), non-cystic status (p = .01), and no prior craniotomy (p = .04) (see Table 3).
Table 3.
Results of multivariable longitudinal model
| Variable | p-value | Association with a greater % reduction in volume |
|---|---|---|
| Volume at GKRS | 0.74 | NA |
| Follow-up time from GKRS | < 0.0001 | Increase in follow-up time |
| Cystic | 0.01 | Non-cystic |
| Prior Craniotomy | 0.04 | No prior craniotomy |
Outcome is the log transformed percent change in volume from GKRS
GKRS Gamma Knife Stereotactic Radiosurgery
Discussion
To the authors’ knowledge, this is the first single-institution report of its size detailing a decrease in tumor volume of hemangioblastomas after GKRS treatment across both von Hippel-Lindau and sporadic populations. It is among the largest hemangioblastoma series in the scientific literature and documents outcomes on a per-lesion basis. Several prior studies have reported favorable treatment outcomes for hemangioblastomas treated with GKRS [6–11]. An advantage of the present study is the consistency of treatment philosophy of treating these patients at time of imaging progression and the longitudinal follow-up of tumor volumes over a clinically significant period of time.
The present study demonstrated that the clinical course of a treated hemangioblastoma over several years after GKRS mirrors that for other benign tumors such as meningiomas [13] and Schwannomas [14]. In contrast untreated lesions grow in a saltatory pattern with periods of quiescence punctuated by periods of growth [4, 5]. The local failure rate after GKRS is low, and for most patients, with a greater follow-up interval, there is a continued decrease in tumor volume. Because the goal of GKRS is to prevent progression of growth or symptoms, the great majority of patients experienced a positive outcome from GKRS. Thus, GKRS likely spares some patients from the need for craniotomy.
Interestingly, 100% of the local failures in our series occurred in only 3 patients (20%). This would suggest that local failure of hemangioblastoma after GKRS is mediated by a more aggressive biological variant. Two of these patients with local failure had VHL (20% of VHL patients) and the other had a sporadic variant (also 20%). Of note, the doses of 18 and 20 Gy used in the cases of local failure are at the higher end of the dose range for hemangioblastoma. Because of the low number of events, we were unable to statistically analyze predictors for local failure. The mechanism by which GKRS treats benign tumors is likely multiple: (1) DNA double stranded breaks [15], (2) apoptosis of tumor cells, and (3) targeting of tumor endothelium [16]. As vascular tumors that derive from endothelium, we would thus expect hemangioblastomas to respond well to GKRS. In other benign tumors such as meningioma, greater mitotic rate and brain invasion are variables that predispose to local failure after GKRS [17]. It remains to be shown whether this is also the case for hemangioblastoma.
Seven patients (47%) experienced new hemangioblastoma formation after GKRS, and all of these patients with new hemangioblastoma formation had VHL (70% of VHL patients). This result is not surprising as VHL patients harbor a germline mutation in the Von Hippel Lindau tumor suppressor gene that predisposes to the formation of hemangioblastomas [18]. The ability of GKRS to non-invasively treat multiple lesions either concurrently or serially at time of new hemangioblastoma formation speaks to its importance as a treatment option in hemangioblastoma management. In our study, there were VHL patients with as many as 26 lesions safely treated with GKRS. There were no major toxicities in our series; lesions were removed preemptively due to tumor or cyst enlargement. In spite of the great majority of lesions in the cerebellum, that individual lesions are generally low volume allows for multifocal treatments without treatments being limited by large integral dose of radiation.
On multivariate analysis, non-cystic tumors and those having no prior craniotomy were associated with greater reductions in volume over time. Both of these predictors are intuitive in a clinical context. Cystic lesions can more quickly expand in volume than purely solid tumors due to their secretory nature [1] and can be considered more severe tumors. Similarly, if a prior craniotomy was required for the same lesion, the very fact it is being re-treated with GKRS attests to its more aggressive nature. It is worth noting that GKRS can be an effective treatment for cystic lesions, particularly if they are in a disadvantageous surgical location or exist in large numbers in a single patient [1].
There are several limitations to this study. As a retrospective review, the series may be subject to inherent patient selection biases. In addition, we included a disproportionately higher number of VHL compared to sporadic patients, with an even greater comparative number of lesions. The low number of local failure events precluded our ability to assess for risk factors of local recurrence. Two patients had imaging follow-up as short as 6 months, and therefore did not contribute much data to the freedom from local failure calculation. All included patients were treated at a single institution, which could decrease the generalizability of this study to alternative populations. Our institution is also a tertiary referral center, which could predispose to a selection bias towards more aggressive clinical cases. Finally, it is possible there is an unexplained interaction between tumors of individual patients. Our multivariate model accounts for an unanticipated, unmeasurable relationship between multiple lesions within one patient. The relationship between lesions within a patient was found to not contribute to the variance, but we retained it in the model to account for this possibility.
Conclusion
GKRS is a safe treatment option for hemangioblastomas that arise as sporadic lesions or in conjunction with VHL. In the populations described in this series GKRS favorably changes the clinical course of saltatory growth of untreated hemangioblastomas and results in long-term decrease in hemangioblastoma volume. Non-cystic status, prior craniotomy, and follow-up time from GKRS are all associated with a greater percent reduction in volume from GKRS treatment to last follow-up. Further studies will contribute to a broader clinical picture of how individual lesions and patients respond to treatment.
