Abstract
This study tested the hypothesis that mid-life intellectual, physical and social activities contribute to cognitive reserve (CR). Two hundred and five individuals (196 with MRI) aged 66-88 from the Cambridge Centre for Ageing and Neuroscience (www.cam-can.com) were studied, with cognitive ability and structural brain health measured as fluid IQ and total grey matter volume, respectively. Mid-life activities were measured using the Lifetime of Experiences Questionnaire.
Multivariable linear regression found that mid-life activities (MA) made a unique contribution to late-life cognitive ability independent of education, occupation and late-life activities. Crucially, MA moderated the relationship between late-life cognitive ability and brain health, with the cognitive ability of people with higher MA less dependent on their brain structure, consistent with the concept of CR.
In conclusion, mid-life activities contribute uniquely to CR. The modifiability of these activities has implications for public health initiatives aimed at dementia prevention.
1. Introduction
Participants, materials and analyses
The concept of cognitive reserve is used to explain why some individuals maintain cognitive ability despite impaired brain health as a consequence of ageing and diseases such as Alzheimer’s disease (Stern 2012, Nilsson & Lövdén 2018). Crucially, the CR concept encompasses the notion that late life cognitive activity is influenced by factors occurring earlier in life. Determination of contributors to cognitive reserve is therefore important for “successful” ageing and prevention of dementia. While epidemiological evidence suggests that education and occupation contribute to cognitive reserve (Richards and Deary 2005), there is increasing interest in the additional contribution of other activities undertaken in midlife, given their potential modifiability. This interest is amplified by evidence that mid-life activities of a social or intellectual nature are associated with higher late-life cognitive ability, after adjusting for childhood cognitive ability (Gow et al. 2017), and by a recent review concluding that low levels of physical and social activity in adulthood represent key risk factors for dementia (Livingston et al. 2017).
Rigorous definitions of cognitive reserve not only predict that lifestyle factors will relate to late-life cognitive ability, but also that such factors will moderate the relationship between cognitive ability and brain structure. Specifically, the cognitive ability of individuals with high cognitive reserve should be less dependent on brain structure than those with low cognitive reserve, possibly as a result of compensatory functional network reorganisation (Stern 2017, see also Nilsson & Lövdén 2018). The present study therefore asked whether mid-life activities (MAs) contribute to cognitive reserve by testing two hypotheses: i) MAs contribute to late-life cognitive ability independent of early-life education, mid-life occupation and late-life activities, and ii) MAs moderate the relationship between cognitive ability and brain structure, such that the relationship is weaker in people with who had engaged in more MAs.
Two hundred and five individuals (93 female) aged 66-88 years were selected from the Cambridge Centre for Ageing and Neuroscience (Cam-CAN, www.cam-can.com, Shafto et al 2014) cohort (see Supplementary Materials for further description of this sample). Cognitive ability was measured using the Cattell Culture Fair test of fluid intelligence (1971) and lifestyle activities by the Lifetime of Experiences Questionnaire (LEQ, Valenzuela and Sachdev 2007), modified for UK participants.
The LEQ measures a broad range of cognitively-stimulating experiences and activities during three life phases: youth, 13-29 years; mid-life, 30-64 years; and late-life, 65 years onwards. Within each phase, activities are subdivided into specific or nonspecific. Specific activities are those that are considered to be undertaken primarily within one particular life phase, such as education or working occupation. In contrast, nonspecific activities such as socializing and playing sports are those can be undertaken at any age, and so are applicable to any life phase. The youth specific score (YS, or education) was derived from the UK’s National Career Service categories, multiplied by number of years at each category. The mid-life specific score (MS, or occupation) was based on the standard occupational classification codes from the UK Office of National Statistics, summed across seven mid-life periods. The late-life specific score (LS, or post-retirement activities) reflected social and intellectual activities such as travel or participation in volunteer organisations. The current LEQ does not cater for some “specific” activities being undertaken at other life stages (eg education in mid- or late life) and so their potential contribution to CR could not be evaluated in the current study.
Scores for non-specific activities in youth (YA), mid-life (MA) and late-life (LA) were summed across seven questions about social, intellectual and physical activities. These addressed participation in i) travel, ii) social outings, iii) playing a musical instrument, iv) artistic pastimes, v) physical activity (mild, moderate, vigorous), vi) reading, vii) speaking a second language. Each of the six resulting scores (two types of activity, specific and non-specific, across the three life phases) was scaled to a score from 0-10.
