Abstract
Background
Non-invasive ventilation (NIV) has been shown to improve survival and quality of life in COPD patients with chronic hypercapnic respiratory failure. However, the proportion of COPD patients with chronic hypercapnia is not yet known and clinical data enabling better identification of patients are scarce. The HOmeVent registry was initiated to determine the prevalence of chronic hypercapnia in COPD in an outpatient setting and to evaluate the predictors of hypercapnia.
Methods
HOmeVent is a multicenter, prospective, observational, non-interventional patient registry that includes COPD patients in GOLD stage 3 or 4. Eligible patients were identified and enrolled in an outpatient setting during routine clinic visits. Assessments included blood gas analyses, pulmonary function testing and quality of life assessment.
Results
Ten outpatient clinics in Germany enrolled 231 COPD patients in the registry (135 in GOLD stage 3 (58%) and 96 in GOLD stage 4 (42%)). Arterial carbon dioxide pressure (PaCO2) was ≥45 mmHg in 58 patients (25%); of these, 20 (9%) had PaCO2 ≥50 mmHg. The prevalence of hypercapnia at both cut-off values was numerically higher for patients in GOLD stage 4 versus 3. An increased body mass index, a decreased forced vital capacity and an increased bicarbonate level were significant independent predictors of hypercapnia. The proportion of patients who received NIV was 6% overall and 22% of those with hypercapnia.
Conclusion
A relevant proportion of COPD patients in GOLD stage 3 and 4 exhibits chronic hypercapnia and might, therefore, be candidates for long-term domiciliary NIV treatment.
Keywords: chronic obstructive pulmonary disease, hypercapnia, non-invasive ventilation, quality of life, registry
Introduction
The number of patients worldwide affected by moderate-to-severe chronic obstructive pulmonary disease (COPD) was estimated to be 174.5 million individuals in 2015, and increased by 44.2% from 1990 to 2015.1 Between 2016 and 2040, COPD is forecasted to rise from the ninth to the fourth leading cause of life-years lost.2 In 2015, 3.2 million people died of COPD globally (an increase of 11.6%) compared with 1990.1 In addition to being an important cause of death, COPD is accompanied by significant morbidity, with a symptom burden similar to that of cancer,3 and a similar impact on health status to cardiovascular disease or diabetes.4 Due to its chronic, and often severe nature, COPD is also associated with significant disease-related health-care costs and economic impact.5
One potential treatment strategy for COPD in some settings is non-invasive ventilation (NIV), a technique that represents one of the most important advances in respiratory medicine over the last decade. Use of NIV is the standard of care for COPD patients hospitalized with acute hypercapnic respiratory failure due to disease exacerbation.6–10 COPD patients who have persistent hypercapnia after an episode of acute hypercapnic respiratory failure have a poor prognosis: almost half do not survive the first year after the index hospitalization, 80% require readmission to hospital and nearly two-thirds will have another life-threatening event.11 Robust data from recent clinical trials suggest that use of NIV at pressures targeted to substantially reduce hypercapnia is of significant benefit in stable hypercapnic COPD and in patients with persistent hypercapnia after acute respiratory failure needing mechanical ventilation.12–14
The results of these trials have been described as practice changing. However, effective planning and implementation of home NIV treatment strategies are difficult without up-to-date information on the prevalence of hypercapnic respiratory failure in patients with stable COPD. In addition, information about clinical predictors with the potential to facilitate better identification of this sub-population of COPD patients in an outpatient setting is currently unavailable. Despite this, and a lack of up-to-date guidelines for the use of home NIV in stable hypercapnic COPD, COPD is the most common indication for domiciliary NIV in Europe.15 This means that it is important to identify the potential population eligible for long-term home NIV in an industrialized country. The option of choice was the design of a prospective patient registry with real-life patient data. Therefore, the current registry-based study was designed to determine the proportion of COPD patients eligible for home NIV. A secondary aim was to better characterize this population in clinical practice and to determine usage of home NIV in chronic stable hypercapnic COPD.
Methods
Design
HOmeVent is a multicenter, prospective, observational, non-interventional patient registry in Germany. Patients were recruited from July 2016 until May 2017. The registry was approved by all local ethic committees (supplementary material) and patients provided written informed consent for access and use of their anonymized clinical data. The study was conducted in accordance with the Declaration of Helsinki and was registered at clinicaltrials.gov (NCT02811588).
