Abstract
Objectives
The purpose of this study was to explore whether pretreatment potential prognostic factors are related to chemotherapeutic outcomes and the prognosis of inpatients with metastatic colorectal cancer (mCRC) undergoing chemotherapy.
Materials and methods
Data from 71 patients with mCRC were analyzed retrospectively. The relationship between the potential prognostic factors before first‐line chemotherapy and the clinicopathological characteristics and chemotherapy response of the patients was calculated using Fisher's exact test and the chi‐square test. The prognostic factors were analyzed using univariate and multivariate analyses. We analyzed the subgroups using the Mann‐Whitney U test.
Results
Four factors were eventually used as prognostic factors, namely the albumin‐to‐globulin ratio (AGR), the fibrinogen‐to‐albumin ratio (FAR), the prealbumin‐to‐globulin ratio (PGR), and the fibrinogen‐to‐prealbumin ratio (FPR); the cutoff values of the four potential prognostic factors were 1.40, 10.63, 5.44, and 18.49, respectively. The high AGR and PGR groups had a higher response rate than that of the low groups. Patients in the low FAR and FPR groups showed a higher objective response rate than the high FAR and FPR groups. Patients with low FPR were associated with a higher disease control rate than patients with high FPR. Higher progression‐free survival (PFS) was observed in the high AGR and PGR and low FAR and FPR groups. The AGR, FAR, PGR, and FPR were considered reliable prognostic factors for PFS in a univariate analysis.
Conclusions
The prechemotherapy AGR, FAR, PGR, and FPR were good prognostic factors to predict the chemotherapy response and PFS in patients with mCRC.
Keywords: chemotherapy response, colorectal cancer, FAR, PGR, prealbumin, prealbumin to globulin
1. BACKGROUND
Colorectal cancer (CRC) is the third most common cancer worldwide and the second most common cause of death according to the 2018 global cancer statistics.1 CRC has the third highest incidence and the fifth highest fatality rate in China.2 TNM staging is an important prognostic factor for patients with CRC. However, patients with metastatic colorectal cancer (mCRC) in the same TNM stage have different survival times. Therefore, there is an urgent need for new clinical predictors to predict chemotherapy efficacy and survival time in patients with mCRC.
Differences in survival time and chemotherapy responses in cancer patients occur due to a host of factors, including systemic inflammation and nutritional status. Previous studies have shown that proinflammatory chemokines and cytokines in the tumor microenvironment promote the occurrence, development, and metastasis of tumors, destroy human immunity, and reduce the therapeutic response of tumors to cytotoxic drugs, ultimately affecting survival.3, 4, 5, 6 At the same time, malnutrition is an important issue in cancer patients and can lead to a series of clinical consequences, such as cachexia, a lower response to treatment, and shorter survival.7 Among several systemic inflammatory and nutritional status biomarkers, fibrinogen, albumin, globulin, the neutrophil‐to‐lymphocyte ratio (NLR), the albumin‐to‐globulin ratio (AGR), the lymphocyte‐to‐monocyte ratio (LMR), the platelet‐to‐lymphocyte ratio (PLR), and the fibrinogen‐to‐albumin ratio (FAR) have been used as prognostic indicators for different types of cancer. Some of these factors are also predictors of therapeutic efficacy in cancer patients receiving cytotoxic drug therapy.
These prognostic factors have also been used to predict the prognosis of CRC patients.8, 9, 10 A relationship between the pretreatment AGR, NLR, LMR, and PLR and the chemotherapy response in patients with CRC has been reported by many studies.10, 11, 12, 13, 14 In addition, the prealbumin produced by the liver has a shorter half‐life and a lower plasma level than albumin, allowing even a slight change in malnutrition to be seen in a short amount of time, making it a more effective biomarker of malnutrition and inflammatory stress than albumin.15, 16 In fact, recent studies have shown that the fibrinogen‐to‐prealbumin ratio (FPR) could be a useful CRC diagnostic and prognostic biomarker.17, 18 However, relatively little research has been performed on the FAR, FPR, and prealbumin‐to‐globulin ratio (PGR) in patients with mCRC.
The objective of this study was to evaluate whether the pretreatment FAR, FPR, PGR, and other potential prognostic factors could be used as markers of chemotherapy response and prognostic factors for progression‐free survival (PFS) in patients with mCRC. A total of seven potential prognostic factors were used in this study, including AGR, FAR, PGR, FPR, LMR, NLR, and PLR.
2. METHODS
2.1. Patient selection
In this retrospective study, 71 patients diagnosed with mCRC at the Cancer Hospital of China Medical University (Liaoning, China) were collected and followed up from June 2015 to February 2019. All patients met the following enrollment criteria: (a) biopsy‐confirmed CRC; (b) an unresectable metastatic lesion diagnosed by imaging; (c) no intestinal obstructions before treatment and no other complications preventing patients from meeting the requirements for chemotherapy; (d) at least two cycles of chemotherapy were completed; (e) the required blood tests were completed within 1‐2 weeks before initial first‐line chemotherapy; (f) an Eastern Cooperative Oncology Group performance status (PS) score of 0‐2; and (g) patients who had preexisting liver disease, chronic renal failure, severe acute or chronic inflammatory disease, or patients who received oral anticoagulation therapy were excluded from this study. All enrolled patients volunteered to participate in this study. This study was approved by the Cancer Hospital of China Medical University Ethics Committee.
2.2. Patient follow‐up
All patients were followed up. After completing the required chemotherapy cycle, all patients were evaluated every 8 weeks until disease progression was detected. The median follow‐up time in this study was 200 days (range, 56‐815 days).
2.3. Clinical data collection
We collected information on patient characteristics before chemotherapy from the hospital records; the location of the tumor in the intestine by colonoscopy and the number of metastatic organs were determined by a computed tomography scan, magnetic resonance imaging, or other imaging examinations. The RAS/BRAF genotype was determined by genetic testing within 1‐2 weeks before chemotherapy. Peripheral blood, measured albumin, prealbumin, globulin, neutrophil count, lymphocyte count, monocyte count, platelet count, hemoglobin, fibrinogen, cancer antigen 19‐9 (CA19‐9), and carcinoembryonic antigen (CEA) were obtained within 1 week of treatment initiation. The reference ranges for plasma albumin, prealbumin, globulin, hemoglobin, and fibrinogen were 35‐50 g/L, 160‐450 mg/L, 20‐35 g/L, 115‐155 g/L, and 2‐4 g/L, respectively. The numbers of neutrophils, lymphocytes, monocytes, and platelets were (1.8‐6.3) × 109/L, (1.1‐3.2) × 109/L, (0.1‐0.6) × 109/L, and (100‐300) × 109/L, respectively. The accepted normal ranges of CA19‐9 and CEA were 0‐37 U/mL and 0‐5 ng/mL, respectively. The AGR, FAR, PGR, FPR, LMR, NLR, and PLR were defined as albumin (g/L)/globulin (g/L), fibrinogen (g/L)/albumin (g/L) × 100, prealbumin (mg/L)/globulin (g/L), fibrinogen (g/L)/prealbumin (mg/L) × 1000, lymphocytes (109/L)/monocytes (109/L), neutrophils (109/L)/lymphocytes (109/L), and platelets (109/L)/lymphocytes (109/L), respectively.
