Abstract
Among women diagnosed with ductal carcinoma in situ (DCIS), we identified factors associated with local invasive cancer (LIC) and regional/metastatic invasive cancer (RMIC) and provide 10-year risks based on clinically relevant factors. We created a retrospective, population-based cohort of 1492 women with an initial diagnosis of DCIS (1983–1996) treated by lumpectomy alone. Histological and molecular markers (Ki67, ER, PR, COX-2, p16, ERBB2) were collected on DCIS cases with a subsequent tumor (DCIS, LIC, or RMIC) and a subsample of frequency-matched controls without subsequent tumors. Competing risks methods were used to identify factors associated with LIC and RMIC and cumulative incidence methods to estimate 10-year risks for combinations of factors. Median follow-up time was 12.6 years (range 0.5–29.5 years). The overall 10-year risk of LIC (11.9 %) was higher than for RMIC (3.8 %). About half of women with initial DCIS lesions are detected by mammography and p16 negative and have a 10-year risk of LIC of 6.2 % (95 % CI 5.8–6.8 %) and RMIC of 1.2 % (95 % CI 1.1–1.3 %). Premenopausal women whose DCIS lesion was p16 positive or p16 negative and detected by palpation had high 10-year risk of LIC of 23.0 % (95 % CI 19.3–27.4 %). Ten-year risk of RMIC was highest at 22.5 % (95 % CI 13.8–48.1 %) for those positive for p16, COX-2, and ERRB2, and negative for ER, but prevalence of this group is low at 3 %. Ten-year risk of LIC and RMIC is low for the majority diagnosed with DCIS. Combinations of molecular markers and method of detection of initial DCIS lesion can differentiate women at low and high risk of LIC and RMIC.
Keywords: Ductal carcinoma in situ, Local invasive cancer, Regional/metastatic invasive cancer, Risk, Recurrence
Introduction
In 2016, more than 60,000 women will be diagnosed with ductal carcinoma in situ (DCIS) [1]. DCIS accounts for 20 % of all newly diagnosed cases of breast cancer in the United States, the vast majority of which are diagnosed by mammography alone [2–5]. By 2020, one million women are expected to be living with the lesion [6]. Due to its low 10-year mortality rate of 1–2.6 %, [4, 7–9] DCIS expert consensus is that the goal of treatment for women with minimal risk of subsequent invasive cancer (IC) should be breast conservation [6]. Estimates of lifetime risk of subsequent IC for women with untreated DCIS range from 30 to 50 % [10–12]. Studies to date have not consistently identified subsets of women with DCIS who are at low (or high) risk of developing local invasive cancer (LIC) versus regional or metastatic invasive cancer (combined, RMIC) [13]. Therefore, women with DCIS are currently treated with mastectomy and radiation at the same rates as those with early stage IC (30 and 50 %, respectively) whose 10-year mortality rates range from 7 to 10 % [14]. Recently it was shown that overall survival rates for women diagnosed with DCIS are highly associated with choice of treatment (either lumpectomy alone, lumpectomy plus radiation, or mastectomy); however, the 10-year disease-free specific survival is not [15]. In fact, the 10-year disease-free specific survival only differs between treatment types by 0.5 %. Thus, the inability to delineate women with DCIS at low (or high) risk of a poor outcome means that the majority is treated aggressively, causing both harm [10, 15–17] (e.g., radiation damage and disfiguring surgery) and significant anxiety [18, 19].
A few studies identified subsets of women with DCIS who can be stratified into risk groups for developing subsequent IC [13]. Silverstein [20] defines three groups based on clinical data, Kerlikowske et al. [21], define four groups using clinical and biomarker data, while Solin et al. [22], define three groups using a formula with seven cancer-related genes and five reference genes. However, to date, no study has separated LIC from RMIC. As breast cancer mortality is typically associated with regional/metastatic events, identifying these women early and accurately is of utmost importance.
We assembled a population-based cohort of 1492 women with an initial diagnosis of DCIS who were subsequently treated by lumpectomy alone. Our primary goal is to define a prognostic signature that accurately delineates those women most likely to have subsequent RMIC from subsequent LIC and from no subsequent IC.
Subjects and methods
Subjects
The study sample and methods are previously described [7, 21]. Briefly, we identified women aged 20 years and older who were diagnosed with DCIS and treated with lumpectomy alone (between January 1, 1983 and December 31, 1996) in one of the nine San Francisco Bay Area counties from the Northern California Surveillance, Epidemiology, and End Results database for a total of 1562 women. We excluded for ineligibility those women treated by mastectomy or by lumpectomy plus radiation within 6 months; with a prior diagnosis of breast cancer; who died within 6 months of initial diagnosis; whose initial DCIS lesion had IC on standardized pathology review; or whose DCIS diagnosis could not be confirmed. Of the eligible women remaining, those who could not be located, refused to participate, or did not speak one of the four languages in which interviews were conducted were also excluded. The final study cohort consisted of 1492 women. This study was reviewed and approved by the UCSF Committee on Human Research. Study participants provided verbal and/or written informed consent.
Telephone interviews and vital status
We obtained demographic information and a breast health history from each woman during a telephone interview, as previously described [7]. Questions included information on any breast procedures, family history of breast cancer, detection method at diagnosis, and menopausal status. For the 212 deceased women, a proxy was interviewed and/or we conducted medical record review. Vital status and underlying cause of death were collected as of December 31, 2010 from the California Department of Vital Statistics and/or death certificates.
Pathology review
For those women with subsequent tumors (case subjects) as well as those without (controls subjects; randomly selected and frequency matched to cases by year of diagnosis), paraffin-embedded tissue samples and/or hematoxylin- and eosin-stained slides of initial DCIS were retrieved from pathology laboratories, as previously described [7, 21]. For case subjects, subsequent tumors were defined as DCIS, LIC (in the ipsilateral breast that contained the initial DCIS lesion), or RMIC (in one or more lymph nodes or in a distant site (e.g., bone, brain, liver, lung, skin) more than 6 months after the initial treatment of DCIS). Study pathologists, blinded to the clinical outcome, reviewed 771 slides of the original DCIS lesions from 153 women who had a subsequent IC (109 LIC and 44 RMIC), 210 who had a subsequent DCIS event or were deceased, and 408 control subjects. In addition to verifying the initial diagnoses as well as subsequent disease, the pathologists determined tumor size, margin width, nuclear grade, and type and quantity of necrosis of the initial DCIS lesion. Women who developed only contralateral breast cancer during the study period (n = 72) were included in the study as control subjects.