Patients
Consecutive patients were evaluated in an outpatient setting by office-based pulmonologists during routine visits for COPD. Inclusion criteria were as follows: age ≥18 years; GOLD stage 3 or 4 COPD; arterial carbon dioxide pressure (PaCO2) value determined within the 30 days prior to enrolment; ability to fully understand the study information; and willingness to provide informed consent. Patients were excluded if they had signs of an acute exacerbation with acute hypercapnic respiratory failure (pH <7.35) and/or were already being treated with NIV. Non-pulmonary comorbidities were not an exclusion criterion.
Office-based pulmonologists used their clinical judgement to make a decision about referring patients with marked hypercapnia (minimum PaCO2 ≥45 mmHg) during their office visit to an NIV center for initiation of NIV. There was no follow-up of normocapnic patients in this registry.
Non-Invasive Ventilation
NIV was initiated according to current guidelines.16,17 The objective of NIV treatment was to improve clinical symptoms and reduce PaCO2 to normocapnic levels. The indication for treatment and choice of ventilator and accessories was at the discretion of the treating physician at the NIV center.
Assessments And Follow-Up
Data on patient demographics, diagnosis, blood gases, ventilation treatment (including type of ventilator, settings and interfaces, and follow-up data including failure rates, side effects and technical issues) were collected during routine clinical care from sources including clinical records, laboratory notes and device data. Baseline arterial blood gases (determined using arterial puncture or from the arterialized ear lobe) were taken during the daytime without NIV while patients were breathing room air (86% of patients) or oxygen (14% of patients; flow rate 2.2±0.9 L/min. Lung function testing was performed according to current guidelines,18 and forced vital capacity (FVC) was measured using spirometry or during full body plethysmography. Body mass index (BMI) was calculated using body weight and height, which were measured in all patients at baseline.
Guidelines state that patient follow-up should be performed 1–2 times per year, and that patients prescribed NIV should have at least one follow-up visit.16,17 Outside the clinical routine framework, patients were asked to complete the EuroQOL five dimensions questionnaire (EQ5D),19 the Severe Respiratory Insufficiency questionnaire (SRI)20 and the COPD Assessment Test™ (CAT) questionnaire21 at baseline. Routine clinical care was not influenced by participation in the registry. Serious adverse events and adverse events of special interest were recorded.
Objectives
The primary objective was to investigate the prevalence and predictors of hypercapnia in patients with severe COPD (GOLD stage 3 or 4). Secondary endpoints included patient flow from an outpatient setting to a NIV center, NIV treatment rates, and patterns of NIV use in this population.
Statistical Analysis
The sample size was calculated based on the ability to determine a reliable estimate of the prevalence of COPD with hypercapnia with an acceptable confidence interval (CI). For a single proportion, a sample size of 200 patients was estimated to be sufficient to identify a hypercapnic COPD prevalence of 20% with a margin error of 11.5% (the final sample size of 231 patients had an associated margin of error of 11.2%).
Data from all sites were pooled for analysis. Standard statistical methods were used to analyze all data. Continuous variables are summarized using the number of observations, mean, median, standard deviation (SD), minimum and maximum values. Categorical variables are summarized using the number of observations and percentages. Two-sided 95% CI values were used to characterize the major parameters. Demographic variables have been tabulated and summarized using descriptive statistics. Spearman’s rank correlations were used to identify relationships between a variety of institutional and patient characteristics. Potential predictors for hypercapnia were tested for significance in a log-linear model. The final model was determined via backward selection.
Results
Subjects
A total of 231 patients were enrolled into the registry by ten office-based pulmonologists; approximately two-thirds of patients were male, 21% were receiving continuous or intermittent home oxygen therapy, and almost all (95%) had not been previously treated with NIV (Tables 1 and 2). A history of sleep apnea was reported by 9% of patients overall, 8% of patients with PaCO2 <45 mmHg, and 10% of patients with PaCO2 ≥45 mmHg.
Table 1.
