Abstract
Background
Chronic rhinosinusitis frequently occurs in people with cystic fibrosis. Several medical interventions are available for treating chronic rhinosinusitis in people with cystic fibrosis; for example, different concentrations of nasal saline irrigations, topical or oral corticosteroids, antibiotics ‐ including nebulized antibiotics, dornase alfa and modulators of the cystic fibrosis transmembrane conductance regulator (CFTR) (such as lumacaftor, ivacaftor or tezacaftor). However, the efficacy of these interventions is unclear.
Objectives
The objective of this review is to compare the effects of different medical interventions in people diagnosed with cystic fibrosis and chronic rhinosinusitis.
Search methods
We searched the Cochrane Cystic Fibrosis Trials Register, compiled from electronic database searches and hand searching of journals and conference abstract books. Date of last search of trials register: 22 May 2019.
We also searched ongoing trials databases, other medical databases and the reference lists of relevant articles and reviews. Date of latest additional searches: 20 May 2019.
Selection criteria
Randomized and quasi‐randomized trials of different medical interventions compared to each other or to no intervention or to placebo.
Data collection and analysis
Two review authors independently assessed trials identified for potential inclusion in the review. We planned to conduct data collection and analysis in accordance with Cochrane methods and to independently rate the quality of the evidence for each outcome using the GRADE guidelines.
Main results
We identified no trials that met the pre‐defined inclusion criteria. The searches identified 47 trials, none of which were eligible for inclusion in the current version of this review.
Authors' conclusions
We identified no eligible trials assessing the medical interventions in people with cystic fibrosis and chronic rhinosinusitis. High‐quality trials are needed which should assess the efficacy of different treatment options detailed above for managing chronic rhinosinusitis, preventing pulmonary exacerbations and improving quality of life in people with cystic fibrosis.
Plain language summary
Medical interventions for chronic inflammation of the nose and sinuses in cystic fibrosis
Review question
We reviewed the evidence for the effect of medical interventions for chronic rhinosinusitis in people with cystic fibrosis.
Background
Chronic rhinosinusitis is long‐term infection and inflammation of the nasal cavity and air‐filled spaces around the eyes and nose. Cystic fibrosis is a genetic condition that makes secretions in the body thick, and hence stagnant. When this occurs in and around the nose, it causes chronic rhinosinusitis. Better care of people with cystic fibrosis has led to them living longer, increasing the chance of developing chronic rhinosinusitis. Early and effective interventions with antibiotics, steroids, drugs that thin the mucus (e.g. dornase alfa) and drugs to improve how the cell membrane channel functions (CFTR modulators) can help to improve quality of life and prevent the development of lower airway disease. Currently, there are no guidelines based on trials to know how best to treat chronic rhinosinusitis in people with cystic fibrosis.
Search date
The evidence is current to: 22 May 2019.
Study characteristics
We found 47 trials in our search, but none of them met our inclusion criteria and they were not included in the current review.
Key results
Although chronic rhinosinusitis is common in people with cystic fibrosis, there is not enough evidence available on its management. This review highlights the need to organize well‐designed trials assessing relevant treatment options to show which treatment is best for managing chronic rhinosinusitis, preventing lower airway disease and improving quality of life in people with cystic fibrosis and chronic rhinosinusitis.
Background
Description of the condition
Cystic fibrosis (CF) is a genetic disorder mainly affecting the nasal cavities, paranasal sinuses (air spaces around eyes and nose), lungs and digestive system. It is an autosomal recessively inherited condition which means that children of carrier parents have a 25% chance of having the disease and 50% chance of being a carrier. Carriers transmit the disease to the next generation without suffering from the disease themselves. It is a relatively common debilitating disorder with its incidence differing across different continents. The highest incidence is noted in Europe (ranging between 1 in 2000 to 1 in 3000 live births) and the lowest in Asia (ranging between 1 in 40,000 to 1 in 100,000 live births) (WHO Human Genetics Programme 2004).
The condition is caused by mutations in a gene on chromosome 7 that encodes the CF transmembrane conductance regulator (CFTR) protein (Collins 1992, Drumm 1993). CFTR is an anion (a negatively‐charged molecule, e.g. chloride (Cl‐) and bicarbonate (HCO3‐)) channel usually present on the plasma membrane of epithelial cells lining the airway, pancreas, liver, intestines, sweat ducts, and epididymis (a tube located at the back of the testicles that stores and carries sperm) (Guggino 2004). The normal function of the anion channel promotes anion secretion to the airway surface (or into the extracellular lumen of the intestine and glandular ducts), which impacts the balance of water and electrolyte transport (Johnson 1995; Rowe 2005).
The most common mutation is F508del, although more than 2000 different potentially disease‐causing CFTR gene mutations have been listed in the Cystic Fibrosis Mutation Database (Cystic Fibrosis Mutation Database 2011). Six different classes of mutations have been identified, based on the way the defective CFTR protein is produced. Class I mutations lead to defective protein production, which in turn leads to the premature termination of the mRNA and a complete absence of CFTR protein in the cell. Class II mutations are mutations in protein processing and trafficking and include the most common mutation ‐ F508del. Class III mutations show defective regulation of CFTR channel gating (e.g. G551D). Class IV mutations reduce the duration of channel opening and also the rate of ion flow. Class V mutations have reduced quantities of normal CFTR protein due to abnormalities of mRNA splicing. Finally, class VI mutations have increased channel turnover at the cell surface due to instability, which results in decreased amounts of functional protein. Due to the difference in the mechanisms involved in the disease process, the amount of healthy CFTR produced varies among individuals.
In a normal airway, hair‐like projections from epithelial cells called cilia beat to move the overlying mucus out of the sinuses. The cilia are covered with 'thin' airway surface liquid (ASL) which is made up of a mucus layer overlying a periciliary fluid layer which is in direct contact with the epithelial cells. Normally, oxygen levels are equal throughout the depths of the ASL. In the airways of people with CF, defective Cl‐ ion transport leads to the excessive absorption of sodium and water and consequently the dehydration of the ASL, resulting in a thin periciliary layer (Rowe 2005). Cilia are not able to function correctly in a thin periciliary layer and mucociliary clearance (the transport of mucus by the cilia towards the sinus openings) is impaired. Mucus is still produced and secreted forming a thick layer of static mucus which the cilia are not able to clear adequately and the thick secretions block the sinus openings. Ventilation of the sinuses decreases and hypoxia (low levels of oxygen in tissue) occurs; this hypoxia impairs mucociliary clearance even more by inhibiting Cl‐ transport and further dehydrating ASL (Blount 2011).
Pseudomonas aeruginosa, Staphylococcus aureus, Haemophilus influenzae, Burkholderia cepacia, Achromobacter xylosoxidans and Stenotrophomonas maltophilia are bacteria which commonly cause infection and inflammation (Brook 2016; Ramsey 1992). These bacteria further impair ciliary motion or co‐ordination (or both), exacerbating impaired mucociliary clearance (Wilson 1987). Inflammation increases mucus secretion by mucosa (Mainz 2012), promotes the formation of neutrophil‐predominant nasal polyps (neutrophils are a type of blood cell that increase during infection) (Ryan 2008) and propagates the cycle of infection and inflammation.
Oxygen levels are highest near the surface of this mucus layer and lowest in its depths near the mucosa. P aeruginosa deposited on the surface penetrates into the hypoxic zones of mucus, adapts to the environment and forms microcolonies (Kim 2015). Hypoxic mucus with P aeruginosa microcolonies resists the natural defence mechanism in the lungs and sinuses, resulting in chronic airway infection.
A single type of mucosa (pseudostratified ciliated columnar epithelium) lines the nasal cavity through the sinuses, larynx, and trachea to the distal lungs (Krouse 2007). The pathophysiology of mucostasis (a mass of thick mucus over the mucosa which cannot be pushed away by cilia), infection and inflammation is the same for upper and lower airway. The 'unified airway model' suggests that infection in the upper airway easily spreads to the lower airway and vice versa (Chang 2014; Illing 2014; Tos 1983). Therefore, chronic rhinosinusitis (CRS) can lead to pulmonary exacerbations, a major cause of death in people with CF. Mucostasis with microcolonies of bacteria in the sinuses form a reservoir of infection. It has been shown that in people who have undergone surgery to open up sinuses and help drain the collected mucus, there is reduced pulmonary disease (Chang 2014; Holzmann 2004; Jones 1993; Rosbe 2001).
According to the European Position Paper on rhinosinusitis and nasal polyps, CRS is diagnosed when a person presents having lived for 12 weeks with one of: nasal obstruction or congestion and/or rhinorrhea; and one of facial pain or pressure and/or reduction in olfaction (sense of smell); and has either positive endoscopic findings of nasal polyps or mucopurulent discharge (yellow‐green sticky secretions of infected mucus) or mucosal edema or radiological findings of disease on a CT scan (or both) (Fokkens 2012).
Description of the intervention
Medical intervention is one of the first steps in managing CRS in people with CF and can include oral and topical treatments which are usually given in combination for a long duration. Available treatment options include nasal saline irrigation, oral and topical antibiotics, oral and topical steroids, anti‐inflammatory agents (such as ibuprofen), dornase alfa and CFTR modulators.