Supplementary Material
Acknowledgments
Funding Funding was provided by Comprehensive Cancer Center at Wake Forest Baptist Medical Center (Grant No. P30 CA012197–40).
Footnotes
Compliance with ethical standards
Conflict of interest We have no conflict of interest to disclose.
Informed consent informed consent was obtained from all human participants in compliance with our IRB standards.
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s11060-019-03118-x) contains supplementary material, which is available to authorized users.
References
- 1.Beitner MM, Winship I, Drummond KJ (2011) Neurosurgical considerations in von Hippel-Lindau disease. J Clin Neurosci 18:171–180. 10.1016/j.jocn.2010.04.054 [DOI] [PubMed] [Google Scholar]
- 2.Capitanio JF, Mazza E, Motta M et al. (2013) Mechanisms, indications and results of salvage systemic therapy for sporadic and von Hippel–Lindau related hemangioblastomas of the central nervous system. Crit Rev Oncol Hematol 86:69–84. 10.1016/J.CRITREVONC.2012.10.001 [DOI] [PubMed] [Google Scholar]
- 3.Conway JE, Chou D, Clatterbuck RE et al. (2001) Hemangioblastomas of the central nervous system in von Hippel-Lindau syndrome and sporadic disease. Neurosurgery 48:55–63. 10.1097/00006123-200101000-00009 [DOI] [PubMed] [Google Scholar]
- 4.Dornbos D, Kim HJ, Butman JA, Lonser RR (2018) Review of the neurological implications of von Hippel–Lindau disease. JAMA Neurol 75:620 10.1001/jamaneurol.2017.4469 [DOI] [PubMed] [Google Scholar]
- 5.Lonser RR, Butman JA, Huntoon K et al. (2014) Prospective natural history study of central nervous system hemangioblastomas in von Hippel-Lindau disease. J Neurosurg 120:1055–1062. https://doi.org/10.317½014.1.JNS131431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kano H, Shuto T, Iwai Y et al. (2015) Stereotactic radiosurgery for intracranial hemangioblastomas: a retrospective international outcome study. J Neurosurg 122:1469–1478. https://doi.org/10.317½014.10.JNS131602 [DOI] [PubMed] [Google Scholar]
- 7.Park YS, Chang JH, Chang JW et al. (2005) Gamma Knife surgery for multiple hemangioblastomas. J Neurosurg 10.3171/JNS.2005.102.S_SUPPLEMENT.0097 [DOI] [PubMed] [Google Scholar]
- 8.Hanakita S, Koga T, Shin M et al. (2014) The long-term outcomes of radiosurgery for intracranial hemangioblastomas. Neuro Oncol 16:429–433. 10.1093/neuonc/not201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sayer FT, Nguyen J, Starke RM et al. (2011) Gamma Knife radiosurgery for intracranial hemangioblastomas-outcome at 3 years. World Neurosurg 75:99–105. 10.1016/j.wneu.2010.09.032discussion45-8. [DOI] [PubMed] [Google Scholar]
- 10.Silva D, Grabowski MM, Juthani R et al. (2016) Gamma Knife radiosurgery for intracranial hemangioblastoma. J Clin Neurosci 31:147–151. 10.1016/j.jocn.2016.03.008 [DOI] [PubMed] [Google Scholar]
- 11.Rajaraman C, Rowe J, Walton L et al. (2004) Treatment options for von Hippel-Lindau’s haemangioblastomatosis: the role of gamma knife stereotactic radiosurgery. Br J Neurosurg 18:338–342. 10.1080/02688690400004944 [DOI] [PubMed] [Google Scholar]
- 12.Liu A, Kuhn EN, Lucas JT et al. (2015) Gamma Knife radiosurgery for meningiomas in patients with neurofibromatosis Type 2. J Neurosurg 122:536–542. https://doi.org/10.317½014.10.JNS132593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kondziolka D, Lunsford LD, Coffey RJ, Flickinger JC (1991) Stereotactic radiosurgery of meningiomas. J Neurosurg 74:552–559. 10.3171/jns.1991.74.4.0552 [DOI] [PubMed] [Google Scholar]
- 14.Kondziolka D, Lunsford LD, McLaughlin MR, Flickinger JC (1998) Long-term outcomes after radiosurgery for acoustic neuromas. N Engl J Med 339:1426–1433. 10.1056/NEJM199811123392003 [DOI] [PubMed] [Google Scholar]
- 15.Baskar R, Dai J, Wenlong N et al. (2014) Biological response of cancer cells to radiation treatment. Front Mol Biosci 1:24 10.3389/fmolb.2014.00024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Truman J-P, García-Barros M, Kaag M et al. (2010) Endothelial membrane remodeling is obligate for anti-angiogenic radiosensitization during tumor radiosurgery. PLoS ONE 5:e12310. 10.1371/journal.pone.0012310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Attia A, Chan MD, Mott RT et al. (2012) Patterns of failure after treatment of atypical meningioma with gamma knife radiosurgery. J Neurooncol 108:179–185. 10.1007/s11060-012-0828-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Calzada MJ (2010) Von Hippel-Lindau syndrome: molecular mechanisms of the disease. Clin Transl Oncol 12:160–165. 10.1007/s12094-010-0485-9 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.