As previously described [21], additional immunohistochemical staining was performed to measure the presence of the following proteins: ER, PR, p53, ERBB2, COX-2, Ki67, and p16. For the first three, if 10 % or more tumor cells showed staining of any intensity, ER and PR were present while p53 was overexpressed. ERBB2 was overexpressed when 10 % or more tumor cells showed moderate or strong membrane staining (?2 or higher) [23]. COX-2 was evaluated on a condensed Allred score [24] with each value corresponding to a combination of Allred classes (0 = Allred class 0; 1 = Allred classes 2, 3, and 4; 2 = 5 and 6; 3 = 7 and 8). p16 was evaluated on a scale of 0 to 3, based on the percentage of positively stained tumor cells, irrespective of staining intensity (0 = no staining, 1 = fewer than 25 % of cells stained, 2 = 25–75 %, 3 = more than 75 % of cells stained) [25]. Tissues with a score of at least 2 were overexpressed for COX-2 or p16. For Ki67, a minimum of 1000 tumor cells were counted from at least three high-powered (0.40) fields in areas that showed the highest labeling. Subsequently, the labeling index was expressed as a percentage as the number of positive cells divided by the number of positive plus negative cells. High Ki67 expression was defined as ≥ 10 % tumor cell staining. In the text, proteins are noted as positive or negative as, e.g., p16+ for p-16 positive.
Van Nuys Prognostic Index (VNPI)
The VNPI was developed to identify women with DCIS who could be treated minimally [20]. By combining five tumor characteristics (tumor size, margin width, nuclear grade, age, and comedonecrosis), three subgroups of women can be delineated, each with varying risks of local recurrence after excision. Women are assigned one-point each for tumor size ≤ 15 mm; tumor grade I-II; margin width ≥ 10 mm; and age > 60. Women are assigned two-points each for tumor size 16–40 mm; tumor grade I-II and necrosis; margin width 1–9 mm; and age 40–60. Women are assigned three points each for tumor size > 40 mm; tumor grade III; margin width < 1 mm; and age < 40. A total score of ≤ 6 is low risk; a score of 7–9 is intermediate risk; and a score of ≥ 10 is high risk.
Statistical analysis
Depending on the outcome of interest (i.e., either LIC or RMIC), subsequent LIC, RMIC, DCIS, and death from causes other than breast cancer were considered competing events. To calculate the appropriate hazard ratio, we used the competing risk package cmprsk in the statistical program R (version 3.2.0) [26] to estimate coefficients in the proportional subdistribution hazards regression model [27].
Initially, we looked at the variables in univariate analyses to assess significance with LIC or RMIC. Subsequently, we built multivariate models by combining variables that were found to be univariately significant and/or previously shown to have a biological basis for association with subsequent tumors [25]. To define risk groups, we employed partDSA [28, 29], a recursive partitioning algorithm for finding combinations of variables in an objective manner. Four risk groups were defined separately for subsequent LIC and RMIC.
To estimate the 10-year probability of subsequent IC for the population-based cohort by variables that were only collected for the subsample, we imputed for those in the cohort who were not in the subsample. The imputed values were based on the observed prevalence in the subsample study stratified by case/control status and type of subsequent tumor as previously described [7, 21]. To estimate either the risk of subsequent LIC or RMIC with competing risks, we estimated the cumulative incidence function (CIF) [30]. This process was repeated 2500 times, each time generating a new imputed value. For each time point t, the 2500 CIF survival estimates were averaged and the 95 % confidence interval (CI) was reported as the 0.025 and 0.975 quantiles. All statistical tests were two-sided. P values less than 0.05 were considered statistically significant.
Results
From January 1, 1983 to May 1, 2013, 446 of the 1492 women in the study cohort (30 % overall or 2.4 % per year) developed a subsequent breast tumor [median follow-up = 12.6 years (range 0.5–29.5 years)]. Of the 1492 women, 210 (14.1 %) had subsequent local DCIS, 236 (15.8 %) had subsequent IC, and 212 (14.2 %) died of a cause other than breast cancer. Of women with subsequent IC, 167 (11.2 %) had LIC, 47 (3.2 %) had regional disease, 14 (0.9 %) had metastatic disease, and 8 (0.5 %) had no reported location. Subsequently, 49 (3.3 %) died of breast cancer [of 167 women with LIC, 20 died (12 %); of 47 with regional disease, 15 died (32 %); and all 14 with metastatic disease died (100 %)]. The 10-year risk of subsequent LIC (11.9 %) was higher than the 10-year risk of subsequent RMIC (3.8 %). Across controls and cases, mammogram screening was comparable (88-94 %) as was the percentage of women taking selective estrogen-receptor modulator prior to recurrence for cases and at the time of last follow-up for controls (12-14 %).
Univariate results of baseline factors associated with subsequent LIC versus RMIC
Premenopausal status was associated with increased risk of subsequent LIC (HR = 1.9, 95 % CI 1.1–3.4) compared to women who did not have a subsequent event (Table 1). Race/ethnicity was also associated with incidence of subsequent LIC; where Hispanic women had an increased risk of LIC compared to Caucasian women (HR = 1.8, 95 % CI 1.1–2.9). Method of initial DCIS detection was associated with incidence of subsequent RMIC; where those women with initial DCIS lesions detected by palpation were at higher risk compared with those that were detected by mammography (HR = 2.6, 95 % CI 1.5–4.8). Family history of breast cancer (Table 1) was not associated with incidence of subsequent LIC or RMIC, and neither was oral contraceptive use, postmenopausal hormone therapy, or body mass index (data not shown).
Table 1.