Baseline Demographics And Characteristics Of Patients Enrolled In The HOmeVent Registry By The Presence Of Hypercapnia Based On Carbon Dioxide Pressure Cut-Off Values Of ≥45 And ≥50 mmHg
| Hypercapnia (PaCO2 ≥45 mmHg) | Hypercapnia (PaCO2 ≥50 mmHg) | |||
|---|---|---|---|---|
| Yes (n=58) | No (n=173) | Yes (n=20) | No (n=211) | |
| Age, years | 66.4±9.1 | 67.5±8.9 | 64.3±8.6 | 67.5±9.0 |
| Male, n (%) | 34 (59) | 111 (64) | 13 (65) | 132 (63) |
| Body mass index, kg/m2 | 28.9±6.2 | 25.3±5.4 | 30.4±6.8 | 25.8±5.6 |
| Smoking status, n (%) | ||||
| Never | 4 (7) | 11 (6) | 2 (10) | 13 (6) |
| Current | 17 (29) | 45 (26) | 5 (25) | 57 (27) |
| Former | 37 (64) | 117 (68) | 13 (65) | 141 (67) |
| GOLD stage 3, n (%) | 21 (36) | 114 (66) | 8 (40) | 127 (60) |
| GOLD stage 4, n (%) | 37 (64) | 59 (34) | 12 (60) | 84 (40) |
| Previous NIV, n (%) | ||||
| None | 50 (86) | 168 (97) | 17 (85) | 201 (95) |
| At home | 1 (2) | 0 | 1 (5) | 0 |
| In hospital | 7 (12) | 5 (3) | 2 (10) | 10 (5) |
| Disease history, n (%) | ||||
| Heart failure | 8 (14) | 14 (8) | 2 (10) | 10 (5) |
| Diabetes mellitus | 15 (26) | 26 (15) | 5 (25) | 36 (17) |
| Hypertension | 34 (59) | 91 (53) | 14 (70) | 111 (53) |
| Coronary artery disease | 9 (16) | 25 (14) | 2 (10) | 32 (15) |
| Depression | 8 (14) | 22 (13) | 4 (20) | 26 (12) |
| Obstructive sleep apnea | 6 (10) | 14 (8) | 3 (15) | 17 (8) |
| Concomitant medication, n (%) | ||||
| Roflumilast | 11 (19) | 18 (12) | 5 (25) | 24 (11) |
| ß2-agonists | 55 (95) | 155 (90) | 19 (95) | 191 (91) |
| Anticholinergics | 52 (90) | 152 (88) | 18 (90) | 186 (88) |
| Methylxanthines | 7 (12) | 19 (11) | 2 (10) | 24 (11) |
| Inhaled corticosteroids | 28 (48) | 89 (51) | 8 (40) | 109 (52) |
| Systemic corticosteroids | 2 (3) | 8 (5) | 2 (10) | 8 (4) |
| Inhaled + systemic corticosteroids | 0 | 3 (2) | 0 | 3 (1) |
| Oxygen therapy, n (%) | ||||
| Day | 0 | 2 (1) | 0 | 2 (1) |
| Night | 0 | 1 (1) | 0 | 1 (<1) |
| Day & night | 20 (34) | 26 (15) | 9 (45) | 37 (18) |
| During exertion | 1 (2) | 0 | 0 | 1 (<1) |
| None | 37 (64) | 143 (83) | 11 (55) | 169 (80) |
Abbreviations: GOLD, global initiative for obstructive lung disease; NIV, non-invasive ventilation; PaCO2, arterial carbon dioxide pressure.
Table 2.
Baseline Lung Function And Blood Gas Data For Patients Enrolled In The HOmeVent Registry By The Presence Of Hypercapnia Based On Carbon Dioxide Pressure Cut-Off Values Of ≥45 And ≥50 mmHg
| Hypercapnia (PaCO2 ≥45 mmHg) | Hypercapnia (PaCO2 ≥50 mmHg) | |||
|---|---|---|---|---|
| Yes (n=58) | No (n=173) | Yes (n=20) | No (n=211) | |
| Pulmonary function | ||||
| FVC, L | 1.7±0.6 | 2.1±0.7 | 1.8±0.6 | 2.1±0.7 |
| FEV1, L/sec | 0.9±0.3 | 1.0±0.3 | 0.9±0.4 | 1.0±0.3 |
| FEV1/FEV1 predicted | 0.3±0.1 | 0.4±0.1 | 0.3±0.1 | 0.4±0.1 |
| FEV1/FVC, % | 56.0±16.5 | 49.6±10.7 | 53.5±3.4 | 51.0±12.6 |
| Residual volume, L | 5.2±1.9 | 5.1±1.4 | 4.9±2.1 | 5.2±1.5 |
| Total lung capacity, L | 7.1±2.3 | 7.4±1.8 | 6.9±2.4 | 7.4±1.9 |
| Blood gases | ||||
| pH | 7.4±0.04 | 7.42±0.03 | 7.39±0.05 | 7.42±0.03 |
| PaCO2, mmHg | 49.4±3.9 | 39.8±3.4 | 53.5±3.8 | 41.2±4.2 |
| PaO2, mmHg | 59.6±11.6 | 64.6±9.5 | 59.1±14.9 | 63.8±9.6 |
| SpO2, % | 89.5±4.8 | 92.4±3.3 | 88.7±6.0 | 91.9±3.5 |
| Base excess, mmol/L | 5.2±3.2 | 1.8±2.3 | 5.9±4.3 | 2.4±2.6 |
| HCO3−, mmol/L | 29.9±3.4 | 26.0±2.3 | 31.8±4.1 | 26.5±2.6 |
| Oxygen during BGA, n (%) | 16 (28) | 16 (9) | 6 (30) | 26 (12) |
| Oxygen flow rate, L/min | 2.4±1.1 | 2.0±0.7 | 2.0±0.6 | 2.2±1.0 |
Abbreviations: BGA, blood gas analysis; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; GOLD, Global Initiative for Obstructive Lung Disease; HCO3−, bicarbonate; PaCO2, arterial carbon dioxide pressure; PaO2, arterial oxygen pressure; SpO2, oxygen saturation.
