Abstract
We present use of a synthetic, injectable matrix-metalloproteinase (MMP)- responsive, bioactive hydrogel as an in situ forming scaffold to deliver thymosin β4 (Tβ4), a pro-angiogenic and pro-survival factor, along with vascular cells derived from human embryonic stem cells (hESC) in ischemic injuries to the heart in a rat model. The gel was found to substitute the degrading extracellular matrix in the infarcted myocardium of rats and to promote structural organization of native endothelial cells, while some of the delivered hESC-derived vascular cells formed de novo capillaries in the infarct zone. Magnetic resonance imaging (MRI) revealed that the microvascular grafts effectively preserved contractile performance 3 d and 6 wk after myocardial infarction, attenuated left ventricular dilation, and decreased infarct size as compared to infarcted rats treated with PBS injection as a control (3 d ejection fraction, + ~7%, P<0.001; 6 wk ejection faction, + ~12%, P<0.001). Elevation in vessel density was observed in response to treatment, which may be due in part to elevations in human (donor)-derived cytokines EGF, VEGF and HGF (1 d). Thus, a clinically relevant matrix for dual delivery of vascular cells and drugs may be useful in engineering sustained tissue preservation and potentially regenerating ischemic cardiac tissue.
Keywords: Hydrogel, Biomimetic material, Matrix metalloproteinase, Cardiac tissue engineering, Vascular grafts, Stem cell
INTRODUCTION
Coronary heart disease, including myocardial infarction, is the leading cause of death worldwide, with estimated health cost of $165 billion in 2009 in the USA alone [1]. A major reason for the high mortality rate is the limited intrinsic regeneration capacity of the adult heart after myocardial infarction accompanied by the shortage of donor hearts for transplantation [2].
Results from recent clinical studies with infarct patients showed improved cardiac function following injection of adult stem cells from different sources including bone marrow and peripheral blood [3,4]. These beneficial effects were mainly attributed to vascular paracrine signaling effects. Despite these promising results, the improvement has been modest (e.g. ventricular ejection fraction < 5%) [3,4]. Most of the injected cells do not integrate into the host cardiac tissue or die within the first days (~ 90%). In addition, the use of adult stem cells may be impaired by ageing or disease (e.g. diabetes) and thus reduce their therapeutic application [5].
Human embryonic stem cells (hESCs) are a potentially unlimited source of cells to preserve or restore cardiac function after myocardial infarction. The first hESC-based preclinical animal studies for repairing infarcted cardiac tissue used cardiomyocytes [6,7] or vascular cells [8,9]. Although promising results have been achieved, hESC-based cardiomyocyte therapy remains complex due to the difficulty of obtaining electrophysiological integration into the host tissue [10], the lack of which can lead to life threatening arrhythmias. hESC vascular-based therapy has shown beneficial short-term efficacy (below 28 d), however requires further improvement in cell delivery and engraftment [6–9].
Here, we explored the potential of hESCs as a source for microvascular cells to preserve cardiac morphology and function following myocardial infarction. We derived both endothelial- and smooth muscle-like cells (ELCs, SMLCs) from hESCs. To increase engraftment, the ELCs and SMLCs were embedded into synthetic injectable hydrogels as in situ forming scaffolds along with thymosin β4 (Tβ4) [11], a pro-angiogenic and pro-survival factor (Fig. S1). These hydrogels are formed by in situ crosslinking of vinyl sulfone-functionalized branched poly(ethylene glycol) (PEG) with a peptide that contains two cysteine residues flanking a matrix metalloproteinase (MMP) substrate site [12]. The hydrogels are bioactive, in that they display adhesion ligands for integrins αvβ3 and α5β1, which are relevant in cardiovascular development and maintenance [13], and, due to the nature of the crosslinking peptide, can be remodeled in response to elevated MMP-levels that occur after myocardial infarction [14]. The synthetic origin enables precisely reproducible manufacturing, and the gels do not provoke adverse immunogenic reactions, as biological matrices may [15]. At 3 d and 6 wk after induction of myocardial infarction in rats, we assessed the potential of these microvascular grafts for preservation of cardiac structure and function.
MATERIALS AND METHODS
Synthesis of PEG-vinylsulfone and peptides (RGDSP, MMP-substrate, Tβ4)
PEG-vinylsulfone (8-arm, Mw = 40,000 g/mol) was synthesized adapting our previous protocol [16]. Synthesis, purification and characterization of the integrin ligand peptide, substrate for MMP and Tβ4 peptide are described in the Supporting Information.
Formation of PEG-hydrogels
Gel formation was performed under physiological conditions as we have previously described [16]. In some cases, Tβ4 40 μg/mL gel) was physically entrapped into PEG-hydrogels by mixing it into the PEG-precursor solution before gelation.
Fluorescent PEG-gels were prepared by adding 2-biotinaminoethanethiol to the gel precursor solution at a concentration of 100 μM (Supporting Methods). The Michael-type addition reaction between the 2-biotinaminoethanethiol and the vinylsulfone groups on the multiarm PEG was continued for 10 min at room temperature in triethanolamine buffer. Incorporation of 2-biotinaminoethanethiol into the hydrogel network was confirmed by staining with streptavidin-Cy3 (Invitrogen), and visualization by fluorescence microscopy.
Isolation and characterization of hESC-derived vascular cells
SMLCs and ELCs were generated according to methodologies previously reported by us [17,18], and further described in the Supporting Information.