T1- and T2-weighted 1mm isotropic MRI scans were available for 196 participants. Brain structure was measured in terms of total grey matter volume (TGM, see Taylor et al. 2017 and Supplementary Materials). Two participants with outlying adjusted TGM values were removed. The analysis used linear regression via the “lm” function in R 3.5.0 (R Core Team, 2016) to relate Cattell scores to the six LEQ scores above, plus age and sex. Data and analysis scripts are available on the Open Science Framework here: https://osf.io/32gme/, which includes individual scores for the 13 LEQ questions that comprise the MA sum score.
2. Results
The covariance and correlation of Cattell, LEQ scores and Age are shown in a Supplementary Table (Supplementary Materials). All LEQ scores were significantly positively correlated with each other, and with Cattell.
Multivariable regression of late-life cognitive ability on the LEQ scores, with age and sex as covariates, showed a strong overall association (adjusted R2=0.355, F(8,196)=15.0, p<.001). In addition to the expected negative effect of age, the coefficients in Table 1 revealed a unique, positive contribution of YS (i.e education), replicating previous studies such as Richards and Deary (2005). More interestingly, midlife nonspecific activities (MA) also made an independent positive contribution after adjustment for all other factors. No other LEQ-based category, including the current late-life activities being performed by the individuals (reflected in both LS and LA), made an independent contribution. After adjusting for age and sex, separate regression of MA on cognitive ability revealed an effect size of R2=16.0, comparable to that for YS (education, R2=15.8%).
Table 1. Results of multivariable linear regression of late-life cognitive ability (Cattell) against six lifetime experience scores from the LEQ, plus age and sex.
| Coefficient | Standard Error | p-value (df=196) | |
|---|---|---|---|
| Young specific activities | +0.465 | 0.128 | <0.0001 |
| Young nonspecific activities | +0.079 | 0.222 | 0.723 |
| Midlife specific activities | +0.218 | 0.156 | 0.164 |
| Midlife nonspecific activities | +0.989 | 0.229 | <0.0001 |
| Late life specific activities | +0.343 | 0.214 | 0.110 |
| Late life nonspecific activities | -0.347 | 0.266 | 0.195 |
| Age | -0.297 | 0.061 | <0.0001 |
| Sex | -1.00 | 0.781 | 0.201 |
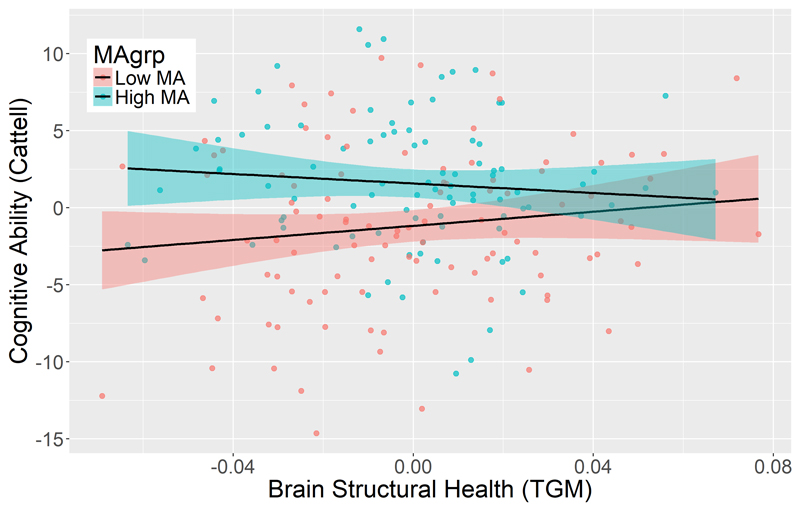
To examine whether MAs contributed not just to late-life cognitive ability but also to cognitive reserve, rigorously defined, we determined whether MAs moderated the relationship between late-life cognitive ability and brain structure (as indexed by TGM). We first adjusted Cattell and TGM scores for 1) Education (YS score), 2) Total Intracranial Volume (TIV) to correct for inter-individual differences in head size, 3) Age and 4) Sex. Linear regression showed an interaction between TGM and MA in predicting Cattell (normalized interaction coefficient = -0.722, standard error = 0.331, p=0.030). This moderating term was negative, meaning that the relationship between cognitive ability and brain health diminished with higher MA, as predicted by cognitive reserve theory. This effect is visualized in Figure 1, where a median split is used to divide participants into low and high MA groups: a more positive slope can be seen in the low MA group than in the high MA group.
Figure 1.
Relationships between cognitive ability and brain structure in the high and low MA groups. Both variates were adjusted for youth-specific activities (education), total intracranial volume, age and sex.
