Abstract
Individuals with schizophrenia display substantial deficits in neurocognition, resulting in poor daily functioning and disability. Recent reports have suggested that neurocognitive dysfunction in this population is linked to increased inflammation. However, there is paucity of evidence supporting this link, as well as lack of information about the putative link of inflammation to daily functioning. We examined neurocognition (MCCB) and daily functioning (SLOF), as well as inflammatory markers (TNF-α, IL-6, IL-1β, and IL-12p70) in 41 individuals with schizophrenia. Poor neurocognition was significantly associated with increased peripheral TNF-α and IL-12p70 (r=−0.44 and r=−0.38, respectively, controlling for BMI, depression and antipsychotic medication). Notably, difficulties with daily functioning were significantly associated with increased peripheral TNF-α (r=−0.51) and a trend with increased IL-12p70. Our findings support previous hypotheses linking neurocognitive impairment to increased inflammation in individuals with schizophrenia. Our results extend these associations in this population, linking inflammation to poor daily functioning in this population.
Keywords: Schizophrenia, neurocognition, cognition, daily functioning, inflammation, IL-12-p70, IL-6, TNF-α, IL-1β
Introduction
Individuals with schizophrenia have a broad range of neurocognitive deficits that contribute to poor functioning and disability (Keefe et al., 2006). However, the exact cause underlying these deficits remains obscure. Several reports have proposed that inflammation may play a role in neurocognitive dysfunction in schizophrenia, as systemic inflammation causes microglia to release inflammatory cytokines, which bind to receptors on neurons leading to changes in mood, cognition and behavior (Khandaker et al., 2015). Furthermore, increased inflammatory cytokines are inversely correlated with cognition (Hope et al., 2015). Consistent with these reports, a meta-analysis of 40 studies of individuals with schizophrenia showed substantial cytokine elevations with IL-12, IFN-γ, TNF-α, soluble IL-2R (Goldsmith et al., 2016; Misiak et al., 2017) and IL-6 (Goldsmith et al., 2016; Miller et al., 2011) were elevated compared to non-clinical controls.
While extensive literature indicates that individuals with schizophrenia display substantial neurocognitive deficits and have increased inflammatory cytokines, there is paucityof evidence supporting this putative link. Furthermore, there is lack of information about whether this link impacts daily functioning in individuals with schizophrenia. While the CNS is distinct from the peripheral circulation due to the blood brain barrier, systemic cytokines do reach the CNS under normal physiologic conditions (Banks, 2005). Thus, peripheral cytokines may provide information about the inflammatory state of the CNS.
To address these gaps in the literature, our aims are to: 1) confirm the link of altered inflammation to neurocognitive deficits in individuals with schizophrenia; and 2) examine the association between inflammation and daily functioning in this population.
Materials and methods:
Participants
Data were obtained from baseline assessments of a clinical trial examining the impact of aerobic exercise on neurocognition in schizophrenia (ClinicalTrials.gov Identifier NCT01897064). The study was approved by the institutional review board and all participants provided written informed consent. The data were collected between 05/2012 and 07/2014.
Forty-one non-acute individuals with schizophrenia were recruited from outpatient mental health clinics in the greater NYC area. The inclusion criteria were a DSM-IV diagnosis of schizophrenia or related disorders; age 18–55 years; English-speaking; taking antipsychotic medication for at least 8 weeks and on current doses for 4 weeks and/or injectable depot antipsychotics with no change in the last 3 months; and medically cleared by a physician to take part in physical exercise training. The exclusion criteria were a DSM-IV diagnosis of alcohol/substance abuse within the past month or alcohol/substance dependence within the past 6 months; recent use of street drugs (confirmed by a urine toxicology test); a history of seizures/head trauma with loss of consciousness resulting in cognitive sequelae/rehabilitation; significant clinical abnormalities in physical examination, EKG, or lab assessments including Compete Blood Count (CBC) and a Basic Metabolic Panel (BMP); untreated hyper- or hypothyroidism; BMI ≥ 40; being pregnant/nursing; having serious suicidal/homicidal risk; presence of moderate or more severe disorganization (SAPS global positive formal thought disorder≥3); more than a mild level of depressive symptoms (BDI>18); and participation in a study involving neurocognitive assessment in the previous 3 months.