Quantitative RT-PCR analysis
To extract total RNA from the rat hearts, the infarcted area, the border zone and the non-infarcted area were manually separated and each homogenized in Trizol Reagent (Invitrogen) following the supplier’s protocol. Total RNA was quantified by a nanodrop spectrometer (Nanodrop 1000, Thermo Scientific). Reverse transcription was performed on 1 μg total RNA by using TaqMan Reverse Transcription Reagents (Applied Biosystems/Roche Molecular Systems) as we recently reported [17]. Quantification of target genes was performed relatively to the reference gene β-actin: relative expression = 2[−(CtSample – Ct/β-actin)] (Table S1). The mean minimal cycle threshold values (Ct) were calculated from quintuplicate reactions.
Infarct Model and Sample Injection
All procedures were approved by the Institutional Animal Care and Use Committee at the Massachusetts Institute of Technology. Male nude rats (NIHRNU, Taconic, 200–250g, n = 68) were anesthetized with 2% isoflurane in 1 L/min oxygen, intubated with a 16g, 2” catheter (Terumo) and mechanically ventilated (Inspira, Harvard Apparatus). The heart was exposed by open thoracotomy between the 3rd and the 4th rib. The left anterior descending (LAD) coronary artery was ligated with a 5–0 silk suture (Ethicon). The infarct was confirmed by the blanching of the infarcted left ventricle as well as a lack of local wall motion. One hour after inducing myocardial infarctions, the animals were randomized for treatment: hESC-derived ELC (0.66 million) and SMLC (0.33 million) in the PEG-hydrogels containing 2.5 μg Tβ4 (n = 10), PEG-hydrogels with 2.5 μg Tβ4 (n = 8), PEG-hydrogels alone (n = 9), or the negative control sample (n = 7), PBS (pH 7.4) (injection volumes: 60 μL). The gel precursor solutions with the ELC/SMLC and/or Tβ4 were mixed in the surgery room around 1 min prior to injection and were then injected across the infarct zone using a tuberculin syringe with a 27g needle (Tyco Healthcare). Positive end-expiratory pressure was applied to re-inflate the lungs. Muscle and skin layers were closed in a standard fashion using 4–0 and 5–0 Vicryl sutures, respectively (both Ethicon). No immunosuppressants were administered. The rats were euthanized with potassium chloride (KCl, 1–2 mmol/kg body weight) i.v. under general anesthesia (2% Isoflurane in 1 L/min oxygen). The surgeon was blinded to treatment group assignment. Cardiac function of the rats was analyzed at 3 d and 6 wk after injection of the gel samples by high-resolution MRI using a small-animal 4.7 T scanner (Bruker) with cardiac gating. Investigators performing MRI acquisition and analysis were blinded to treatment group assignment. MRI protocol is described in the Supporting Information.
Histology and Microscopy
After euthanizing the rats (see above), the hearts were explanted and embedded in O.C.T. (Tissue Tek). The samples were frozen and sectioned with a cryostat (ThermoShandon) at −15° C. Transverse sections (5 μm - 15 μm thickness) were stained with hematoxylin and eosin (PolyScientific) and Masson’s Trichrome (Rowley) to determine the infarct area, or after permeabilizing (in some cases) for 60 min at 4° C (1% BSA, 0.3% TritonX-100 in PBS, pH 7.4) stained with the following primary antibodies: anti-human PECAM1 (1:20, Dako), anti α-SMA (1:50, Sigma), anti-human nuclei (1:50, Millipore), anti-rat endothelial cell antibody-1 (RECA-1, 1:100, Abcam), and anti-rat/human PECAM-1+ (1: 100, Abcam). As a secondary antibody, goat anti-mouse IgG Cy5 conjugate was used (1:500, Invitrogen) followed by DAPI nuclear staining (Invitrogen). The procedures for imaging and subsequent measurements are described in the Supporting Information.
Cytokine expression
To analyse the expression of rat and human cytokines in the infarcted rats hearts, a Procarta Cytokine assay was used (Affymetrix). The infarcted area of the left ventricle was cut off, homogenized and prepared according to the manufacturer’s protocol (n = 3) for both control and sample injection, per each time point). Results were normalized to the respective baseline control at 0 d, and results are shown relative to the response in the control group treated with PBS. To assess the content of Thymosin β4, a peptide immunoassay was applied using the same homogenized samples (Peninsula Laboratories). The samples were prepared and analyzed according to the manufacturer’s protocol.
Statistical analysis
Normal distribution was tested using the method of Kolmogorov and Smirnov. To assess equal standard deviations of the groups, Bartlett’s test was applied. For analysis involving three or more groups ANOVA was used followed by a Student-Newman-Keuls post hoc test. For analysis of time course changes, a paired Student’s t-test analysis was performed. Results are presented as means ± SEM unless otherwise stated. Comparative analysis was done using GraphPadPrism software. Differences between data sets were considered statistically different when P < 0.05.