Prevalence Of Hypercapnia
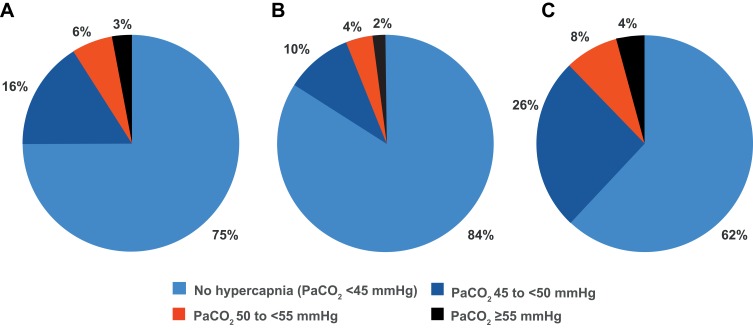
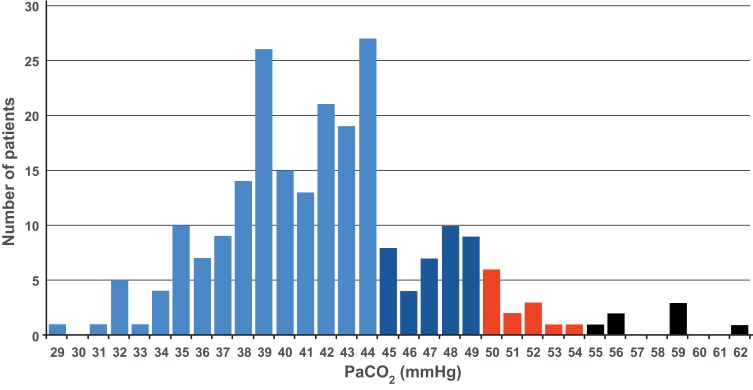
Overall, 58/231 patients (25%) had PaCO2 ≥45 mmHg, and 20 (9%) had PaCO2 ≥50 mmHg (Figure 1). The 95% CI for the true proportion of patients with PaCO2 ≥45 mmHg was therefore 19.4–31.5%, and for PaCO2 ≥50 mmHg was 5.7–13.9%. The prevalence of hypercapnia at both these cut-off values was higher in patients with GOLD stage 4 disease compared with stage 3 (Figure 1). The proportions of patients with different PaCO2 levels are shown in Figure 2.
Figure 1.
Prevalence of hypercapnia overall (A), and by GOLD stage 3 (B) or 4 (C).
Figure 2.
Distribution of patients by arterial carbon dioxide pressure (PaCO2). Light blue = normocapnia (PaCO2 <45 mmHg); Dark blue = hypercapnic (PaCO2 45 to <50 mmHg); Red & black = hypercapnic with an indication for non-invasive ventilation in Germany (red= PaCO2 ≥50 mmHg; black = PaCO2 ≥ 50 mmHg).
Predictors Of Hypercapnia
BMI, forced vital capacity (FVC) and bicarbonate (HCO3−) levels were identified as being significantly associated with the presence of hypercapnia (defined as PaCO2 ≥45 mmHg) (Table 3). Increases in BMI and bicarbonate were associated with a significant increase in the risk of hypercapnia, whereas the risk of hypercapnia decreased as FVC increased. There were no significant interactions between these variables.