Nasal saline irrigation
Nasal saline irrigation involves washing the nasal cavity with saline delivered via squeeze bottles and netti pots. It helps to soften dried mucous crusts and clears them out of the sinonasal cavity. It is usually used in two concentrations: hypertonic (more concentrated than serum, ranging from 3% to 7%); and isotonic (as concentrated as our serum, i.e. 0.9%, such as NasaMist®). Nasal saline irrigation can also include low‐volume treatments like Sterimar® (natural sea water nasal spray).
Corticosteroids
Corticosteroids break the infection‐inflammation cycle, preventing the progression of CRS in people with CF. They are strong immunosuppressants and decrease the inflammatory response to the retained mucus or bacterial microcolonies. Oral corticosteroids are given as tablets, usually in the morning to match the circadian rhythm (bodily rhythm of morning and night). Topical corticosteroids are usually administered as nasal sprays inhaled in the morning. Topical application increases the local delivery of the drug and prevents the potential side effects that could arise by systemic administration.
Antibiotics
Antibiotics are traditionally given as oral tablets or intravenous drips. In CF, nebulized tobramycin and colistin have been used to prevent the formation of bacterial micro‐colonies in the mucus lining the sinuses.
Dornase alfa
Dornase alfa (recombinant human DNase) is a relatively newly developed medicine which is administered topically. It breaks long gene chains present in mucus and decreases its stickiness. It enhances the clearance of mucus from the sinonasal cavities.
CFTR modulators
CFTR modulators (e.g. ivacaftor, lumacaftor and tezacaftor) act on the cause of CF, the defective CFTR protein. They increase the efficacy of these proteins and thereby increase their activity partially. Ivacaftor was approved for individuals with G551D mutations, but has been expanded for use in people with other Class III and residual function mutations (Guigui 2016). Combination treatment of lumacaftor and ivacaftor has been used in children with homozygous F508del‐CFTR with good results (Ratjen 2017). Tezacaftor is a newly approved CFTR modulator widely studied for its efficacy in different CFTR mutations including non‐F508del mutations of CFTR (Sala 2018).
Of these medical interventions, many interventions including saline irrigations, antibiotics and steroids are used empirically and have now become the standard of care.
How the intervention might work
Nasal saline irrigation
Nasal saline irrigation physically removes viscous mucus that impairs the clearance of debris and bacteria. Hypertonic saline could have the additional benefit of creating an osmotic gradient, where water is drawn into the mucus thus decreasing its viscosity. As water transfers from inflamed mucosa to the airway surface, edematous (swollen with fluid) mucosa shrink, thus acting as a decongestant (Kang 2015). Although hypertonic saline increases the osmotic gradient further and is the preferred saline in people with CF, it has been recognized to decrease the function of cilia (ciliostasis) (Boek 1999).
Corticosteroids
Short courses of oral steroids in tapering doses are sometimes given with antibiotics in initial treatment, but their use is controversial. There is a risk of pulmonary exacerbation with the use of oral steroids in people with CF, although long‐term oral steroids have been shown to slow the progression of lung disease, decrease hospitalization rates for respiratory exacerbations and improve quality of life with no effect on sino‐nasal symptoms (Cheng 2015). The short‐term use of oral steroids has not been analyzed.
Topical corticosteroids are commonly prescribed and have the advantage of enhanced local effects without systemic complications. Owing to their anti‐inflammatory properties, topical corticosteroids have been used to decrease the burden associated with nasal polyps (Costantini 1990; Hadfield 2000). As inflammation decreases, edema improves and paranasal sinuses remain open longer, allowing secretions to clear.
Antibiotics
Early aggressive anti‐pseudomonal antibiotic therapy has been advocated to delay the onset of chronic P aeruginosa infection (Doring 2000; Langton Hewer 2017). The duration of antibiotic use for eradication is variable and depends on patient selection criteria, the source of respiratory secretion (upper versus lower airway) and the type of therapy (Gibson 2003). Ratjen reported a 12‐month pathogen‐free period after the use of an inhaled antibiotic (Ratjen 2001).
Anti‐pseudomonal antibiotics are also used as maintenance therapy. These can include inhaled anti‐pseudomonal antibiotics, oral quinolones and macrolides. Advantages of using an inhaled antibiotic, include a greater amount of drug reaching the nose and sinuses directly and limited systemic absorption and toxicity. Inhaled antibiotics that have been studied in people with CF include tobramycin (Ramsey 1999), colistin (Hodson 2002) and aztreonam (Fernandez 1994). Continuous anti‐staphylococcal antibiotics are not used as they propagate the formation of methicillin‐resistant S aureus, increase the colonisation of P aeruginosa and increase the formation of small colony variants (cell wall‐deficient strains that have increased capability to survive defence mechanisms). Use of these antibiotics are appropriate only when they are given intermittently for respiratory symptoms (Gibson 2003).
Disease exacerbations are usually treated by targeting antibiotic therapy to the results of bacterial and antibiotic sensitivities. Antibiotics such as amoxicillin with clavulinic acid, second and third generation cephalosporins (such as cefuroxime axetil, cefprozil, cefixime, cefpodoxime proxetil and loracarbef) are chosen when culture grows H influenzae. Co‐trimoxazole, doxycycline and minocycline are chosen when the culture grows B cepacia. Aminoglycosides are given once daily in order to achieve a high peak blood concentration to enhance anti‐bacterial activity and a low trough blood concentration to minimize toxic effects to the ears and kidneys.
Dornase alfa
The extensive neutrophil degradation observed in mucus from the CF airway results in the accumulation of long‐chain, extracellular DNA. This increases the viscosity of the mucus, contributing to the disease. Dornase alfa targets the highly viscous mucus which lines the mucus membranes (Shak 1990); it cleaves the DNA and decreases the viscosity, helping in its clearance (Lindig 2013).
CFTR modulators
Ivacaftor and lumacaftor are new drugs designed to target the CFTR protein. Ivacaftor is a CFTR potentiator that improves the open probability of the defective Cl‐ channel in those with at least one copy of the mutant G551D‐CFTR allele (Accurso 2010); this mutation is present in 4% to 5% of people with CF (Kaiser 2012). It has also been used successfully in people with other class III mutations such as G178R, S549N, S549R, G551S, G970R, G1244E, S1251N, S1255P, or G1349D (De Boeck 2014). Lumacaftor is a corrector which improves the folding and trafficking of some protein to the cell surface. It is used with ivacaftor as a combination treatment for people with CF aged 12 years or older with two copies of the F508del mutation (Castellani 2008; Liou 2001). The use of these medications improve the function of the CFTR protein. It increases Cl‐ transport and thus decreases the viscosity of the mucus.
Why it is important to do this review
Due to advancements in treating people with CF, many are surviving to an older age and CRS is common, reaching 100% when examined clinically or radiographically (Liang 2014). The unified airway model suggests that the lining of nose and sinuses is in continuity with that of lower respiratory tract. The bacteria that can be found in the upper airway, can also be found in the lower airway. Therefore, if there is an infection in the upper airway, it will easily spread to the lower airway; this can be life‐threatening in people with CF (Tipirneni 2017).
Medical treatment is the initial management for people with CRS, and several treatment options are currently available. Given the diversity of treatments, it is important to know which combination of intervention and which method of delivery provides the best quality of life for people with CF.
This review will help provide high‐quality information regarding medical interventions for CRS in people with CF to researchers, medical professionals, people with CF, parents and the public.
Objectives
The objective of this review is to compare the effects of different medical interventions in people diagnosed with CF and CRS.
Methods
Criteria for considering studies for this review
Types of studies
We planned to include randomized controlled trials (RCTs), quasi‐RCTs and cluster‐RCTs. We also planned to include RCTs of cross‐over design if data from the first treatment phase were available. Trials were included only if an adequate follow‐up period (i.e. a minimum of three months) was present.
We excluded trials that were randomized by the side of the nose.
Types of participants
Participants diagnosed with CF by sweat test or genetic testing for CFTR mutations and further diagnosed with CRS according to either the International Consensus Statement on Allergy and Rhinology: Rhinosinusitis (ICAR:RS) (Orlandi 2016) or the European Position Paper on Rhinosinusitis and Nasal Polyps (Fokkens 2012).
CRS was defined as persistent sinonasal inflammation lasting at least 12 weeks, with two or more of the following symptoms: nasal blockage or obstruction or congestion; nasal discharge; facial discomfort; decreased sense of smell; and an objective finding by endoscopic or CT imaging showing nasal polyps or mucopurulent discharge or mucosal edema.
Asymptomatic individuals were excluded from this analysis.
Types of interventions
We planned to include both short courses of treatment (up to 28 days) and long courses of treatment (longer than four weeks).
As the Cochrane Review 'Topical nasal steroids for treating nasal polyposis in people with cystic fibrosis' assesses trials addressing the effect of topical nasal steroids on nasal polyposis in CF (Beer 2015), such trials are excluded from this review. We also excluded trials that evaluated the effect of medical interventions on surgical outcomes; the medical interventions that were considered for this review are listed below.