RESULTS
Upregulation of MMP-2 and MMP-9 after myocardial infarction
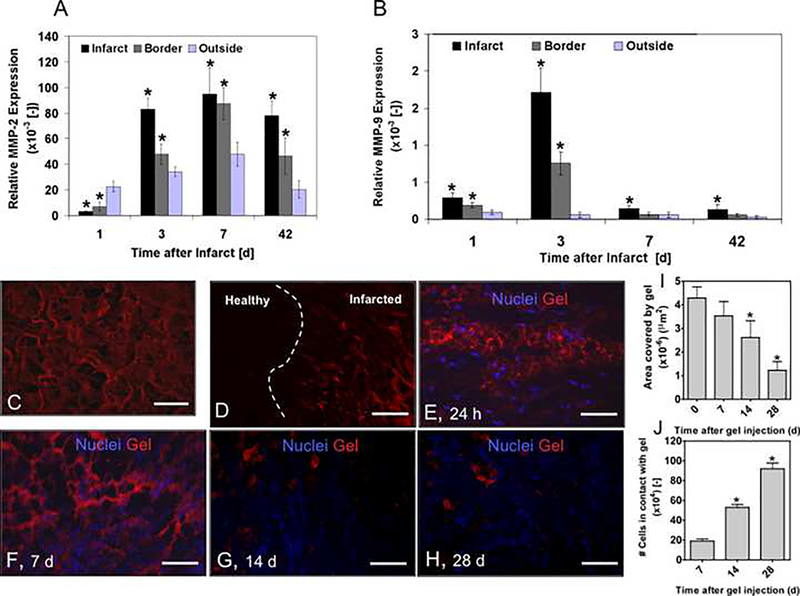
We have previously demonstrated that MMP-2 and MMP-9 trigger degradation of the employed gel in vitro [19]. To examine the in vivo potential of the synthetic MMP-responsive hydrogels for biomolecular and cellular delivery, we first analyzed the temporal gene expression profile of MMP-2 (Fig. 1A) and MMP-9 (Fig. 1B) in infarcted rat hearts by quantitative RT-PCR.
Figure 1. Temporal profiles of MMP-2 and MMP-9 gene expression and gel degradation after myocardial infarction in rats.
(A, B) MMP-2 (A) and MMP-9 (B) gene expression. Results are Mean ± SD, n=4. *denotes statistical significance, P < 0.05. (C) Fluorescently labeled gel in vitro. The gel was labeled with 2-biotinaminoethanethiol/streptavidin-Cy3. Scale bar: 50 μm. (D) In vivo, the gel (red) infiltrates the degrading extracellular matrix in the infarcted cardiac tissue (dashed white line depicts border zone between infarcted and non-infarcted myocardium). Scale bar: 500 μm. (E) At 24 h after injection into the infarcted rat hearts. Attachment of individual rat cells on the bioactive gels. Scale bar: 25 μm (F) Rat cell infiltration and gel degradation at 7 (G), 14 (H) and 28 d. No gel was detected at d 42. Scale bar: 50 μm. (I) Quantified gel degradation. (J) Increased cell attachment while gel was degrading over time. In I and J, results are Mean ± SD, n= 3; * denote significant differences, * P < 0.05.
The results show two distinct profiles for MMP-2 and MMP-9. While MMP-2 demonstrated prolonged, significantly higher expression between 3 and 42 d (around 30-fold from 1 d level), MMP-9 expression peaked at 3 d (around 5-fold from 1 d level), before leveling off at 7 d after myocardial infarction.
Degradation of the MMP-responsive gels in rats with myocardial infarctions
To assess gel degradation in vivo, imaging of biotinylated gels with fluorescently-conjugated streptavidin was employed (Fig. 1C). The labeled gels were injected as liquid precursors into infarcted rats (two liquid components of the gel were mixed immediately prior to injection) followed by in situ gel formation in the infarcted area within a few minutes. Gel degradation was assessed by fluorescence microscopy. At 24 h, the gel had partially substituted the degrading extracellular matrix of the infarcted myocardium (Fig. 1D), and individual rat cells were found within the gel (Fig. 1E). Over time the gel was remodeled (Fig. 1E–H), leaving around 25% of the injected gel at 28 d (Fig. 1I). As the gel degraded, the number of cells in contact with gel significantly increased (Fig. 1J). By 42 d, the gel could not be detected anymore.
Release profile of Tβ4 from MMP-responsive gels in rats with myocardial infarctions
To evaluate the in vivo delivery of Tβ4 by MMP-responsive hydrogels, a gel-precursor solution containing the biomolecule (2.5 μg of Tβ4 per 60 μL of gel) was injected into the infarcted hearts. At 1 d, 3 d and 6 wk the hearts were digested and the concentration of Tβ4 was assessed by ELISA (Fig. S2). Our results show that Tβ4 was released from the gel over a few days, leaving around 20% of the initial amount at 3 d, and around 5% at 6 wk.
Improved cardiac performance and structure after injection of MMP-responsive gel with encapsulated hESC-derived vascular cells and Tβ4
We next examined delivery of hESC-derived ELCs and SMLCs combined with Tβ4 to preserve cardiac structure and function after myocardial infarction. As a first component, gel delivery alone was investigated. The bioactive MMP-responsive gel displaying key characteristics of collagenous extracellular matrices [15] was injected into the infarcted hearts. As a second component, the same bioactive MMP-responsive gel served as a biomolecule delivery vehicle for physically entrapped Tβ4 (2.5 μg per 60 μL of gel). Finally, in its full implementation, gel containing Tβ4 (2.5 μg) and a mixture of ELCs (0.66 × 106) and SMLCs (0.33 × 106) was injected (Fig. S3).
To examine the in vivo effects of these treatments on preservation of cardiac structure and function, 6 wk animal studies were performed using rats with surgically-induced chronic myocardial infarction. Myocardial infarctions were induced by permanent ligation of the left anterior descending coronary artery (LAD) (mortality rate: ~7%). The gel precursor solutions with the ELCs/SMLCs and/or Tβ4 were mixed in the surgery room and injected into the infarct area 1 h following infarction.