Table 3.
Predictors Of Hypercapnia (Arterial Carbon Dioxide ≥45 mmHg) Tested In A Log-Linear Model
| Odds Ratio (95% CI) | p-value | |
|---|---|---|
| BMI (per 5 kg/m2 increase) | 2.05 (1.462.95) | <0.01 |
| TLC (per 1 L increase) | 1.2 (0.96–1.52) | n.s. |
| FVC (per 1 L increase) | 0.35 (0.16–0.7) | <0.001 |
| Bicarbonate (per 1 mmol/L increase) | 1.72 (1.46–2.09) | <0.01 |
Abbreviations: BMI, body mass index; CI, confidence interval; n.s., not statistically significant; TLC, total lung capacity.
Use Of NIV
Of the 58 patients with hypercapnia (PaCO2≥45 mmHg) enrolled by office pulmonologists, 19 patients were referred to an NIV center and 18 out of 19 presented at the NIV center. Of those referred, 10 patients (53%) had PaCO2 ≥50 mmHg. 10 patients with PaCO2 ≥50 mmHg were not referred to the NIV center. NIV therapy was not indicated for four patients and one patient refused the treatment at the NIV center. Therefore, 13/231 patients (6%) with COPD GOLD stage 3 and 4 received NIV (22.4% of those with hypercapnia). Length of stay for NIV initiation was 4.8±2.8 days. Suggested NIV use was nocturnal in 9 patients (69%), diurnal and nocturnal in 3 patients (23%) and daytime in 1 patient (8%). Humidification was used during NIV in all patients and 8 patients (62%) were treated with oxygen during NIV. Interface was a standard oronasal/full face mask in 12 patients (92%) and a standard nasal mask in 1 patient (8%). Mean inspiratory positive airway pressure (IPAP) was 21.5±4.1 cmH2O, mean expiratory positive airway pressure (EPAP) was 8.1±3.4 cmH2O, and mean respiratory rate was 14.8±3.0 breaths/min.
Quality Of Life
Quality of life was not significantly different between patients with and without hypercapnia. For patients with versus without hypercapnia, EQ5D scores were 48.7±21.3 versus 53.3±21.1, CAT scores were 22.8±7.8 versus 22.4±7.7, and SRI summary scale scores were 52.3±19.0 versus 55.5±18.0. Findings were similar at a PaCO2 cut-off value of ≥50 mmHg.
Discussion
Data from the HOmeVent registry provide up-to-date information on the prevalence of hypercapnia in severe COPD and use of NIV in Germany. The proportion of stable COPD patients in GOLD stage 3 and 4 who had hypercapnia (defined as PaCO2 ≥45 mmHg) was relatively high (25%), and 9% had a PaCO2 ≥50 mmHg. Lower FVC and higher BMI were independent predictors of hypercapnia. Only a minority of hypercapnic patients (13/58 [22%]) went on to receive long-term NIV, and half of those with severe hypercapnia (PaCO2 ≥50 mmHg) were not referred for NIV.
The Eurovent study analyzed patterns of home mechanical ventilation provision in Europe in 2001–2002,15 but this did not specifically relate to COPD and, to the best of our knowledge, no other registry data on this topic have been published to date. Given that COPD rates are expected to rise over the coming decade, the number of COPD patients with an indication for chronic NIV is expected to increase, highlighting the importance of having a good understanding of disease incidence, patient presentation and treatment options.
