Abstract
Background
In Australia, evidence-based guidelines recommend that women consider taking selective oestrogen receptor modulators (SERMs) to reduce their risk of breast cancer. In practice, this requires effective methods for communicating the harms and benefits of taking SERMs so women can make an informed choice.
Aim
To evaluate how different risk presentations influence women’s decisions to consider taking SERMs.
Design and setting
Cross-sectional, correlational study of Australian women in general practice.
Method
Three risk communication formats were developed that included graphics, numbers, and text to explain the reduction in breast cancer risk and risk of side effects for women taking SERMs (raloxifene or tamoxifen). Women aged 40–74 years in two general practices were shown the risk formats using vignettes of hypothetical women at moderate or high risk of breast cancer and asked to choose ‘If this was you, would you consider taking a SERM?’ Descriptive statistics and predictors (risk format, level of risk, and type of SERM) of choosing SERMs were determined by logistic regression.
Results
A total of 288 women were recruited (an 88% response rate) between March and May 2017. The risk formats that showed a government statement and an icon array were associated with a greater likelihood of considering SERMs relative to one that showed a novel expected frequency tree. Risk formats for raloxifene and for the high-risk vignettes were also more strongly associated with choosing to consider SERMs. No associations were found with any patient demographics.
Conclusion
Specific risk formats may lead to more women considering taking SERMs to reduce breast cancer risk, especially if they are at high risk of the condition. Raloxifene may be a more acceptable SERM to patients.
Keywords: breast neoplasms, cancer, preventive therapy, primary care, raloxifene hydrochloride, tamoxifen
INTRODUCTION
Breast cancer was the most commonly diagnosed cancer in Australian women in 2017 and the fourth commonest cause of cancer. An estimated 18 235 Australians (13.2% of all new cancer cases) were diagnosed with breast cancer in 2018, with 3157 people dying from breast cancer in the same year.1
A number of selective oestrogen receptor modulators (SERMs) have been demonstrated in randomised controlled trials to significantly reduce the incidence of breast cancer in women at increased risk.2–5 This has led to international guidelines recommending that women who are at moderate or high risk of breast cancer should consider taking SERMs (that is, women with one first-degree relative affected before the age of 50 or with two affected first-degree relatives).2,6 Use of SERMs is not without potential side effects including an increased likelihood of developing uterine cancer, blood clots, and menopausal symptoms. As a consequence, the benefits of treatment must be balanced against the risk of harms, to allow women to make an informed decision.2,5 Two of the most common SERMs prescribed are tamoxifen and raloxifene. Tamoxifen is slightly more effective in reducing breast cancer incidence but raloxifene has fewer serious adverse effects.2 At this time, raloxifene is recommended for postmenopausal women, while tamoxifen may be used in both premenopausal and postmenopausal women.6
Despite recommendations for women at risk of breast cancer to take SERMs, the awareness and uptake of SERMs has been universally low.7–9 Previous research suggests this is because women at increased risk and their clinicians have insufficient knowledge about the benefits and harms of taking SERMS.7 Currently, there are no guidelines for how to communicate the benefits and harms of taking SERMs, and it is clear that women would benefit from high-quality evidence-based information presented in a way that would assist their understanding of the risks and benefits of all options.10
The formats in which disease risk and risk reduction are presented can impact people’s preventive treatment choices.11 There are some well-accepted recommendations on best practice when presenting risk numerically: an absolute risk reduction format should be used as it produces a more accurate comprehension of risk than either relative risk reduction or number needed to treat.10,12–16 Risk presentations require the reference class and time frame to be clear, and this is particularly necessary when presenting risk information in terms of frequencies, percentages, or probabilities.11,17,18 Natural frequencies are better understood and result in significantly increased rates of correct inferences from risk communication material compared with conditional probabilities.19,20 When presenting risk verbally, qualitative risk terms should always be given with quantitative information because this type of presentation can lead to greater accounting of numeric information as well as more accurate interpretations and use of the numbers provided.21
How this fits in
| Selective oestrogen receptor modulators (SERMs) have been demonstrated to reduce the risk of developing breast cancer in women who are at increased risk, and Australian guidelines recommend that GPs consider prescribing SERMs for eligible patients. However, SERMs increase the risk of developing uterine cancer (tamoxifen), blood clots (tamoxifen and raloxifene), and menopausal symptoms (tamoxifen and raloxifene), so an understanding of the balance of harms and benefits is needed to make an informed decision. This study explored established and novel ways of presenting the harms and benefits of taking SERMs to reduce breast cancer risk, and evaluated the response women have to the different risk formats, and different medications, at different levels of hypothetical risk. |
Some of the most studied risk communication methods include icon arrays (also known as pictograms), and graphs depicting risk over time. Although there is some disagreement in the literature regarding the most effective visual aid, previous studies have consistently demonstrated that inclusion of a visual aid promotes comprehension and accuracy.22–27 Although less well studied, expected frequency trees or natural frequency trees have been previously useful to communicate multiple conditional probabilities and therefore were chosen as the reference group for the analysis.28,29
The authors wanted to explore different methods of communicating the benefits and harms of taking SERMs to women in primary care. Cancer Australia6 and Royal Australian College of General Practitioners guidelines30 recommend that women at moderate and high risk of breast cancer consider taking SERMs to reduce their risk. Initial discussions about taking SERMs could potentially occur in general practice, especially for women at moderate risk of breast cancer. Therefore these communication methods were tested in a general practice setting.