Magnetic resonance imaging (MRI) was used to provide in vivo analysis of cardiac structure and function (Fig. 2A–E and Fig. S4). We assessed cardiac performance, left ventricular dilation and infarct size at 3 d and 6 wk after inducing the infarcts, enabling the repetitive monitoring of cardiac structure and function of the same rats. Treatment conditions and the PBS injection as a control were randomly assigned, and rats were excluded from the study after the 3 d MRI measurement if infarcts were smaller than 10% of the left ventricle or if rats did not show infarcts after histological analysis [6].
Figure 2. Cardiac performance after myocardial infraction.
(A) MRI analysis (for n=11 infarcted rats receiving PBS, n=9 infarcted rats receiving gel, n=8 infarcted rats receiving gel + Tβ4, and n=10 infarcted rats receiving gel + Tβ4 + cells) at 3 d and 6 wk after inducing myocardial infarctions. Dashed lines mark infarcted areas. (B) Infarct size. (C) End-diastolic volume (EDV). (D) End-systolic volume (ESV). (E) Ejection fraction (EF). Results are presented as Mean ± SEM. * denote significant differences within time point, * P < 0.05, ** P < 0.01, *** P < 0.001; # denote significant differences between time point comparing the respective control/treatment groups, # P < 0.05, ## P < 0.01, ### P < 0.001.
We did not detect significant differences in infarct size at 3 d between the 3 treatment groups and the control group, which received PBS injection (Fig. 2B). At 6 wk, significantly decreased infarct size was found for the treatment group with the combined Tβ4 and cell delivery as compared with the control group. The other treatment conditions showed significantly reduced infarct size at 6 wk as compared to their respective treatment group at 3 d.
To investigate the effects of the three treatments on left ventricular dilation, the end-diastolic volume (EDV) was analyzed (Fig. 2C). Attenuated left ventricular dilation was detected between the combined Tβ4 and cell delivery treatment and the PBS-treated control group at 3 d as well as at 6 wk. No other pair-wise comparisons showed statistically significant attenuation of left ventricular dilation. Significant left ventricular dilation was found for all conditions at 6 wk as compared to their respective group at 3 d representing the normal course of remodeling after myocardial infarction [20].
To further examine cardiac function, the left ventricular end-systolic volume (ESV) was analyzed at 3 d and 6 wk (Fig. 2D). The results show a significantly decreased ESV for all treatment conditions already at 3 d, as compared to the controls. Importantly, at 6 wk, we report significantly reduced ESV for all treatment conditions as compared to the control group. In addition, at 6 wk the combined Tβ4 and cell delivery condition differed significantly from the gel alone condition as well as the condition with the Tβ4 delivery. Furthermore, significantly smaller ESV was detected for the Tβ4 delivery condition as compared to the gel alone condition, indicating beneficial effects of the released biomolecule. Significantly higher ESV was demonstrated for all conditions as compared to their counterparts at 3 d, which reflects the normal remodeling of the post-infarcted heart.
Finally, the ejection fraction (EF) was assessed (Fig. 2E). Significant differences were found at 3 d between each treatment condition and the PBS-treated control group, as well as for the 6 wk time point (with ejection fraction being preserved by 12% relative to the PBS-treated control group). Furthermore, the combined Tβ4 and cell delivery condition differed significantly from the gel alone and Tβ4 delivery conditions. For all analyses, the observed experimental variance (reflected in the S.E.M.) was similar to those reported in other studies using surgically-induced myocardial infarctions in rats [6].
Preserved myocytes and neovascularization
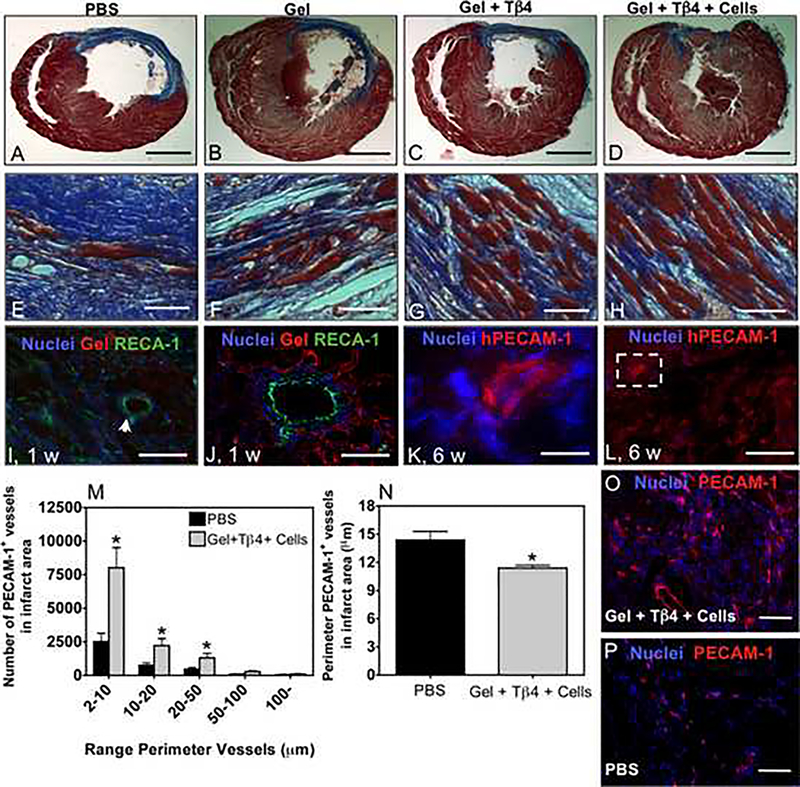
To better understand the functional improvement as assessed by MRI, we histologically analyzed the rat hearts at 6 wk to evaluate cardiomyocyte organization and potential neovascularization (Fig. 3). Trichrome staining enabled the analysis of the structure of the preserved force-generating cardiomyocytes and of the collagen content deposited in the infarcted area (Fig. 3A–H). The cardiomyocytes appeared to be better preserved and aligned in bundles in hearts treated with the combined Tβ4 and cell delivery (Fig. 3D,H), as compared to the rats treated with PBS (Fig. 3A,E), gel alone (Fig. 3 B,F) or Tβ4 delivery alone (Fig. 3C,G).
