Abstract
Background
Despite increasing use of computed tomography (CT), chest X-ray remains the first-line investigation for suspected lung cancer in primary care in the UK. No systematic review evidence exists as to the sensitivity of chest X-ray for detecting lung cancer in people presenting with symptoms.
Aim
To estimate the sensitivity of chest X-ray for detecting lung cancer in symptomatic people.
Design and setting
A systematic review was conducted to determine the sensitivity of chest X-ray for the detection of lung cancer.
Method
Databases including MEDLINE, EMBASE, and the Cochrane Library were searched; a grey literature search was also performed.
Results
A total of 21 studies met the eligibility criteria. Almost all were of poor quality. Only one study had the diagnostic accuracy of chest X-ray as its primary objective. Most articles were case studies with a high risk of bias. Several were drawn from non-representative groups, for example, specific presentations, histological subtypes, or comorbidities. Only three studies had a low risk of bias. Two primary care studies reported sensitivities of 76.8% (95% confidence interval [CI] = 64.5 to 84.2%) and 79.3% (95% CI = 67.6 to 91.0%). One secondary care study reported a sensitivity of 79.7% (95% CI = 72.7 to 86.8%).
Conclusion
Though there is a paucity of evidence, the highest-quality studies suggest that the sensitivity of chest X-ray for symptomatic lung cancer is only 77% to 80%. GPs should consider if further investigation is necessary in high-risk patients who have had a negative chest X-ray.
Keywords: diagnostic imaging, early diagnosis, lung cancer, primary care, X-rays
INTRODUCTION
Lung cancer is the single largest cause of cancer mortality both worldwide1 and in the UK.2 Compared with many other cancers, improvements in lung cancer survival over recent decades have been modest. The age-standardised 5-year survival rate has only increased from approximately 5% to 10% since 1971,2 compared with improvements from 53% to 87% in the 5-year survival rate for breast cancer in the same period.3
Diagnosis of lung cancer at earlier stages of disease is associated with improved survival. Optimising early detection is therefore considered an important strategy in improving outcomes.4 Chest X-ray is comparatively cheap, accessible,5 and has a low radiation dose.6 It remains the first-line investigation for lung cancer in primary care and the most common radiological route to diagnosis.7 This is reflected in current National Institute for Health and Care Excellence lung cancer guidelines, which recommend chest X-ray for initial evaluation in all patients, aside from those aged >40 years who have unexplained haemoptysis.8 Outcomes for lung cancer in the UK remain poor compared to other advanced economies,9 where modalities such as computed tomography (CT) are used more extensively.10
Despite its predominance in guidelines and clinical practice, no systematic review has determined the sensitivity of chest X-ray alone for lung cancer in patients presenting with symptoms, which is the aim of the present study.
METHOD
A systematic review was conducted in June 2017 and updated in December 2018.
The sensitivity of chest X-ray for lung cancer was estimated by identifying studies that:
reported the number of patients who were investigated with chest X-ray owing to symptoms in the year before their diagnosis of lung cancer; and
reported the contemporaneous results of the chest X-rays.
Screening studies were not included. The authors registered the study protocol with PROSPERO.11 An amendment to the protocol was subsequently made to correct an error. In addition, articles were screened based on their title and abstract, rather than on the basis of title only, as reported in the protocol.
Search strategy
In July 2017 the authors searched CINAHL, Cochrane Database of Systematic Reviews (CDSR), Cochrane Controlled Register of Trials (CENTRAL), Database of Abstracts of Reviews of Effects (DARE), Health Technology Assessment (HTA), NHS Economic Evaluation Database (NHS EED), EMBASE, MEDLINE, MEDLINE in process, MEDLINE Epub ahead of print, PubMed, and Science Citation Index (SCI). These resources were searched with no language restrictions from 1999 using a search strategy with subject headings and free-text words for the concepts ‘chest X-ray’ and ‘lung cancer’. Only studies published after 1999 were included in order to ensure that evidence reflected contemporary radiological technology and practice. The searches were peer reviewed and updated in December 2018 in all the databases. The full search strategies are available from the authors on request. The reference lists of included articles were screened. The websites of several organisations12–26 were manually searched to identify any potentially eligible reports, guidelines, and audits (grey literature search).
How this fits in
| Chest X-ray remains the first-line investigation for suspected lung cancer in the UK. Outcomes for lung cancer are relatively poor compared with the healthcare systems of many other advanced economies, which make more extensive use of other imaging modalities such as computed tomography (CT). This systematic review found that there is limited high-quality evidence published on the diagnostic accuracy of chest X-ray. The few high-quality studies identified suggest that chest X-ray misses (at least initially) lung cancer in >20% of people. As earlier diagnosis is closely associated with improved survival, it is therefore possible that the use of chest X-ray in UK practice may delay the diagnosis of lung cancer in some patients. These findings support calls to increase open-access CT for GPs, but, given resource restrictions and the potential to cause harm through overdiagnosis, further research is required to help identify which patients who have had a non-diagnostic chest X-ray should be referred for additional investigation. |
Inclusion and exclusion criteria
The authors considered any study that reported the number of adult patients who had a chest X-ray following a symptomatic presentation to a clinician in the year before diagnosis with lung cancer. The period of 1 year was selected with reference to estimates of detectable pre-clinical phase of lung cancer (mean sojourn time),27 estimated to be between 5.5 months28 and 2.2 years.29 Studies where it was unclear if the duration between chest X-ray and diagnosis was <1 year were excluded. Studies based on screening populations were excluded. Studies of patients aged <18 years, other intrathoracic malignancies such as mesothelioma and lymphoma, metastatic lung disease from a non-lung cancer primary tumour, and imaging undertaken for staging or diagnostic surveillance for recurrent lung cancer were also excluded. In order to evaluate the diagnostic accuracy of chest X-ray in clinical practice, the authors excluded studies that examined the proportion of chest X-rays where lesions were ‘missed’ but identified in retrospect.