3. Discussion
This study tested the hypothesis that lifestyle activities in mid-life contribute to the cognitive reserve that supports cognitive ability in late-life. Consistent with this, we found that general mid-life activities (MA) make an independent contribution to late-life cognition over and above age, sex, education, occupation and current (late-life) activities, replicating the findings of Gow et al (2017). However, a rigorous determination of cognitive reserve requires further evidence that it moderates the association between a brain state and cognitive outcome (e.g., Brayne et al 2010). Importantly, we found such a moderation by MA on the relationship between total grey matter volume and cognitive ability, such that cognitive ability in those older individuals who had been involved in rich and varied lifestyle activities in mid-life were less dependent on their current structural brain health.
This evidence that midlife activities contribute to cognitive reserve may have ramifications for the primary prevention of dementia. These activities reflect lifestyle choices, and are therefore amenable to modification. Our observation that the impact of MA was independent of educational attainment and occupational status, suggests that a public health initiative aimed at boosting cognitive reserve via enhancement of MA is generalizable to the entire adult population. The importance of such initiatives for primary prevention of dementia is underscored by the total failure to date to identify interventions for secondary prevention of dementia.
This study does not address the relative contribution to cognitive reserve of the various intellectual, social and physical components of the LEQ midlife activities score. This is because the LEQ non-specific scores are a composite across 13 questions that are not designed to separate these different components. However, other studies shed some light on this issue. While some studies have suggested that physical activity reduces future dementia risk (Richards et al 2003, Middleton et al 2010), the UK Whitehall II study found no association between physical activity and subsequent 15 year cognitive decline (Sabia et al 2017). Similarly, Gow et al (2017) found that mid-life intellectual and social activities, but not physical activity, were associated with late-life cognitive health. Other studies have also highlighted the benefits of intellectual and social activity (Akbaraly et al. 2009, Köhncke et al 2016). Future work, complementing the LEQ with specific measures for each of these MA components, will be needed to clarify this issue.
Another key objective for future research is identification of the biological mechanisms by which MA exert an effect on late life cognitive ability. On a molecular level, it is possible that the effect is mediated via BDNF-induced synaptogenesis and neurogenesis. There is evidence that the BDNF val66met polymorphism moderates the relationship between CR and cognition (Ward et al. 2015), and high levels of BDNF expression in postmortem brain are associated with slower rates of cognitive decline (Buchman et al 2016). At systems level, it has long been speculated that a CR effect may be mediated via alterations in functional connectivity. Task-free fMRI studies have identified CR-related changes in network topology (Marques et al. 2016), while there is emerging evidence from task-related fMRI work that CR may be associated with a functional network that is task-invariant (Stern 2017). Complementing these whole brain approaches, others have identified regional connectivity changes associated with CR (Franzmeier et al. 2017).
There are important limitations of this study. First, LEQ measures are self-report, raising the possibility that more cognitively healthy older people remember more lifetime activities. Second, a limitation of cross-sectional studies is the possibility of reverse causation, namely that a higher cognitive ability throughout life triggers the pursuit of more beneficial lifestyle activities, rather than vice versa. Third, the study cohort did not undergo testing for AD biomarkers and so the potential confounding effect of AD pathology could not be addressed. Finally, LEQ scores were highly positively correlated, so we cannot infer that other variables (e.g, occupation) play no role in late-life cognitive ability. These limitations could be addressed by intervention studies targeting specific aspects of mid-life activity, with later life cognitive ability as the outcome measure, though such studies would require a timeframe of 20-30 years.
In conclusion, our findings suggest that lifestyle activities in mid-life can contribute to cognitive reserve and support late-life cognition. The potential modifiability of these activities has important implications for public health initiatives aimed at reducing the risk of dementia.
Supplementary Material
Acknowledgments
DC is funded by the Cambridge National Institute for Health Research (NIHR) Biomedical Research Centre. FM is part-funded by Medical Research Council (MC U105292687). MV is funded by a National Health and Medical Research Council of Australia Career Development Fellowship. The Cambridge Centre for Ageing and Neuroscience (Cam-CAN) research was supported by the Biotechnology and Biological Sciences Research Council (grant number BB/H008217/1). Additional support to RH and RK was from the Medical Research Council (grants SUAG/010 RG91365 and SUAG/014 RG91365) and the European Union’s Horizon 2020 research and innovation programme under grant agreement No 732592. We are grateful to the Cam-CAN respondents and their primary care teams in Cambridge for their participation in this study. The Cam-CAN corporate author consists of the people named here: http://www.cam-can.com/index.php?content=corpauth#12.