Measures
The Structured Clinical Interview (SCID) was used to establish DSM-IV diagnoses. Symptoms were assessed using the Scale for the Assessment of Positive and Negative Symptoms (SAPS/SANS) and The Beck Depression Inventory (BDI). Neurocognition was assessed using the MATRICS Consensus Cognitive Battery (MCCB; Nuechterlein, et al., 2008) with the MCCB composite score serving as a primary variable of interest (adjusted for age, gender). All MCCB tests were administered on weekdays between 9–11am to minimize the influence of diurnal variation on neurocognition (Hufford et al., 2014). Daily functioning was assessed using the Specific Level of Functioning Scale (SLOF; Schneider & Struening, 1983) via informants, typically first-degree relatives residing with the participant. All tests and clinical interviews were administered by a Masters’-level or above clinician.
For cytokine analysis, a sample of venous blood was collected on weekdays at ~9am following overnight fasting. All samples were collected within a week of the administration of the MCCB and SLOF. Samples were collected into serum separating tubes and were allowed to coagulate at room temperature for 30 min. Serum samples were collected through 10 min centrifugation at 4°C using a Clay Adam Compact II Centrifuge (3200rpm). Blood samples were then stored at −80°C. All samples were analyzed using ELISA kits according to the manufacturer’s protocol (Millipore HSTCMAG-28SK). The intra- and inter-assay coefficients of variation for each of the cytokines are: IL-12(p70) <5%, <15%; TNFα <5%, <15%; IL-6 <5%, <20%; and IL-1β <5%, <15%, respectively.
Statistical Analyses
Data analyses were conducted using IBM SPSS ver. 22. Tests were 2-tailed with significance level set at α=.05. Associations between variables were examined using zero-order and partial correlations controlling for depression (BDI), Body Mass Index (BMI) and antipsychotic medication (chlorpromazine equivalents), as chlorpromazine equivalents as well as depression and BMI (Ambrosio et al., 2018) may impact outcome variables.
Results:
The sample was 36% female with an average age of 37.0 (SD=10.0). Diagnoses were Schizophrenia 30 (73%), Schizoaffective 7 (17%), and Psychosis NOS 4 (10%). The sample’s average BMI was 31.1 (SD=5.9). Twenty-four percent of participants were smokers. All participants were taking antipsychotic medications with average Chlorpromazine equivalents of 374.8 (SD=318.4). The sample’s average BDI score was 7.7 (SD=7.7).
Poorer neurocognition, as indexed by the MATRICS Composite Score, was significantly associated with increased peripheral TNF-α and IL-12p70 levels (see Table 1). For TNF-α, these findings were driven primarily by significant associations with poorer neurocognitive performance in the domains of speed of processing (r=−0.43, p=0.02), visual learning (r=−0.41, p= 0.02), and reasoning and problem solving (r=−0.41, p=0.03). Similarly, the association between neurocognition and IL-12p70 was driven primarily by decreased speed of processing (r=−0.55, p<0.01) and visual learning (r=−0.36, p=0.05).
Table 1:
Correlations between inflammatory markers and overall neurocognition and daily functioning.
| Daily Functioning | Neurocog. | TNF-α | IL-6 | IL-1β | IL-12p70 | |
|---|---|---|---|---|---|---|
| -- | 0.46* | −0.49** | −0.18 | 0.02 | −0.31 | |
| 0.46* | -- | −0.35* | −0.31 | −0.21 | −0.38* | |
| TNF-α | −0.51** | −0.44* | -- | 0.48** | −0.05 | 0.46** |
| −0.18 | −0.33 | 0.49* | -- | 0.17 | 0.63*** | |
| IL-1β | −0.07 | −0.28 | −0.05 | 0.17 | -- | 0.20 |
| IL-12p70 | −0.35 | −0.38* | 0.47* | 0.63*** | 0.27 | -- |
N=41;
<0.05,
<0.01,
<0.001;
Zero order correlations are presented above the diagonal. Partial correlations (controlling for BMI, depression and chlorpromazine equivalents are presented below the diagonal. Daily Functioning - SLOF Informant Total; Neurocognition – MCCB Composite Score.