Nasal saline irrigation ‐ isotonic (0.9%) saline, hypertonic saline (3% to 7%)
Corticosteroids ‐ oral or intravenous (e.g. prednisolone, hydrocortisone); or topical (e.g. beclomethasone, triamcinalone, budesonide, fluticasone, mometasone, betamethasone)‐ considered on people diagnosed with CF and CRS without nasal polyps
Antibiotics ‐ anti‐staphylococcal (e.g. dicloxacillin, cephalexin, amoxicillin with clavulinic acid, macrolides (azithromycin, erythromycin, clarithromycin), nafcicillin and vancomycin (for MRSA)), anti‐pseudomonal (e.g. ciprofloxacin, inhaled tobramycin, inhaled colistin, ceftazidime, combination treatment of ticarcillin, piperacillin, imipenem, meropenem, aztreonam, amikacin), for treating H influenzae (e.g. amoxicillin with clavulinic acid, second and third generation cephalosporin (cefuroxime axetil, cefprozil, cefixime, cefpodoxime proxetil, loracarbef) and for treating B cepacia (e.g. co‐trimoxazole, doxycycline, minocycline)
Ibuprofen ‐ high dose (more than 2400 mg/day)
Dornase alfa
CFTR modulators ‐ ivacaftor, lumacaftor or tezacaftor
Methods of delivery for topical application include nebulizers (jet or ultrasonic), soft mist, dry powder inhaler, pressured metered dose inhaler, nasal drops, nasal spray, squeeze bottles and netti pots.
We planned to compare the interventions listed above to no intervention, to placebo or to another medical intervention or class of medical intervention. We also planned to compare the same medical intervention given at a different dose, for a different duration or via a different route. We planned to include comparisons of combinations of the treatments listed above. Concurrent treatments were allowed if they are used in both treatment arms.
Types of outcome measures
If a trial had been found which measured outcomes other than those mentioned below, the trial would have been included if it had met our inclusion criteria and if the outcome had been measured objectively and was relevant to our review. The inclusion of any such additional outcomes would have been noted as a post hoc change in the 'Difference between review and protocol' section.
Primary outcomes
QoL measured by questionnaires (e.g. Rhinosinusitis Outcome Measure‐31 (Tipirneni 2017) and Sinonasal Outcome Test‐22 (SNOT‐22) (Tipirneni 2017) for adults; Sinonasal‐5 for children (Tipirneni 2017))
Refractory to treatment ‐ defined by persistent symptoms during intervention or recurrence of symptoms soon after stopping the intervention, requiring higher dose or concentration or more potent intervention to obtain any or sustained benefit as measured by Chronic Sinusitis Survey or visual analogue scale
-
Treatment‐related adverse events
mild (an adverse event that does not require treatment, does not require the medication to be stopped, e.g. nausea)
moderate (an adverse event that needs treatment, does not require the medication to be stopped; or needs the treatment to be stopped for a short duration, e.g. intractable vomiting)
severe (an adverse event that is life‐threatening and requires the medication to be stopped permanently, e.g. allergy to an antibiotic)
Secondary outcomes
Periods of improved symptoms (as measured by number of days of benefit)
-
Change in nasal endoscopic findings
nasal polyps
mucopurulent discharge (discharge containing both mucus and pus)
mucosal edema or change
Change in Lund‐Mackay scores (scoring based on nose and sinus appearances as seen in CT scans)
-
Pulmonary function tests (as measured by either a change from baseline, post‐treatment values or both)
forced expiratory volume in one second (FEV1)
functional vital capacity (FVC)
-
Change in nutritional status
height (cm) (in children)
weight (kg) (in children)
body mass index (BMI) (in adults)
Results were reported at the end of treatment or at one month (or both), up to three months, three to six months, six to 12 months and over 12 months.
Search methods for identification of studies
We searched for all relevant published and unpublished trials without restrictions on language, year or publication status.
Electronic searches
To identify relevant studies, we searched the Cochrane Cystic Fibrosis and Genetic Disorder Group's Cystic Fibrosis Trials Register using the term: sinusitis. The Cystic Fibrosis Trials Register is compiled from electronic searches of the Cochrane Central Register of Controlled Trials (CENTRAL) (updated each new issue of the Cochrane Library), weekly searches of MEDLINE, a search of Embase to 1995 and the prospective handsearching of two journals ‐ Pediatric Pulmonology and the Journal of Cystic Fibrosis. Unpublished work was identified by searching the abstract books of three major CF conferences: the International Cystic Fibrosis Conference; the European Cystic Fibrosis Conference and the North American Cystic Fibrosis Conference. For full details of all searching activities for the register, please see the relevant section of the Cochrane Cystic Fibrosis and Genetic Disorders Group's website.
Date of latest search: 22 May 2019.
We searched the following databases:
Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library (www.cochranelibrary.com/);
PubMed (www.ncbi.nlm.nih.gov/pubmed);
Embase Ovid (1974 to present).
We also searched the following trials registries and search engines:
ClinicalTrials.gov (www.clinicaltrials.gov/);
WHO ICTRP (http://apps.who.int/trialsearch/Default.aspx);
EU Clinical Trials Register (www.clinicaltrialsregister.eu/);
See Appendix 1 for the full search strategies.
Date of latest additional searches: 20 May 2019.
Searching other resources
We checked the bibliographies of included trials and any relevant systematic reviews identified for further references to relevant trials. We contacted trial authors if we deemed this to be necessary.
Data collection and analysis
Selection of studies
Two authors (TK and VK) independently reviewed all identified abstracts and reports retrieved from databases and other sources. If the reference appeared relevant to the review topic, they obtained a full text copy. The same two authors assessed and selected any trials which they find to be relevant according to the review's inclusion and exclusion criteria. We resolved disagreements by discussion with a third author (BAW or LK). Any review author who is an investigator on a trial was not active in the selection process for that trial.
Data extraction and management
The review authors created a data extraction sheet based on the 'Checklist of items to consider in data collection or data extraction' as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). Two review authors (TK and VK) planned to independently extract data from the included trials and resolve any disagreements by discussion with a third review author (BAW or LK). If a review author was an author on a trial, that author would not extract data for the trial.
The review authors planned to include key characteristics of the trial, such as trial design, setting, sample size, population, how outcomes were defined and collected in the trial. We also planned to collect baseline information on prognostic factors or effect modifiers, e.g. baseline symptom scores and Lund‐Mackay scores. We planned to note down separately if participants were selected based on bacterial colonization.
For any trials of multiple interventions, the review authors planned to make pair‐wise summaries. If a common intervention group was present, we planned to split the sample size and mark this on the data extraction sheet.
For the outcomes of interest, the review authors planned to extract the findings of the trials on an available‐case‐analysis basis. We planned to include all participants from the point of randomization, irrespective of the treatment they actually received.
The review authors planned to present all the results for each of the broad comparison groups (i.e. nasal saline irrigation, antibiotics, corticosteroids, etc.) together and planned to undertake subgroup analyses to look at the effects of individual agents to investigate any identified heterogeneity in the results (see below).
The review authors planned to extract the following statistics for each trial and each outcome:
for dichotomous data ‐ the number of participants experiencing an event and number of participants assessed at that time point;
for continuous data ‐ mean values, standard deviations (SDs) and number of participants in each treatment arm, if available, we planned to report absolute values and change from baseline.
We planned to report results at the end of treatment or at one month (or both), up to three months, three to six months, six to 12 months and over 12 months; we would also report the number of days of benefit both at the end of treatment and at one month.
Assessment of risk of bias in included studies
Two review authors (TK and VK) planned to independently assess the risk of bias in each included trial based on the following six components: sequence generation, allocation concealment, blinding or masking, incomplete outcome data, selective outcome reporting, and other biases. For each of these components, we planned to assign a judgment regarding the risk of bias as high, low or unclear (Higgins 2011b). We planned to make these judgments separately for objectively and subjectively ascertained measures for the domains of blinding and incomplete outcome data. The review authors planned to record assessments in the standard risk of bias table for each included trial and planned to use these assessments in making judgments on overall trial quality while preparing a summary of findings table (see below). When methodological details were unclear, the review authors would attempt to contact the trial authors for clarification. The review authors planned to resolve any differences by discussion.
We will include in the review any trials on which the authors themselves are investigators, if these are relevant to the review question, but will note this fact in the section on 'Declarations of interest'. In such cases, the review author who is also an investigator on the trial, will not make any risk of bias judgments for that trial.
Measures of treatment effect
Dichotomous data
For dichotomous data, (refractory to treatment, presence or absence of abnormalities on nasal endoscopy and need for surgery, adverse events), we planned to present results as summary risk ratios (RR) with 95% confidence intervals (CI).
Continuous data
For continuous data (QoL measurements, pulmonary function tests, change in nutritional status and Lund‐Mackay scores), we planned to calculate the mean difference (MD) between groups with 95% CIs if outcomes were measured in the same way in trials. When trials used different assessment scales, the authors planned to calculate the standardized mean difference (SMD) with 95% CIs.
Unit of analysis issues
Cluster‐randomized trials
We planned to include cluster‐randomized trials in this review along with individually randomized trials. We planned to use the methods described in the Cochrane Handbook of Systematic Reviews of Interventions to account for any unit of analysis error (Higgins 2011c). We planned to derive an estimate of the intracluster correlation coefficient (ICC) from the trial, from a similarly designed trial or from a trial of a similar population. If planned to use ICCs from other sources in the review, we planned to report this and conduct a sensitivity analysis to recognize the effect of the variation of the ICCs. If planned to identify both cluster‐randomized trials and individually‐randomized trials with little heterogeneity between their methodology, and further if we considered an interaction between the effect of the intervention and choice of randomization unit is unlikely, we planned to pool the relevant information and combine the results. We planned to acknowledge any heterogeneity that may occur in the unit of randomization and planned to conduct a sensitivity analysis to investigate the effects of this.