Prevalence of risk factors among women initially treated for ductal carcinoma in situ (DCIS) by lumpectomy alone according to type of subsequent tumor event (local or metatstatic/regional invasive cancer)
| Variable‡ | No subsequent tumor† (N = 829) % (No.) | Local invasive (N = 167) % (No.) | Risk of local invasive HR(95 % CI) | Regional/metastatic invasive (N = 61) % (No.) | Risk of regional/metastic invasive HR (95 % CI) |
|---|---|---|---|---|---|
| Age at diagnosis (years) | |||||
| 20–39 | 2 (21) | 3 (5) | 1.0 (0.4–2.5) | 3 (2) | 0.9 (0.2–3.8) |
| 40–49 | 21 (175) | 25 (41) | 1.3 (0.8–1.9) | 30 (18) | 1.2 (0.7–2.4) |
| 50–59 | 25 (209) | 22 (36) | 1.1 (0.7–1.7) | 16 (10) | 0.7 (0.3–1.4) |
| 60–69 | 23 (187) | 24 (40) | 1.2 (0.7–1.6) | 18 (11) | 0.7 (0.3–1.4) |
| ≥ 70 | 29 (237) | 27 (45) | 1.0 (referent) | 33 (20) | 1.0 (referent) |
| Race and/or ethnicity | |||||
| White | 79 (629) | 75 (114) | 1.0 (referent) | 83 (44) | 1.0 (referent) |
| African American |
6 (49) | 6 (10) | 1.2 (0.6–2.4) | 9 (5) | 1.6 (0.6–4.0) |
| Hispanic | 8 (63) | 12 (18) | 1.8 (1.1–2.9) | 4 (2) | 0.5 (0.1–2.0) |
| Asian | 7 (58) | 7 (11) | 1.0 (0.6–2.0) | 4 (2) | 0.5 (0.1–2.0) |
| Family history of breast cancer‖ | |||||
| Negative | 73 (520) | 66 (84) | 1.0 (referent) | 71 (32) | 1.0 (referent) |
| Positive | 27 (191) | 34 (43) | 1.3 (0.9–1.9) | 29 (13) | 1.0 (0.5–2.0) |
| Menopausal status¶ | |||||
| Postmenopausal | 95 (784) | 90 (144) | 1.0 (referent) | 98 (53) | 1.0 (referent) |
| Premenopausal | 5 (42) | 10 (16) | 1.9 (1.1–3.4) | 2 (1) | 0.2 (0.03–1.8) |
| Detection method | |||||
| Mammography | 82 (580) | 78 (95) | 1.0 (referent) | 65 (30) | 1.0 (referent) |
| Palpation** | 18 (125) | 22 (27) | 1.4 (0.9–2.2) | 35 (16) | 2.6 (1.5–4.8) |
Patients with subsequent DCIS event or who died of cause other than breast cancer are considered competing risks but data not shown (N = 435)
Control subjects were a random sample of women with ductal carcinoma in situ who did not have a subsequent tumor event and were frequency matched by year of diagnosis to the case subjects who were women who had a subsequent tumor event
Of the total 1492 patients (including DCIS subsequent events and dead of cause other than breast cancer), 10 % of subjects had missing data for race/ethnicity; 28 % of subjects had missing data for family history, 0.2 % for menopausal status, and 29 % for detection method
Defined as at least one first-degree relative (mother, sister, or daughter) with breast cancer
Women were considered to be postmenopausal if both ovaries had been removed, if they reported their periods had stopped permanently for reasons other than hysterectomy, if they were currently using postmenopausal hormone therapy, or if they were aged 55 or older
Palpable mass found by the woman or by her physician upon physical examination at the time of diagnosis
Although no measured histopathologic characteristics were associated with subsequent RMIC (Supplementary Table 1), several tended toward an increased risk of subsequent LIC: poor cell polarity, cribriform architectural growth pattern, and psammomatous calcification (data not shown). The VNPI was not associated with increased risk of either type of subsequent IC (Supplementary Table 1).
Risk of subsequent LIC was associated with p16+ (HR = 1.9, 95 % CI 1.2–3.2; Table 2), the combination of p16+COX-2− (HR = 2.3, 95 % CI 1.3–4.1) as well as p16+COX-2+ER+ERBB2− (HR = 1.9, 95 % CI 1.0–3.7). Risk of subsequent RMIC was associated with p16+ (HR = 2.8, 95 % CI 1.4–5.8), as well as: p16+Ki67+ (HR = 2.9, 95 % CI 1.3–7.1); p16+COX-2+Ki67+ (HR = 3.3, 95 % CI 1.2–9.0); and p16+COX-2+ER+ERBB2+ (HR = 3.6, 95 % CI 1.1–11.7); and p16+COX-2+ER−ERBB2+ (HR = 6.5, 95 % CI 2.3–18.2). Univariate associations with subsequent DCIS or death from disease other than breast cancer are shown in Supplementary Tables 2, 3, and 4.
Table 2.