Figure 3. Effects of different delivery strategies on cardiac structure 6 weeks after injection.
(A-D) Representative trichrome stains of transverse heart sections. Collagen in the infarct areas is shown in blue, whereas myocytes are in red. Animals were treated by (A) PBS, (B) gel, (C) gel + Tβ4 and (D) gel + Tβ4 + cells. Low (A-D) and high (E-H) magnification. Bar corresponds to 2.5 mm (A-D) and 100 μm (E-H). (I) Bioactive MMP-responsive hydrogel supports structural aligning of rat cardiomyocytes 24 h after inducing myocardial infarctions. Dashed white line separates regions with gel (left) and without gel (right). Scale bar: 50 μm. (J) Gel supports rat vessel formation (arrow) involving rat endothelial cells at 7 d after inducing myocardial infarctions. Scale bar: 50 μm. (K) Rat arteriole integrated in bioactive MMP-responsive hydrogel at 7 d after inducing myocardial infarctions. Scale bar: 25 μm. (L-N) Human PECAM-1+ cells contribute to the formation of capillary-like networks. Bar corresponds to 50 μm (L and N) or 10 μm (M). (O) Number of PECAM-1+ (rat and human) vessels within the infarcted area. (P) Average diameter of PECAM-1+ vessels within infarcted area. In O and P,. results are presented as Mean ± SD, n= 3. * denote significant differences * P < 0.05. (Q) Vessels within infarcted area 42 d after injection of gel, drug and hESC-derived ELC and SMLC. Scale bar: 100μm. (R) Vessels within infarcted area 42 d after injection of PBS only. Scale bar: 100μm.
To examine whether the hydrogel is able to promote structural organization of the host endothelial cells and cardiomyocytes, the hearts were histologically analyzed with biotin/streptavidin-Cy3 labeled gels 24 h after injection in the infarcted hearts (Fig. 3I–K) and after 1 wk. The gel was able to structurally support the native cardiomyocytes aligning in muscle-like bundles as well as infiltration of native arterioles with lumen-forming endothelial cells (Fig. 3J,K). When evaluating the blood vessel density within the infarct (staining both rat and human cells for PECAM+), our treatment resulted in higher numbers of small vessels (< 50 μm) than in infarcted hearts treated with PBS injection (Fig. 3O-R). To further assess the neovascularization potential of the hESC-derived vascular cells, we histologically analyzed the combined delivery of Tβ4 and cells by using an antibody against human PECAM-1. De novo capillary-like structures were found, mainly at the borders of the infarct but also to a lesser extent within the infarct area (Fig. 3L–N).
Vascular and pro-survival factors support preservation of cardiac structure and function
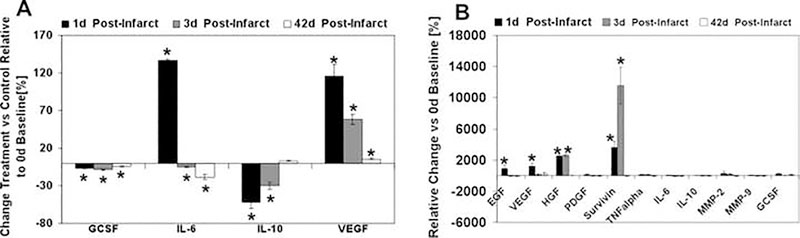
To analyze the underlying mechanisms potentially mediating cardiac preservation in the treatment conditions described above, we assessed the expression of relevant vascular and pro-survival factors over time until 6 wk (Fig. 4A,B). As compared to the PBS-treated control group, animals receiving the combined treatment with hESC-derived vascular cells and Tβ4 showed a 100% upregulation of rat VEGF at 1 d, around 50% at 3 d and still around 10% at 42 d (Fig. 4A). Similarly, human vascular cytokines were significantly upregulated 1 d after injection (VEGF, EGF, HGF), however were not detectable at 3 d after infarction/injection, except HGF. Human survivin, an apoptosis inhibitor [21], was strongly up-regulated at 1 d (30×), and even more at 3 d (120×) (Fig. 4B).
Figure 4. Pro-survival factors in the infarcted left ventricle.
(A) Expression of rat (host) growth factors and cytokines over time as evaluated by a Multiplex assay. (B) Expression of human (donor) growth factors (VEGF, EGF, HGF, PDGF, TNFa, GCSF), apoptosis inhibitors (survivin), enzymes (MMP-2 and MMP-9) and cytokines (IL-6 and IL-10) as assessed by a Multiplex assay. Results are presented as Mean ± SD, n= 3. * denote significant differences * P < 0.05.