Cross‐over trials
We planned to include cross‐over trials if we could perform paired analysis on data according to Elbourne (Elbourne 2002). If not, we planned to include the first phase of a cross‐over trial if an adequate follow‐up period (i.e. a minimum of three months) was available.
Trials with multiple treatment groups
The authors planned to include multi‐arm trials in the review if they can make pair‐wise comparisons on the intervention groups and if when investigated alone, the comparison would meet the review's inclusion criteria. The authors would only analyze interventions or groups meeting the inclusion criteria in this review. The authors planned to assess the risk of bias based on the completeness of available data and absence of selective reporting of comparisons of intervention arms. The authors planned to attempt to overcome any unit of analysis errors by combining groups to create a single pair‐wise comparison.
Dealing with missing data
Whenever possible, we tried to contact original investigators to request missing data. We planned to assess missing data to try and establish if they were missing at random or if they had a particular value. Where possible, we planned to extract data to allow an intention‐to‐treat analysis, where all randomized participants were analyzed in the groups to which they were originally assigned (Higgins 2011c). We planned to calculate discrepancies in the numbers randomized and numbers analyzed in each treatment group and report these as the percentage lost to follow‐up. If more than 10% have been lost to follow‐up for any trial, for dichotomous outcomes, we planned to assign the worst outcome and analyze the impact of this assignment using a sensitivity analysis.
If SDs are found to be missing in a small proportion of trials, we planned to impute these from other trials for which full data are available (Higgins 2011c). We planned to carry out sensitivity analyses to assess the impact of changing the assumptions made. If the majority of trials included in this review were missing SDs, we planned not to impute these values. We planned to not make any assumptions regarding loss to follow‐up for continuous data and they will analyze the results for those who completed the trial.
Assessment of heterogeneity
The review authors planned to assess heterogeneity between trials for the outcomes of interest both visually using a forest plot and using the I² statistic as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b). For multiple‐intervention trials, we planned to split the sample size for the common intervention group in order to prevent a unit‐of‐analysis error, to perform investigations of heterogeneity.
The interpretation of I² will be as follows:
0% to 40%: might not be important;
30% to 60%: may represent moderate heterogeneity;
50% to 90%: may represent substantial heterogeneity;
75% to 100%: considerable heterogeneity (Higgins 2011b).
Assessment of reporting biases
The review authors planned to use funnel plots to assess publication and reporting bias, if the number of trials permitted this. We planned to measure funnel plot asymmetry with a linear regression approach on the logarithm scale of the RR. If the funnel plot was asymmetrical, we planned to explore alternative causes in addition to publication bias.
Data synthesis
The review authors planned to carry out statistical analysis using the Review Manager software (Review Manager 2014). If we found that trials estimate the same underlying treatment effect, we planned to use a fixed‐effect model to combine the data. Alternatively, if there was a high degree of heterogeneity between the trials' populations and methods, or if substantial statistical heterogeneity was detected (I² lies between 60% and 90%), we planned to use a random‐effects model and determine the average treatment effect to decide if the treatment effect is clinically meaningful. The review authors planned to treat the random‐effects model summary as the average range of possible treatment effects and present the results as the average treatment effect with 95% CIs together with the estimates of I².
Subgroup analysis and investigation of heterogeneity
If data had permitted, we planned to carry out subgroup analyses as follows:
age (children ‐ aged up to 18 years versus adults ‐ aged more than 18 years);
surgical status of participants (pre‐ and post‐surgery);
presence of co‐morbidities (e.g. any other chronic disease such as diabetes and hypothyroidism);
effects of each category of a type of medical intervention (e.g. fluticasone and mometasone; of inhaled corticosteroids).
Sensitivity analysis
To ensure the conclusions drawn during the review process were robust and representative of reality, if there were sufficient comparable trials and if we identified methodological differences between trials, we planned to conduct sensitivity analyses excluding those trials with clearly inadequate randomisation, concealment of allocation or blinding (high risk of bias) (Deeks 2011).
We also planned to explore the impact of including trials with high levels of missing data in the overall assessment of treatment effect. As stated above, if more than 10% of participants have been lost to follow‐up for any trial and we had assigned the worst outcome for dichotomous outcomes, we planned to analyze the impact of this assignment using a sensitivity analysis. We also planned to assess the effect of any imputed SDs we had used in the analyses.
Furthermore, if we used ICCs from other sources in the review, we planned to report this and conduct a sensitivity analysis to recognize the effect of the variation of the ICCs. We planned to acknowledge heterogeneity that may occur in the randomization unit and we planned to conduct a sensitivity analysis to investigate the effects of the randomization unit.
Summary of findings tables
The authors planned to create a summary of findings table for each comparison presented and use the GRADE approach to interpret the findings. The authors planned to include in each table the following outcomes of this review:
QoL questionnaires;
refractoriness to treatment;
treatment‐related adverse events;
periods of improved symptoms;
nasal endoscopic findings;
Lund‐Mackay scores; and
pulmonary function test values.
For each, we planned to state the population, setting, intervention, and comparison. We planned to assess the quality of the body of evidence by considering the overall risk of bias of the included trials, the directness of the evidence, the inconsistency of the results, the precision of the estimates, and the risk of publication bias (Schünemann 2011a; Schünemann 2011b).
Results
Description of studies
Results of the search
A total of 14 references to six trials were identified from the Cochrane Cystic Fibrosis and Genetic Disorders (CFGD) Group's CF trials register; four of these trials were assessed as potentially relevant for inclusion, but later excluded. We identified 80 ongoing trials from the ongoing trial registries; we deemed five to be potentially relevant, but on further inspection one was excluded and one was listed in the review as ongoing (NCT02888730). No additional trials were identified on searching PubMed, Ovid Embase and the Cochrane Library as described in the appendices (Appendix 2).
A 'Preferred Reporting Items for Systematic Reviews and Meta‐Analysis' (PRISMA) flow diagram depicting search results is shown in Figure 1.
1.
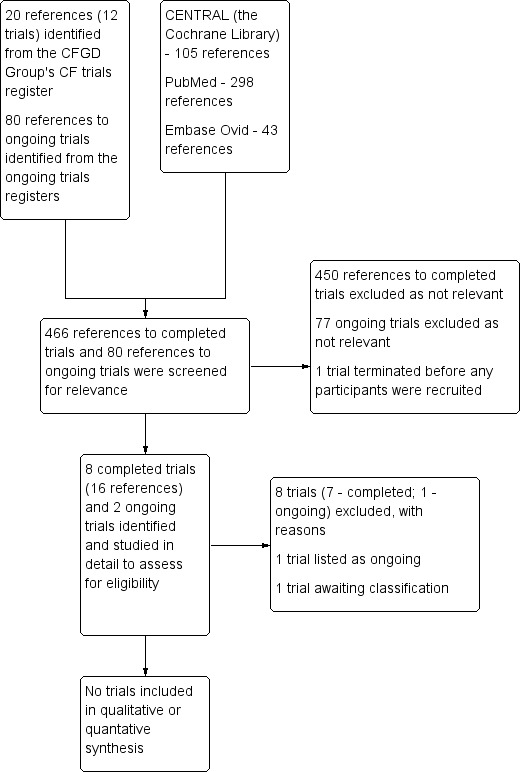
Study flow diagram.
Detail of these trials can be found in the tables (Characteristics of excluded studies; Characteristics of ongoing studies).
Included studies
No trials met the inclusion criteria for this review.
Excluded studies
We excluded eight trials. The reasons for exclusion were inadequate follow‐up period in four trials (Mainz 2011,Mainz 2014; Mainz 2016; NCT03439865), technique of randomisation by the side of the nose in two trials (Wagner 1999; Wagner 2002), usage of an intervention not considered in our protocol (ACTRN12613000214730) and assessing the effects of medical intervention on surgical outcomes (NCT00416182).
Ongoing studies
The protocol of the ongoing trial describes a parallel‐assigned triple‐blinded multicentre RCT based in France (NCT02888730). People aged seven years and older with diagnosed CF (by positive sweat test or identification of two CF‐causing mutations) and CRS with bacteria sensitive to tobramycin (as confirmed by an otolaryngologist by endoscopy and culture) will be randomly assigned to receive either tobramycin or 0.9% sodium chloride via nebulizer twice daily for 15 days. Participants will be assessed on day 0, day 15, day 30 and day 90. The trial outcomes include the density of bacteria in sinus ostia, minimum inhibitory concentration of bacteria to antibiotics, symptoms (nasal obstruction, rhinorrhea, mucopurulent secretion, facial pain, dysosmia), endoscopic scores, QoL questionnaires and lung function tests.
Risk of bias in included studies
We included no trials and therefore could not assess methodological quality.
Effects of interventions
We identified no trials assessing effects of medical intervention for CRS in people with CF.
Discussion
Summary of main results
No trials were identified which met our inclusion criteria for this review.
Although CRS is seen in nearly 100% of people with CF (Liang 2014), adequate trials are not available in order to come to a consensus on the medical management of CRS in people with CF.
Overall completeness and applicability of evidence
We identified no trials which met our inclusion criteria. There is a paucity of robust trials that address medical interventions in people diagnosed with CF and CRS. One ongoing trial identified during our searches looks promising and will be assessed fully once completed (NCT02888730). We may have a better consensus on medical management of CRS in people with CF in future updates of this review.