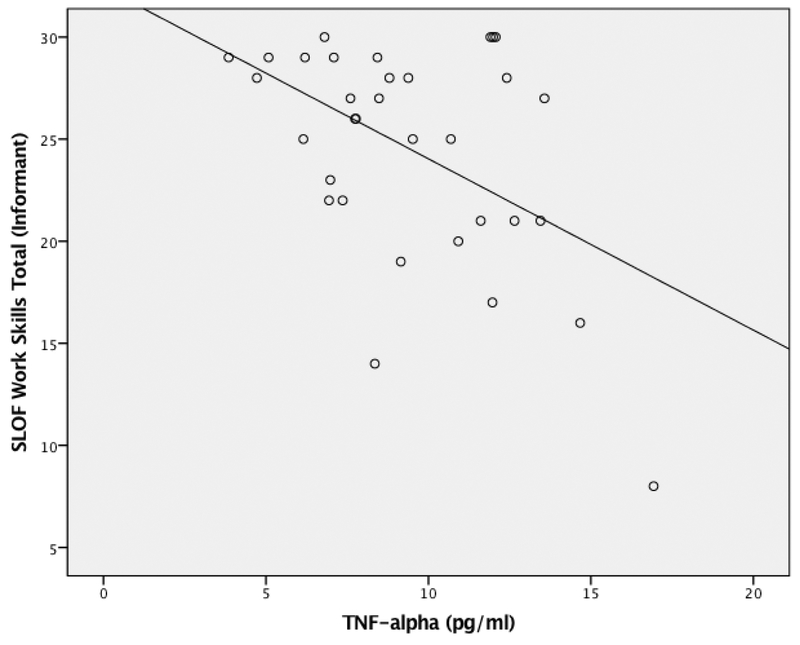
Difficulty in daily functioning, as indexed by the SLOF via informants, was significantly correlated with increased peripheral TNF-α expression (see Table 1). This association was driven largely by functional difficulties in the domains of work skills (r=−0.54, p<0.01; see Figure 1) and personal care (r=−0.41, p=0.03). There was a trend association between elevations in IL-12p70 and decreased daily functioning (r=−.35, p=0.06). There were no significant differences in inflammatory markers between smokers and non-smokers, as well as between males and females. Likewise, there were no significant associations between inflammation markers and SANS-assessed negative symptoms, or and age.
Figure 1: Association Between TNF-α and Work Skills.
N=41; Serum TNF-α; SLOF - Specific Level of Functioning Scale (informant); Results were controlled for Body Mass Index, depression and antipsychotic medication dosage.
Discussion:
To the best of our knowledge, this is the first report concurrently examining the associations between neurocognition, daily functioning and inflammation in individuals with schizophrenia. The findings demonstrate that elevations in inflammation markers are significantly associated with poor daily functioning, specifically in the domains of work skills and personal care. These findings extend previous reports examining inflammation and neurocognition in this population. Our results confirm previous studies indicating a link between increased inflammation and neurocognitive deficits in schizophrenia. Specifically, individuals with schizophrenia with increased peripheral TNF-α and IL-12p70 expression displayed more severe neurocognitive deficits. Exploratory analyses indicated that these associations were driven largely by poor performance on the domains of speed of processing, reasoning and problem solving, and visual learning.
Previous reports linking TNF-α and cognition in individuals with schizophrenia have been inconsistent (Goldsmith et al., 2018; Lv et al., 2015; Misiak et al., 2017; Zhang et al., 2016), potentially due to the regulation of TNF-α, which could be analyzed at the transcriptional, translational and post-translational levels (Brenner et al., 2015). Variability in cognitive measures used in previous studies may have also contributed to the uneven results – for example, Zhang et al. (2016) assessed cognition via the the interview-based PANSS cognitive factor, which previous reports have suggested measures verbal skills rather than neurocognition (see Nielsen et al., 2014; Ehmann et al., 2004). Likewise, Goldsmith et al., 2018 showed increased TNF-α in individuals with deficit schizophrenia who often exhibit cognitive deficits. However, their study did not examine neurocognition. The presence of depression may have also contributed to the variability in published results - as our study criteria excluded individuals with more than mild depressive symptoms, the links between neurocognition, daily functioning, and inflammation are not likely attributed to depression, which has been found to be independently associated with increased inflammation, particularly TNF-α (Bhattacharya et al., 2016). Such exclusion has not been consistent in previous studies.