DISCUSSION
Our goal was to preserve tissue morphology and function following myocardial infarction using hESC-based microvascular grafts delivered in a bioactive matrix containing a pro-angiogenic and pro-survival factor. We used a combination of ELCs and SMLCs in the cellular transplant, since both cell types are needed to form stable microvessels [22]. Both hESC-derived vascular-like cells [21,22] were embedded into a synthetic bioactive carrier that has been designed to be responsive to the elevated MMP-levels occurring after myocardial infarction [14,23] thereby facilitating remodeling by cells in the infarct zone as well as the encapsulated vascular-like cells and slow release of the entrapped Tβ4 peptide as the matrix degrades. Tβ4 was used in this study due to its cytoprotective properties on vascular cells and cardiomyocytes in ischemic environments, as well as its ability to induce neovascularization processes and reduce inflammatory responses [11].
While our results demonstrate that the bioactive gel alone can enhance cardiac function, the highest efficacy is provided by the combined delivery of Tβ4 and cells in the gel scaffold. It appears that after 6 wk the host cardiomyocytes are better aligned in muscle-like bundles in the infarct zone in the cell-treated rats (Fig. 3H), as compared to PBS-treated control rats (Fig. 3E). This could arise from the gel providing structural support (Fig. 3B,F), the hESC-derived vascular cells contributing to preserving the viability of host cardiomyocytes, either by the formation of capillary-like vessels (Fig. 3L–N) and/or stabilization of host vessels (Fig. 3J,K), or it could originate from the secretion of paracrine factors from the transplanted vascular-like cells (Fig. 4B) or the native rat cells in response to the hESC-derived vascular cells (Fig. 4A).
Our results suggest that the improvement in cardiac function by the combined delivery of Tβ4 and cells in the gel scaffold is mainly mediated by a paracrine effect and a neovascularization process. Our immunohistochemical data shows higher density of microvessels in hearts treated by gels containing vascular cells and Tβ4 than in PBS-treated hearts. Although most of the neovessels are from rat origin, some hESC-derived vascular cells survived over 6 wk after transplantation, mainly located at the borders of the infarct (Fig. 3L–N) and to a lesser extent within the infarct area. Because of the larger number of rat than human cells present in the microvascular structures, we conclude that a paracrine effect dominates. Measurements of donor and host cytokines support that a paracrine effect may be present: the human (donor) cytokines EGF, VEGF and HGF were elevated in the infarct at 1 d and HGF was still at 3 d (Fig. 4B), and the rat (host) cytokine VEGF was elevated at 1 d and 3 d (Fig. 4A). Evidence of elevation of apoptosis inhibition in the donor cells was also present, in that survivin levels were elevated at d 1 and 3. In addition to these pro-angiogenic and pro-survival influences, a local diminution of secretion of IL-6, a pro-inflammatory cytokine [24], was observed in the animals treated by the combined delivery of Tβ4 and cells in the gel.
Studies with bone marrow- or adipose tissue-derived stem cells and progenitor cells in animals and also in human clinical evaluation suggest that both neovascularization and paracrine effects play an important role in protecting native cardiomyocytes from death, enhancing the healing processes and preserving cardiac function [25,26]. Paracrine factors enhance angiogenesis and modulate inflammatory responses, which are relevant processes in infarcted hearts (Fig. 4A,B) [27–29]. Importantly, although significant advances have been made in the use of cardiomyocytes for heart regeneration, there is no clear evidence that these cells improve heart function due to their ability to contract [10]. In fact, a recent study indicates that hESC-derived cardiomyocytes transplanted into infarcted hearts contribute to high ejection fractions due to their ability to increase the vascularization at the infarcted heart area [30].
The beneficial effect of the hydrogel alone is in line with recent studies injecting alginate [31] and collagen [32] matrices into infarcted cardiac muscle; the materials were thought to decrease wall stress in the remaining myocardium after an infarct and thus contribute to prevent left ventricular dilation and preserve cardiac function. Alginate is only very slowly resorbed, if at all, and collagen is typically derived from animal sources, whereas the hydrogel we report is completely synthetic yet is biomimetic and yields to MMP-driven remodeling. Indeed, our synthetic MMP-sensitive gels were found to be infiltrated within and potentially substitute the degrading extracellular matrix of the infarcted myocardium and promoted structural organization of host endothelial cells, rat arterioles and cardiomyocytes (Fig. 3I–K). The arterioles engulfed by our gel (Fig. 3I,J) may have been preexisting vessels, rather than being grown within a short time of 7 d. However, we show that an increasing number of cells invade the gel over time, suggesting that indeed the gel is permissive to cell ingrowth (Fig. 1E–H). In addition, previous work performed by us showed that MMP-sensitive gels can be invaded by endothelial cells, and that endothelial cells are able to survive within the gel [19]. In addition, our gel, mimicking key degradation characteristics of collagen (with collagen-derived, MMP-sensitive crosslinking peptides [15]), was fully degraded within 6 wk. It is unclear to what extent the degradation profile of our gel benefits cardiac structure and function.
We have demonstrated [19] that our synthetic hydrogel, mimicking key biochemical characteristics of collagen matrices [15], is able to retain the physically entrapped Tβ4 over time, and to release it on-demand as MMP-2 and MMP-9 enzymes trigger gel degradation and release in vitro. This possibly exposes the delivered hESC-derived vascular cells to Tβ4 remaining in the gel, simultaneously exerting a cardioprotective effect on the cells in the vicinity of the gel by the released portion (Fig. S2).