Quality of the evidence
We identified no trials that met our inclusion criteria. Hence, we could not assess the quality of the evidence.
Potential biases in the review process
No bias was encountered. We applied no language restriction on publication, including during identification of ongoing trials. We used broad search terms in this review which identified a large number of trials. We also conducted searches in other medical databases and online trial registries.
Agreements and disagreements with other studies or reviews
There is a paucity of trials assessing medical interventions for people with CRS and CF.
Three systematic reviews have been published (Liang 2014; Shah 2018; Virgin 2017). All of these reviews evaluate the four completed RCTs that have been listed as excluded in this review. They also have included non‐RCT studies for analysis.
Further investigation of the excluded studies of our Cochrane Review revealed that the five RCTs addressed different aspects of medical management of CRS in people with CF (Mainz 2011, Mainz 2014, Mainz 2016; Wagner 1999; Wagner 2002). In 2011, Mainz conducted a trial to compare the effects of inhaled dornase alfa over normal saline (Mainz 2011). Dornase alfa significantly improved the QoL. However, the trial was conducted on a small sample size of five participants and no difference were found in SNOT 20 scores, rhinoscopy findings, aerated volume of maxillary sinus as per magnetic resonance imaging and pulmonary function tests. The 2014 publication by Mainz suggested that daily inhalation of dornase alfa produced significant improvement in nasal symptoms, SNOT‐20 scores and pulmonary function tests at the end of 28 days of treatment (Mainz 2014); but similar benefit was not observed with hypertonic saline (6%) (Mainz 2016). Both Wagner trials evaluated the efficacy of adeno‐associated virus vector‐cystic fibrosis trans‐membrane regulator (AAV‐CFTR) in CF maxillary sinus, firstly as a phase I and then as a phase II trial. Both failed to show statistically significant improvement in rate of relapse of sinusitis (Wagner 1999; Wagner 2002).
Authors' conclusions
Implications for practice.
Currently, there is no evidence from randomized controlled trials (RCTs) or quasi‐RCTs that meet our inclusion criteria to support or refute any form of medical management for chronic rhinosinusitis (CRS) in people with cystic fibrosis (CF). Treatment protocols are to be based on available RCTs and non‐RCT evidence.
Implications for research.
As the treatment of CF advances, many individuals survive to an older age. The incidence of CRS and the need for effective treatments continues to increase. Hence, it is essential to conduct robust clinical trials with long‐term follow‐up. We recommend that the following questions are addressed in the upcoming trials.
Are CF transmembrane regulator (CFTR) modulators effective in managing CRS in people with CF?
Is dornase alfa helpful to improve the quality of life of people with CRS and CF?
Which type of corticosteroid and which mode of delivery provides the best outcome for CRS in people with CF?
Is any particular combination treatment effective in managing CRS in people with CF?
Are inhaled antibiotics helpful in preventing pulmonary exacerbations in people with CRS and CF?
Acknowledgements
We thank the management of Kasturba Medical College, Johns Hopkins University School of Medicine, and University of Alabama for giving us the opportunity to be involved in the completion of this protocol and review. We also thank Nikki Jahnke from the Cochrane Cystic Fibrosis and Genetic Disorders Group for her help with this protocol and review.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Cystic Fibrosis and Genetic Disorders Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Appendices
Appendix 1. Search Methods – Electronic Searching
| Database or Resource | Search strategy |
| ClinicalTrials.gov (www.clinicaltrials.gov/) |
(Cystic Fibrosis OR CFTR) AND (rhinitis OR sinusitis OR rhinosinusitis) |
| WHO ICTRP (apps.who.int/trialsearch/Default.aspx) |
(Cystic Fibrosis OR CFTR) AND (rhinitis OR sinusitis OR rhinosinusitis) |
| European Union (EU) Clinical Trials Register (www.clinicaltrialsregister.eu/) |
(Cystic Fibrosis OR CFTR) AND (rhinitis OR sinusitis OR rhinosinusitis) |
| PubMed | (Cystic Fibrosis OR CFTR) AND (rhinitis OR sinusitis OR rhinosinusitis) AND (randomised control trial OR RCT OR review OR narrative review OR systematic review OR meta‐analysis) |
| Ovid Embase | (Cystic Fibrosis OR CFTR) AND (rhinitis OR sinusitis OR rhinosinusitis) AND (randomised control trial OR RCT OR review OR narrative review OR systematic review OR meta‐analysis) |
| the Cochrane Library | (Cystic Fibrosis OR CFTR) AND (rhinitis OR sinusitis OR rhinosinusitis) AND (randomised control trial OR RCT OR review OR narrative review OR systematic review OR meta‐analysis) |
| Google Scholar | (Cystic Fibrosis OR CFTR) AND (rhinitis OR sinusitis OR rhinosinusitis) AND (randomised control trial OR RCT OR review OR narrative review OR systematic review OR meta‐analysis) |
Appendix 2. Revised electronic search strategies
| Database or resource | Search strategy |
| CENTRAL (the Cochrane Library) (www.cochranelibrary.com/) |
ADVANCED SEARCH ‐ SEARCH MANAGER FORM #1 cystic next fibros* #2 MeSH descriptor: [Cystic Fibrosis] this term only #3 MeSH descriptor: [Cystic Fibrosis Transmembrane Conductance Regulator] this term only #4 cftr #5 fibrocystic and pancrea* #6 mucoviscido* #7 #1 or #2 or #3 or #4 or #5 or #6 #8 rhinitis or sinusitis or rhinosinusitis #9 MeSH descriptor: [Sinusitis] explode all trees #10 #8 or #9 #11 #7 and #10 |
| PubMed (www.ncbi.nlm.nih.gov/pubmed) | (Cystic Fibrosis OR mucoviscidosis OR cystic fibrosis transmembrane conductance regulator OR cftr) AND (rhinitis OR sinusitis OR rhinosinusitis) AND (randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized [tiab] OR placebo [tiab] OR drug therapy [sh] OR randomly [tiab] OR trial [tiab] OR groups [tiab] NOT (animals [mh] NOT humans [mh])) |
| Embase Ovid | (Cystic Fibrosis OR mucoviscidosis OR cystic fibrosis transmembrane conductance regulator OR cftr) AND (rhinitis OR sinusitis OR rhinosinusitis) AND (randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized [tiab] OR placebo [tiab] OR drug therapy [sh] OR randomly [tiab] OR trial [tiab] OR groups [tiab] NOT (animals [mh] NOT humans [mh])) |
| ClinicalTrials.gov (www.clinicaltrials.gov/) |
RECRUITMENT STATUS: All studies CONDITION OR DISEASE: cystic fibrosis OTHER TERMS: rhinitis OR sinusitis OR rhinosinusitis |
| WHO ICTRP (apps.who.int/trialsearch/Default.aspx) |
SEARCH 1: cystic fibrosis AND rhinitis SEARCH 2: cystic fibrosis AND sinusitis SEARCH 3: cystic fibrosis AND rhinosinusitis |
| EU Clinical Trials Register (www.clinicaltrialsregister.eu/) |
(cystic fibrosis) AND (rhinitis OR sinusitis OR rhinosinusitis) |
Characteristics of studies
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| ACTRN12613000214730 | Intervention undertaken was not considered in our protocol. In this trial, study group underwent therapeutic ultrasound insonation over each maxillary sinus for five minutes. This intervention has not been considered in our protocol. Hence, we excluded this trial. |
| Mainz 2011 | Follow‐up period was not adequate for analysis. In this trial, included participants were treated with inhaled dornase alfa or normal saline for 28 days. Ther were assessed on 28th day. After a washout period of another 28 days, they were crossed over to the alternative treatment. The minimum period of follow‐up needed to include a trial in this review in 3 months. Hence, we excluded this trial. |
| Mainz 2014 | Follow‐up period was not adequate for analysis. In this trial, included participants were treated with inhaled dornase alfa or normal saline for 28 days. They were assessed on the 28th day. After a washout period of another 28 days, they were crossed over to the alternative treatment. The minimum period of follow‐up needed to include a trial in this review is 3 months. Hence, we excluded this trial. |
| Mainz 2016 | Follow‐up period was not adequate for analysis. In this trial, included participants were treated with inhaled hypertonic saline (6%) or normal saline for 28 days. They were assessed on the 28th day. After a washout period of another 28 days, they were crossed over to the alternative treatment. The minimum period of follow‐up needed to include a trial in this review is 3 months. Hence, we excluded this trial. |
| NCT00416182 | Study evaluated the effect of medical interventions on surgical outcomes. In this trial, participants were randomized to receive dornase alfa or placebo via nasal inhalation one week following a nasal surgery, to assess improvement in post‐operative sinusitis symptoms. We have planned to exclude trials evaluating effect of medical interventions on surgical outcomes. Hence, we excluded this trial. |
| NCT03439865 | Follow‐up period was not adequate for analysis. In the protocol of this ongoing trial, participants were planned to be treated with ivacaftor along with standard of care treatment (topical nasal steroid spray and culture‐directed antibiotics) or standard of care alone for 14 days. Outcomes are planned to be assessed on day 1, day 14 and day 30. The minimum period of follow‐up needed to include a trial in this review is 3 months. Hence, we excluded this trial. |
| Wagner 1999 | Methodology of randomisation was not acceptable as per our protocol. Investigators preformed a prospective, randomized, phase I clinical trial by instilling adeno‐associated virus vector ‐ CF transmembrane conductance regulator (AAV‐CFTR) into a maxillary sinus with a surgically‐created antrostomy. The contralateral sinus served as a control. According to our protocol, we planned to exclude trials randomized by the side of the nose. Hence, we excluded this trial. |
| Wagner 2002 | Methodology of randomisation was not acceptable as per our protocol. Investigators performed a phase II double‐blind randomized placebo‐controlled trial by instilling 100,000 replication units of targeted genetics adeno‐associated virus vector – CF (tgAAVCF) into one of the maxillary sinuses. The contralateral maxillary sinus acted as a control. According to our protocol, we planned to exclude trials randomized by the side of the nose. Hence we excluded this trial. |
CF: cystic fibrosis
Characteristics of studies awaiting assessment [ordered by study ID]
NCT03145051.