Our findings invite speculation about the underlying link between inflammation and neurocognitive dysfunction in schizophrenia. Neuroinflammation is characterized by activation of microglial cells, which are similar to macrophages. These cells are activated by injury, local or systemic inflammation and upregulate surface receptors, which trigger the release of inflammatory cytokines. Inflammatory cytokines, including TNF-α, modulate the balance between synaptogenesis and neurodegeneration, a process critical to normal neurocognitive function (Park & Bowers, 2010; Monji et al., 2009). Consistent with our findings, a recent study has linked elevated soluble TNF receptor 2 to hippocampal volume and neurocognitive performance in individuals with schizophrenia (Kudo, et al., 2018) and another showed that TNF- α levels were correlated with deficit schizophrenia, a phenotype which has impaired cognition (Goldsmith et al., 2018). Similarly, evidence from animal models of schizophrenia implicates impaired N-methyl-D-aspartate (NMDA) signaling. NMDA antagonists such as phencyclidine and ketamine have been found to induce microglial activation in rodent brains (Nakki, et al., 1995, 1996). Additionally, increased microglial activation in grey matter and the hippocampus is seen during acute exacerbations in individuals with schizophrenia, suggesting that inflammation may contribute to grey matter loss and cognitive deterioration (van Berckel, et al., 2008).
One intervention that has anti-inflammatory properties along with beneficial impact on neurocognitive functioning is aerobic exercise (AE). Although intense acute AE temporarily increases inflammation (Karstoft & Pedersen, 2016), long-term chronic AE training exerts anti-inflammatory effects in key insulin-responsive tissues (e.g., skeletal muscle, liver, and adipose tissue) by modulating immune cell function and production of anti-inflammatory cytokines (Gleeson et al., 2011). AE has also been found to increase hippocampal neurogenesis in rodents, which has been linked with improvements in memory tasks (Hotting & Roder, 2013) and has been found to increase hippocampal volume in individuals with schizophrenia and healthy subjects (Pajonk et al., 2010). We have previously described AE with active-play video games as a feasible and safe method to improve cognitive deficits in individuals with schizophrenia (Kimhy et al., 2015, 2016). AE upregulates BDNF in individuals with schizophrenia, which may enhance neurocognition by increasing neurogenesis and neuroplasticity (Kimhy et al., 2015; Vakhrusheva et al., 2016). Interestingly, TNF-α has been found to be inversely correlated with BDNF in individuals with schizophrenia and may be involved in BDNF regulation (Zhang et al., 2017). Future work should elucidate the links between AE, BDNF and inflammation and their impact on neurocognition in schizophrenia.
Further support for the link between inflammation and neurocognition, tocilizumab, an IL-6 receptor monoclonal antibody, improved neurocognition in a small open-label trial in individuals with schizophrenia (Miller et al., 2016). In another study, adding celecoxib (a COX-2 inhibitor) to antipsychotics improved (trend-level) PANSS-assessed cognitive scales in individuals with schizophrenia (Muller et al., 2002). Furthermore, a double-blind randomized controlled trial targeting positive symptoms using minocycline (an antibiotic with anti-inflammatory properties) as an augmentation to antipsychotics, reported improvement in executive function compared to placebo as measured by CANTAB (Levkovitz, et al., 2010). Further studies are needed to assess the potential effects and mechanisms of anti-inflammation medications on neurocognition and functioning in schizophrenia.
While inflammation is linked to neurocognition, targeting inflammation may also improve physical health as people with schizophrenia have increased risk of cardiovascular disease (Kimhy et al., 2014), obesity, and type 2 diabetes, all of which are associated with increased peripheral inflammation (Gregor & Hotamisligil, 2011; Hotamisligil, 2017). Our group have previously shown that AE can improve cardiopulmonary function (Armstrong et al., 2016). As such, AE may be used as an intervention improve inflammatory profiles in individuals with schizophrenia, which could lead to improvements in cognition, daily functioning, as well as physical health.
The present study has a number of limitations including the relatively modest sample size (n=41). Additionally, the exclusion of participants with active substance use and more than mild depressive symptoms make it less representative of all people with schizophrenia. Future studies should include more detailed characterization of the inflammatory phenotypes of individuals with schizophrenia, including characteristics of circulating monocytes and T cells, and the effects of different inflammatory cytokines on different cognitive domains in schizophrenia. Though schizophrenia is more common in men than women (Saha et al., 2005), women have higher rates of inflammation and higher rates of autoimmune diseases (Yang & Kozloski, 2011). As such, it would be clinically important to determine whether the effects of inflammation on neurocognition are sex specific and if this change following menopause. Similarly, aging is also associated with decreases in neurocognition and changes in inflammation. Future studies should employ larger samples to address the impact of potential biological mediators on neurocognition and functioning in schizophrenia. In summary, our findings support previous hypotheses linking neurocognitive impairment to increased inflammation, particularly peripheral TNF-α and IL-12p70, in individuals with schizophrenia. Our results suggest that these associations also extend to poor daily functioning in this population.