Chest X-rays were considered positive if any abnormality considered suspicious for lung cancer was noted at the time of reporting and were considered negative if no features suspicious of lung cancer were noted at the time of reporting. Where the findings of chest X-ray were not reported in a way that could be classified as positive or negative according to this definition, the authors reported the presence or absence of abnormalities on the chest X-rays.
The authors did not exclude any studies based on the reference standard used.
Study selection
Titles and abstracts of all studies were screened with reference to the inclusion and exclusion criteria. A random 20% of all titles and abstracts were independently screened by a second author. As it was anticipated that relevant data in some cases would have been reported incidentally, rather than as a primary finding of studies, the reviewers maintained a low threshold for selecting citations for full-text review. In the case of disagreements or uncertainty, a third reviewer was consulted. A full-text review of all selected texts was undertaken by the first author to determine final eligibility.
Data extraction
Data from included studies were extracted using a form including demographics and presenting symptoms of participants, sensitivity of chest X-ray, sample size, setting, for example, primary or secondary care, and the reference standard implemented to determine true disease status.
Analysis
The outcome was the sensitivity of chest X-ray for the detection of lung cancer. This was determined by evaluating the stated numbers of patients in each study who presented with symptoms, those who had had chest X-ray in the year before diagnosis with lung cancer, and for those for whom the chest X-ray had yielded a positive result. Ninety-five per cent confidence intervals (CI) for each within-study sensitivity estimate were also calculated. Meta-analysis was planned to be undertaken if possible. In the event of high between-study heterogeneity or a low quality of eligible studies, the authors planned to proceed with a descriptive synthesis of the studies only. A modified version of the QUADAS-2 tool30 for diagnostic accuracy studies was used for quality assessment.
RESULTS
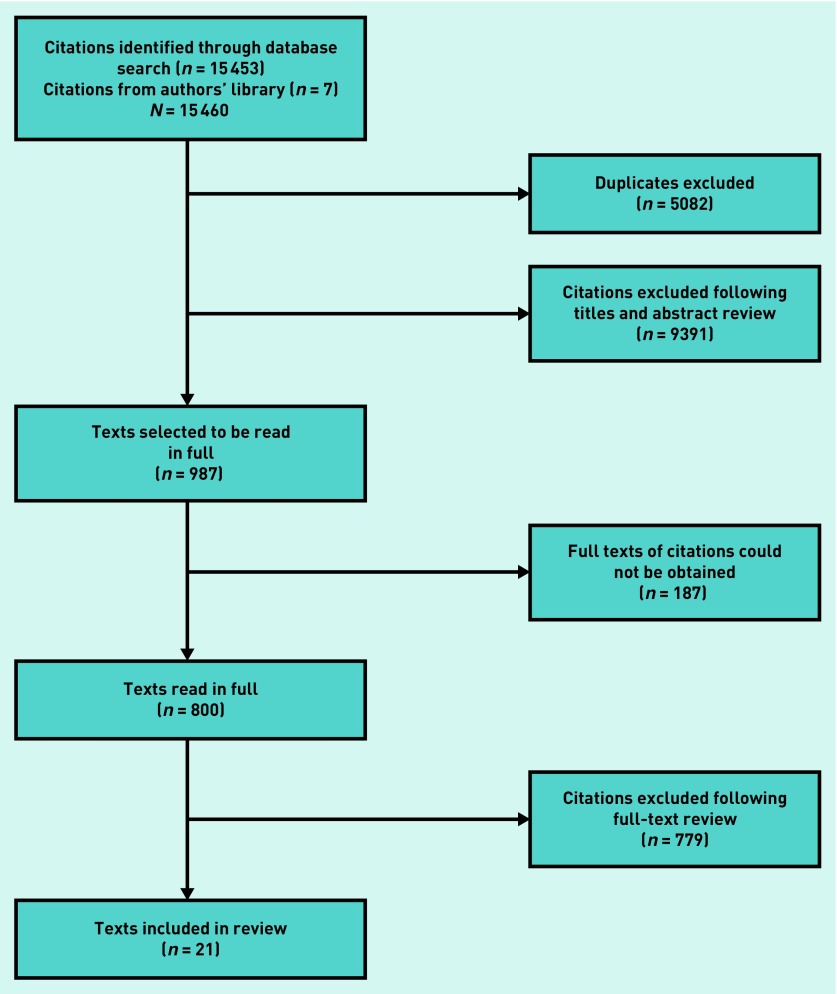
The selection of the 21 studies31–51 included in this review is presented in the PRISMA diagram in Figure 1. Though 987 citations were selected for full-text review, 187 citations could not be obtained despite attempts to contact authors by email. The majority of the citations that were not obtained were in non-English publications (n = 119, 63.6%), while a substantial proportion (n = 90, 48.1%) of these citations reported no clinical data at all in their abstracts, but were selected for full-text review owing to the comprehensive approach taken by the reviewers.