Although approaches to cellular and protein-based treatments in myocardial infarction in comparable animal models are varied, thus rendering it difficult to precisely compare and contrast outcomes, we do point out the following studies, which allow some degree of comparison. Cardiomyocytes derived from hESC encapsulated in Matrigel together with pro-survival morphogens resulted in attenuated ventricular dilation and enhanced cardiac structure and function in an ischemia/reperfusion rat model, showing an increase in ejection fraction 4 w after treatment compared with controls (ejection fraction: + ~8%, P < 0.05) [6]. In another key study, adult cardiac stem cells were injected into the infarcted hearts of rats, 5 h after permanently ligating the LAD, resulting in enhanced cardiac function at 20 d (ejection fraction: + ~11%, P < 0.05), and the injected cells contributed to blood-carrying new vessels and myocytes [33].
CONCLUSIONS
In the study described here, we show that the injection of hESC-derived vascular cells embedded in synthetic MMP-responsive bioactive hydrogels together with physically entrapped Tβ4 enhanced contractile performance 3 d (ejection fraction: + ~7%, P < 0.001) and 6 wk (ejection fraction: + ~12%, P < 0.001) after myocardial infarction as compared to infarcted rats receiving PBS injection as a control. As contrasted with studies with complex biomatrices such as Matrigel®, the synthetic matrix presented here is highly clinically relevant. The matrix provided for survival of some of the transplanted hESC-derived vascular cells, and their inclusion in the gel along with Tβ4 provided a statistical benefit in terms of contractile performance compared to gels with Tβ4 alone. Measurements of cytokines present in the lesion over time suggest that at least one mechanism of the transplanted cells’ effect is through paracrine action of released angiogenic and survival factors. Future studies may examine the effects of our treatments in reperfusion infarct models, as well as comparing the injection of hESC-based microvascular grafts versus endothelial progenitor cell (EPC)-derived grafts.
Supplementary Material
ACKNOWLEDGEMENTS
We greatly acknowledge Dr. Marisa Jaconi (University of Geneva, Switzerland) for practical support in establishing the myocardial infarction models, Hester Shieh (Massachusetts Institute of Technology, MIT) for help with hESC culture, Dr. David Sosnovik, Gregory Wojtkiewicz and Jenny Chan for technical help in MR imaging and analysis (Massachusetts General Hospital, Boston). We are grateful to Chakib Boussahmain, Laura Crankshaw and Weijia Zhang (all MIT) for support with histological processing.
This work was supported in part by NIH (grant HL060435), the European Union’s 6th Framework Program Expertissues, and the European Union’s 7th Framework Program Angioscaff. TPK was supported by the Swiss National Science Foundation (grant numbers 120938 and PBELP3–127902) and a Rotary Ambassadorial Scholarship. LSF was supported by a Marie Curie-Reintegration Grant, MIT-Portugal program, FCT (PTDC/SAU-BEB/098468/2008) and Crioestaminal.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].American Heart Association. Heart Disease and Stroke Statistics. 2009.
- [2].Pasumarthi KB, Field LJ. Cardiomyocyte cell cycle regulation. Circ Res 2002; 90: 1044–54. [DOI] [PubMed] [Google Scholar]
- [3].Passier R, van Laake LW, Mummery CL. Stem-cell-based therapy and lessons from the heart. Nature 2008; 453: 322–9. [DOI] [PubMed] [Google Scholar]
- [4].Segers VFM, Lee RT. Stem cell-therapy for cardiac disease. Nature 2008; 451: 937. [DOI] [PubMed] [Google Scholar]
- [5].Caballero S, Sengupta N, Afzal A, Chang KH, Li Calzi S, Guberski DL, et al. Ischemic vascular damage can be repaired by healthy, but not diabetic, endothelial progenitor cells. Diabetes 2007; 56:960–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Laflamme MA, Chen KY, Naumova AV, Muskheli V, Fugate JA, Dupras SK, et al. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol 2007; 25: 1015–24. [DOI] [PubMed] [Google Scholar]
- [7].Van Laake LW, Passier R, Monshouwer-Kloots J, Verkleij AJ, Lips DJ, Freund C, et al. Human embryonic stem cell-derived cardiomyocytes survive and mature in the mouse heart and transiently improve function after myocardial infarction. Stem Cell Research 2007; 1: 9–24. [DOI] [PubMed] [Google Scholar]
- [8].Yang L, Soonpaa MH, Adler ED, Roepke TK, Kattman SJ, Kennedy M, et al. Human cardiovascular progenitor cells develop from a KDR+ embryonic-stem-cell-derived population. Nature 2008; 453: 524–8. [DOI] [PubMed] [Google Scholar]
- [9].Li Z, Wilson KD, Smith B, Kraft DL, Jia F, Huang M, et al. Functional and transcriptional characterization of human embryonic stem cell-derived endothelial cells for treatment of myocardial infarction. PLoS One 2009; 4: e8443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Rubart M, Field LJ. ES cells for troubled hearts. Nat Biotechnol 2007; 25: 993–4. [DOI] [PubMed] [Google Scholar]
- [11].Smart N, Risebro CA, Melville AA, Moses K, Schwartz RJ, Chien KR, et al. Thymosin beta4 induces adult epicardial progenitor mobilization and neovascularization. Nature 2007; 445: 177–82. [DOI] [PubMed] [Google Scholar]