Univariate results of molecular markers associated with type of subsequent tumor event (invasive cancer or ductal carcinoma in situ [DCIS])
| Factor‡ | No subsequent tumor event† (N = 408) % (No.) | Local invasive (N = 109) % (No.) | Risk of local invasive HR† (95 % CI) | Regional/metastatic invasive (N = 44) % (No.) | Risk of regional/metastic invasive HR (95 % CI) |
|---|---|---|---|---|---|
| Estrogen receptor (ER) | |||||
| Negative | 19 (37) | 17 (12) | 0.7 (0.4–1.3) | 31 (10) | 1.7 (0.8—3.6) |
| Positive | 81 (158) | 83 (60) | 1.0 (referent) | 69 (22) | 1.0 (referent) |
| Progesterone receptor (PR) | |||||
| Negative | 21 (35) | 31 (23) | 1.2 (0.8–2.0) | 34 (10) | 1.5 (0.7—3.2) |
| Positive | 79 (131) | 69 (52) | 1.0 (referent) | 66 (19) | 1.0 (referent) |
| p53 | |||||
| Positive | 10 (16) | 13 (9) | 1.0 (0.5–2.0) | 18 (6) | 1.6 (0.7—4.0) |
| Negative | 90 (146) | 87 (62) | 1.0 (referent) | 82 (27) | 1.0 (referent) |
| ERBB2 oncoprotein | |||||
| Positive | 13 (27) | 17 (13) | 0.9 (0.5–1.7) | 29 (10) | 1.9 (0.9—3.9) |
| Negative | 87 (175) | 83 (63) | 1.0 (referent) | 71 (24) | 1.0 (referent) |
| Ki67 | |||||
| Positive§ | 36 (59) | 57 (30) | 1.5 (0.9–2.6) | 61 (11) | 1.8 (0.7–4.3) |
| Negative | 64 (103) | 43 (23) | 1.0 (referent) | 39 (7) | 1.0 (referent) |
| p16 | |||||
| Positive | 31 (42) | 56 (34) | 1.9 (1.2–3.2) | 66 (21) | 2.8 (1.4—5.8) |
| Negative | 69 (91) | 44 (27) | 1.0 (referent) | 34 (11) | 1.0 (referent) |
| Cyclooxygenase-2 (COX-2) | |||||
| Positive | 45 (63) | 41 (24) | 0.9 (0.5–1.5) | 52 (13) | 1.4 (0.7–3.1) |
| Negative | 55 (76) | 59 (35) | 1.0 (referent) | 48 (12) | 1.0 (referent) |
| p16/Ki67 | |||||
| Positive/positive | 9 (12) | 23 (11) | 1.4 (0.7–3.0) | 33 (8) | 2.9 (1.3–7.1) |
| Positive/negative | 20 (25) | 19 (9) | 1.5 (0.7–3.1) | 21 (5) | 1.7 (0.6–4.8) |
| All other groupings | 71 (91) | 57 (27) | 1.0 (referent) | 46 (11) | 1.0 (referent) |
| COX-2/Ki67 | |||||
| Positive/positive | 17 (22) | 20 (11) | 1.0 (0.5–2.0) | 30 (7) | 1.8 (0.7–4.4) |
| All other groupings | 83 (106) | 80 (45) | 1.0 (referent) | 70 (16) | 1.0 (referent) |
| Ki67/COX-2 | |||||
| Positive/positive | 17 (22) | 20 (11) | 1.0 (0.5–2.0) | 30 (7) | 1.8 (0.7–4.4) |
| Negative/positive | 23 (30) | 18 (10) | 0.9 (0.5–1.8) | 17 (4) | 1.0 (0.4–3.0) |
| All other groupings | 59 (76) | 62 (35) | 1.0 (referent) | 52 (12) | 1.0 (referent) |
| p16/COX-2 | |||||
| Positive/negative | 8 (11) | 27 (16) | 2.3 (1.3–4.1) | 21 (6) | 1.7 (0.7–4.1) |
| All other groupings | 92 (124) | 73 (44) | 1.0 (referent) | 79 (22) | 1.0 (referent) |
| p16/COX-2/Ki67 | |||||
| Positive/positive/negative | 11 (13) | 10 (5) | 0.9 (0.3–2.2) | 20 (4) | 2.5 (0.8–7.6) |
| Negative/negative/positive | 17 (20) | 20 (10) | 0.6 (0.2–1.2) | 5 (1) | 0.3 (0.04–2.3) |
| Negative/negative/negative | 29 (33) | 14 (7) | 1.0 (0.8–1.3) | 5 (1) | 1.1 (0.8–1.6) |
| Positive/positive/positive | 7 (8) | 12 (6) | 0.9 (0.4–2.1) | 30 (6) | 3.3 (1.2–9.0) |
| All other groupings | 36 (41) | 45 (23) | 1.0 (referent) | 40 (8) | 1.0 (referent) |
| P16/Cox-2/ER/ERBB2 | |||||
| Pos/pos/pos/neg | 11 (15) | 19 (11) | 1.9 (1.0–3.7) | 4 (1) | 0.4 (0.05–2.6) |
| Pos/pos/neg/neg | 2 (2) | 2 (1) | 0.7 (0.1–5.3) | 8 (2) | 4.6 (0.98–21.8) |
| Pos/pos/pos/pos | 3 (4) | 3 (2) | 0.8 (0.2–3.8) | 11 (3) | 3.6 (1.1–11.7) |
| Pos/pos/neg/pos | 2 (3) | 0 (0) | 0 | 11 (3) | 6.5 (2.3–18.2) |
| All other groupings | 82 (109) | 76 (44) | 1.0 (referent) | 65 (17) | 1.0 (referent) |
Adjusted for diagnosis age
Patients with subsequent DCIS event or who died of cause other than breast cancer are considered competing risks but data not shown (N = 435)
Control subjects were a random sample of women with ductal carcinoma in situ who did not have a subsequent tumor event and were frequency matched by year of diagnosis to the case subjects, who were women who had a subsequent tumor event
Of the 774 patients (including DCIS subsequent events and dead of cause other than breast cancer), 17 % had missing data for ER status, 17 % for PR status, 17 % for p53 status, 17 % for ERBB2, 60 % for Ki67, 60 % for p16, 62 % for COX-2
More than 10 % positive cells
Multivariable results of factors associated with subsequent LIC versus RMIC
DCIS lesions that were detected by palpation and those that were p16+COX-2+ER−ERBB2+ were statistically associated with subsequent RMIC (Table 3). Of the DCIS lesions associated with subsequent RMIC, 35 % were detected by palpation (Table 1) while 11 % were p16+COX-2+ER−ERBB2+ (Table 2). The 10-year risk of subsequent RMIC was highest for those women with an initial DCIS lesion that was p16+COX-2+ER−ERBB2+ (22.7 %; Table 4) and somewhat less elevated for those who detected their lesion by palpation (8.7 %). For LIC, significant associations were p16+ and premenopausal status, and detection by palpation (Table 3). Of the DCIS lesions associated with subsequent LIC, 18 % were detected by palpation and 2 % were p16+ and premenopausal, with corresponding 10-year risk of LIC of 13.6 and 27.8 %, respectively (Table 4).