Figure 1.
PRISMA diagram of study inclusion.
The most common reason for exclusion (n = 739) was that the study did not contain research or data that were pertinent to the study question. This included a large number (n = 117) of general texts, such as reviews, correspondence, and educational articles that did not address the study question.
Some citations (n = 59) were excluded because the interpretation of the imaging was undertaken retrospectively, when the diagnosis of lung cancer was already known. Seventeen studies were not eligible because patients had been chosen for inclusion on the basis of a chest X-ray that was known to be positive or negative for lung cancer. Four studies were ineligible because they evaluated individual performance at interpreting chest X-rays using films, where the presence or absence of lesions was already known to the study investigators. Other studies were excluded because: the cancers considered were not a primary lung cancer (n = 44), they were case reports of a single patient (n = 53), the duration between chest X-ray and diagnosis was >1 year or unclear (n = 28), they were drawn from screening data (n = 22), or patients were <18 years of age (n = 2).
Given the high heterogeneity between studies included and their low quality, meta-analysis was not appropriate.
Summary of eligible studies
A final total of 21 studies met the inclusion criteria (Table 1). The number of patients in each study varied notably (range n = 2 to n = 208). Study estimates of sensitivity ranged from 40% to 100%. Most of the studies were case series. Only one study had the primary objective of estimating the diagnostic accuracy of chest X-ray for lung cancer.43
Table 1.
Summary of studies included in reviewa
| Study, year of publication | Participants n | CXR positive, n (%) | 95% CI for percentage | Total CXR negative (%) | CXR negative: normal (%) | CXR negative: abnormal (%) | Histology (%) | Mean age, years | Male,% | Population characteristics | Setting (primary or hospital) | Country |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hamada et al,31 1999 | 31 | 22 (71.0) | 52.0 to 89.9% | 9 (29.0) | — | — | NSCLC (74.2), SCLC (25.8) | 60.6 | 100.0 | Asbestos exposure | Hospital | Japan |
| Tanaka et al,32 1999 | 3 | 3 (100.0) | — | 0 (0.0) | — | — | NSCLC (33.3), pleomorphic (33.3), unknown (33.3) | 72 | 100.0 | Gingival metastasis | Hospital | Japan |
| Bini et al,33 2001 | 2 | 2 (100.0) | — | 0 (0.0) | — | — | Pulmonary blastoma (100) | 62.5 | 100.0 | Pulmonary blastoma | Hospital | Italy |
| Lee et al,34 2001 | 6 | 4 (66.7) | 20.4 to 100% | 2 (33.3) | — | — | SCLC (100) | 62.5 | 50.0 | Paraneoplastic GI dysfunction | Hospital | US |
| Haro et al, 200235 | 208b | 185b (88.9) | 84.4 to 93.5% | 23b (11.1) | — | — | — | 62b | 84.4b | Haemoptysis | Hospital | Spain |
| Losa Gaspà et al,36 2002 | 93 | 84 (90.3) | 84.0 to 96.6% | 9 (9.7) | — | — | — | 63.0 | 72.4b | Metastatic cancer | Hospital | Spain |
| Abraham et al,37 2003 | 23 | 19 (82.6) | 65.6 to 99.7% | 4 (17.4) | — | — | — | 53.4 | 47.8 | Presented with facial pain | Not known | US |
| Gomez et al,38 2004 | 41 | 36 (87.8) | 77.1 to 98.5% | 5 (12.2) | — | — | Carcinoid (100) | 50.0 | 66 | Bronchial carcinoid | Hospital | Spain |
| Kitazaki et al,39 2005 | 2 | 2 (100.0) | — | 0 (0.0) | — | — | NSCLC (100) | 71.5 | 0.00 | Bronchioalveolar treated with gefitinib | Hospital | Japan |
| Bando et al,40 2006c | 15 | 12 (80.0) | 57.3 to 100% | 3 (20.0) | — | — | SCLC (33.3), NSCLC (26.7), others (13.3), unknown (26.7) | 68.3b | 73.3 | Vocal cord paralysis | Hospital | Japan |
| Bjerager et al,41 2006 | 58 | 46 (79.3) | 67.6 to 91.0% | 12 (20.7) | — | — | — | 66d | 64.3b | Patients who were diagnosed with lung cancer in primary care | Primary | Denmark |
| Brock et al,42 2006 | 30 | 12 (40.0) | 12.3 to 67.7% | 18 (60.0) | 9 (30.0) | 9c (30.0) | NSCLC (85.9), SCLC (8.7), other (5.4)b | 46d | 67.4b | Patients with HIV | Hospital | US |
| Stapley et al,43 2006 | 164 | 126 (76.8) | 64.5 to 84.2% | 38 (23.2) | 17 (10.4) | 21 (12.8) | NSCLC (64.0), SCLC (21.1), unspecified carcinoma (10.9), unknown (4.0)b,f | 70.8a | 68.8a | Patients who were diagnosed with lung cancer in primary care | Primary | England |
| Fernandez et al,44 2007c | 102 | 97 (95.1) | 90.8 to 99.4% | 5 (4.9) | 5 (4.9) | — | NSCLC (68.8), SCLC (20.5), anaplastic (9.9), unknown (1.8)b,f | 68b | 85.4b | Patients who were diagnosed with lung cancer in hospital | Hospital | Spain |