All patients received systemic first‐line chemotherapy combined with or without molecular‐targeted therapy. Different chemotherapy and molecular‐targeted therapy regimens were used depending on the patient's PS, genetic test results, and previous treatment regimens. Each patient received at least two cycles of chemotherapy. The response to chemotherapy was assessed radiologically every two cycles and at the completion of therapy. This study used the Response Evaluation Criteria in Solid Tumors (RECIST 1.1) criteria to assess the patient's chemotherapy response as follows: progression of disease (PD), stable disease (SD), partial response (PR), and complete remission (CR). Disease control rate (DCR) = CR + PR + SD. Objective response rate (ORR) = CR + PR. The PFS time period was equal to the time of disease progression or death minus the initiation time for first‐line chemotherapy.
2.4. Data analysis methods
Statistical Package for Social Sciences version 20 software (SPSS Inc) was used to complete the data analyses of this study. We used the cutoff values of the seven potential prognostic factors determined by a receiver operating characteristic (ROC) curve analysis to divide all patients into high and low groups for each factor. The relationship between the seven potential prognostic factors before first‐line chemotherapy and the clinicopathological characteristics and the chemotherapy response of the patients was evaluated by Fisher's exact test and the chi‐square test. We used the Kaplan‐Meier method to plot PFS curves, and the PFS rates were analyzed using the log‐rank test. Univariate and multivariate analyses were performed with the variables using a Cox proportional hazards model. The correlation between the prognostic factors and PFS was assessed by Pearson's correlation analysis. The Mann‐Whitney U test was used for the subgroup analysis. A P‐value <0.05 was considered statistically significant.
3. RESULTS
3.1. Identification of the optimal cutoff value for the seven potential prognostic factors
The ROC curve was used to determine the cutoff values of the seven factors. The seven factors were used as the test variables, and the median PFS time of 200 days was the state variable. The results showed that the optimal cutoff values of AGR, FAR, PGR, FPR, LMR, NLR, and PLR were 1.397 (area under the curve [AUC] = 0.913, P < 0.001), 10.631 (AUC = 0.804, P < 0.001), 5.444 (AUC = 0.844, P < 0.001), 18.490 (AUC = 0.811, P < 0.001), 2.617 (AUC = 0.630, P = 0.059), 4.719 (AUC = 0.598, P = 0.157), and 200.833 (AUC = 0.591, P = 0.186), respectively. Although the AUC of FPR was slightly larger than that of FAR, the AUC of AGR was larger than that of PGR. These mixed results did not show that PGR and FPR were better than AGR and FAR for predicting the prognosis of patients. However, because the AUC value of AGR was 0.913, which is the largest of the four indicators, its prognostic value for PFS in mCRC patients may be superior to the other three indicators (Figure 1).
Figure 1.
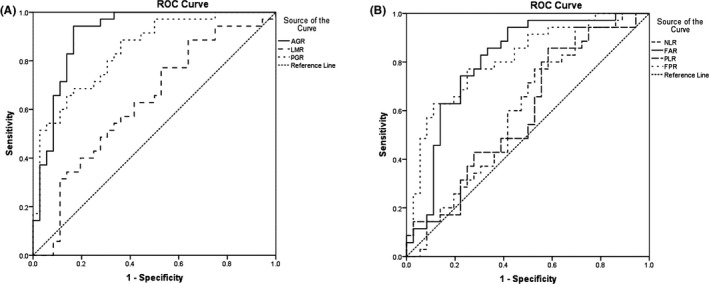
Receiver operating characteristic curve analysis of the albumin‐to‐globulin ratio (AGR), the lymphocyte‐to‐monocyte ratio (LMR), and the prealbumin‐to‐globulin ratio (PGR) (A), and the neutrophil‐to‐lymphocyte ratio (NLR), the fibrinogen‐to‐albumin ratio (FAR), the platelet‐to‐lymphocyte ratio (PLR), and the fibrinogen‐to‐prealbumin ratio (FPR) (B) in patients with metastatic colorectal cancer
As the AUCs of the three potential prognostic factors, NLR, LMR, and PLR, were smaller and P > 0.05, these three indicators were excluded, and AGR, FAR, PGR, and FPR were used in the following study. The cutoff values of AGR, FAR, PGR, and FPR were set to 1.40, 10.63, 5.44, and 18.49, respectively.
3.2. Patient characteristics
Female patients accounted for 38.03% of the population in this study. Sixty years was the median age of the patients. The colorectum was divided into the right‐sided colon (RSC), including the cecum, ascending colon, and right transverse colon, and the left‐sided colorectum (LSC), including the left transverse colon, descending colon, sigmoid colon, and rectum. The proportions of patients with tumors in the RSC and LSC were 26.76% and 73.24%, respectively. The histological type was poorly or mucinous adenocarcinoma in 24 (33.80%) patients. Thirty (42.25%) patients had at least two organs affected by metastasis. RAS/BRAF gene testing was performed in 50 patients, of which 22 had the mutation. Thirty‐six patients received appropriate targeted therapy (bevacizumab or cetuximab). The proportions of patients with CA19‐9 and CEA levels greater than normal were 56.34% and 78.87%, respectively. The patients mainly received the following chemotherapy regimens: CapeOX (oxaliplatin + capecitabine), FOLFOX (oxaliplatin + leucovorin + 5‐fluorouracil), FOLFOXIRI (oxaliplatin + irinotecan + leucovorin + 5‐fluorouracil), and XELIRI (capecitabine + irinotecan). According to the RECIST 1.1 criteria, patients with PR and SD accounted for 39.44% and 52.11% of all patients, respectively (Table 1).
Table 1.