Table 3.
Hazard ratios (HRs) and 95 % confidence intervals (CIs) from final multivariable models of clinical and histopathological characteristics and molecular markers independently associated with subsequent tumor events
| Variable | Local invasive cancer HR (95 % CI) | Regional/metastatic invasive HR (95 % CI) |
|---|---|---|
| Age at diagnosis (years) | 1.0 (0.98–1.03) | 1.01 (1.0–1.1) |
| Detection by palpation† (vs. mammography) | 2.1 (1.1–4.0) | 2.4 (0.9–6.6) |
| Menopausal Status and p16 | ||
| Post- and negative | Reference | |
| Post- and positive | 1.7 (0.9–3.0) | |
| Pre- and negative | 1.2 (0.2–9.6) | |
| Pre- and positive | 5.8 (1.9–17.6) | |
| p16/COX-2/ER/ERBB2 | ||
| Pos/Pos/Neg/Pos | 5.2 (1.4–19.5) | |
| All other groupings | 1.0 (referent) | |
Univariately significant variables were included in a backward/forward model selection approach
Palpable mass found by the woman or by her physician upon physical examination
Table 4.
Estimate of 10-year risks of local invasive cancer and metastatic/regional invasive cancer for characteristics of women initially diagnosed with DCIS that were independently associated with subsequent invasive cancer
| Variable | 10-year risk of local invasive cancer % (95 % CI) | 10-year risk of regional/metastatic invasive cancer % (95 % CI) |
|---|---|---|
| Overall | 9.96 (8.3, 11.6) | 3.23 (2.3–4.2) |
| Menopausal status | ||
| Premenopausal | 18.4 (17.4–19.2) | Only 2 women |
| Postmenopausal | 9.5 (9.4–9.6) | 3.4 (2.4–4.4) |
| Detection method | ||
| Palpation† | 13.6 (12.3–15.1) | 8.7 (7.2–11.8) |
| Mammography | 8.9 (8.7–9.2) | 2.7 (2.6–2.8) |
| Estrogen receptor (ER) | ||
| Negative | 6.9 (6.1–7.9) | 4.7 (4.2–5.6) |
| Positive | 10.6 (10.2–11.1) | 2.9 (2.7–3.0) |
| ERBB2 oncoprotein | ||
| Negative | 9.0 (8.0–10.2) | 5.2 (4.6–6.0) |
| Positive | 9.9 (9.5–10.3) | 2.8 (2.7–3.0) |
| p16 | ||
| Positive | 14.4 (13.5–15.6) | 5.7 (5.3–6.1) |
| Negative | 6.9 (6.4–7.4) | 1.8 (1.7–2.0) |
| p16/menopausal status | ||
| Negative/post- | 6.6 (6.1–7.2) | 1.9 (1.8–2.1) |
| Positive/post- | 13.4 (12.4–14.5) | 6.0 (5.6–6.5) |
| Negative/pre- | 7.2 (5.8–9.2) | Too few women |
| Positive/pre- | 27.8 (21.7–37.2) | Too few women |
| p16/Ki67 | ||
| Positive/positive | 16.3 (14.6–18.4) | 7.1 (6.3–8.2) |
| Positive/negative | 11.6 (10.1–13.7) | 4.4 (3.8–5.4) |
| All other groupings | 6.9 (6.4–7.5) | 1.8 (1.7–2.0) |
| p16/COX-2 | ||
| Positive/negative | 14.3 (12.9–16.4) | 4.3 (3.8–5.0) |
| All other groupings | 8.4 (8.0–8.9) | 3.0 (2.9–3.1) |
| Ki67/COX-2 | ||
| Positive/positive | 11.4 (10.2–13.1) | 5.4 (4.7–6.6) |
| Negative/positive | 7.0 (6.1–8.2) | 2.7 (2.3–3.3) |
| All other groupings | 10.5 (9.5–10.7) | 2.8 (2.6–3.1) |
| p16/COX-2/Ki67 | ||
| Positive/positive/negative | 11.0 (8.9–14) | 5.3 (4.3–6.8) |
| Negative/negative/positive | 8.1 (7.0–9.4) | 2.1 (1.8–2.6) |
| Negative/negative/negative | 5.5 (4.8–6.6) | 1.2 (0.9–1.6) |
| Positive/positive/positive | 16 (13.5–19.5) | 8.9 (7.4–11.4) |
| All other groupings | 10.1 (9.4–11.0) | 3.1 (2.9–3.5) |
| P16/Cox-2/ER/ERBB2 | ||
| Positive/positive/positive/negative | 15.7 (13.2–18.9) | 3.0 (2.4–4.1) |
| Positive/positive/negative/negative | 8.5 (5.9–12.8) | 10.9 (7.6–15.8) |
| Positive/positive/positive/positive | 10.7 (8.0–14.8) | 10.5 (8.0–14.1) |
| Positive/positive/negative/positive | 4.6 (0–9.9) | 22.7 (13.6–51.3) |
| All other groupings | 9.0 (8.6–9.4) | 2.5 (2.4–2.7) |
| Van Nuys Index | ||
| 4,5,6 | 8.1 (7.3–8.9) | 3.0 (2.8–3.3) |
| 7,8,9 | 10.1 (9.7–10.5) | 3.5 (3.4–3.7) |
| 10,11,12 | 16.6 (12.9–21.8) | 4.2 (3.2–5.6) |
CI confidence interval; COX-2 cyclooxygenase-2; ER estrogen receptor; ERBB2 human epidermal growth factor receptor 2 (HER2/neu-oncoprotein)
Palpable mass found by the woman or by her physician upon physical examination
For subsequent LIC, the 10-year risk was highest for women who were premenopausal (18 %) and those who were premenopausal and p16+ (27.8 %). The 10-year risk of LIC was lowest for women whose initial DCIS lesion was p16−COX-2−Ki67− or p16+COX-2+ER−ERBB2+ (5.5 and 4.6 %, respectively). For subsequent RMIC, the 10-year risk was highest (22.7 %) for women whose initial DCIS lesion was p16+COX-2+ER−ERBB2+ and lowest (1.2 %) for when the initial DCIS lesion was p16−COX-2−Ki67−.