It was hypothesised that the methods used to present the harms and benefits of taking SERMs may influence women’s decisions about taking tamoxifen or raloxifene to prevent breast cancer, and should be examined and included in decision-making tools used in primary care. This study therefore aimed to examine three different risk communication formats to find the format/s that correlate with an increased likelihood of women considering taking SERMs to reduce their risk of developing breast cancer.
METHOD
Study design
This was a cross-sectional, correlational study of women aged 40 to 74 years who were consecutively sampled from two Australian general practice waiting rooms between March and May 2017.
Setting
The study was conducted using an electronic tablet with a touchscreen in the waiting rooms of general practice clinics in metropolitan Melbourne. The general practices were purposively recruited based on the Socio-Economic Indexes for Areas (SEIFA)31 score for their location to increase the diversity of the recruited sample.
Women between 40 and 74 years attending an appointment with their GP were approached consecutively and invited to participate in the study. The women were given an electronic tablet to self-complete, starting with a digital consenting process. Once recruited, participants completed demographic questions (age, education, marital status, language spoken at home, country of origin, and personal or family history of breast cancer), followed by 12 risk presentations that appeared one by one in a random order. For each risk presentation (one page each for each different medication, risk format, and level of risk), participants chose between two options:
Yes, I would consider taking [raloxifene/tamoxifen] to reduce my risk of breast cancer, or
No, I would not consider taking [raloxifene/tamoxifen] to reduce my risk of breast cancer.
Participants
Women were eligible to participate if they were aged between 40 and 74 years old and were waiting for a doctor’s appointment. They were excluded if they were too unwell, too visually or hearing impaired, were non-English speaking or reading, or had severe intellectual disability or psychiatric illness. Because of the hypothetical nature of this study, all participants regardless of their menopausal status were presented with information on tamoxifen and raloxifene.
This study was exploratory and therefore sampling was pragmatic.
Risk presentations
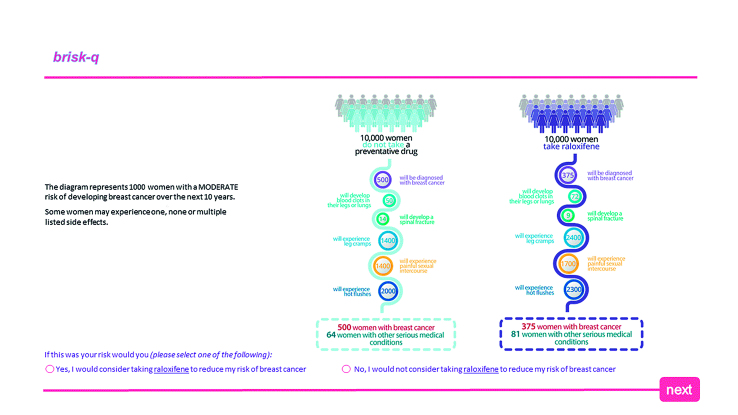
Three risk communication formats were developed and adapted to provide information about the reduction in breast cancer incidence and frequency of side effects for tamoxifen and raloxifene in women at moderate or high risk of breast cancer (further information about the facts used in the risk presentations is available from the authors on request). These formats showed the absolute risk reduction alongside a government statement (Figure 1); icon arrays (Figure 2); and a novel expected frequency tree (Figure 3). The 12 risk format combinations included a combination of one of the three risk formats for one of each level of risk and for both medications. The risk presentations were displayed in a random order using a computer-generated sequence to reduce learning and ordering effects.