| Methods | RCT
Parallel assignment, cross‐over trial Single blinded Duration: 8 weeks |
| Participants | 50 participants, aged between 11 and 65 years Inclusion criteria
Exclusion criteria
|
| Interventions |
Treatment arm Nasal irrigation with Respimer Netiflow mineral salts solution, 4 times/day during 8 weeks using Respimer Netiflow class I medical device Control arm Nasal irrigation with saline solution, 4 times/day during 8 weeks using Respimer Netiflow class I medical device |
| Outcomes |
Primary outcome
Secondary outcome
|
| Notes | This trial was undertaken by Laboratoire de la Mer between January 2015 and December 2017. The results of this trial are not retrievable. We are trying to contact the primary investigator for further information. |
RCT: randomized controlled trial
Characteristics of ongoing studies [ordered by study ID]
NCT02888730.
| Trial name or title | Efficacy of Antibiotic (Tobramycin) Delivered by Nebulized Sonic Aerosol for Chronic Rhinosinusitis Treatment of Cystic Fibrosis Patients: A Multicenter Double‐blind Randomized Controlled Trial |
| Methods | RCT Parallel assignment Triple blinded Duration: 15 days Multicenter: 6 centers (Créteil, Marseille, Nantes, Toulouse, Clermont‐Ferrand and Nice) |
| Participants | Estimated enrolment: 86 participants aged 7 years or older Inclusion criteria
Exclusion criteria
|
| Interventions |
Treatment arm 1 ampoule tobramycin (5 mL containing 300 mg of tobramycin and 11.25 mg of sodium chloride) nebulized nasally 2x per day (morning and evening with a maximum of 12 hours but not less than 6 hours between the 2 doses) each sonic nebulization lasting approximately 15 minutes; dosage will not be adjusted to body weight. Active intervention is manufactured by the "Pharmacie à Usage Interne" of the Henri Mondor Hospital prepared with Base Tobramycin and excipients in accordance with TOBI's composition Control arm 1 ampoule placebo (5 mL containing sodium chloride 0.9%) nebulized nasally 2x per day (morning and evening with a maximum of 12 hours but not less than 6 hours between the 2 doses) each sonic nebulization lasting approximately 15 minutes; dosage will not be adjusted to body weight. Placebo is manufactured by the "Pharmacie à Usage Interne" of the Henri Mondor Hospital so that it has the same colour (light yellow transparent) as tobramycin |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Starting date | February 2017 |
| Contact information | Virginie ESCABASSE, MD Tel: 1 45 17 55 97 ext 33 virginie.escabasse@chicreteil.fr |
| Notes |
CF: cystic fibrosis CFU: colony forming units CRS: chronic rhinosinusitis CT: computer tomography FEV: forced expiratory volume FVC: forced vital capacity RCT: randomized controlled trial SNOT: sino‐nasal outcomes test
Differences between protocol and review
As the Cochrane Review 'Topical nasal steroids for treating nasal polyposis in people with cystic fibrosis' has been published previously (Beer 2015), trials addressing the effect of topical nasal steroids on nasal polyposis are excluded from this review.
With the advise of our information specialist, we have revised the search strategies to those as listed in Appendix 2 to further identify ongoing and completed trials.
Contributions of authors
| Task | Author(s) responsible |
| Protocol stage: draft the protocol | All five authors. |
| Review stage: select which trials to include (2 + 1 arbiter) | Tulasi Kota Karanth and Dr KVL Karanth will independently select trials. Disagreements will be discussed with Dr BA Woodworth and Dr L Karanth. |
| Review stage: extract data from trials (2 people) | Tulasi Kota Karanth and Dr KVL Karanth will independently extract data from trials. |
| Review stage: enter data into RevMan | Tulasi Kota Karanth. |
| Review stage: carry out the analysis | Dr L Karanth, Dr KVL Karanth, Tulasi Kota Karanth. |
| Review stage: interpret the analysis | Dr L Karanth, Dr KVL Karanth, Tulasi Kota Karanth. |
| Review stage: draft the final review | All five authors. |
| Update stage: update the review | All five authors. |
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Institutes of Health (NIH)/National Heart, Lung, and Blood Institute (1 R01 HL133006‐01), USA.
National Institutes of Health (NIH)/National Heart, Lung, and Blood Institute (1 R01 HL133006‐01)
-
National Institute for Health Research, UK.
This systematic review was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Cystic Fibrosis and Genetic Disorders Group.
Declarations of interest
TK: none known.
VK: none known.
BKW has received payment from Oakstone, LLC. for providing educational reviews of recent medical literature on topics of interest to general otolaryngologists and has received reimbursement for attending a conference on inner ear disorders from Med‐El Corporation.
BAW is a consultant for Olympus and Cook Medical and has grant support from the NHLBI and Cook Medical.
LK: none known
New
References
References to studies excluded from this review
ACTRN12613000214730 {published data only}
- ACTRN12613000214730. A randomised trial of therapeutic ultrasound for chronic rhinosinusitis in adults with cystic fibrosis. www.anzctr.org.au (date submitted 20 February 2013).
Mainz 2011 {published data only}
- Mainz JG, Schiller I, Ritschel C, Mentzel HJ, Riethmüller J, Koitschev A, et al. Sinonasal inhalation of dornase alfa in CF: A double‐blind placebo‐controlled cross‐over pilot trial. Auris Nasus Larynx 2011;38(2):220‐7. [PUBMED: 21030168] [DOI] [PubMed] [Google Scholar]
Mainz 2014 {published data only}
- Karadag A, Catal F. Nasal saline as a placebo in chronic rhinosinusitis. Journal of Cystic Fibrosis 2014;13(5):601. [CFGD Register: BD154c] [DOI] [PubMed] [Google Scholar]
- Mainz JG, Schien C, Schiller I, Schadlich K, Koitschev A, Koitschev C, et al. Sinonasal inhalation of dornase alfa administered by vibrating aerosol to cystic fibrosis patients: a double‐blind placebo‐controlled cross‐over trial. Journal of Cystic Fibrosis 2014;13(4):461‐70. [CFGD Register: BD154b; PUBMED: 24594542] [DOI] [PubMed] [Google Scholar]
- Mainz JG, Schiller I, Koitschev A, Koitschev C, Riethmuller J, Wiedemann B, et al. Sinonasal inhalation of dornase alfa reduces rhinosinusitis symptoms in CF. Results of a DBPC‐cross‐over‐study. Journal of Cystic Fibrosis 2010;9 Suppl 1:S23. [Abstract no.: 88; CFGD Register: BD154a] [Google Scholar]
Mainz 2016 {published data only}
- Mainz JG, Schaedlich K, Hentschel J, Schumacher U, Koitschev C, Koitschev A, et al. Sinonasal inhalation of isotonic vs. hypertonic saline (6.0%) in CF patients with chronic rhinosinusitis ‐ results of a multicentre, double‐blind, controlled prospective trial. Journal of Cystic Fibrosis 2015;14 Suppl 1:S95. [Abstract no.: 146; CFGD Register: CO59a] [DOI] [PubMed] [Google Scholar]
- Mainz JG, Schumacher U, Schadlich K, Hentschel J, Koitschev C, Koitschev A, et al. Sino nasal inhalation of isotonic versus hypertonic saline (6.0%) in CF patients with chronic rhinosinusitis ‐ Results of a multicenter, prospective, randomized, double‐blind, controlled trial. Journal of Cystic Fibrosis 2016;15(6):e57‐e66. [CFGD Register: CO59b; PUBMED: 27267518] [DOI] [PubMed] [Google Scholar]
NCT00416182 {published data only}
- NCT00416182. Nasally delivered pulmozyme for sinusitis in cystic fibrosis. www.clinicaltrials.gov/show/nct00416182 (first posted 27 December 2006).
NCT03439865 {published and unpublished data}
- NCT03439865. Ivacaftor for acquired CFTR dysfunction in chronic rhinosinusitis. www.clinicaltrials.gov/ct2/show/NCT03439865 (first posted 20 February 2018).