Highlights:
Increased TNF-α and IL-12p70 were significantly associated with decreased neurocognition.
Increased TNF-α was significantly associated with poor daily functioning.
Inflammation may be a novel pathway of cognitive impairment in schizophrenia.
Targeting inflammation in people with schizophrenia may improve neurocognition and functioning.
Acknowledgements:
Funding - The National Institute of Mental Health, Bethesda, MD (1R21MH096132 to Dr. Kimhy).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest disclosures - Dr. Kimhy serves as a consultant to NeuroCog Trials (Durham, NC) relating to another project.
References
- Ambrosio G, Kaufmann FN, Manosso L, Platt N, Ghisleni G, Rodrigues ALS, … Kaster MP, 2018. Depression and peripheral inflammatory profile of patients with obesity. Psychoneuroendocrinology. 91, 132–141. [DOI] [PubMed] [Google Scholar]
- Armstrong HF, Bartels MN, Paslavski O, Cain D, Shoval HA, Ballon JS, Khan S, Sloan RP, Kimhy D, 2016. The impact of aerobic exercise training on cardiopulmonary functioning in individuals with schizophrenia. Schizophr Res, 173, 116–177. [DOI] [PubMed] [Google Scholar]
- Banks WA, 2005. Blood-brain barrier transport of cytokines: a mechanism for neuropathology. Curr Pharm Des. 11, 973–984. [DOI] [PubMed] [Google Scholar]
- Bhattacharya A, Derecki NC, Lovenberg TW, Drevets WC, 2016. Role of neuroimmunological factors in the pathophysiology of mood disorders. Psychopharmacology, 233, 1623–1636. [DOI] [PubMed] [Google Scholar]
- Brenner D, Blaser H, & Mak TW 2015. Regulation of tumour necrosis factor signalling: live or let die. Nat Rev Immunol, 15, 362–374. [DOI] [PubMed] [Google Scholar]
- Debnath M, 2015. Adaptive Immunity in Schizophrenia: Functional Implications of T Cells in the Etiology, Course and Treatment. J Neuroimmune Pharmacol, 10, 610–619. [DOI] [PubMed] [Google Scholar]
- Ehmann TS, Khanbhai I, Macewan GW, Smith GN, Honer WG, Flynn S, Altman S, 2004. Neuropsychological correlates of the PANSS Cognitive Factor. Psychopathology. 37, 253–258. [DOI] [PubMed] [Google Scholar]
- Gleeson M, Bishop NC, Stensel DJ, Lindley MR, Mastana SS, & Nimmo MA, 2011. The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat Rev Immunol, 11, 607–615. [DOI] [PubMed] [Google Scholar]
- Goldsmith DR, Haroon E, Miller AH, Strauss GP, Buckley PF, & Miller BJ, 2018. TNF-alpha and IL-6 are associated with the deficit syndrome and negative symptoms in patients with chronic schizophrenia. Schizophr Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith DR, Rapaport MH, & Miller BJ, 2016. A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Mol Psychiatry, 21, 1696–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope S, Hoseth E, Dieset I, Morch RH, Aas M, Aukrust P, … Andreassen OA 2015. Inflammatory markers are associated with general cognitive abilities in schizophrenia and bipolar disorder patients and healthy controls. Schizophr Res, 165, 188–194. [DOI] [PubMed] [Google Scholar]
- Hotting K, Roder B 2013. Beneficial effects of physical exercise on neuroplasticity and cognition. Neurosci Biobehav Rev, 37, 2243–2257. [DOI] [PubMed] [Google Scholar]
- Karstoft K, Pedersen BK 2016. Exercise and type 2 diabetes: focus on metabolism and inflammation. Immunol Cell Biol, 94, 146–150. [DOI] [PubMed] [Google Scholar]
- Keefe RS, Poe M, Walker TM, Harvey PD 2006. The relationship of the Brief Assessment of Cognition in Schizophrenia (BACS) to functional capacity and real-world functional outcome. J Clin Exp Neuropsychol, 28, 260–269. [DOI] [PubMed] [Google Scholar]
- Khandaker GM, Cousins L, Deakin J, Lennox BR, Yolken R, Jones PB 2015. Inflammation and immunity in schizophrenia: implications for pathophysiology and treatment. Lancet Psychiatry, 2, 258–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimhy D, Khan S, Ayanrouh L, Chang RW, Hansen MC, Lister A, Ballon JS, Vakhrusheva J, Armstrong HF, Bartels MN, Sloan RP 2016. Use of Active-Play Video Games to Enhance Aerobic Fitness in Schizophrenia: Feasibility, Safety, and Adherence. Psychiatr Serv; 67, 240–243. [DOI] [PubMed] [Google Scholar]