Baseline characteristics of the patients
| Characteristics | No. of patients | Percentage |
|---|---|---|
| Sex | ||
| Male | 44 | 61.97% |
| Female | 27 | 38.03% |
| Age (y) | ||
| ≤60 | 36 | 50.70% |
| >60 | 35 | 49.30% |
| Body mass index (kg/m2) | ||
| <18.5 or >25 | 19 | 26.76% |
| ≥18.5 to ≤25 | 52 | 73.24% |
| Location of primary tumor | ||
| Left‐sided colon | 52 | 73.24% |
| Right‐sided colon | 19 | 26.76% |
| Histological type | ||
| Well, moderately | 47 | 66.20% |
| Poorly, mucinous | 24 | 33.80% |
| Detection of unresectable tumor | ||
| Synchronous | 53 | 74.65% |
| Metachronous | 18 | 25.35% |
| Number of organs affected by metastasis | ||
| One organ | 41 | 57.75% |
| More than one organ | 30 | 42.25% |
| Regimen of first‐line chemotherapy | ||
| CapeOX | 24 | 33.80% |
| FOLFOXIRI | 13 | 18.31% |
| FOLFOX | 9 | 12.68% |
| XELIRI | 5 | 7.04% |
| Others | 20 | 28.17% |
| Molecular‐targeted therapy | ||
| No | 35 | 49.30% |
| Yes | 36 | 50.70% |
| RAS/BRAF gene detection | ||
| No | 21 | 29.58% |
| Wild type | 28 | 39.44% |
| RAS/BRAF mutations | 22 | 30.98% |
| Best response | ||
| CR | 0 | – |
| PR | 28 | 39.44% |
| SD | 37 | 52.11% |
| PD | 6 | 8.45% |
| ORR | 28 | 39.44% |
| DCR | 65 | 91.55% |
| Tumor biomarkers | ||
| CEA > 5 ng/mL | 56 | 78.87% |
| CA19‐9 > 37 U/mL | 40 | 56.34% |
| AGR | ||
| ≤1.40 | 33 | 46.48% |
| >1.40 | 38 | 53.52% |
| FAR | ||
| ≤10.63 | 48 | 67.61% |
| >10.63 | 23 | 32.39% |
| PGR | ||
| ≤5.44 | 27 | 38.03% |
| >5.44 | 44 | 61.97% |
| FPR | ||
| ≤18.49 | 36 | 50.70% |
| >18.49 | 35 | 49.30% |
3.3. Relationship between the four pretreatment prognostic factors and the first‐line chemotherapy response
The relationship between the levels of the four prognostic factors before chemotherapy and the treatment response rates was evaluated.
The prechemotherapy levels of AGR, PGR, and FPR in the 71 patients had significant effects on PR, SD, and PD. Although there was a higher proportion of patients in the PR and SD groups from the low FAR (≤10.63) subgroup, this relationship did not achieve statistical significance (P > 0.05; Table 2).
Table 2.
Relationship between first‐line chemotherapy and progression of disease, complete remission, and stable disease according to four prognostic factors
| Progressive disease | Partial response | Stable disease | Fisher χ 2 | P‐value | |
|---|---|---|---|---|---|
| AGR | |||||
| ≤1.40 | 6 | 7 | 20 | 13.044 | 0.001 |
| >1.40 | 0 | 21 | 17 | ||
| FAR | |||||
| ≤10.63 | 3 | 23 | 22 | 4.829 | 0.067 |
| >10.63 | 3 | 5 | 15 | ||
| PGR | |||||
| ≤5.44 | 5 | 5 | 17 | 10.842 | 0.004 |
| >5.44 | 1 | 23 | 20 | ||
| FPR | |||||
| ≤18.49 | 0 | 20 | 16 | 11.860 | 0.002 |
| >18.49 | 6 | 8 | 21 | ||
Patients in the high AGR (>1.4) and PGR (>5.44) groups had higher ORRs and DCRs than those in the low groups (AGR: 55.3% vs 44.7% and 100.0% vs 81.8%; and PGR: 52.3% vs 18.5% and 97.7% vs 81.5%, respectively). Compared with patients in the high FAR (>10.63) and FPR (>18.49) groups, patients in the low FAR and FPR groups had higher ORRs (FAR: 47.9% vs 21.7%, and FPR: 55.6% vs 22.9%). The low FPR group had a higher DCR than the high FPR group (100.0% vs 82.9%). Although patients in the low FAR group tended to have a higher DCR, this relationship did not achieve statistical significance (P > 0.05). This observation suggests that FPR may be superior to FAR in predicting DCR (Table 3).
Table 3.
Treatment response to first‐line chemotherapy according to four prognostic factors
| Objective response rate | χ 2 | P‐value | Disease control rate | P‐value* | |
|---|---|---|---|---|---|
| AGR | |||||
| ≤1.40 | 44.7% | 8.574 | 0.003 | 81.8% | 0.008 |
| >1.40 | 55.3% | 100.0% | |||
| FAR | |||||
| ≤10.63 | 47.9% | 4.461 | 0.035 | 93.8% | 0.381 |
| >10.63 | 21.7% | 87.0% | |||
| PGR | |||||
| ≤5.44 | 18.5% | 7.982 | 0.005 | 81.5% | 0.027 |
| >5.44 | 52.3% | 97.7% | |||
| FPR | |||||
| ≤18.49 | 55.6% | 7.944 | 0.005 | 100.0% | 0.011 |
| >18.49 | 22.9% | 82.9% | |||
Fisher χ 2.
3.4. Relationship between four pretreatment prognostic factors and progression‐free survival
The median follow‐up was 200 days. Thirty‐two of the 71 patients (50.7%) experienced tumor progression during follow‐up. The median PFS values for the low AGR and high AGR groups were 131 and 236 days (P = 0.002), respectively. The median PFS values for the low FAR and high FAR groups were 208 and 130 days (P = 0.012), respectively. The median PFS values for the low PGR and high PGR groups were 138 and 221 days (P < 0.001), respectively. The median PFS values for the low FPR and high FPR groups were 214 and 158 days (P = 0.015), respectively. The results showed that the low AGR and PGR groups had lower PFS rates than the high AGR and PGR groups. The high FAR and FPR groups had lower PFS rates than the low FAR and FPR groups (Figure 2).
Figure 2.