The HOmeVent registry findings are the first to provide data on the size of the severe COPD patient population who could benefit from home NIV therapy. This is clinically important given the documented beneficial effects of home NIV on hard clinical endpoints in recent well-designed randomized clinical trials.12–14 The results of these studies showed that long-term use of NIV targeted to adequately reduce CO2 levels in stable chronic COPD patients with hypercapnia significantly improved 1-year survival and quality of life compared with standard treatment,12 and that the addition of home NIV to oxygen therapy significantly improved 1-year admission-free survival versus home oxygen therapy alone (hazard ratio 0.54, 95% CI 0.34–0.84; p=0.007).14 These improvements in morbidity and mortality achieved with home NIV therapy are relevant for both patients and the healthcare system.22
The ability to predict which stable severe COPD patients are likely to have chronic hypercapnia can facilitate the identification of patients who could benefit from delivery of home NIV. In addition, it is important to note that NIV in this setting needs to be targeted to achieve adequate reductions in carbon dioxide levels,12,13 and that the NIV pressures required to facilitate this can be attained without a negative impact on quality of life.13
Although predictors of mortality in COPD patients with hypercapnic respiratory failure receiving NIV have been more widely studied,23,24 there is a relative lack of data on predictors of chronic hypercapnia development in stable severe COPD. In our study, BMI, FVC and bicarbonate level were identified as being significantly and independently associated with the presence of hypercapnia in patients with chronic stable COPD. BMI has been shown to be an independent predictor of mortality in patients with stable severe COPD and chronic hypercapnia,23,25 and base excess was particularly relevant as a predictor of mortality in these patients when the BMI was ≥25 kg/m2.23,26 It is also possible that patients with a high BMI might have had coexisting obesity hypoventilation syndrome, which results in hypercapnia27,28 and could potentially influence our results. FVC has been shown to be significantly lower in COPD patients with versus without lung hyperinflation.29 Chronic hyperinflation associated with conditions such as COPD is an important cause of diaphragmatic dysfunction.30–32 Therefore, the association between FVC and hypercapnia in our study seems plausible. Data on parameters related to hyperinflation (e.g. lung volumes determined using body plethysmography) would be helpful to better understand this association. With respect to serum bicarbonate, increased levels are a physiological consequence of hypercapnia. Elevated bicarbonate levels in the presence of hypercapnia are therefore expected. Higher levels of bicarbonate reflect more severe disease and are most likely a consequence, rather than a predictor, of hypercapnia in stable severe COPD patients.
Our study has an observational, registry-based design and the patients included are representative of routine clinical care. Therefore, the findings are likely to have good external validity. An important limitation is the small sample size, and the limited number of office-based pneumologists and NIV centers that participated in this registry. Therefore, additional registries with larger numbers of participants and centers are encouraged to better define the prevalence of chronic hypercapnia in patients with COPD. In addition, the patients were recruited in Germany, meaning that the decision to start NIV was primarily made based on the German guideline for home mechanical ventilation.16,17
Conclusions
Data from the HOmeVent registry showed that more than a quarter of all patients with GOLD stage 3 or 4 COPD had chronic hypercapnia. Given the associations identified in our population, stable COPD patients with low FVC or high BMI in the outpatient setting should undergo blood gas assessment. The prevalence of chronic hypercapnia identified in this registry is clinically relevant given the fact that these patients are potential candidates for long-term home NIV treatment. Furthermore, efforts are required to increase the use of NIV in these patients given that fewer than 25% of those who had hypercapnia actually received NIV in our study, and half of those with an indication for NIV based on the German guidelines were not referred to an NIV center.
Acknowledgments
An abstract describing interim data from the HOmeVent registry was presented as a poster at the European Respiratory Society Conference 2017 (Eur Respir J; 2017 50: OA4426; DOI: 10.1183/1393003.congress-2017.OA4426) and at the American Thoracic Society Conference 2018 (Am J Respir Crit Care Med; 2018;197:A3265). The authors thank Katarina Ortner for her help regarding the study management. Medical writing assistance with manuscript preparation was provided by Nicola Ryan, independent medical writer, funded by ResMed.
Funding Statement
The HOmeVent registry is funded by ResMed. Representatives and scientists from the ResMed participated in the study including design, data collation, data analysis, and critical review of the paper.
Data Sharing Statement
The data sets supporting the conclusion of this article are included within the article. All data requests should be submitted to the corresponding author for consideration.
Author Contributions
TK, MD, WW, DM, HW, TF and PCN were involved in the conception, hypotheses delineation and design of the study. GH and AGroeschel considerably contributed to patient recruitment and data acquisition. AGraml, MD, and PCN analyzed and interpreted the data. MD and PCN wrote the article. All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
MD has received speaking and advisor fees from Phillips, Weinmann, and Heinen und Löwenstein, during the conduct of the study. WW has received speaking fees and advisory fees from Weinmann, Vivisol, Heinen und Löwenstein, VitalAire (all in Germany) and from Philips-REspironics (USA) ; in addition, the Cologne study group has received open research grants from Weinmann, Vivisol, Heinen und Löwenstein and VitalAire (all in Germany), and from Philips-Respironics (USA). HW is a paid consultant to ResMed. TF is Chief Executive Officer at the Clinical Research Institute which was in charge of the HOmeVent registry. PCN, DM and A Graml are employees of ResMed. A Gröschel reports personal fees from ResMed, during the conduct of the study. TK and GH have no conflicts of interest to disclose beyond financial support for the HOmeVent registry. The authors report no other conflicts of interest in this work.