- [12].Lutolf MP, Hubbell JA. Synthesis and physicochemical characterization of end-linked poly(ethylene glycol)-co-peptide hydrogels formed by Michael-type addition. Biomacromolecules 2003; 4:713–22. [DOI] [PubMed] [Google Scholar]
- [13].Francis SE, Goh KL, Hodivala-Dilke K, Bader BL, Stark M, Davidson D, et al. Central roles of alpha5beta1 integrin and fibronectin in vascular development in mouse embryos and embryoid bodies. Arterioscler Thromb Vasc Biol 2002; 22: 927–33. [DOI] [PubMed] [Google Scholar]
- [14].Heymans S, Luttun A, Nuyens D, Theilmeier G, Creemers E, Moons L, et al. Inhibition of plasminogen activators or matrix metalloproteinases prevents cardiac rupture but impairs therapeutic angiogenesis and causes cardiac failure. Nat Med 1999; 5: 1135–42. [DOI] [PubMed] [Google Scholar]
- [15].Lutolf MP, Weber FE, Schmoekel HG, Schense JC, Kohler T, Mueller R, et al. Repair of bone defects using synthetic mimetics of collagenous extracellular matrices. Nat Biotechnol 2003; 21: 513–8. [DOI] [PubMed] [Google Scholar]
- [16].Kraehenbuehl TP, Zammaretti P, Van der Vlies AJ, Schoenmakers RG, Lutolf MP, Jaconi ME, et al. Three-dimensional extracellular matrix-directed cardioprogenitor differentiation: systematic modulation of a cell-responsive PEG-hydrogel. Biomaterials 2008; 29: 2757–66. [DOI] [PubMed] [Google Scholar]
- [17].Ferreira LS, Gerecht S, Shieh HF, Watson N, Rupnick MA, Dallabrida SM, et al. Vascular progenitor cells isolated from human embryonic stem cells give rise to endothelial and smooth muscle like cells and form vascular networks in vivo. Circ Res 2007; 101: 286–94. [DOI] [PubMed] [Google Scholar]
- [18].Levenberg S, Golub JS, Amit M, Itskovitz-Eldor J, Langer R. Endothelial cells derived from human embryonic stem cells. Proc Natl Acad Sci U S A 2002; 99: 4391–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Kraehenbuehl TP, Ferreira LS, Zammaretti P, Hubbell JA, Langer R. Cell-responsive hydrogel for encapsulation of vascular cells. Biomaterials 2009; 30:4318–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Nahrendorf M, Wiesmann F, Hiller KH, Hu K, Waller C, Ruff J, et al. Serial cine-magnetic resonance imaging of left ventricular remodeling after myocardial infarction in rats. J Magn Reson Imaging 2001; 14: 547–55. [DOI] [PubMed] [Google Scholar]
- [21].Mita AC, Mita MM, Nawrocki ST, Giles FJ. Survivin: key regulator of mitosis and apoptosis and novel target for cancer therapeutics. Clin Cancer Res 2008; 14:5000–5. [DOI] [PubMed] [Google Scholar]
- [22].Darland DC, D’Amore PA. Blood vessel maturation: vascular development comes of age. J Clin Invest 1999; 103:157–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Spinale FG. Myocardial matrix remodeling and the matrix metalloproteinases: influence on cardiac form and function. Physiol Rev 2007; 87: 1285–342. [DOI] [PubMed] [Google Scholar]
- [24].Frangogiannis NG, Smith CW, Entman ML. The inflammatory response in myocardial infarction. Cardiovasc Res 2002; 53:31–47. [DOI] [PubMed] [Google Scholar]
- [25].Gnecchi M, He H, Liang OD, Melo LG, Morello F, Mu H, et al. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med 2005; 11: 367–8. [DOI] [PubMed] [Google Scholar]
- [26].Ebelt H, Jungblut M, Zhang Y, Kubin T, Kostin S, Technau A, et al. Cellular cardiomyoplasty: improvement of left ventricular function correlates with the release of cardioactive cytokines. Stem Cells 2007; 25: 236–44. [DOI] [PubMed] [Google Scholar]
- [27].Kinnaird T, Stabile E, Burnett MS, Lee CW, Barr S, Fuchs S, et al. Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms. Circ Res 2004; 94: 678–85. [DOI] [PubMed] [Google Scholar]
- [28].Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo JL, et al. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med 2007; 204: 3037–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Swirski FK, Nahrendorf M, Etzrodt M, Wildgruber M, Cortez-Retamozo V, Panizzi P, et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science 2009; 325: 612–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].van Laake LW, Passier R, den Ouden K, Schreurs C, Monshouwer-Kloots J, Ward-van Oostwaard D, et al. Improvement of mouse cardiac function by hESC-derived cardiomyocytes correlates with vascularity but not graft size. Stem Cell Res 2009; 3:106–12. [DOI] [PubMed] [Google Scholar]
- [31].Landa N, Miller L, Feinberg MS, Holbova R, Shachar M, Freeman I, et al. Effect of injectable alginate implant on cardiac remodeling and function after recent and old infarcts in rat. Circulation 2008; 117: 1388–96. [DOI] [PubMed] [Google Scholar]
- [32].Dai W, Wold LE, Dow JS, Kloner RA. Thickening of the infarcted wall by collagen injection improves left ventricular function in rats: a novel approach to preserve cardiac function after myocardial infarction. J Am Coll Cardiol 2005; 46: 714–9. [DOI] [PubMed] [Google Scholar]
- [33].Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, et al. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell 2003; 114: 763–76. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.