Figure 1.
Example of government guideline risk communication format for raloxifene and patients with a moderate risk of breast cancer.
Figure 2.
Example of icon array risk communication format for raloxifene and patients with a moderate risk of breast cancer.
Figure 3.
Example of expected frequency tree risk communication format for raloxifene and patients with a moderate risk of breast cancer.
Outcome measures and analysis
The primary outcome was the intention to consider taking tamoxifen or raloxifene. Descriptive statistics were used to summarise participants’ characteristics. Age and education were collapsed into binary variables: younger than 50 years old/50 to 74 years old to reflect the average age of the onset of menopause, and those who had completed at least a Bachelor’s degree compared with those who had not. Multivariable logistic regression was used to examine the association between intention and risk format, risk category, and medication, adjusted for responders’ education, language spoken at home, age, country of origin, and family history of breast cancer. Odds ratios with robust standard errors were calculated using the survey command in Stata (version 13.1) to allow for the repeated responses provided by the participant to the 12 scenarios.
RESULTS
Women were recruited from two general practices — one was located in a lower socioeconomic area (with a SEIFA score of 925.8, which is relatively more disadvantaged compared with the Australian average of 1000), and one was located in a higher socioeconomic area (with a SEIFA score of 1097.6).31 Of the 368 women approached in the waiting rooms of these clinics, 328 were eligible and 288 participants were recruited (88% response rate). A total of 87 (30%) women had a family history of breast cancer. Eleven women who had previously been diagnosed with breast cancer were removed from the final analysis after reflection because it was considered quite likely that their perceptions and beliefs would be different from women who had not had breast cancer when considering preventive medication. The final dataset therefore included 277 women. Table 1 describes the demographics of the participants.
Table 1.
Summary of participants’ characteristics
| Demographics | Participants, n (%) |
|---|---|
| Age (years) | |
| 40–49 | 98 (34) |
| 50–59 | 81 (28) |
| 60–69 | 79 (27) |
| 70–74 | 30 (10) |
|
| |
| Country of origin | |
| Australia | 191 (66) |
| Other | 97 (34) |
|
| |
| Language spoken at home | |
| English | 247 (86) |
| Other | 41 (14) |
|
| |
| Marital status | |
| Single | 68 (24) |
| In a relationship | 160 (56) |
| Separated/divorced | 47 (16) |
| Widowed | 13 (5) |
|
| |
| Education | |
| Never completed high school | 67 (23) |
| Completed high school only | 79 (27) |
| TAFE or similar | 62 (22) |
| ≥ University degree | 80 (28) |
|
| |
| Previous breast cancer diagnosis | |
| Yes | 11 (4) |
| No | 277 (96) |
|
| |
| Family history of breast cancer | |
| Yes | 87 (30) |
| No | 201 (70) |
TAFE = technical and further education.
Presentations showing the government statement with absolute risk and the icon array were associated with similar increases in willingness to consider taking SERMs when compared with the presentation showing expected frequency trees (P = 0.04 and P = 0.03, respectively) (Table 2). Vignettes of women at high risk of breast cancer were associated with a greater willingness to consider SERMs compared with those of women at moderate risk (P<0.001) (Table 2). Participants were significantly more likely to consider using raloxifene than tamoxifen (P<0.001) (Table 2). No demographics were associated with an increased likelihood of considering taking SERMs (further information about univariate analysis of predictors of willingness to consider SERMs is available from the authors on request).
Table 2.