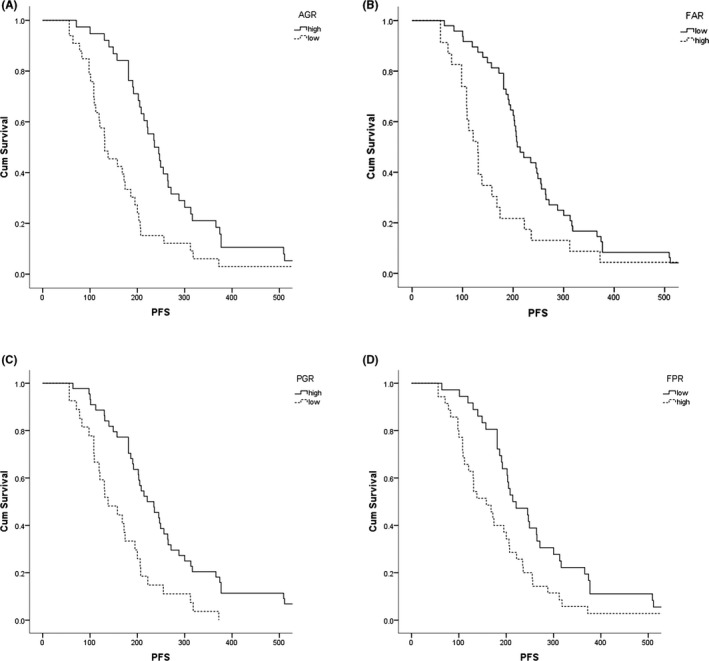
Kaplan‐Meier survival curve analysis of the relationship between the pretreatment albumin‐to‐globulin ratio (AGR) (A), the fibrinogen‐to‐albumin ratio (FAR) (B), the prealbumin‐to‐globulin ratio (PGR) (C), and the fibrinogen‐to‐prealbumin ratio (FPR) (D), and progression‐free survival (PFS)
Univariate Cox regression analyses showed that the risk of disease progression was lower in the low FAR and FPR groups and the high AGR and PGR groups. Moreover, the univariate analysis showed that PFS was significantly related to CA19‐9, hemoglobin, histological type, metachronous cancer, peritoneal dissemination, and the number of organs affected by metastasis. After adjusting for the effects of these parameters in the multivariate analysis, pretreatment CA19‐9, hemoglobin, and histological type were independent predictors for PFS, which was consistent with the findings of previous research19, 20, 21, 22, 23, 24 (Table 4).
Table 4.
Correlations between progression‐free survival (PFS) and various clinicopathological factors
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | P‐value | Hazard ratio | 95% CI | P‐value | |
| Age (>60 y) | 1.242 | 0.775‐1.992 | 0.368 | |||
|
Body mass index (<18.5 or >25 kg/m2) |
1.008 | 0.594‐1.711 | 0.976 | |||
|
Location of primary tumor (Right‐sided colon) |
1.554 | 0.908‐2.660 | 0.108 | |||
|
Histological type (Poorly, mucinous) |
2.269 | 1.361‐3.784 | 0.002 | 2.671 | 1.564‐4.562 | <0.001 |
| Detection of unresectable tumor (metachronous) | 0.556 | 0.320‐0.968 | 0.038 | |||
| Peritoneal dissemination (Yes) | 2.058 | 1.239‐3.417 | 0.005 | |||
| Number of organs affected by metastasis (≥2) | 1.907 | 1.146‐3.176 | 0.013 | |||
| RAS/BRAF gene mutations (Yes) | 1.190 | 0.673‐2.106 | 0.550 | |||
| Molecular‐targeted therapy (Yes) | 0.727 | 0.452‐1.167 | 0.186 | |||
| CEA (>5 ng/mL) | 1.523 | 0.854‐2.719 | 0.154 | |||
| CA19‐9 (>37 U/mL) | 2.344 | 1.409‐3.898 | 0.001 | 2.440 | 1.434‐4.149 | 0.001 |
| Hemoglobin (<115 g/L) | 5.299 | 2.820‐9.955 | <0.001 | 4.761 | 2.523‐8.986 | <0.001 |
| AGR ≤ 1.40 | 2.140 | 1.322‐3.465 | 0.002 | |||
| FAR > 10.63 | 1.911 | 1.143‐3.197 | 0.014 | |||
| PGR ≤ 5.44 | 2.425 | 1.458‐4.032 | 0.001 | |||
| FPR > 18.49 | 1.786 | 1.108‐2.880 | 0.017 | |||
Pearson's correlation analysis implied that the AGR and PGR were positively correlated with PFS in patients with mCRC (Spearman's r = 0.847 and 0.671, respectively; P < 0.001). The FAR and FPR were inversely correlated with PFS in mCRC patients (Spearman's r = −0.561 and −0.576, respectively; P < 0.001; Figure 3).
Figure 3.
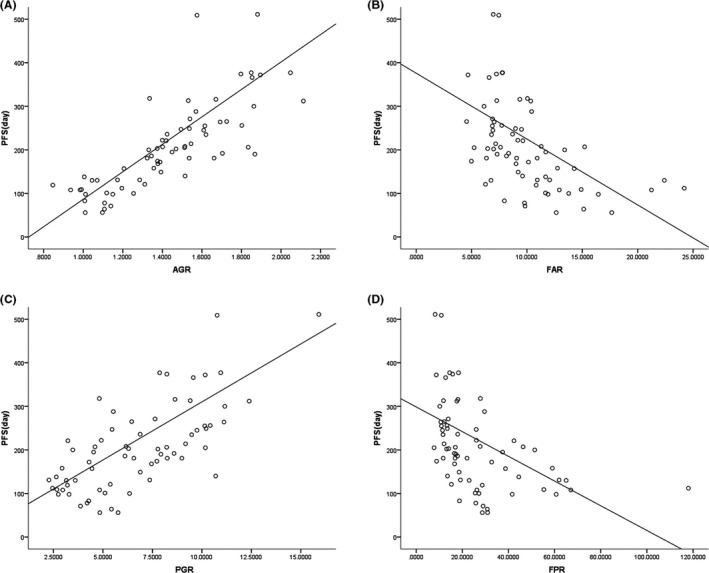
Pearson's correlation analysis for the linear relationship between the albumin‐to‐globulin ratio (AGR) (A), the fibrinogen‐to‐albumin ratio (FAR) (B), the prealbumin‐to‐globulin ratio (PGR) (C), and the fibrinogen‐to‐prealbumin ratio (FPR) (D), and progression‐free survival (PFS)
3.5. Correlation between clinicopathological factors and the four prognostic factors
The analysis indicated that a high AGR was closely related to CA19‐9 ≤ 37 U/mL, hemoglobin ≥ 115 g/L, well and moderate histological types, and metachronous cancer. High PGR was associated with age <60 years, CA19‐9 ≤ 37 U/mL, and hemoglobin ≥ 115 g/L. A correlation was observed between low FAR and only one organ affected by metastasis and hemoglobin ≥ 115 g/L. Correlations were detected between low FPR and only one organ affected by metastasis, CA19‐9 ≤ 37 U/mL, CEA ≤ 5 ng/mL, and hemoglobin ≥ 115 g/L. Interestingly, we found that patients with left‐sided colorectal cancer (LSCC) were more likely to have high AGRs and PGRs and low FARs and FPRs, while patients with right‐sided colon cancer (RSCC) were more likely to have low AGRs and high FPRs, although these results were not significant (P > 0.05; Tables 5 and 6).