- Kimhy D, Vakhrusheva J, Bartels MN, Armstrong HF, Ballon JS, Khan S, Chang RW, Hansen MC, Ayanruoh L, Lister A, Castrén E, Smith EE, Sloan RP 2015. The Impact of Aerobic Exercise on Brain-Derived Neurotrophic Factor and Neurocognition in Individuals With Schizophrenia: A Single-Blind, Randomized Clinical Trial. Schizophr Bull, 41, 859–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimhy D, Vakhrusheva J, Bartels MN, Armstrong HF, Ballon JS, Khan S, Chang RW, Hansen MC, Ayanruoh L, Smith EE, Sloan RP 2014. Aerobic fitness and body mass index in individuals with schizophrenia: Implications for neurocognition and daily functioning. Psychiatry Res, 220, 784–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv MH, Tan YL, Yan SX, Tian L, Chen DC, Tan SP, … Zhang XY 2015. Decreased serum TNF-alpha levels in chronic schizophrenia patients on long-term antipsychotics: correlation with psychopathology and cognition. Psychopharmacology, 232, 165–172. [DOI] [PubMed] [Google Scholar]
- Miller BJ, Buckley P, Seabolt W, Mellor A, Kirkpatrick B 2011. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry, 70, 663–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BJ, Dias JK, Lemos HP, Buckley PF 2016. An open-label, pilot trial of adjunctive tocilizumab in schizophrenia. J Clin Psychiatry, 77, 275–276. [DOI] [PubMed] [Google Scholar]
- Misiak B, Stanczykiewicz B, Kotowicz K, Rybakowski JK, Samochowiec J, Frydecka D, 2017. Cytokines and C-reactive protein alterations with respect to cognitive impairment in schizophrenia and bipolar disorder: A systematic review. Schizophr Res. [DOI] [PubMed] [Google Scholar]
- Monji A, Kato T, Kanba S 200) Cytokines and schizophrenia: Microglia hypothesis of schizophrenia. Psychiatry Clin Neurosci, 63, 257–265. [DOI] [PubMed] [Google Scholar]
- Muller N, Riedel M, Scheppach C, Brandstatter B, Sokullu S, Krampe K, … Schwarz MJ 2002. Beneficial antipsychotic effects of celecoxib add-on therapy compared to risperidone alone in schizophrenia. Am J Psychiatry, 159, 1029–1034. [DOI] [PubMed] [Google Scholar]
- Nielsen RE, Lindström E, Telléus GK, Levander S, 2014. Is the PANSS cognitive scale measuring cognition? Nord J Psychiatry, 68:573–578. [DOI] [PubMed] [Google Scholar]
- Park KM, Bowers WJ 2010. Tumor necrosis factor-alpha mediated signaling in neuronal homeostasis and dysfunction. Cell Signal, 22, 977–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha S, Chant D, Welham J, McGrath J 2005. A systematic review of the prevalence of schizophrenia. PLoS Med, 2:e141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider LC, Struening EL 1983. SLOF: a behavioral rating scale for assessing the mentally ill. Soc Work Res Abstr, 19, 9–21. [DOI] [PubMed] [Google Scholar]
- Vakhrusheva J, Marino B, Stroup TS, Kimhy D 2016. Aerobic Exercise in People with Schizophrenia: Neural and Neurocognitive Benefits. Curr Behav Neurosci Rep, 3, 165–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Kozloski M 2011. Sex differences in age trajectories of physiological dysregulation: inflammation, metabolic syndrome, and allostatic load. J Gerontol A Biol Sci Med Sci, 66, 493–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XY, Tan YL, Chen DC, Tan SP, Yang FD, Wu HE, … Soares JC 2016. Interaction of BDNF with cytokines in chronic schizophrenia. Brain Behav Immun, 51, 169–175. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Fang X, Fan W, Tang W, Cai J, Song L, Zhang C, 2017. Interaction between BDNF and TNF-alpha genes in schizophrenia. Psychoneuroendocrinology, 89, 1–6. [DOI] [PubMed] [Google Scholar]