Subsequent LIC or RMIC by risk group
We defined four risk groups for LIC and for RMIC (Tables 5, 6; Fig. 1). For LIC, the four groups were defined using method of detection, p16, and menopausal status (Table 5). Among the 1492 women, 52 % were in the lowest risk group and had a 10-year risk of LIC of 6.2 % (95 % CI 5.8–6.8 %). Ten percent were in the low-risk group with a 10-year risk of 9.5 % (95 % CI 7.9–12 %). One-third were in the intermediate risk group with a 10-year risk of 13.4 % (95 % CI 12.4–14.5 %), respectively. Three percent were in the highest risk group with a 10-year risk of 23 % (95 % CI 19.3–27.4 %). For subsequent RMIC, the four groups were defined using method of detection, p16, COX-2, ER, and ERBB2 (Table 6). Fifty-one percent of the women were in the lowest risk group with a 10-year risk of RMIC of 1.2 % (95 % CI 1.1–1.3 %). Forty-two percent were in the next to lowest risk group with a 10-year risk of 4.3 % (95 % CI 4.0–4.7 %). The intermediate and high-risk groups contained 4 and 3 %, respectively, with a 10-year risk of 12.2 % (95 % CI 9.9–15.3 %) and 22.5 % (95 % CI 13.75–48.1 %), respectively. Statistically significant difference in risk estimates can be inferred from the nonoverlapping confidence intervals. The non-significance between the intermediate and high-risk groups in RMIC can be explained by the similarity in survival curves until 60 months when the two groups separate (Fig. 1b).
Table 5.
Stratification of women into low, intermediate, and high 10-year risk for subsequent local invasive cancer
| Risk category | Description | Prevalence in cohort %† | 10-year risk of local cancer % (95 % CI) |
|---|---|---|---|
| Lowest | p16− and detected by mammo | 52 | 6.2 (5.8–6.8) |
| Low | p16−, detected by palpation, and postmenopausal | 10 | 9.5 (7.9–12) |
| Intermediate | p16+ and postmenopausal | 35 | 13.4 (12.4–14.5) |
| High | p16−, detected by palpation, and premenopausal; or p16 + and premenopausal | 3 | 23 (19.3–27.4) |
Average prevalence estimated among 2500 cohorts of 1490 women with missing measures imputed as described in the statistical section
Table 6.
Stratification of women into low, intermediate, and high 10-year risk for subsequent metastatic/regional cancer
| Risk category | Definition | Prevalence in cohort %† | 10-year risk of met/reg cancer % (95 % CI) |
|---|---|---|---|
| Lowest | p16− and detected by mammogram | 51 | 1.2 (1.1–1.3) |
| Low | p16− and detected by palpation; or p16+COX-2+ER+ERBB2−; or p16+COX-2− | 42 | 4.3 (4.0–4.7) |
| Intermediate | p16+COX-2+ER−ERBB2−; or p16+COX-2+ER+ERBB2+ | 4 | 12.2 (9.9–15.3) |
| High | p16+COX-2+ER−ERBB2+ | 3 | 22.5 (13.8–48.1) |
Average prevalence estimated among 2500 cohorts of 1490 women with missing measures imputed as described in the statistical section
Fig. 1.
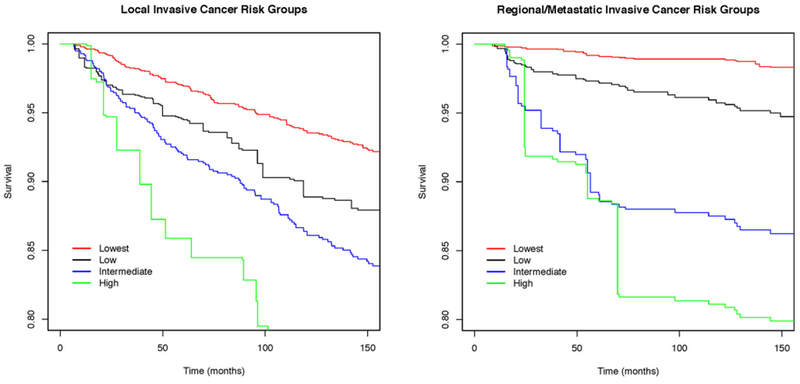
Survival experiences of four risk groups for LIC (a) and RMIC (b). See Tables 5 and 6 for definitions of groups. Curves are averaged cumulative incidence function survival estimates at each of 2500 time points
Discussion
Standard multimodal treatments of DCIS have limited impact on survival since differences in overall survival rates are attributed to comorbidities at diagnosis rather than treatment benefit [15]. Thus, women should be treated less aggressively and research should focus on differentiating women who will benefit from minimal treatment or surveillance only. At present there are no published prognostic models that differentiate LIC from RMIC in women diagnosed with DCIS. Thus, our results have important implications for informing personalized treatment. In a population-based cohort of 1492 women with an initial diagnosis of DCIS who were subsequently treated by lumpectomy alone, we found that the risk of subsequent RMIC is low and we were able to define a prognostic signature to delineate women at low and high risk of RMIC. Women at lowest risk of RMIC (51 % of women with 10-year risk of 1.2 %) had DCIS lesions that were mammographically detected and p16−, while women at highest risk (3 % of women with 10-year risk of 22.5 %) had lesions that were p16+COX-2+ER−ERBB2+.