Wagner 1999 {published data only}
- Wagner JA, Messner AH, Moran ML, Daifuku R, Kouyama K, Desch JK, et al. Safety and biological efficacy of an adeno‐associated virus vector‐cystic fibrosis transmembrane regulator (AAV‐CFTR) in the cystic fibrosis maxillary sinus. Laryngoscope 1999;109(2 Pt 1):266‐74. [CFGD Register: BD8b] [DOI] [PubMed] [Google Scholar]
- Wagner JA, Nepomuceno IB, Shah N, Messner AH, Moran ML, Norbash AM, et al. Maxillary sinusitis as a surrogate model for CF gene therapy clinical trials in patients with antrostomies. Journal of Gene Medicine 1999;1(1):13‐21. [CFGD Register: BD8c] [DOI] [PubMed] [Google Scholar]
- Wagner JA, Reynolds T, Moran ML, Moss RB, Wine JJ, Flotte TR, et al. Efficient and persistent gene transfer of AAV‐CFTR in maxillary sinus [letter]. Lancet 1998;351(9117):1702‐3. [CFGD Register: BD8a] [DOI] [PubMed] [Google Scholar]
Wagner 2002 {published data only}
- Wagner JA, Messner AH, Friborg S, Reynolds T, Guggino WB, Moss RB, et al. A phase II, double‐blind, randomized, placebo‐controlled clinical trial of tgAAVCF in the maxillary sinus of CF patients. Pediatric Pulmonology 1998;Suppl 17:260. [CFGD Register: BD9a] [Google Scholar]
- Wagner JA, Messner AH, Moran ML, Guggino WB, Flotte TR, Wine JJ, et al. A phase II, double‐blind, randomized, placebo‐controlled clinical trial of tgAAVCF using maxillary sinus delivery in CF patients with antrostomies. Pediatric Pulmonology 1999;Suppl 19:223‐4. [CFGD Register: BD9b] [Google Scholar]
- Wagner JA, Moran ML, Messner AH, Daifuku R, Conrad CK, Reynolds T, et al. A phase I/II study of tgAAV‐CF for the treatment of chronic sinusitis in patients with cystic fibrosis. Human Gene Therapy 1998;9(6):889‐909. [CFGD Register: BD9d] [DOI] [PubMed] [Google Scholar]
- Wagner JA, Nepomuceno IB, Messner AH, Moran ML, Batson EP, Dimiceli S, et al. A phase II, double‐blind, randomized, placebo‐controlled clinical trial of tgAAVCF using maxillary sinus delivery in patients with cystic fibrosis with antrostomies. Human Gene Therapy 2002;13(11):1349‐59. [CFGD Register: BD9c; PUBMED: 12162817] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
NCT03145051 {published data only}
- NCT03145051. Efficacy of nasal irrigation with Respimer® Netiflow® vs saline among patients with cystic fibrosis and chronic rhinosinusitis. www.clinicaltrials.gov/show/NCT03145051 (first posted 09 May 2017).
References to ongoing studies
NCT02888730 {published and unpublished data}
- NCT02888730. Tobramycin delivered by nebulized sonic aerosol for chronic rhinosinusitis treatment of cystic fibrosis patients (AVASMUC). www.clinicaltrials.gov/ct2/show/NCT02888730 (first posted 05 September 2016).
Additional references
Accurso 2010
- Accurso FJ, Rowe SM, Clancy JP, Boyle MP, Dunitz JM, Durie PR, et al. Effect of VX‐770 in persons with cystic fibrosis and the G551D‐CFTR mutation. New England Journal of Medicine 2010;363(21):1991‐2003. [PUBMED: 21083385] [DOI] [PMC free article] [PubMed] [Google Scholar]
Beer 2015
- Beer H, Southern KW, Swift AC. Topical nasal steroids for treating nasal polyposis in people with cystic fibrosis. Cochrane Database of Systematic Reviews 2015, Issue 6. [DOI: 10.1002/14651858.CD008253.pub4; PUBMED: 26098667] [DOI] [PMC free article] [PubMed] [Google Scholar]
Blount 2011
- Blount A, Zhang S, Chestnut M, Hixon B, Skinner D, Sorscher EJ, et al. Transepithelial ion transport is suppressed in hypoxic sinonasal epithelium. Laryngoscope 2011;121(9):1929‐34. [PUBMED: 22024847] [DOI] [PMC free article] [PubMed] [Google Scholar]
Boek 1999
- Boek WM, Keleş N, Graamans K, Huizing EH. Physiologic and hypertonic saline solutions impair ciliary activity in vitro. Laryngoscope 1999;109(3):396‐9. [PUBMED: 10089964] [DOI] [PubMed] [Google Scholar]
Brook 2016
- Brook I. Microbiology of chronic rhinosinusitis. European Journal of Clinical Microbiology and Infectious Disease 2016;35(7):1059‐68. [PUBMED: 27086363] [DOI] [PubMed] [Google Scholar]
Castellani 2008
- Castellani C, Cuppens H, Macek M Jr, Cassiman JJ, Kerem E, Durie P, et al. Consensus on the use and interpretation of cystic fibrosis mutation analysis in clinical practice. Journal of Cystic Fibrosis 2008;7(3):179‐96. [PUBMED: 18456578] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chang 2014
- Chang EH. New insights into the pathogenesis of cystic fibrosis sinusitis. International Forum of Allergy & Rhinology 2014;4(2):132‐7. [PUBMED: 24282147] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cheng 2015
- Cheng K, Ashby D, Smyth RL. Oral steroids for long‐term use in cystic fibrosis. Cochrane Database of Systematic Reviews 2015, Issue 12. [DOI: 10.1002/14651858.CD000407.pub4; PUBMED: 23794322] [DOI] [PMC free article] [PubMed] [Google Scholar]
Collins 1992
- Collins FS. Cystic fibrosis: molecular biology and therapeutic implications. Science 1992;256(5058):774. [PUBMED: 1375392] [DOI] [PubMed] [Google Scholar]
Costantini 1990
- Costantini D, Cicco M, Giunta A, Amabile G. Nasal polyposis in cystic fibrosis treated by beclomethasone dipropionate. Acta Universitatis Carolinae. Medica 1990;36(1‐4):220‐1. [PUBMED: 2130702] [PubMed] [Google Scholar]
Cystic Fibrosis Mutation Database 2011
- Cystic Fibrosis Mutation Database. www.genet.sickkids.on.ca/StatisticsPage.html (accessed prior to 20 June 2017).
De Boeck 2014
- Boeck K, Munck A, Walker S, Faro A, Hiatt P, Gilmartin G, et al. Efficacy and safety of ivacaftor in patients with cystic fibrosis and a non‐G551D gating mutation. Journal of Cystic Fibrosis 2014;13(6):674‐80. [PUBMED: 25266159] [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JP, Altman DG on behalf of the CSMG. Chapter 9: Analysing data and undertaking meta‐analysis. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Doring 2000
- Doring G, Conway SP, Heijerman HG, Hodson M, Hoiby N, Smyth AR, et al. Antibiotic therapy against Pseudomonas aeruginosa in cystic fibrosis: a European consensus. European Respiratory Journal 2000;16(4):749–67. [PUBMED: 11106223] [DOI] [PubMed] [Google Scholar]
Drumm 1993
- Drumm ML, Collins FS. Molecular biology of cystic fibrosis. Molecular Genetic Medicine 1993;3:33. [PUBMED: 7693108] [DOI] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vaillancourt JM. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. [DOI] [PubMed] [Google Scholar]
Fernandez 1994
- Fernandez JD, Santiago RT, Matacon MP, Mayo RC, Sanchez GT. Inhaled aztreonam therapy in patients with cystic fibrosis colonized with Pseudomonas aeruginosa. Anales Espanoles De Pediatria 1994;40:185‐8. [Google Scholar]
Fokkens 2012
- Fokkens WJ, Lund VJ, Mullol J, Bachert C, Alobid I, Baroody F, et al. EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012. A summary for otorhinolaryngologists. Rhinology 2012;50(1):1‐12. [PUBMED: 22469599] [DOI] [PubMed] [Google Scholar]
Gibson 2003
- Gibson RL, Burns JL, Ramsey BW. Pathophysiology and management of pulmonary infections in cystic fibrosis. American Journal of Respiratory and Critical Care Medicine 2003;168(8):918‐51. [PUBMED: 14555458] [DOI] [PubMed] [Google Scholar]
Guggino 2004
- Guggino WB, Banks‐Schlegel SP. Macromolecular interactions and ion transport in cystic fibrosis. American Journal of Respiratory and Critical Care Medicine 2004;170(7):815‐20. [PUBMED: 15447951] [DOI] [PubMed] [Google Scholar]
Guigui 2016
- Guigui S, Wang J, Cohen RI. The use of ivacaftor in CFTR mutations resulting in residual functioning protein. Respiratory Medicine Case Reports 2016;19:193‐5. [PMCID: PMC5079351] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hadfield 2000
- Hadfield PJ, Rowe‐Jones JM, Mackay IS. A prospective treatment trial of nasal polyps in adults with cystic fibrosis. Rhinology 2000;38(2):63‐5. [PUBMED: 10953842] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JP, Deeks JJ, editor(s). Chapter 7: Selecting studies and collecting data. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
- Higgins JP, Altman DG, Sterne JA, editor(s) on behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011c
- Higgins JP, Deeks JJ, Altman DG, editor(s) on behalf of the Cochrane Statistical Methods Group. Chapter 16: Special topics in statistics. In: Higgins JP, Green S, editor(s). Cochrane Handbook of Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hodson 2002