Table 5.
Relationship between the pretreatment AGR and FAR and clinicopathological factors
| AGR | FAR | |||||||
|---|---|---|---|---|---|---|---|---|
| Low | High | χ 2 | P‐value | Low | High | χ 2 | P‐value | |
| Age (y) | ||||||||
| >60 | 19 | 16 | 1.691 | 0.193 | 23 | 12 | 0.113 | 0.737 |
| ≤60 | 14 | 22 | 25 | 11 | ||||
| Performance status | ||||||||
| 2 | 2 | 2 | – | 1.000* | 3 | 1 | – | 1.000* |
| 0/1 | 31 | 36 | 45 | 22 | ||||
| Body mass index (kg/m2) | ||||||||
| <18.5 or >25 | 8 | 11 | 0.199 | 0.655 | 14 | 5 | 0.438 | 0.508 |
| ≥18.5 to <25 | 25 | 27 | 34 | 18 | ||||
| Location of primary tumor | ||||||||
| Left‐sided colon | 22 | 30 | 1.359 | 0.244 | 34 | 18 | 0.438 | 0.508 |
| Right‐sided colon | 11 | 8 | 14 | 5 | ||||
| Histological type | ||||||||
| Poorly/mucinous | 17 | 7 | 8.645 | 0.003 | 14 | 10 | 1.423 | 0.233 |
| Well/moderately | 16 | 31 | 34 | 13 | ||||
| Unresectable tumor | ||||||||
| Synchronous | 29 | 24 | 5.703 | 0.017 | 34 | 19 | 1.139 | 0.286 |
| Metachronous | 4 | 14 | 14 | 4 | ||||
| Number of organs affected by metastasis | ||||||||
| More than one organ | 18 | 12 | 3.818 | 0.051 | 15 | 15 | 7.353 | 0.007 |
| One organ | 15 | 26 | 33 | 8 | ||||
| RAS/BRAF gene | ||||||||
| Mutations | 10 | 12 | 0.034 | 0.854 | 17 | 5 | 0.542 | 0.462 |
| Wild type | 12 | 16 | 19 | 9 | ||||
| CEA (ng/mL) | ||||||||
| >5 | 29 | 27 | 3.001 | 0.083 | 35 | 21 | – | 0.120* |
| ≤5 | 4 | 11 | 13 | 2 | ||||
| CA19‐9 (U/mL) | ||||||||
| >37 | 25 | 15 | 9.453 | 0.002 | 24 | 16 | 2.420 | 0.120 |
| ≤37 | 8 | 23 | 24 | 7 | ||||
| Hemoglobin (g/L) | ||||||||
| <115 | 20 | 4 | 19.796 | <0.001 | 10 | 14 | 11.138 | 0.001 |
| ≥115 | 13 | 34 | 38 | 9 | ||||
| Targeted therapy | ||||||||
| No | 19 | 16 | 1.691 | 0.193 | 25 | 10 | 0.461 | 0.497 |
| Yes | 14 | 22 | 23 | 13 | ||||
Fisher χ 2.
Table 6.
Relationship between the pretreatment PGR and FPR and clinicopathological factors
| PGR | FPR | |||||||
|---|---|---|---|---|---|---|---|---|
| Low | High | χ 2 | P‐value | Low | High | χ 2 | P‐value | |
| Age (y) | ||||||||
| >60 | 18 | 17 | 5.260 | 0.022 | 15 | 20 | 1.701 | 0.192 |
| ≤60 | 9 | 27 | 21 | 15 | ||||
| Performance status | ||||||||
| 2 | 1 | 3 | – | 1.000* | 3 | 1 | – | 0.614* |
| 0/1 | 26 | 41 | 33 | 34 | ||||
| Body mass index (kg/m2) | ||||||||
| <18.5 or >25 | 5 | 14 | 1.510 | 0.219 | 13 | 6 | 3.258 | 0.071 |
| ≥18.5 to <25 | 22 | 30 | 23 | 29 | ||||
| Location of primary tumor | ||||||||
| Left‐sided colon | 19 | 33 | 0.183 | 0.669 | 27 | 25 | 0.115 | 0.734 |
| Right‐sided colon | 8 | 11 | 9 | 10 | ||||
| Histological type | ||||||||
| Poorly/mucinous | 12 | 12 | 2.205 | 0.138 | 11 | 13 | 0.344 | 0.557 |
| Well/moderately | 15 | 32 | 25 | 22 | ||||
| Unresectable tumor | ||||||||
| Synchronous | 23 | 30 | 2.556 | 0.110 | 24 | 29 | 2.458 | 0.117 |
| Metachronous | 4 | 14 | 12 | 6 | ||||
| Number of organs affected by metastasis | ||||||||
| More than one organ | 15 | 15 | 3.159 | 0.075 | 11 | 19 | 4.096 | 0.043 |
| One organ | 12 | 29 | 25 | 16 | ||||
| RAS/BRAF gene | ||||||||
| Mutations | 8 | 14 | 0.045 | 0.833 | 10 | 12 | 0.325 | 0.569 |
| Wild type | 11 | 17 | 15 | 13 | ||||
| CEA (ng/mL) | ||||||||
| >5 | 24 | 32 | 2.623 | 0.105 | 25 | 31 | 3.896 | 0.048 |
| ≤5 | 3 | 12 | 11 | 4 | ||||
| CA19‐9 (U/mL) | ||||||||
| >37 | 23 | 17 | 14.739 | <0.001 | 14 | 26 | 9.039 | 0.003 |
| ≤37 | 4 | 27 | 22 | 9 | ||||
| Hemoglobin (g/L) | ||||||||
| <115 | 16 | 8 | 12.617 | <0.001 | 5 | 19 | 12.942 | <0.001 |
| ≥115 | 11 | 36 | 31 | 16 | ||||
| Targeted therapy | ||||||||
| No | 15 | 20 | 0.683 | 0.409 | 16 | 19 | 0.688 | 0.407 |
| Yes | 12 | 24 | 20 | 16 | ||||
Fisher χ 2.