Multivariable logistic regression analysis to determine associations between risk presentation format, type of medication, and breast cancer risk category, unadjusted and adjusted
| Predictor | OR (95% CI) | P-value | AOR | P-value |
|---|---|---|---|---|
| Format | ||||
| EFT | Ref | Ref | ||
| Government statement | 1.14 (1.01 to 1.30) | 0.04 | 1.15 (1.01 to 1.31) | 0.04 |
| Icon array | 1.12 (1.01 to 1.25) | 0.03 | 1.13 (1.01 to 1.26) | 0.03 |
|
| ||||
| Medication | ||||
| Tamoxifen | Ref | Ref | ||
| Raloxifene | 1.48 (1.30 to 1.69) | <0.001 | 1.50 (1.31 to 1.71) | <0.001 |
|
| ||||
| Risk category | ||||
| Moderate risk | Ref | Ref | ||
| High risk | 1.35 (1.20 to 1.52) | <0.001 | 1.36 (1.20 to 1.54) | <0.001 |
AOR = Adjusted odds ratio (adjusted for having a relative with breast cancer, country of birth, language spoken, relationship status, education attained). CI = confidence interval. EFT = expected frequency tree. OR = odds ratio. Ref = Reference group.
DISCUSSION
Summary
This is the first study in Australia to examine women’s intentions about taking SERMs for breast cancer. Women were more likely to consider taking SERMs if risk information is presented in specific formats (as government statement or icon array), if they are at high risk of breast cancer, and if raloxifene is the medication offered.
The expected frequency trees also presented information on a greater range of side effects, including the more common but less serious ones. It may be that this further highlighted the potential harms of taking SERMs compared with the other formats and therefore made participants less willing to consider taking SERMs.
Strengths and limitations
The main strength of the study was the broad sample of women attending general practice and the high response rate (88% of women approached agreed to participate in the study). Women were recruited from different socioeconomic backgrounds, and with an even distribution of age and education level, therefore increasing the external validity of the results.
A major limitation of the study was its hypothetical nature, which meant that responses were based on participants imagining that each risk format related to them. It is important to recognise that hypothetical uptake rates are usually higher than real uptake rates and intentions are only a moderate predictor of actual behaviour,9,32 and therefore the results may well be an overestimate of how many women would consider taking SERMs.
Comparison with existing literature
Previous studies have found that using absolute risk instead of relative risk to convey information about risk and risk reduction allowed patients to more accurately draw correct inferences and make better informed choices.10,12–16 The accuracy of patients’ risk perception is further increased when the baseline risk is included with the risk reduction figure,11,12,16 and when combining numerical and verbal information.10 Furthermore, including an evaluative label with numeric information produces larger changes in behavioural intent than using numeric information alone.21 The current study’s findings are consistent with this evidence: the absolute risk and government statement (Figure 1) presented the absolute risk reduction with baseline risk and provided additional qualitative information to set the risk information in context.
Previous research conducted by the authors of this study exploring risk communication to show the harms and benefits of colorectal cancer screening methods also supported the use of a government statement but had less convincing evidence for the use of icon arrays.33 This was in contrast with the findings from the current study, suggesting that risk communication might need to be modified depending on the clinical decision required.
Many studies have shown that icon arrays improve comprehension of risk and risk reduction.10,11,22–27 Icon arrays visually demonstrate the numerator and denominator in one diagram, making clear the number of affected compared with the reference class or population at risk.11 In the current study, icon arrays were also associated with greater intention to consider taking SERMs than were the expected frequency trees. Expected frequency trees are potentially a visually engaging representation using natural frequencies but there are limited data about their effect on understanding risk.10,17 In this study, the expected frequency trees were less likely to result in women considering SERMs than either the absolute risk and government guideline or the icon array. It is possible that the expected frequency trees used in this study were too complex or presented too much information, particularly in the limited time women had to review them and with a lack of clinical discussion about their meaning.
Although low numeracy can affect people of all educational levels, it is traditionally thought to be more prevalent in people of lower educational attainment.11,22,34 Use of visual aids such as icon arrays may reduce the effect of low numeracy22 when presenting complex risk information, and the findings from the current study are consistent with this. Alternatively, it may be that education itself is not an important predictor of taking SERMs, although evidence about this is inconsistent.9
Similarly, there is conflicting evidence about patient and physician preference for tamoxifen compared with raloxifene to reduce risk.34–38 There are no data available about Australian prescribing rates of either of these medications but US data indicate that raloxifene is prescribed more frequently than tamoxifen for breast cancer risk reduction.7 The current study found that participants were significantly more likely to consider raloxifene than tamoxifen, potentially due to the more acceptable side effect profile of raloxifene. Specifically, raloxifene has two advantages compared with tamoxifen: it does not increase the risk of endometrial cancer and it reduces the risk of spinal fractures.