References
- 1.GBD 2015 Chronic Respiratory Disease Collaborators. Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Respir Med. 2017;5(9):691–706. doi: 10.1016/S2213-2600(17)30293-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Foreman KJ, Marquez N, Dolgert A, et al. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: reference and alternative scenarios for 2016-40 for 195 countries and territories. Lancet. 2018;392(10159):2052–2090. doi: 10.1016/S0140-6736(18)31694-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Joshi M, Joshi A, Bartter T. Symptom burden in chronic obstructive pulmonary disease and cancer. Curr Opin Pulm Med. 2012;18(2):97–103. doi: 10.1097/MCP.0b013e32834fa84c [DOI] [PubMed] [Google Scholar]
- 4.Janson C, Marks G, Buist S, et al. The impact of COPD on health status: findings from the BOLD study. Eur Respir J. 2013;42(6):1472–1483. doi: 10.1183/09031936.00153712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sullivan SD, Ramsey SD, Lee TA. The economic burden of COPD. Chest. 2000;117(2 Suppl):5s–9s. doi: 10.1378/chest.117.2_suppl.5S [DOI] [PubMed] [Google Scholar]
- 6.Brochard L, Mancebo J, Wysocki M, et al. Noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease. N Engl J Med. 1995;333(13):817–822. doi: 10.1056/NEJM199509283331301 [DOI] [PubMed] [Google Scholar]
- 7.Chandra D, Stamm JA, Taylor B, et al. Outcomes of noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease in the United States, 1998-2008. Am J Respir Crit Care Med. 2012;185(2):152–159. doi: 10.1164/rccm.201106-1094OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lightowler JV, Wedzicha JA, Elliott MW, Ram FS. Non-invasive positive pressure ventilation to treat respiratory failure resulting from exacerbations of chronic obstructive pulmonary disease: cochrane systematic review and meta-analysis. BMJ. 2003;326(7382):185. doi: 10.1136/bmj.326.7402.1329-b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meyer TJ, Hill NS. Noninvasive positive pressure ventilation to treat respiratory failure. Ann Intern Med. 1994;120(9):760–770. doi: 10.7326/0003-4819-120-9-199405010-00008 [DOI] [PubMed] [Google Scholar]
- 10.Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis management and prevention of COPD. January 2015. Available from: http://www.goldcopd.org/uploads/users/files/GOLD_Report_2015_Feb18.pdf Accessed October30, 2018.
- 11.Chu CM, Chan VL, Lin AW, Wong IW, Leung WS, Lai CK. Readmission rates and life threatening events in COPD survivors treated with non-invasive ventilation for acute hypercapnic respiratory failure. Thorax. 2004;59(12):1020–1025. doi: 10.1136/thx.2004.024307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kohnlein T, Windisch W, Kohler D, et al. Non-invasive positive pressure ventilation for the treatment of severe stable chronic obstructive pulmonary disease: a prospective, multicentre, randomised, controlled clinical trial. Lancet Respir Med. 2014;2(9):698–705. doi: 10.1016/S2213-2600(14)70153-5 [DOI] [PubMed] [Google Scholar]
- 13.McEvoy RD, Pierce RJ, Hillman D, et al. Nocturnal non-invasive nasal ventilation in stable hypercapnic COPD: a randomised controlled trial. Thorax. 2009;64(7):561–566. doi: 10.1136/thx.2008.108274 [DOI] [PubMed] [Google Scholar]
- 14.Murphy PB, Rehal S, Arbane G, et al. Effect of home noninvasive ventilation with oxygen therapy vs oxygen therapy alone on hospital readmission or death after an acute COPD exacerbation: a randomized clinical trial. JAMA. 2017;317(21):2177–2186. doi: 10.1001/jama.2017.4451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lloyd-Owen SJ, Donaldson GC, Ambrosino N, et al. Patterns of home mechanical ventilation use in Europe: results from the Eurovent survey. Eur Respir J. 2005;25(6):1025–1031. doi: 10.1183/09031936.05.00066704 [DOI] [PubMed] [Google Scholar]
- 16.Windisch W, Geiseler J, Simon K, Walterspacher S, Dreher M. German national guideline for treating chronic respiratory failure with invasive and non-invasive ventilation - revised edition 2017: part 1. Respiration. 2018;96(2):66–97. doi: 10.1159/000488001 [DOI] [PubMed] [Google Scholar]
- 17.Windisch W, Geiseler J, Simon K, Walterspacher S, Dreher M. German national guideline for treating chronic respiratory failure with invasive and non-invasive ventilation - revised edition 2017: part 2. Respiration. 2018;96(2):171–203. doi: 10.1159/000488667 [DOI] [PubMed] [Google Scholar]
- 18.Criee CP, Baur X, Berdel D, et al. Standardization of spirometry: 2015 update. Published by German Atemwegsliga, German respiratory society and German society of occupational and environmental medicine. Pneumologie. 2015;69(3):147–164. doi: 10.1055/s-0034-1391345 [DOI] [PubMed] [Google Scholar]
- 19.EuroQol Research Foundation. EQ-5D. Available from: Accessed May 13, 2019.