References
- Stern Y. Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol. 2012;11:1006–1012. doi: 10.1016/S1474-4422(12)70191-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson J, Lövdén M. Naming is not explaining: future directions for the "cognitive reserve" and "brain maintenance" theories. Alzheimers Res Ther. 2018;10:34. doi: 10.1186/s13195-018-0365-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards M, Deary IJ. A life course approach to cognitive reserve: a model for cognitive aging and development? Ann Neurol. 2005;58:617–22. doi: 10.1002/ana.20637. [DOI] [PubMed] [Google Scholar]
- Gow AJ, Pattie A, Deary IJ. Lifecourse Activity Participation From Early, Mid, and Later Adulthood as Determinants of Cognitive Aging: The Lothian Birth Cohort 1921. J Gerontol B Psychol Sci Soc Sci. 2017;72:25–37. doi: 10.1093/geronb/gbw124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston G, Sommerlad A, Orgeta V, et al. Dementia prevention, intervention, and care. Lancet. 2017 Jul 19;2017 doi: 10.1016/S0140-6736(17)31363-6. pii: S0140-6736(17)31363-6. [DOI] [PubMed] [Google Scholar]
- Stern Y. An approach to studying the neural correlates of reserve. Brain Imaging Behav. 2017;11:410–416. doi: 10.1007/s11682-016-9566-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafto MA, Tyler LK, Dixon M, et al. The Cambridge Centre for Ageing and Neuroscience (Cam-CAN) study protocol: a cross-sectional, lifespan, multidisciplinary examination of healthy cognitive ageing. BMC Neurol. 2014 Oct 14;14:204. doi: 10.1186/s12883-014-0204-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattell RB. Abilities: their Structure, Growth, and Action. Houghton-Mifflin; 1971. [Google Scholar]
- Valenzuela MJ, Sachdev P. Assessment of complex mental activity across the lifespan: development of the Lifetime of Experiences Questionnaire (LEQ) Psychol Med. 2007;37:1015–25. doi: 10.1017/S003329170600938X. [DOI] [PubMed] [Google Scholar]
- Taylor JR, Williams N, Cusack R, et al. The Cambridge Centre for Ageing and Neuroscience (Cam-CAN) data repository: Structural and functional MRI, MEG, and cognitive data from a cross-sectional adult lifespan sample. NeuroImage. 2017;144:262–269. doi: 10.1016/j.neuroimage.2015.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EClipSE Collaborative Members. Brayne C, Ince PG, et al. Education, the brain and dementia: neuroprotection or compensation? Brain. 2010;133:2210–6. doi: 10.1093/brain/awq185. [DOI] [PubMed] [Google Scholar]
- Richards M, Hardy R, Wadsworth ME. Does active leisure protect cognition? Evidence from a national birth cohort. Soc Sci Med. 2003;56:785–92. doi: 10.1016/s0277-9536(02)00075-8. [DOI] [PubMed] [Google Scholar]
- Middleton LE, Barnes DE, Lui LY, et al. Physical activity over the life course and its association with cognitive ability and impairment in old age. J Am Geriatr Soc. 2010;58:1322–6. doi: 10.1111/j.1532-5415.2010.02903.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbaraly TN, Portet F, Fustinoni S, et al. Leisure activities and the risk of dementia in the elderly: results from the Three-City Study. Neurology. 2009;73:854–61. doi: 10.1212/WNL.0b013e3181b7849b. [DOI] [PubMed] [Google Scholar]
- Köhncke Y, Laukka EJ, Brehmer Y, et al. Three-year changes in leisure activities are associated with concurrent changes in white matter microstructure and perceptual speed in individuals aged 80 years and older. Neurobiol Aging. 2016;41:173–186. doi: 10.1016/j.neurobiolaging.2016.02.013. [DOI] [PubMed] [Google Scholar]
- Ward DD, Summers MJ, Saunders NL, et al. The BDNF Val66Met polymorphism moderates the relationship between cognitive reserve and executive function. Transl Psychiatry. 2015 Jun 30;5:e590. doi: 10.1038/tp.2015.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchman AS, Yu L, Boyle PA, et al. Higher brain BDNF gene expression is associated with slower cognitive decline in older adults. Neurology. 2016;86:735–41. doi: 10.1212/WNL.0000000000002387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques P, Moreira P, Magalhães R, et al. The functional connectome of cognitive reserve. Hum Brain Mapp. 2016;37:3310–22. doi: 10.1002/hbm.23242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzmeier N, Hartmann JC, Taylor ANW, et al. Left frontal hub connectivity during memory performance supports reserve in aging and mild cognitive impairment. J Alzheimers Dis. 2017;59:1381–1392. doi: 10.3233/JAD-170360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone IB, Leung KK, Clegg S, Barnes J, Whitwell J, Ashburner J, Fox NC, Ridgway GR. Accurate automatic estimation of total intracranial volume: A nuisance variable with less nuisance. Neuroimage. 2015;104:366–372. doi: 10.1016/j.neuroimage.2014.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.