Participants in the current study were more willing to consider SERMs when presented with a high-risk scenario as opposed to a moderate-risk scenario. This makes intuitive sense given that the absolute benefits of SERMs are greater for high-risk women and therefore more likely to outweigh concerns about side effects than for moderate-risk women.
Implications for research and practice
This study could inform the development of decision aids to support informed decision making and potentially increase the uptake of SERMs to reduce women’s risk of developing breast cancer. Further research is required to test risk communication tools with women who are at increased risk, for whom SERMs should be considered. Modification of the expected frequency trees could be considered to simplify them and reduce potential information overload. Outcomes should be expanded to include risk comprehension, cancer worry, informed choice, and actual uptake of SERMs.
Tamoxifen was added to the Australian Pharmaceutical Benefits Scheme (PBS) in 2016,39 and is now subsidised by the Australian Government for women who are at increased risk of breast cancer; however, raloxifene is not currently listed for breast cancer risk reduction in asymptomatic women. This creates an important barrier to physician prescribing and may contribute to low uptake rates of SERMs.40 The preferable side effect profile may mean a greater uptake of raloxifene in postmenopausal women at increased risk of breast cancer, especially if it were to be made available on the PBS for risk reduction.
Acknowledgments
Dr Patty Chondros assisted with the statistical analysis.
Funding
Professor Jon D Emery is funded by an NHMRC Practitioner Fellowship and Sibel Saya’s PhD is supported by an Australian Government Research Training Program Scholarship.
Ethical approval
The study was approved by the Human Ethics Advisory Group at the Department of General Practice, University of Melbourne (Ethics Approval ID 1647929.2).
Provenance
Freely submitted; externally peer reviewed.
Competing interests
The authors have declared no competing interests.
Discuss this article
Contribute and read comments about this article: bjgp.org/letters
REFERENCES
- 1.Australian Institute of Health and Welfare. Cancer in Australia. 2017. https://www.aihw.gov.au/reports/cancer/cancer-in-australia-2017/contents/table-of-contents (accessed 6 Sep 2019)
- 2.Cuzick J, DeCensi A, Arun B, et al. Preventive therapy for breast cancer: a consensus statement. Lancet Oncol. 2011;12(5):496–503. doi: 10.1016/S1470-2045(11)70030-4. [DOI] [PubMed] [Google Scholar]
- 3.Cuzick J, Sestak I, Bonanni B, et al. Selective oestrogen receptor modulators in prevention of breast cancer: an updated meta-analysis of individual participant data. Lancet. 2013;381(9880):1827–1834. doi: 10.1016/S0140-6736(13)60140-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Euhus DM, Diaz J. Breast cancer prevention. Breast J. 2015;21(1):76–81. doi: 10.1111/tbj.12352. [DOI] [PubMed] [Google Scholar]
- 5.Visvanathan K, Hurley P, Bantug E, et al. Use of pharmacologic interventions for breast cancer risk reduction: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2013;31(23):2942–2962. doi: 10.1200/JCO.2013.49.3122. [DOI] [PubMed] [Google Scholar]
- 6.Cancer Australia. Risk reducing medication for women at increased risk of breast cancer due to family history. 2011. https://canceraustralia.gov.au/publications-and-resources/cancer-australia-publications/risk-reducing-medication-women-increased-risk-breast-cancer-due-family-history (accessed 6 Sep 2019)
- 7.Bambhroliya A, Chavez-MacGregor M, Brewster AM. Barriers to the use of breast cancer risk reduction therapies. J Natl Compr Canc Netw. 2015;13(7):927–935. doi: 10.6004/jnccn.2015.0107. [DOI] [PubMed] [Google Scholar]