- 20.Windisch W, Freidel K, Schucher B, et al. The Severe Respiratory Insufficiency (SRI) Questionnaire: a specific measure of health-related quality of life in patients receiving home mechanical ventilation. J Clin Epidemiol. 2003;56(8):752–759. doi: 10.1016/s0895-4356(03)00088-x [DOI] [PubMed] [Google Scholar]
- 21.Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the COPD Assessment Test. Eur Respir J. 2009;34(3):648–654. doi: 10.1183/09031936.00102509 [DOI] [PubMed] [Google Scholar]
- 22.Suh ES, Murphy PB, Hart N. Home mechanical ventilation for chronic obstructive pulmonary disease: what next after the HOT-HMV trial? Respirology. 2019. doi: 10.1111/resp.13484 [DOI] [PubMed] [Google Scholar]
- 23.Budweiser S, Jorres RA, Riedl T, et al. Predictors of survival in COPD patients with chronic hypercapnic respiratory failure receiving noninvasive home ventilation. Chest. 2007;131(6):1650–1658. doi: 10.1378/chest.06-2124 [DOI] [PubMed] [Google Scholar]
- 24.Raveling T, Wijkstra P, Bladder G, Nieuwenhuis J, Duiverman M. Predictors of survival in severe COPD patients initiated on chronic NIV. Eur Respir J. 2016;48:PA2154. [Google Scholar]
- 25.Celli BR, Cote CG, Marin JM, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004;350(10):1005–1012. doi: 10.1056/NEJMoa021322 [DOI] [PubMed] [Google Scholar]
- 26.Budweiser S, Jorres RA, Riedl T, Heinemann F, Pfeifer M. Base excess, a marker of chronic hypercapnic respiratory failure and predictor of survival in COPD. Eur Respir Rev. 2006;15:194–196. doi: 10.1183/09059180.00010118 [DOI] [Google Scholar]
- 27.Mokhlesi B, Kryger MH, Grunstein RR. Assessment and management of patients with obesity hypoventilation syndrome. Proc Am Thorac Soc. 2008;5(2):218–225. doi: 10.1513/pats.200708-122MG [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Masa JF, Pepin JL, Borel JC, Mokhlesi B, Murphy PB, Sanchez-Quiroga MA. Obesity hypoventilation syndrome. Eur Respir Rev. 2019;28(151). doi: 10.1183/16000617.0097-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen C, Jian W, Gao Y, Xie Y, Song Y, Zheng J. Early COPD patients with lung hyperinflation associated with poorer lung function but better bronchodilator responsiveness. Int J Chron Obstruct Pulmon Dis. 2016;11:2519–2526. doi: 10.2147/COPD.S110021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Similowski T, Yan S, Gauthier AP, Macklem PT, Bellemare F. Contractile properties of the human diaphragm during chronic hyperinflation. N Engl J Med. 1991;325(13):917–923. doi: 10.1056/NEJM199109263251304 [DOI] [PubMed] [Google Scholar]
- 31.Polkey MI, Kyroussis D, Hamnegard CH, Mills GH, Green M, Moxham J. Diaphragm strength in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1996;154(5):1310–1317. doi: 10.1164/ajrccm.154.5.8912741 [DOI] [PubMed] [Google Scholar]
- 32.Rochester DF, Braun NM. Determinants of maximal inspiratory pressure in chronic obstructive pulmonary disease. Am Rev Respir Dis. 1985;132(1):42–47. doi: 10.1164/arrd.1985.132.1.42 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis management and prevention of COPD. January 2015. Available from: http://www.goldcopd.org/uploads/users/files/GOLD_Report_2015_Feb18.pdf Accessed October30, 2018.