To date, there are two common approaches to identify women at risk of subsequent tumors: the VNPI [20] and Oncotype DX breast cancer assay [22, 31]. For both, the stratification is based on diagnosis of subsequent local tumor, which includes DCIS and LIC. In our subsample data, the VNPI was not associated with either LIC or RMIC in competing risk models (Supplementary Table 1). In the cohort (with imputation), the VNPI was able to differentiate the 10-year risk of LIC: 8.1 % for the low-, 10.1 % for the intermediate-, and 16.6 % for the high-risk groups. Interestingly, the 10-year risk CI for the VNPI low-risk group does not overlap that of our lowest risk group (Tables 4, 5). The VNPI was not able to differentiate 10-year risk for RMIC (range 3.0–4.2 %; Table 4). The range of 10-year risks for an ipsilateral breast event based on the Oncotype DX assay ranges from 10.6 to 25.9 %; while the range for invasive ipsilateral breast events is from 3.7 to 19.2 %. The latter is similar to the range of our four risk groups for LIC (6.2–23 %). It should be noted that a more complicated algorithm/formula is needed to calculate Oncotype DX scores; while ours can be stratified based on one marker measured in clinical laboratories by immunohistochemical staining, method of detection, and menopausal status. In addition, Oncotype DX does not assess risk of regional or metastatic ICs.
As expected, women are at a higher risk of LIC if their DCIS lesion is positive for ER while they are at a higher risk of RMIC if their lesion is negative for ER. A similar pattern is observed for ERBB2. In combinations of markers, a woman with a DCIS lesion that is p16+COX-2+ER−ERBB2+ is at low risk of LIC but high risk of RMIC. This is consistent with the literature, where a greater proportion of initially diagnosed with LIC tend to be positive for ER and ERBB2; whereas, with RMIC, they are more likely to be negative for ER and ERBB2 [32, 33]; as well as the biologic justification of p16+Cox-2+ being associated with RMIC [25].
Our study has several strengths, first, it is a large, population-based study and includes women diagnosed with DCIS and treated only by lumpectomy. Importantly, the distributions of nuclear grade and detection by palpation, and rate of subsequent invasive cancer in this study, illustrate that the women included do not comprise a low-risk cohort even though they were all only treated by lumpectomy alone. Additional strengths include that this study examines clinical, histopathologic, and molecular markers; has a median follow-up of over 12 years; and has limited selection bias due to recruitment of women from a wide range of hospitals (n = 63). Limitations include the retrospective nature of collection of clinical variables increases potential for recall bias (however, none of the variables in the risk prediction are collected from women’s recall); exclusion of treatments other than lumpectomy prohibits assessing association of variables with response to adjuvant therapies; imputation for missing biomarker data could lead to over-/under-estimation of risk estimates; and a restricted list of biomarkers due to limited tissue available. Validation in independent cohorts is needed.
In summary, we found that the 10-year risk of LIC was substantially higher than that of RMIC and combinations of clinical and biomarker data could delineate risk groups for both LIC and RMIC. The treatment implications for these risk groups are significant, in that those women at high risk of subsequent RMIC may benefit from mastectomy and/or systemic therapy, while those at high risk of subsequent LIC may choose less aggressive treatment, lumpectomy with or without radiation therapy [10, 12, 15]. Those women at low risk of either LIC or RMIC, which comprise about half of women diagnosed with DCIS, could be treated conservatively with lumpectomy only and active imaging surveillance [15, 34].
Supplementary Material
Acknowledgments
National Cancer Institute-funded: Research Grant (R01 CA163687) and University of California, San Francisco Breast Cancer SPORE (P50 CA58207); California Breast Cancer Research Program (2RB-0197). Technical support was received from the UCSF Cancer Center (P30 CA82103), UCSF Cancer Center Tissue and Immunohistochemistry Cores, and Northern California Surveillance, Epidemiology, and End Results Program.
Abbreviations
- CI
Confidence interval
- CIF
Cumulative incidence function
- DCIS
Ductal carcinoma in situ
- IC
Invasive cancer
- LIC
Local invasive cancer
- RMIC
Regional/metastatic invasive cancer
- VNPI
Van Nuys Prognostic Index
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s10549-016-3814-z) contains supplementary material, which is available to authorized users.
Compliance with ethical standards
Conflict of interest The authors have no conflict of interest.
References
- 1.Siegel R, Miller K, Jemal A (2015) Cancer statistics, 2015. CA Cancer J Clin 65(1):5–29 [DOI] [PubMed] [Google Scholar]
- 2.American Cancer Society (2013) Breast cancer facts & figures (2013-2014) In: American Cancer Society, Inc, Atlanta [Google Scholar]
- 3.Ernster VL, Ballard-Barbash R, Barlow WE, Zheng Y, Weaver DL, Cutter G, Yankaskas BC, Rosenberg R, Carney PA, Kerlikowske K et al. (2002) Detection of ductal carcinoma in situ in women undergoing screening mammography. J Natl Cancer Inst 94(20):1546–1554 [DOI] [PubMed] [Google Scholar]
- 4.Kerlikowske K (2010) Epidemiology of DCIS. JNCI 2010(41):139–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Virnig BA, Tuttle TM, Shamliyan T, Kane RL (2010) Ductal carcinoma in situ of the breast: a systematic review of incidence, treatment, and outcomes. JNCI 102(3):170–178 [DOI] [PubMed] [Google Scholar]
- 6.Allegra CJ, Aberle DR, Ganschow P, Hahn SM, Lee CN, Millon-Underwood S, Pike MC, Reed SD, Saftlas AF, Scarvalone SA et al. (2010) National Institutes of Health State-of-the-Science Conference Statement: diagnosis and management of DCIS September 22-24, 2009. JNCI 102(3):161–169 [DOI] [PubMed] [Google Scholar]
- 7.Kerlikowske K, Molinaro A, Cha I, Ljung BM, Ernster VL, Stewart K, Chew K, Moore DH, Waldman F (2003) Characteristics associated with recurrence among women with DCIS treated by lumpectomy. JNCI 95(22):1692–1702 [DOI] [PubMed] [Google Scholar]