- 8.Livaudais-Toman J, Karliner LS, Tice JA, et al. Impact of a primary care based intervention on breast cancer knowledge, risk perception and concern: a randomized, controlled trial. Breast. 2015;24(6):758–766. doi: 10.1016/j.breast.2015.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ropka ME, Keim J, Philbrick JT. Patient decisions about breast cancer chemoprevention: a systematic review and meta-analysis. J Clin Oncol. 2010;28(18):3090–3095. doi: 10.1200/JCO.2009.27.8077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trevena LJ, Davey HM, Barratt A, et al. A systematic review on communicating with patients about evidence. J Eval Clin Pract. 2006;12(1):13–23. doi: 10.1111/j.1365-2753.2005.00596.x. [DOI] [PubMed] [Google Scholar]
- 11.Trevena LJ, Zikmund-Fisher BJ, Edwards A, et al. Presenting quantitative information about decision outcomes: a risk communication primer for patient decision aid developers. BMC Med Inform Decis Mak. 2013;13(suppl 2):S7. doi: 10.1186/1472-6947-13-S2-S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akl EA, Oxman AD, Herrin J, et al. Using alternative statistical formats for presenting risks and risk reductions. Cochrane Database Syst Rev. 2011;(3):CD006776. doi: 10.1002/14651858.CD006776.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Covey J. A meta-analysis of the effects of presenting treatment benefits in different formats. Med Decis Making. 2007;27(5):638–654. doi: 10.1177/0272989X07306783. [DOI] [PubMed] [Google Scholar]
- 14.McGettigan P, Sly K, O’Connell D, et al. The effects of information framing on the practices of physicians. J Gen Intern Med. 1999;14(10):633–642. doi: 10.1046/j.1525-1497.1999.09038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moxey A, O’Connell D, McGettigan P, Henry D. Describing treatment effects to patients: how they are expressed makes a difference. J Gen Intern Med. 2003;18(11):948–959. doi: 10.1046/j.1525-1497.2003.20928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zipkin DA, Umscheid CA, Keating NL, et al. Evidence-based risk communication: a systematic review. Ann Intern Med. 2014;161(4):270–280. doi: 10.7326/M14-0295. [DOI] [PubMed] [Google Scholar]
- 17.Gigerenzer G, Gaissmaier W, Kurz-Milcke E, et al. Helping doctors and patients make sense of health statistics. Psychol Sci Public Interest. 2007;8(2):53–96. doi: 10.1111/j.1539-6053.2008.00033.x. [DOI] [PubMed] [Google Scholar]
- 18.Gigerenzer G, Galesic M. Why do single event probabilities confuse patients? BMJ. 2012;344:e245. doi: 10.1136/bmj.e245. [DOI] [PubMed] [Google Scholar]
- 19.Gigerenzer G, Hoffrage U. How to improve Bayesian reasoning without instruction: frequency formats. Psychol Rev. 1995;102(4):684–704. [Google Scholar]
- 20.Hoffrage U, Gigerenzer G. Using natural frequencies to improve diagnostic inferences. Acad Med. 1998;73(5):538–540. doi: 10.1097/00001888-199805000-00024. [DOI] [PubMed] [Google Scholar]
- 21.Zikmund-Fisher BJ, Fagerlin A, Keeton K, Ubel PA. Does labeling prenatal screening test results as negative or positive affect a woman’s responses? Am J Obstet Gynecol. 2007;197(5):528.e1–528.e6. doi: 10.1016/j.ajog.2007.03.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garcia-Retamero R, Galesic M. Who proficts from visual aids: overcoming challenges in people’s understanding of risks. Soc Sci Med. 2010;70(7):1019–1025. doi: 10.1016/j.socscimed.2009.11.031. [DOI] [PubMed] [Google Scholar]
- 23.Garcia-Retamero R, Hoffrage U. Visual representation of statistical information improves diagnostic inferences in doctors and their patients. Soc Sci Med. 2013;83:27–33. doi: 10.1016/j.socscimed.2013.01.034. [DOI] [PubMed] [Google Scholar]