3.6. Subgroup analysis
To investigate whether molecular‐targeted drugs can prolong PFS in patients in the high and low subgroups, we analyzed the prognostic significance of targeted therapy stratified by the pretreatment values of the four prognostic factors for mCRC. As a result, the high AGR, low FAR, and low FPR groups and the use of molecular‐targeted therapy tended to prolong patient PFS, while molecular‐targeted therapy had no clinical benefit in the low AGR, high FAR, and high FPR groups. However, this result was not statistically significant (P > 0.05; Table 7).
Table 7.
Correlations between PFS and molecular‐targeted therapy according to four prognostic factors
| PFS (d) | Z | P‐value | ||
|---|---|---|---|---|
| Molecular‐targeted therapy (Yes) | Molecular‐targeted therapy (No) | |||
| AGR | ||||
| ≤1.40 | 134.00 (98.75, 206.00) | 131.00 (108.00, 193.00) | −0.090 | 0.928 |
| >1.40 | 252.00 (205.75, 359.50) | 221.50 (146.75, 266.50) | −1.959 | 0.050 |
| FAR | ||||
| ≤10.63 | 246.00 (200.25, 316.50) | 195.00 (146.75, 264.25) | −1.862 | 0.063 |
| >10.63 | 125.50 (98.00, 104.00) | 131.00 (93.00, 198.00) | −0.031 | 0.975 |
| PGR | ||||
| ≤5.44 | 168.00 (115.00, 283.50) | 125.00 (81.75, 180.75) | −1.480 | 0.139 |
| >5.44 | 245.00 (192.00, 316.00) | 221.00 (144.50, 268.00) | −0.881 | 0.378 |
| FPR | ||||
| ≤18.49 | 245.00 (197.00, 345.00) | 190.00 (149.00, 271.00) | −1.139 | 0.255 |
| >18.49 | 168.00 (109.00, 288.00) | 144.50 (102.00, 218.00) | −0.817 | 0.414 |
4. DISCUSSION
Several recent studies have suggested that the pretreatment AGR, FPR, PLR, NLR, and LMR predict chemotherapeutic outcomes in patients with CRC.11, 12, 13, 14, 18 However, studies that have considered FAR and PGR are rare. At the beginning of this study, we used seven potential prognostic factors across a wide range of areas, including AGR, FAR, PGR, FPR, LMR, NLR, and PLR. At the end of this study, the prognostic factors AGR, FAR, PGR, and FPR were considered accurate markers in mCRC patients receiving first‐line chemotherapy. Since the AUC value of AGR was larger than the other 3 markers, AGR may be more valuable for predicting the PFS of mCRC patients than the other 3 markers.
Nutrition and inflammatory status play a pivotal role in the development of CRC. Poor nutritional status and physical condition are proportional to the levels of inflammation in patients.25 The presence of inflammation is considered to be a prognostic factor associated with cancer complications and reduced survival.4, 26 Studies have shown that malnutrition can decrease immunity and accelerate the development of malignant tumors, eventually leading to the deterioration of cancer patients and a decline in the quality of life.7
The albumin level can reflect the nutritional status of cancer patients.27 Albumin is an important endogenous antioxidant that can help facilitate the transport of substances and maintain osmotic pressure in the blood vessels. Hypoproteinemia may be associated with cancer, which inhibits the synthesis of albumin by the liver and allows it to reside in pleural and peritoneal effusions.28 Tumor cells in cancer patients are characterized by rapid cell proliferation, which accelerates cell metabolism, consumes albumin, and severely reduces its storage capacity. Patients with gastrointestinal tumors are at a higher risk of hypoproteinemia than other cancer patients due to possible gastrointestinal obstruction and malabsorption.29 As a sensitive marker, the nutritional status of cancer patients can be evaluated by albumin. Hypoalbuminemia is related to poor prognosis in cancer patients.30
Globulin is a major protein produced by immune organs that reflects the inflammatory and immune status of the human body.31 Globulin includes not only C‐reactive protein but also a variety of acute‐phase proteins, such as C3 and serum amyloid A. In chronic inflammation, the level of globulin gradually increases, which reflects the inflammatory state of the body.32 Studies have shown that the prognosis of cancer patients is closely related to the level of C‐reactive protein.33
Fibrinogen is synthesized by the liver. When the human body is injured, infected, or inflamed, fibrinogen is converted into fibrin.34 A hypercoagulable state was found in 50% of cancer patients, suggesting a close association between this state and the progression of cancer.35 The promotion of inflammation, tumor angiogenesis, cell proliferation, aggregation, and metastasis are the main reasons why fibrinogen promotes the development of cancer.36, 37 The prognosis of cancer patients could be assessed by the level of fibrinogen.38
Albumin, globulin, and fibrinogen can also be used to indicate the prognosis of patients. However, the results are susceptible to various factors. The accuracy of the albumin test results is affected by physiological and pathological changes, such as dehydration or fluid retention in cancer patients. When a single plasma globulin level is used as a predictor, hemodilution, hemoconcentration, and redundant components in the globulin can affect the actual globulin value, which can lead to inaccurate results. Physiological and pathological factors, including advanced age and cardiovascular and cerebrovascular disease, can affect the actual fibrinogen results. Albumin and fibrinogen have a relatively stable half‐life of approximately 20 days and 5 days, respectively, but different globulins have half‐lives of several hours to several days, which leads to their unstable values. Although it is possible to test both globulin and albumin in patients at the same time, the specific values may reflect the inflammatory and nutritional status of patients at different times. This study combined albumin, globulin, and fibrinogen as predictors to mitigate the abovementioned adverse effects and minimize the impact of the inaccuracy of a single value on the final result. The results of this study are consistent with those of previous studies.39, 40
Prealbumin, as an indicator of malnutrition, has a lower plasma concentration and a 2‐day half‐life. Although both prealbumin and albumin are negative acute‐phase reactants, and prealbumin has the following advantages compared with albumin. First, although albumin is a malnutrition indicator, it is a late indicator, and patients need to be treated with nutritional intervention for approximately 3 weeks before albumin levels increase. However, the prealbumin level will change after 2‐3 days of nutritional treatment, as it is a sensitive biomarker for monitoring the timely nutritional and inflammatory status in cancer patients. Second, any change in blood volume can lead to false albumin results. Some diseases, such as liver disease, bowel disease, kidney disease, lupus, and other diseases that increase capillary permeability, can confound the albumin test results. However, prealbumin results are not easily affected by physiological or pathological factors and provide more current information. In the end, albumin and prealbumin have been used as biomarkers to indicate the long‐ or short‐term nutritional and inflammatory status in cancer patients, respectively.15, 16 Based on these advantages of prealbumin, in this study, we determined whether PGR and FPR have greater advantages as prognostic factors than AGR and FAR. When the cutoff value of the predictors was determined by the ROC curve, the AUC of FPR was slightly larger than that of FAR, and the AUC of AGR was larger than that of PGR. The association between the predictors and the chemotherapy response suggests that FPR may be superior to FAR for predicting the DCR. Based on these mixed results, we found that only AGR may be superior to the other 3 markers regarding its prognostic value in mCRC patients because its AUC value was the largest. The results showed that PGR and FPR are more accurate prognostic factors for mCRC than AGR, and FAR could not be obtained. This may be because there is a large amount of time between the earliest tumor stages and the later stages of mCRC, leaving patients in a state of chronic malnutrition and inflammation for a long period. Therefore, prealbumin with a shorter half‐life as a prognostic factor did not show a benefit over albumin. Based on this analysis, we speculate that PGR and FPR are superior to AGR and FAR as prognostic factors in patients with early cancer or in those undergoing a nutritional intervention, but more research is needed to confirm this hypothesis.