| Kato et al,45 2010 | 3 | 3 (100.0) | — | 0 (0.0) | — | — | Squamous cell (100) | 64.7 | 100.0 | Squamous cell carcinoma with necrotic cavities | Hospital | Japan |
| Kikuchi et al,46 2010 | 2 | 2 (100.0) | — | 0 (0.0) | — | — | Pleomorphic carcinoma (100) | 71.0 | 100.0 | Pleomorphic carcinoma | Hospital | Japan |
| Uzun et al,47 2010c | 51 | 50 (98.0) | 94.2 to 100% | 1 (1.9) | — | — | NSCLC (90.2), SCLC (5.9), other (3.9) | 54.3b | 76.4b | Haemoptysis | Hospital | Turkey |
| Mao et al,48 2011 | 10 | 6 (60.0) | 39.2 to 99.2% | 4 (40.0) | — | — | NSCLC (70.0), SCLC (30.0) | 58.7 | 50.0 | Diabetes insipidus from pituitary metastases | Hospital | China |
| Okazaki et al,49 2012 | 2 | 2 (100.0) | — | 0 (0.0) | — | — | SCLC (100) | 75.0 | 50.0 | Gastric metastases from lung primary | Hospital | Japan |
| Barry et al,50 2015 | 158 | 126 (79.7) | 72.7 to 86.8% | 32 (20.2) | 23g (14.6) | 9 (5.7) | — | — | — | Hospital | Hospital | Ireland |
| Ghimire et al,51 2016 | 7 | 7 (100.0) | — | 0 (0.0) | — | — | NSCLC (100) | 54.7b | 76.0b | Patients undergoing bronchoscopy | Hospital | Nepal |
Studies assessed as having low risk of bias are indicated in bold.
Number includes cases that were not eligible, which could not be excluded.
Interpretation of CXR reported as ‘abnormal’ or ‘normal’ but authors did not state which abnormalities were contemporaneously considered to be suspicious for lung cancer at time of reporting CXR. ‘Abnormal’ processed as ‘positive’ for this review.
Median.
‘Non-specific infiltrates’. Authors did not state if these were considered positive or negative.
Percentages are >100.0% due to rounding.
Abnormal but no follow-up recommended. CI = confidence interval. CXR = chest X-ray. GI = gastrointestinal. NSCLC = non-small-cell lung carcinoma. SCLC = small-cell lung carcinoma.
Many of the studies only included particular subgroups of the relevant patient population, such as atypical tumour histology, or specific comorbidities and symptom presentations. Only four studies41,43,44,50 were based on representative populations of patients with lung cancer, rather than particular subgroups.
A population-based observational case series identified all patients in the Danish county of Aarhus who had a diagnosis of lung cancer during a 6-month period in 2003.41 The purpose of the study was to explore reasons for diagnostic delay in lung cancer. Of 58 patients who had a chest X-ray arranged from general practice, 46 (79.3%; 95% CI = 67.6 to 91.0) had chest X-rays that suggested the possibility of lung cancer, including two cases in which infection was identified with a recommendation for repeat imaging after an appropriate interval. In the remaining 12 (20.7%), chest X-rays were reported as ‘raised no suspicion of lung cancer’.
An English retrospective cohort study examined chest X-ray results of 164 patients from general practices in a primary care trust diagnosed with lung cancer between January 1998 and September 2002 (patients aged ≥40 years).43 In over three-quarters (n = 126, 76.8%; 95% CI = 64.5 to 84.2%) the chest X-ray indicated the possibility of lung cancer, while 38 (23.17%) patients had a ‘negative’ chest X-ray. Of the 38 ‘negative’ chest X-rays, 21 (12.8%) were categorised as abnormal but not suspicious of malignancy, while 17 (10.4%) were reported as ‘normal’.
A retrospective case note review of all patients diagnosed with lung cancer in a Spanish centre from January 2001 to September 2006 included 102 patients who had a chest X-ray before diagnosis.44 An ‘abnormality’ was present on 97 (95.1%) of the patients’ chest X-rays; however, this could not be considered synonymous with ‘sensitivity’ as the authors did not indicate which of the abnormalities were considered to be suspicious for lung cancer when they were reported. The abnormalities were nodules or masses in 53 cases (52.0%), pleural effusions in 16 (15.7%), an enlarged hilum in 16 (15.7%), multiple pulmonary metastasis in six (5.9%), a widened mediastinum in four (3.9%), and an interstitial infiltration in two (2.0%).
Finally, a conference abstract reported a retrospective review of chest X-ray reports in a secondary care setting in the Republic of Ireland.50 The authors identified 126 (79.7%, 95% CI = 72.7 to 86.8%) of 158 patients as likely to have a lung malignancy and/or advised to have repeat imaging. A further 23 (14.6%) patients had a chest X-ray in which the authors refer to ‘lesion not identified’ and nine (5.7%) in which an abnormality was identified but no follow-up recommended.