- 8.Ernster VL, Barclay J, Kerlikowske K, Wilkie H, Ballard-Barbash R (2000) Mortality among women with DCIS of the breast in the population-based SEER program. Arch Intern Med 160(7):953–958 [DOI] [PubMed] [Google Scholar]
- 9.Fisher B, Dignam J, Wolmark N, Mamounas E, Costantino J, Poller W, Fisher ER, Wickerham DL, Deutsch M, Margolese R et al. (1998) Lumpectomy and radiation therapy for the treatment of intraductal breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-17. J Clin Oncol 16(2):441–452 [DOI] [PubMed] [Google Scholar]
- 10.Welch HG, Woloshin S, Schwartz LM (2008) The sea of uncertainty surrounding ductal carcinoma in situ—the price of screening mammography. JNCI 100(4):228–229 [DOI] [PubMed] [Google Scholar]
- 11.Allred DC (2010) Ductal carcinoma in situ: terminology, classification, and natural history. JNCI Monogr 2010(41):134–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sanders ME, Schuyler PA, Dupont WD, Page DL (2005) The natural history of low-grade DCIS of the breast in women treated by biopsy only revealed over 30 years of long-term follow-up. Cancer 103(12):2481–2484 [DOI] [PubMed] [Google Scholar]
- 13.Smith BD (2015) When is good enough really good enough? Defining the role of radiation in low-risk DCIS. JCO 33(7):686–691 [DOI] [PubMed] [Google Scholar]
- 14.Ries L, Eisner M, Kosary C, Hankey B, Miller B, Clegg L, Edwards B (2000) SEER cancer statistics review, 1973-1997. In: Bethesda MD: (ed) National Cancer Institute [Google Scholar]
- 15.Worni M, Akushevich I, Greenup R, Sarma D, Ryser MD, Myers ER, Hwang ES (2015) Trends in treatment patterns and outcomes for ductal carcinoma in situ. J Natl Cancer Inst 107(12):djv263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Owen D, Tyldesley S, Alexander C, Speers C, Truong P, Nichol A, Wai ES (2013) Outcomes in patients treated with mastectomy for ductal carcinoma in situ. Int J Radiat Oncol Biol Phys 85(3):e129–e134 [DOI] [PubMed] [Google Scholar]
- 17.Harris JR, Morrow M (2009) Clinical dilemma of DCIS. JCO 27(32):5303–5305 [DOI] [PubMed] [Google Scholar]
- 18.Partridge A, Adloff K, Blood E, Dees EC, Kaelin C, Golshan M, Ligibel J, de Moor JS, Weeks J, Emmons K et al. (2008) Risk perceptions and psychosocial outcomes of women with ductal carcinoma in situ: longitudinal results from a cohort study. J Natl Cancer Inst 100(4):243–251 [DOI] [PubMed] [Google Scholar]
- 19.Rakovitch E, Franssen E, Kim J, Ackerman I, Pignol J-P, Paszat L, Pritchard K, Ho C, Redelmeier D (2003) A comparison of risk perception and psychological morbidity in women with ductal carcinoma in situ and early invasive breast cancer. Breast Cancer Res Treat 77(3):285–293 [DOI] [PubMed] [Google Scholar]
- 20.Silverstein M (2003) The USC/Van Nuys Prognostic Index for DCIS of the breast. Am J Surg 186(4):337–343 [DOI] [PubMed] [Google Scholar]
- 21.Kerlikowske K, Molinaro AM, Gauthier ML, Berman HK, Waldman F, Bennington J, Sanchez H, Jimenez C, Stewart K, Chew K et al. (2010) Biomarker expression and risk of subsequent tumors after initial DCIS diagnosis. JNCI 102(9):627–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Solin LJ, Gray R, Baehner FL, Butler SM, Hughes LL, Yoshizawa C, Cherbavaz DB, Shak S, Page DL, Sledge GW et al. (2013) A multigene expression assay to predict local recurrence risk for ductal carcinoma in situ of the breast. JNCI 105(10):701–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tamimi R, Baer H, Marotti J, Galan M, Galaburda L, Fu Y, Deitz A, Connolly J, Schnitt S, Colditz G et al. (2008) Comparison of molecular phenotypes of ductal carcinoma in situ and invasive breast cancer. Breast Cancer Res 10(4):R67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Allred D, Harvey J, Berardo M, Clark G (1998) Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol 11(2):155–168 [PubMed] [Google Scholar]
- 25.Gauthier ML, Berman HK, Miller C, Kozakeiwicz K, Chew K, Moore D, Rabban J, Chen YY, Kerlikowske K, Tlsty TD (2007) Abrogated response to cellular stress identifies DCIS associated with subsequent tumor events and defines basal-like breast tumors. Cancer Cell 12(5):479–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gray R (2014) cmprsk: Subdistribution analysis of competing risks In: R package version 2.2, 7 edn [Google Scholar]
- 27.Fine J, Gray R (1999) A proportional hazards model for the subdistribution of a competing risk. JASA 5:190–207 [Google Scholar]
- 28.Lostritto K, Strawderman RL, Molinaro AM (2012) A partitioning deletion/substitution/addition algorithm for creating survival risk groups. Biometrics 68(4):1146–1156 [DOI] [PubMed] [Google Scholar]
- 29.Molinaro AM, Lostritto K, van der Laan M (2010) partDSA: deletion/substitution/addition algorithm for partitioning the covariate space in prediction. Bioinformatics 26(10):1357–1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pepe MS, Mori M (1993) Kaplan-Meier, marginal or conditional probability curves in summarizing competing risks failure time data? Stat Med 12(8):737–751 [DOI] [PubMed] [Google Scholar]
- 31.Rakovitch E, Nofech-Mozes S, Hanna W, Baehner F, Saskin R, Butler S, Tuck A, Sengupta S, Elavathil L, Jani P et al. (2015) A population-based validation study of the DCIS score predicting recurrence risk in individuals treated by breast-conserving surgery alone. Breast Cancer Res Treat 152(2):389–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Phipps A, Buist DM, Malone K, Barlow W, Porter P, Kerlikowske K, Li C (2011) Family history of breast cancer in first-degree relatives and triple-negative breast cancer risk. Breast Cancer Res Treat 126(3):671–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li C, Beaber E, Tang M-T, Porter P, Daling J, Malone K (2013) Reproductive factors and risk of estrogen receptor positive, triplenegative, and HER2-neu overexpressing breast cancer among women 20–44 years of age. Breast Cancer Res Treat 137(2):579–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Esserman L, Shieh Y, Thompson I (2009) Rethinking screening for breast cancer and prostate cancer. JAMA 302(151):1685–1692 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