The splenic curvature divides CRC into LSCC and RSCC, which have significantly different biological behaviors. The mechanism underlying this difference is related to microsatellite and chromosomal instability, genetic mutations, and the CpG island methylator phenotype. The clinical symptoms of LSCC are mainly altered bowel habits, bloody stools, and acute and chronic intestinal obstruction, while the tumor complications of RSCC are mostly anemia, cachexia, and enterobrosis. Chemotherapy and molecular‐targeted therapy also have different therapeutic responses to LSCC and RSCC. Thus, although the prevalence of LSCC is higher than that of RSCC, the survival time of patients with LSCC is significantly longer than that of patients with RSCC.41, 42, 43, 44 In this study, the univariate analysis suggested that patients with RSCC tend to have a worse prognosis (HR: 1.55). The relationship between pretreatment prognostic factors and clinicopathological factors implied that the proportion of patients with LSCC was higher in the subgroups with a high AGR and PGR and a low FAR and FPR, while the proportion of patients with RSCC was higher in the subgroups with a low AGR and a high FPR, which was consistent with the findings of previous research, although the sample size collected in this study may have been too small to achieve statistical significance (P > 0.05).
The RAS and BRAF genes have become the most important molecular markers for the targeted therapy of CRC. The RAS and BRAF genes are involved in the occurrence, proliferation, and progression of tumors, and patients with RAS‐ and BRAF‐mutated tumors have a worse prognosis. Clinical studies have confirmed that patients with CRC and RAS mutations cannot benefit from targeted therapy with epidermal growth factor receptor (EGFR) targets, such as the EGFR monoclonal antibodies cetuximab, panitumumab, or the small molecule tyrosinase inhibitors erlotinib and gefitinib.42, 45 The univariate analysis indicated that an RAS or a BRAF gene mutation can be used as a predictor of a poor prognosis (HR: 1.19), which was consistent with the results of previous research, although our results were not statistically significant (P > 0.05).
Cetuximab and bevacizumab are two molecular‐targeted drugs widely used in patients with CRC. The pharmacokinetic characteristics of cetuximab and bevacizumab increase the clearance rates of these two drugs to 20% in patients with low albumin.46, 47 Exposure to lower therapeutic levels of cetuximab and bevacizumab could adversely impact the molecular‐targeted therapeutic response. Therefore, we analyzed the therapeutic effect of targeted therapy on patients with a poor mCRC prognosis. As a result, the two molecular‐targeted drugs prolonged PFS in patients with a better prognosis (high AGR and low FAR and FPR subgroups), while these two targeted drugs did not seem to have a therapeutic effect in patients with a poor prognosis (low AGR and high FAR and FPR subgroups). Although the results were not statistically significant because of the small sample size, the same results were reported by a pancreatic cancer study.48 More similar studies are needed in the future.
Several strengths of this study should be mentioned. First, this is the first report to conceive of PGR as a prognostic factor, specifically, as a prognostic factor for chemotherapeutic response. Second, the results of this study suggest that the AGR may be superior to the FAR, PGR, and FPR in terms of prognostic value in mCRC patients. Third, the pretreatment AGR, FAR, PGR, and FPR may be correlated with RSCC and LSCC and prognosis. Fourth, there may be some relationship between the pretreatment AGR, FAR, PGR, and FPR and the effect of targeted therapy in mCRC patients. Fifth, these four clinical prognostic indicators are inexpensive, easy to obtain, less invasive, and easily accepted by patients.
This study also had some limitations. First, this study was a single‐center retrospective study in which selective bias may arise. Second, this study was different from other studies in that the NLR, LMR, and PLR were not used as prognostic factors. Moreover, the cutoff values of the AGR, FAR, and FPR were inconsistent with those in previous studies, which may have been caused by the small sample size or the different types of tumors. Third, the AGR, FAR, PGR, and FPR were not independent predictors for PFS in the multivariate analysis, suggesting that these four predictors are susceptible to multiple factors.29 However, as mentioned above, the AGR, FAR, PGR, and FPR reflect two states of inflammation and nutrition compared with albumin, globulin, fibrinogen, and prealbumin alone, making them more powerful prognostic predictors. Fourth, the follow‐up time was relatively short, and the patient's overall survival time was not obtained.
5. CONCLUSIONS
The pretreatment AGR, FAR, PGR, and FPR had significant prognostic value for patients with mCRC who received first‐line chemotherapy.
ETHICAL APPROVAL
This study was approved by the Cancer Hospital of China Medical University, Liaoning Cancer Hospital & Institute Ethics Committee and was performed in accordance with the ethical standards established in the 1964 Declaration of Helsinki and its later amendments.
INFORMED CONSENT
Informed consent was obtained from all participants included in the study.