Quality assessment
Assessment of quality was undertaken by two of the authors using a modified version of the QUADAS-2 tool,30 with disagreements between reviewers resolved through discussion. Three studies41,43,50 were deemed to have a low risk of bias. A further study was deemed to have a low risk of bias in the selection of patients;44 however, the reporting of chest X-ray result as normal or abnormal, rather than suspicious or not suspicious for lung cancer, introduced bias that limited applicability for this review. The majority of studies (n = 17, 81.0%) were deemed to have a high risk of bias. In particular, these studies included distinct subgroups of the relevant patient population, such as atypical tumour histology, or specific comorbidities and symptom presentations.
DISCUSSION
Summary
This systematic review identified three studies that reported sensitivity of chest X-ray and that had a low risk of bias. The sensitivity estimates for these studies were: 79.3% (95% CI = 67.6 to 91.0%),41 76.8% (95% CI = 64.5 to 84.2%),43 and 79.7% (95% CI = 72.7 to 86.8%).50
These results suggest that chest X-ray fails to identify lung cancer (at least initially) in >20% of people who are subsequently diagnosed with lung cancer. All three of these studies were conducted in countries with broadly similar primary care systems (Denmark, England, Republic of Ireland). Two of these studies41,43 were derived from primary care settings and, though the remaining study was from a secondary care radiology department,50 it is likely that many of the chest X-rays performed resulted from primary care referrals.
Strengths and limitations
This review featured a sensitive and comprehensive search of bibliographic databases and grey literature in order to identify published and unpublished sources. This study is highly relevant both to national cancer policy and everyday clinical practice. With approximately 46 700 new diagnoses of lung cancer in the UK per year,2 of which approximately 56% are diagnosed following referral for chest X-ray,7 these findings suggest that false-negative chest X-rays could contribute to a delayed diagnosis for several thousand patients each year.
Diagnostic accuracy was the stated primary outcome of only one study; in most included studies a value of sensitivity was estimated from data reported. These studies were therefore at high risk of bias. Indeed, none conformed to the conventional standards of diagnostic accuracy studies.52 While the best available evidence was selected for analysis, many other eligible studies were of poor quality making meta-analysis inappropriate. In order to identify all relevant evidence, the present review included studies from different settings. The different disease prevalence in primary and secondary care is known to impact on test performance,53 which could not be accounted for in this review. However, the consistency in the sensitivity estimates from the higher-quality studies is striking. Due to the large number of citations, selection was peer reviewed in only 20% of cases and data extraction was conducted by one researcher. Notably, 187 citations could not be obtained, reflecting the broad search strategy used and the low threshold used for selection for full-text review. Only about half of those articles (n = 97, 51.2%) contained any study data in their abstracts.
Comparison with existing literature
Several studies have evaluated the performance of chest X-ray by re-examining radiographs in the light of a known lung cancer diagnosis. Although such studies were not eligible for this review, that literature provides an important context. Notably, a Dutch retrospective review of radiographs of non-small-cell lung cancer cases (n = 495) reported that 19% had a nodular lesion that had been ‘missed’.54
It is possible that lung cancers may not have been present when imaging occurred (interval cancers). A large screening trial concluded that, of those cancers that were not detected on screening chest X-ray but subsequently diagnosed within 1 year, the lung cancer was not visible, even in retrospect, in 65% of cases.55
Separate literature has explored the role of ‘observer error’ in failing to recognise cancers that were evident in retrospect. Inexperience, poor technique in visual scanning of the image, failures in recognising abnormalities, and of decision making along with lapses of concentration have all been identified as factors contributing to missed lung cancers on chest X-ray.56,57
Other studies have considered the characteristics of lesions, which may make them less identifiable. Smaller tumours are identified much less frequently; lesions measuring <1 cm in diameter are particularly likely to be missed on chest X-ray compared with other modalities such as CT.54
Location is also important, with missed lung cancers frequently located in the upper lobes54,58–61 or obscured by overlying anatomy such as ribs, lung vasculature, and the heart. Many missed cancers are located in the hilar regions, where the confluence of complex anatomy makes diagnosis particularly challenging.56 The technical quality of the radiograph itself and the positioning of the patient are additional factors that can influence the likelihood of successful detection of lung cancer on chest X-ray.62
Implications for research and practice
Chest X-ray retains a predominant role in UK clinical practice and guidance for the diagnosis of lung cancer.63 Most lung cancers are diagnosed following suspicious findings on chest X-ray7 and increasing the use of chest X-ray in primary care has been associated with diagnosis at an earlier stage and reduced mortality.64 However, this review suggests that chest X-ray may have a false-negative rate of at least 20%. GPs should take limited reassurance from a non-diagnostic chest X-ray and consider additional imaging or referral of those at high risk, or re-imaging in the face of continuing symptoms. If chest X-ray were a novel technology, it is debatable whether the available evidence would be deemed sufficient to support its implementation as a diagnostic test for lung cancer. In order to improve the UK’s lung cancer outcomes, diagnostic strategies may necessitate widening access to more definitive modalities, such as CT. Although this study has demonstrated a significant false-negative rate for chest X-ray, it is important to recognise that the benefits of increased rates of CT investigation must be balanced against known harms including overdiagnosis and false-positives.65 Future work is required to determine which patients can be reasonably followed up by safety netting following an unremarkable chest X-ray and which patients require further investigation.