- Hodson ME, Gallagher CG, Govan JR. A randomised clinical trial of nebulised tobramycin or colistin in cystic fibrosis. European Respiratory Journal 2002;20(3):658‐64. [PUBMED: 12358344] [DOI] [PubMed] [Google Scholar]
Holzmann 2004
- Holzmann D, Speich R, Kaufmann T, Laube I, Russi EW, Simmen D, et al. Effects of sinus surgery in patients with cystic fibrosis after lung transplantation: a 10‐year experience. Transplantation 2004;77(1):134‐6. [PUBMED: 14724449] [DOI] [PubMed] [Google Scholar]
Illing 2014
- Illing EA, Woodworth BA. Management of the upper airway in cystic fibrosis. Current Opinion in Pulmonary Medicine 2014;20(6):623‐31. [PUBMED: 25250804] [DOI] [PMC free article] [PubMed] [Google Scholar]
Johnson 1995
- Johnson LG, Boyles SE, Wilson J, Boucher RC. Normalization of raised sodium absorption and raised calcium‐mediated chloride secretion by adenovirus‐mediated expression of cystic fibrosis transmembrane conductance regulator in primary human cystic fibrosis airway epithelial cells. Journal of Clinical Investigation 1995;95(3):1377. [PUBMED: 7533790] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jones 1993
- Jones JW, Parsons DS, Cuyler JP. The results of functional endoscopic sinus (FES) surgery on the symptoms of patients with cystic fibrosis. International Journal of Pediatric Otorhinolaryngology 1993;28(1):25‐32. [PUBMED: 8300311] [DOI] [PubMed] [Google Scholar]
Kaiser 2012
- Kaiser J. Personalized medicine. New cystic fibrosis drug offers hope, at a price. Science 2012;335(6069):645. [PUBMED: 22323790 ] [DOI] [PubMed] [Google Scholar]
Kang 2015
- Kang SH, Dalcin Pde T, Piltcher OB, Migliavacca Rde O. Chronic rhinosinusitis and nasal polyposis in cystic fibrosis: update on diagnosis and treatment. Jornal Brasileiro de Pneumologia 2015;41(1):65‐76. [PUBMED: 25750676] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2015
- Kim RJ, Park L, Wood AJ, Yin T, Jain R, Douglas RG. Chronic rhinosinusitis and cystic fibrosis: the interaction between sinus bacteria and mucosal immunity. International Forum of Allergy & Rhinology 2015;5(5):380‐5. [PUBMED: 25778791] [DOI] [PubMed] [Google Scholar]
Krouse 2007
- Krouse JH, Brown RW, Fineman SM, Han JK, Heller AJ, Joe S, et al. Asthma and the unified airway. Otolaryngology and Head and Neck Surgery 2007 May;136(5 Suppl):S75‐106. [PUBMED: 17462497] [DOI] [PubMed] [Google Scholar]
Langton Hewer 2017
- Langton Hewer SC, Smyth AR. Antibiotic strategies for eradicating Pseudomonas aeruginosa in people with cystic fibrosis. Cochrane Database of Systematic Reviews 2017, Issue 4. [DOI: 10.1002/14651858.CD004197.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Liang 2014
- Liang J, Higgins T, Ishman SL, Boss EF, Benke JR, Lin SY. Medical management of chronic rhinosinusitis in cystic fibrosis: a systematic review. Laryngoscope 2014;124(6):1308‐13. [PUBMED: 24338982 ] [DOI] [PubMed] [Google Scholar]
Lindig 2013
- Lindig J, Steger C, Beiersdorf N, Michl R, Beck JF, Hummel T, et al. Smell in cystic fibrosis. European Archives of Oto‐rhino‐laryngology 2013;270(3):915‐21. [PUBMED: 22890694] [DOI] [PubMed] [Google Scholar]
Liou 2001
- Liou TG, Adler FR, Fitzsimmons SC, Cahill BC, Hibbs JR, Marshall BC. Predictive 5‐year survivorship model of cystic fibrosis. American Journal of Epidemiology 2001;153(4):345‐52. [PUBMED: 11207152] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mainz 2012
- Mainz JG, Koitschev A. Pathogenesis and management of nasal polyposis in cystic fibrosis. Current Allergy and Asthma Reports April;12(2):163‐74. [DOI: 10.1007/s11882-012-0250-y; PUBMED: 22350539] [DOI] [PubMed] [Google Scholar]
Orlandi 2016
- Orlandi RR, Kingdom TT, Hwang PH. International Consensus Statement on Allergy and Rhinology: Rhinosinusitis Executive Summary. International Forum of Allergy & Rhinology 2016;6 Suppl 1:S3‐S21. [PUBMED: 26878819] [DOI] [PubMed] [Google Scholar]
Ramsey 1992
- Ramsey B, Richardson MA. Impact of sinusitis in cystic fibrosis. Journal of Allergy and Clinical Immunology 1992;90(3 pt 2):547‐52. [PUBMED: 1527348] [DOI] [PubMed] [Google Scholar]
Ramsey 1999
- Ramsey B, Pepe M, Quan JM, Otto KL, Montgomery AB, Williams‐Warren J, et al. Intermittent administration of inhaled tobramycin in patients with cystic fibrosis. Cystic Fibrosis Inhaled Tobramycin Study Group. New England Journal of Medicine 1999;340(1):23‐30. [PUBMED: 9878641] [DOI] [PubMed] [Google Scholar]
Ratjen 2001
- Ratjen F, Comes G, Paul K, Posselt HG, Wagner TO, Harms K, German Board of the European Registry for Cystic Fibrosis (ERCF). Effect of continuous antistaphylococcal therapy on the rate of P. aeruginosa acquisition in patients with cystic fibrosis. Pediatric Pulmonology 2001;31(1):13‐6. [PUBMED: 25383937] [DOI] [PubMed] [Google Scholar]
Ratjen 2017
- Ratjen F, Hug C, Marigowda G, Tian S, Huang X, Stanojevic S, et al. Efficacy and safety of lumacaftor and ivacaftor in patients aged 6‐11 years with cystic fibrosis homozygous for F508del‐CFTR: a randomised, placebo‐controlled phase 3 trial. Lancet Respiratory Medicine 2017;5(7):557‐67. [PUBMED: 28606620] [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rosbe 2001
- Rosbe KW, Jones DT, Rahbar R, Lahiri T, Auerbach AD. Endoscopic sinus surgery in cystic fibrosis: do patients benefit from surgery?. International Journal of Pediatric Otorhinolaryngology 2001 Nov 1;61(2):113‐119. [PUBMED: 11589977] [DOI] [PubMed] [Google Scholar]
Rowe 2005
- Rowe SM, Miller S, Sorscher EJ. Cystic Fibrosis. New England Journal of Medicine 2005;352(19):1992‐2001. [PUBMED: 15888700] [DOI] [PubMed] [Google Scholar]
Ryan 2008
- Ryan MW. Diseases associated with chronic rhinosinusitis: what is the significance?. Current Opinion in Otolaryngology & Head and Neck Surgery 2008;16(3):231‐6. [PUBMED: 18475077] [DOI] [PubMed] [Google Scholar]
Sala 2018
- Sala MA, Jain M. Tezacaftor for the treatment of cystic fibrosis. Expert Reviews in Respiratory Medicine 2018;12(9):725‐32. [PUBMED: 30073878] [DOI] [PubMed] [Google Scholar]
Schünemann 2011a
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Guyatt GH on behalf of the CA and RMG and the CSMG. Chapter 11: Presenting results and ‘Summary of findings’ tables. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Schünemann 2011b
- Schünemann HJ, Oxman AD, Vist GE, Higgins JP, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Shah 2018
- Shah GB, Keyzer L, Russell JA, Halderman A. Treatment of chronic rhinosinusitis with dornase alfa in patients with cystic fibrosis: a systematic review. International Forum of Allergy & Rhinology 2018;8(6):729‐36. [PUBMED: 29323796] [DOI] [PubMed] [Google Scholar]
Shak 1990
- Shak S, Capon DJ, Hellmiss R, Marsters SA, Baker CL. Recombinant human DNase I reduces the viscosity of cystic fibrosis sputum. Proceedings of the National Academy of Sciences of the United States of America 1990;87(23):9188‐92. [PUBMED: 2251263] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tipirneni 2017
- Tipirneni KE, Woodworth BA. Medical and surgical advancements in the management of cystic fibrosis chronic rhinosinusitis. Current Otorhinolaryngology Reports 2017;5(1):24–34. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tos 1983
- Tos M. Distribution of mucus producing elements in the respiratory tract. Differences between upper and lower airway. European Journal of Respiratory Diseases. Supplement 1983;128(Pt 1):269‐79. [PUBMED: 6578075] [PubMed] [Google Scholar]
Virgin 2017
- Virgin FW. Clinical chronic rhinosinusitis outcomes in pediatric patients with cystic fibrosis. Laryngoscope Investig Otolaryngol. 2017 May 31;2(5):276‐280. [PUBMED: 29094071] [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO Human Genetics Programme 2004
- WHO Human Genetics Programme. The molecular genetic epidemiology of cystic fibrosis: report of a joint meeting of WHO/IECFTN/ICF(M)A/ECFS, Genoa, Italy 19 June 2002. http://apps.who.int/iris/handle/10665/68702 (accessed prior to 20 June 2017).
Wilson 1987
- Wilson R, Pitt T, Taylor G, Watson D, MacDermot J, Sykes D, et al. Pyocyanin and 1‐hydroxyphenazine produced by Pseudomonas aeruginosa inhibit the beating of human respiratory cilia in vitro. Journal of Clinical Investigation 1987;79(1):221‐9. [PUBMED: 3098783] [DOI] [PMC free article] [PubMed] [Google Scholar]