- 24.Martin RW, Brower ME, Geralds A, et al. An experimental evaluation of patient decision aid design to communicate the effects of medications on the rate of progression of structural joint damage in rheumatoid arthritis. Patient Educ Couns. 2012;86(3):329–334. doi: 10.1016/j.pec.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 25.Tait AR, Voepel-Lewis T, Zikmund-Fisher BJ, Fagerlin A. Presenting research risks and benefits to parents: does format matter? Anesth Analg. 2010;111(3):718–723. doi: 10.1213/ANE.0b013e3181e8570a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Waters EA, Weinstein ND, Colditz GA, Emmons K. Formats for improving risk communication in medical tradeoff decisions. J Health Commun. 2006;11(2):167–182. doi: 10.1080/10810730500526695. [DOI] [PubMed] [Google Scholar]
- 27.Waters EA, Weinstein ND, Colditz GA, Emmons KM. Reducing aversion to side effects in preventive medical treatment decisions. J Exp Psychol Appl. 2007;13(1):11–21. doi: 10.1037/1076-898X.13.1.11. [DOI] [PubMed] [Google Scholar]
- 28.Kurz-Milcke E, Gigerenzer G, Martignon L. Transparency in risk communication: graphical and analog tools. Ann N Y Acad Sci. 2008;1128(1):18–28. doi: 10.1196/annals.1399.004. [DOI] [PubMed] [Google Scholar]
- 29.Spiegelhalter D, Gage J. What can education learn from real-world communication of risk and uncertainty? Mathemathics Enthusiast. 2015;12:4–10. [Google Scholar]
- 30.Royal Australian College of General Practitioners. Guidelines for preventive activities in general practice. 9th edn. East Melbourne: RACGP; 2018. https://www.racgp.org.au/FSDEDEV/media/documents/Clinical%20Resources/Guidelines/Red%20Book/Guidelines-for-preventive-activities-in-general-practice.pdf (accessed 1 Oct 2019) [Google Scholar]
- 31.Australian Bureau of Statistics. 2033.055.001 — Census of population and housing: Socio-Economic Indexes for Areas (SEIFA). 2013. http://www.abs.gov.au/ausstats/abs@.nsf/Lookup/bySubject/2033.0.55.001~2011~MainFeatures~MainPage~1 (accessed 6 Sep 2019)
- 32.Persky S, Kaphingst KA, Condit CM, McBride CM. Assessing hypothetical scenario methodology in genetic susceptibility testing analog studies: a quantitative review. Genet Med. 2007;9(11):727–738. doi: 10.1097/gim.0b013e318159a344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim GY, Walker J, Bickerstaffe A, et al. The CRISP-Q study: communicating the risks and benefits of colorectal cancer screening. Aust J Gen Pract. 2018;47(3):139–145. doi: 10.31128/AFP-04-17-4195. [DOI] [PubMed] [Google Scholar]
- 34.Visschers VH, Meertens RM, Passchier WW, de Vries NN. Probability information in risk communication: a review of the research literature. Risk Anal. 2009;29(2):267–287. doi: 10.1111/j.1539-6924.2008.01137.x. [DOI] [PubMed] [Google Scholar]
- 35.Fagerlin A, Zikmund-Fisher BJ, Nair V, et al. Women’s decisions regarding tamoxifen for breast cancer prevention: responses to a tailored decision aid. Breast Cancer Res Treat. 2010;119(3):613–620. doi: 10.1007/s10549-009-0618-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oppong BA, King TA. Recommendations for women with lobular carcinoma in situ (LCIS) Oncology. 2011;25(11):1051–1058. [PubMed] [Google Scholar]
- 37.Roetzheim RG, Lee JH, Fulp W, et al. Acceptance and adherence to chemoprevention among women at increased risk of breast cancer. Breast. 2015;24(1):51–56. doi: 10.1016/j.breast.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aktas B, Sorkin M, Pusztai L, Hofstatter EW. Uptake of exemestane chemoprevention in postmenopausal women at increased risk for breast cancer. Eur J Cancer Prev. 2016;25(1):3–8. doi: 10.1097/CEJ.0000000000000124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pharmaceutical Benefits Scheme. Public summary document — March 2016 PBAC meeting. 2016. http://www.pbs.gov.au/industry/listing/elements/pbac-meetings/psd/2016-03/files/tamoxifen-psd-march-2016.pdf (accessed 6 Sep 2019)
- 40.Keogh LA, Hopper JL, Rosenthal D, Phillips KA. Australian clinicians and chemoprevention for women at high familial risk for breast cancer. Hered Cancer Clin Pract. 2009;7(1):9. doi: 10.1186/1897-4287-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]