Acknowledgments
The authors are extremely grateful to Monica Koo, Marie Bourne, Nazia Ahmed, Sibel Saya, and Dorota Karasek for their assistance in translation of non-English studies; Judy Wright and Natalie King for their advice regarding the systematic review; and Tracey Farragher for providing additional advice regarding the design of the study. The authors would also like to thank the many authors who kindly provided additional information in order to determine eligibility of their studies for this review.
Funding
Stephen H Bradley and Bethany Shinkins are funded by the multi-institutional CanTest Collaborative, which is funded by Cancer Research UK (reference number: C8640/A23385). Richard D Neal and William T Hamilton are Associate Directors of the multi-institutional CanTest Collaborative, which is funded by Cancer Research UK (reference number: C8640/A23385).
Ethical approval
Ethical approval is not required.
Provenance
Freely submitted; externally peer reviewed.
Competing interests
Matthew EJ Callister is the chief Investigator for Yorkshire Lung Screening Trial and co-Investigator for Yorkshire Enhanced Stop Smoking Study. Both studies are funded by Yorkshire Cancer Research. The other authors have no competing interests.
Discuss this article
Contribute and read comments about this article: bjgp.org/letters
REFERENCES
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Cancer Research UK Lung cancer statistics. 2019. https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/lung-cancer (accessed 19 Sep 2019)
- 3.Cancer Research UK Breast cancer survival statistics. 2014. https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/breast-cancer/survival#heading-Two (accessed 19 Sep 2019)
- 4.Rubin G, Berendsen A, Crawford SM, et al. The expanding role of primary care in cancer control. Lancet Oncol. 2015;16(12):1231–1272. doi: 10.1016/S1470-2045(15)00205-3. [DOI] [PubMed] [Google Scholar]
- 5.Hamilton W. Cancer diagnosis in primary care. Br J Gen Pract. 2010. [DOI] [PMC free article] [PubMed]
- 6.Public Health England. Patient dose information: guidance; X-ray examinations. 2008. https://www.gov.uk/government/publications/medical-radiation-patient-doses/patient-dose-information-guidance (accessed 19 Sep 2019)
- 7.Aslam R, Kennedy MP, Bhartia B, et al. The radiological route to diagnosis of lung cancer patients. Thorax. 2018;73(Suppl 4):A70–A71. [Google Scholar]
- 8.National Institute for Health and Care Excellence . Lung cancer: diagnosis and management CG121. London: NICE; 2011. https://www.nice.org.uk/guidance/cg121/chapter/1-Guidance (accessed 19 Sep 2019) [PubMed] [Google Scholar]
- 9.Arnold M, Rutherford MJ, Bardot A, et al. Progress in cancer survival, mortality, and incidence in seven high-income countries 1995–2014 (ICBP SURVMARK-2): a population-based study. Lancet Oncol. 2019 doi: 10.1016/S1470-2045(19)30456-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eurostat, Statistics Explained. Healthcare resource statistics-technical resources and medical technology. 2019. https://ec.europa.eu/eurostat/statistics-explained/index.php/Healthcare_resource_statistics_-_technical_resources_and_medical_technology#Availability_of_technical_resources_in_hospitals. (accessed Oct 2019)
- 11.Bradley S, Grice A, Lopez RR, et al. Diagnostic accuracy of low dose CT versus chest x-ray and false negative rates for chest x-ray in lung cancer. 2019. https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=69629 (accessed 19 Sep 2019)
- 12.Royal College of Radiologists. https://www.rcr.ac.uk (accessed 19 Sep 2019)
- 13.American College of Radiology https://www.acr.org/ (accessed 19 Sep 2019)
- 14.American Society of Clinical Oncology. https://www.asco.org/ (accessed 19 Sep 2019)
- 15.ASTRO: American Society of Radiation Oncology. https://www.astro.org (accessed 19 Sep 2019)
- 16.British Institute of Radiology. https://bir.org.uk (accessed 19 Sep 2019)
- 17.European Society for Radiotherapy Oncology. https://www.estro.org (accessed 19 Sep 2019)
- 18.European Society for Medical Oncology. https://www.esmo.org (accessed 19 Sep 2019)
- 19.International Society of Radiology. http://isradiology.org/2017/isr/index.php (accessed 19 Sep 2019)
- 20.International Association for the Study of Lung Cancer. https://www.iaslc.org (accessed 19 Sep 2019)
- 21.British Thoracic Society. https://www.brit-thoracic.org.uk (accessed 19 Sep 2019)
- 22.British Thoracic Oncology Group. https://www.btog.org (accessed 19 Sep 2019)
- 23.Public Health England National Cancer Registration Analysis Service. 2016. https://www.gov.uk/guidance/national-cancer-registration-and-analysis-service-ncras (accessed 19 Sep 2019)
- 24.European Respiratory Society. https://www.ersnet.org (accessed 19 Sep 2019)
- 25.American Thoracic Society. http://www.thoracic.org (accessed 19 Sep 2019)
- 26.Cancer and Primary Care Research International Network. http://www.ca-pri.org (accessed 19 Sep 2019)
- 27.Detterbeck FC, Gibson CJ. Turning gray: the natural history of lung cancer over time. J Thorac Oncol. 2008;3(7):781–792. doi: 10.1097/JTO.0b013e31817c9230. [DOI] [PubMed] [Google Scholar]
- 28.Chien C-R, Lai M-S, Chen TH-H. Estimation of mean sojourn time for lung cancer by chest X-ray screening with a Bayesian approach. Lung Cancer. 2008;62(2):215–220. doi: 10.1016/j.lungcan.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 29.Wu D, Erwin D, Rosner GL. Sojurn time and lead time projection in lung cancer screening. Lung Cancer. 2011;72(3):322–326. doi: 10.1016/j.lungcan.2010.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Whiting PF, Rutjes AWS, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155(8):529–536. doi: 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- 31.Hamada K, Tokuyama T, Okamoto Y, et al. A clinicopathological study of lung cancer patients with occupational exposure to chrysotile asbestos fibers. Intern Med. 1999;38(10):780–784. doi: 10.2169/internalmedicine.38.780. [DOI] [PubMed] [Google Scholar]
- 32.Tanaka M, Sawada M, Inase N, et al. Cases of gingival metastasis from lung cancer and a review of the literature. (In Japanese) Japanese Journal of Lung Cancer. 1999;39(3):323–329. [Google Scholar]
- 33.Bini A, Ansaloni L, Grani G, et al. Pulmonary blastoma: report of two cases. Sur Today. 2001;31(5):438–442. doi: 10.1007/s005950170136. [DOI] [PubMed] [Google Scholar]
- 34.Lee HR, Lennon VA, Camilleri M, Prather CM. Paraneoplastic gastrointestinal motor dysfunction: clinical and laboratory characteristics. Am J Gastroenterol. 2001;96(2):373–379. doi: 10.1111/j.1572-0241.2001.03454.x. [DOI] [PubMed] [Google Scholar]
- 35.Haro M, Jimenez J, Tornero A, et al. Usefulness of computerized tomography and bronchoscopy in patients with hemoptysis. Analysis of 482 cases. (In Spanish) An Med Interna. 2002;19(2):59–65. [PubMed] [Google Scholar]
- 36.Losa Gaspà F, Germá JR, Albareda JM, et al. Metastatic cancer presentation. Validation of a diagnostic algorithm with 221 consecutive patients. (In Spanish) Rev Clin Esp. 2002;202(6):313–319. doi: 10.1016/s0014-2565(02)71065-5. [DOI] [PubMed] [Google Scholar]
- 37.Abraham PJ, Capobianco DJ, Cheshire WP. Facial pain as the presenting symptom of lung carcinoma with normal chest radiograph. Headache. 2003;43(5):499–504. doi: 10.1046/j.1526-4610.2003.03097.x. [DOI] [PubMed] [Google Scholar]
- 38.Gomez A, Zalacain R, Cabriada V, et al. Bronchial carcinoid tumors. Analysis of 41 cases. (In Spanish) Rev Clin Esp. 2004;204(4):202–205. doi: 10.1157/13060271. [DOI] [PubMed] [Google Scholar]
- 39.Kitazaki T, Fukuda M, Soda H, Kohno S. Novel effects of gefitinib on mucin production in bronchioloalveolar carcinoma; two case reports. Lung Cancer. 2005;49(1):125–128. doi: 10.1016/j.lungcan.2004.11.027. [DOI] [PubMed] [Google Scholar]
- 40.Bando H, Nishio T, Bamba H, et al. Vocal fold paralysis as a sign of chest diseases: a 15-year retrospective study. World J Surgery. 2006;30(3):293–298. doi: 10.1007/s00268-005-7959-x. [DOI] [PubMed] [Google Scholar]
- 41.Bjerager M, Palshof T, Dahl R, et al. Delay in diagnosis of lung cancer in general practice. Br J Gen Pract. 2006;56(532):863–868. [PMC free article] [PubMed] [Google Scholar]
- 42.Brock MV, Hooker CM, Engels EA, et al. Delayed diagnosis and elevated mortality in an urban population with HIV and lung cancer: implications for patient care. J Acquir Immume Defic Syndr. 2006;43(1):47–55. doi: 10.1097/01.qai.0000232260.95288.93. [DOI] [PubMed] [Google Scholar]
- 43.Stapley S, Sharp D, Hamilton W. Negative chest X-rays in primary care patients with lung cancer. Br J Gen Pract. 2006;56(529):570–573. [PMC free article] [PubMed] [Google Scholar]
- 44.Fernandez V, Alonso JL, Munuera L, et al. Analysis of lung cancer cases diagnosed in an internal medicine department: from January 2001 to September 2006. (In Spanish) An Sist Sanit Navar. 2007;30(3):353–362. doi: 10.4321/s1137-66272007000500004. [DOI] [PubMed] [Google Scholar]
- 45.Kato T, Narita K, Ohara K. Three cases of squamous cell carcinomas which enlarged rapidly with necrotic cavities after bronchoscopy. (In Japanese) Japanese Journal of Lung Cancer. 2010;50(6):822–827. [Google Scholar]
- 46.Kikuchi R, Isowa N, Tokuyasu H, et al. Three cases of resected pleomorphic carcinoma. Ann Thorac Cardiovasc Surg. 2010;16(4):264–269. [PubMed] [Google Scholar]
- 47.Uzun O, Atasoy Y, Findik S, et al. A prospective evaluation of hemoptysis cases in a tertiary referral hospital. Clin Resp J. 2010;4(3):131–138. doi: 10.1111/j.1752-699X.2009.00158.x. [DOI] [PubMed] [Google Scholar]
- 48.Mao JF, Zhang JL, Nie M, et al. Diabetes insipidus as the first symptom caused by lung cancer metastasis to the pituitary glands: clinical presentations, diagnosis, and management. J Postgrad Med. 2011;57(4):302–306. doi: 10.4103/0022-3859.90080. [DOI] [PubMed] [Google Scholar]
- 49.Okazaki A, Araya T, Sakai A, et al. Two cases of small cell lung cancer with metastasis to the stomach at initial diagnosis. (In Japanese) Japanese Journal of Lung Cancer. 2012;52(2):220–225. [Google Scholar]
- 50.Barry C, Bergin D. Non-detected primary lung cancers on chest x-ray: 3 year retrospective review in university hospital. Ir J Med Sci. 2015;1:S262. [Google Scholar]
- 51.Ghimire RH, Bhatta N, Koirala P, et al. Outcomes bronchoscopic evaluation in a university hospital. JNMA J Nepal Med Assoc. 2016;55(204):51–54. [PubMed] [Google Scholar]
- 52.Bossuyt PM, Reitsma JB, Bruns DE, et al. STARD 2015: an updated list of essential items for reporting diagnostic accuracy studies. BMJ. 2015;351:h5527. doi: 10.1136/bmj.h5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bentley TG, Catanzaro A, Ganiats TG. Implications of the impact of prevalence on test thresholds and outcomes: lessons from tuberculosis. BMC Res Notes. 2012;5(1):563. doi: 10.1186/1756-0500-5-563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Quekel LGBA, Kessels AGH, Goei R, van Engelshoven JMA. Miss rate of lung cancer on the chest radiograph in clinical practice. Chest. 1999;115(3):720–724. doi: 10.1378/chest.115.3.720. [DOI] [PubMed] [Google Scholar]
- 55.Kvale PA, Johnson CC, Tammemägi M, et al. Interval lung cancers not detected on screening chest X-rays: how are they different? Lung Cancer. 2014;86(1):41–46. doi: 10.1016/j.lungcan.2014.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Del Ciello A, Franchi P, Contegiacomo A, et al. Missed lung cancer: when, where, and why? Diag Interv Radiol. 2017;23(2):118–126. doi: 10.5152/dir.2016.16187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kundel HL, La Follette PS., Jr Visual search patterns and experience with radiological images. Radiology. 1972;103(3):523–528. doi: 10.1148/103.3.523. [DOI] [PubMed] [Google Scholar]
- 58.Austin JH, Romney BM, Goldsmith LS. Missed bronchogenic carcinoma: radiographic findings in 27 patients with a potentially resectable lesion evident in retrospect. Radiology. 1992;182(1):115–122. doi: 10.1148/radiology.182.1.1727272. [DOI] [PubMed] [Google Scholar]
- 59.Shah PK, Austin JHM, White CS, et al. Missed non-small cell lung cancer: radiographic findings of potentially resectable lesions evident only in retrospect. Radiology. 2003;226(1):235–241. doi: 10.1148/radiol.2261011924. [DOI] [PubMed] [Google Scholar]
- 60.Wu MH, Gotway MB, Lee TJ, et al. Features of non-small cell lung carcinomas overlooked at digital chest radiography. Clin Radiology. 2008;63(5):518–528. doi: 10.1016/j.crad.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 61.Theros EG. 1976 Caldwell Lecture: varying manifestation of peripheral pulmonary neoplasms: a radiologic-pathologic correlative study. AJR Am J Roentgen. 1977;128(6):893–914. doi: 10.2214/ajr.128.6.893. [DOI] [PubMed] [Google Scholar]
- 62.Brogdon B, Kelsey C, Moseley RD., Jr Factors affecting perception of pulmonary lesions. Radiol Clin North Am. 1983;21(4):633–654. [PubMed] [Google Scholar]
- 63.National Institute for Health and Care Excellence . Suspected cancer: recognition and referral NG12. London: NICE; 2015. https://www.nice.org.uk/guidance/ng12 (accessed 19 Sep 2019) [PubMed] [Google Scholar]
- 64.Kennedy MPT, Cheyne L, Darby M, et al. Lung cancer stage-shift following a symptom awareness campaign. Thorax. 2018;73(12):1128–1136. doi: 10.1136/thoraxjnl-2018-211842. [DOI] [PubMed] [Google Scholar]
- 65.Heleno B, Siersma V, Brodersen J. Estimation of overdiagnosis of lung cancer in low-dose computed tomography screening: a secondary analysis of the Danish lung cancer screening trial. JAMA Intern Med. 2018;178(10):1420–1422. doi: 10.1001/jamainternmed.2018.3056. [DOI] [PMC free article] [PubMed] [Google Scholar]