Zhang L, Zhang J, Wang Y, et al. Potential prognostic factors for predicting the chemotherapeutic outcomes and prognosis of patients with metastatic colorectal cancer. J Clin Lab Anal. 2019;33:e22958 10.1002/jcla.22958
REFERENCES
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394‐424. [DOI] [PubMed] [Google Scholar]
- 2. Zheng RS, Sun KX, Zhang SW, et al. Report of cancer epidemiology in China, 2015. Zhonghua zhong liu za zhi [Chin J Oncol]. 2019;41(1):19‐28. [DOI] [PubMed] [Google Scholar]
- 3. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646‐674. [DOI] [PubMed] [Google Scholar]
- 4. Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860‐867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet (London, England). 2001;357(9255):539‐545. [DOI] [PubMed] [Google Scholar]
- 6. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer‐related inflammation. Nature. 2008;454(7203):436‐444. [DOI] [PubMed] [Google Scholar]
- 7. Gupta D, Lis CG. Pretreatment serum albumin as a predictor of cancer survival: a systematic review of the epidemiological literature. Nutr J. 2010;9:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Walsh SR, Cook EJ, Goulder F, Justin TA, Keeling NJ. Neutrophil‐lymphocyte ratio as a prognostic factor in colorectal cancer. J Surg Oncol. 2005;91(3):181‐184. [DOI] [PubMed] [Google Scholar]
- 9. Kwon H‐C, Kim SH, Oh SY, et al. Clinical significance of preoperative neutrophil‐lymphocyte versus platelet‐lymphocyte ratio in patients with operable colorectal cancer. Biomarkers. 2012;17(3):216‐222. [DOI] [PubMed] [Google Scholar]
- 10. Azab B, Kedia S, Shah N, et al. The value of the pretreatment albumin/globulin ratio in predicting the long‐term survival in colorectal cancer. Int J Colorectal Dis. 2013;28(12):1629‐1636. [DOI] [PubMed] [Google Scholar]
- 11. Shibutani M, Maeda K, Nagahara H, et al. The pretreatment albumin to globulin ratio predicts chemotherapeutic outcomes in patients with unresectable metastatic colorectal cancer. BMC Cancer. 2015;15:347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Artaç M, Uysal M, Karaağaç M, et al. Prognostic impact of neutrophil/lymphocyte ratio, platelet count, CRP, and albumin levels in metastatic colorectal cancer patients treated with FOLFIRI‐bevacizumab. J Gastrointest Cancer. 2017;48(2):176‐180. [DOI] [PubMed] [Google Scholar]
- 13. Shibutani M, Maeda K, Nagahara H, et al. Prognostic significance of the lymphocyte‐to‐monocyte ratio in patients with metastatic colorectal cancer. World J Gastroenterol. 2015;21(34):9966‐9973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wu Y, Li C, Zhao J, et al. Neutrophil‐to‐lymphocyte and platelet‐to‐lymphocyte ratios predict chemotherapy outcomes and prognosis in patients with colorectal cancer and synchronous liver metastasis. World J Surg Oncol. 2016;14(1):289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Douville P, Talbot J, Lapointe R, Belanger L. Potential usefulness of serum prealbumin in total parenteral nutrition. Clin Chem. 1982;28(7):1706‐1707. [PubMed] [Google Scholar]
- 16. Collins N. The difference between albumin and prealbumin. Adv Skin Wound Care. 2001;14(5):235‐236. [DOI] [PubMed] [Google Scholar]
- 17. Sun F, Tan Y‐A, Gao Q‐F, et al. Circulating fibrinogen to pre‐albumin ratio is a promising biomarker for diagnosis of colorectal cancer. J Clin Lab Anal. 2019;33(1):e22635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sun F, Peng HX, Gao QF, et al. Preoperative circulating FPR and CCF score are promising biomarkers for predicting clinical outcome of stage II‐III colorectal cancer patients. Cancer Manage Res. 2018;10:2151‐2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Xu W‐Y, Zhang H‐H, Yang X‐B, et al. Prognostic significance of combined preoperative fibrinogen and CA199 in gallbladder cancer patients. World J Gastroenterol. 2018;24(13):1451‐1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Panagopoulos ND, Karakantza M, Koletsis E, et al. Influence of blood transfusions and preoperative anemia on long‐term survival in patients operated for non‐small cell lung cancer. Lung Cancer (Amsterdam, Netherlands). 2008;62(2):273‐280. [DOI] [PubMed] [Google Scholar]
- 21. Ott C, Gerken M, Hirsch D, et al. Advanced mucinous colorectal cancer: epidemiology, prognosis and efficacy of chemotherapeutic treatment. Digestion. 2018;98(3):143‐152. [DOI] [PubMed] [Google Scholar]
- 22. Ghiringhelli F, Hennequin A, Drouillard A, Lepage C, Faivre J, Bouvier AM. Epidemiology and prognosis of synchronous and metachronous colon cancer metastases: a French population‐based study. Dig Liver Dis. 2014;46(9):854‐858. [DOI] [PubMed] [Google Scholar]
- 23. Arakawa K, Kawai K, Ishihara S, et al. Prognostic significance of peritoneal metastasis in stage IV colorectal cancer patients with R0 resection: a multicenter, retrospective study. Dis Colon Rectum. 2017;60(10):1041‐1049. [DOI] [PubMed] [Google Scholar]
- 24. Saltz LB, Cox JV, Blanke C, et al. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. N Engl J Med. 2000;343(13):905‐914. [DOI] [PubMed] [Google Scholar]
- 25. Wei X‐L, Wang F‐H, Zhang D‐S, et al. A novel inflammation‐based prognostic score in esophageal squamous cell carcinoma: the C‐reactive protein/albumin ratio. BMC Cancer. 2015;15:350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Alexandre J, Gross‐Goupil M, Falissard B, et al. Evaluation of the nutritional and inflammatory status in cancer patients for the risk assessment of severe haematological toxicity following chemotherapy. Ann Oncol. 2003;14(1):36‐41. [DOI] [PubMed] [Google Scholar]
- 27. Daniele A, Divella R, Abbate I, et al. Assessment of nutritional and inflammatory status to determine the prevalence of malnutrition in patients undergoing surgery for colorectal carcinoma. Anticancer Res. 2017;37(3):1281‐1287. [DOI] [PubMed] [Google Scholar]
- 28. Andersson CE, Lonnroth IC, Gelin LJ, Moldawer LL, Lundholm KG. Pretranslational regulation of albumin synthesis in tumor‐bearing mice. The role of anorexia and undernutrition. Gastroenterology. 1991;100(4):938‐945. [DOI] [PubMed] [Google Scholar]
- 29. Alkan A, Koksoy EB, Utkan G. Albumin to globulin ratio, a predictor or a misleader? Ann Oncol. 2015;26(2):443‐444. [DOI] [PubMed] [Google Scholar]
- 30. Djaladat H, Bruins HM, Miranda G, Cai J, Skinner EC, Daneshmand S. The association of preoperative serum albumin level and American Society of Anesthesiologists (ASA) score on early complications and survival of patients undergoing radical cystectomy for urothelial bladder cancer. BJU Int. 2014;113(6):887‐893. [DOI] [PubMed] [Google Scholar]
- 31. Ballow M. Mechanisms of action of intravenous immune serum globulin in autoimmune and inflammatory diseases. J Allergy Clin Immunol. 1997;100(2):151‐157. [DOI] [PubMed] [Google Scholar]
- 32. Du X‐J, Tang L‐L, Mao Y‐P, et al. The pretreatment albumin to globulin ratio has predictive value for long‐term mortality in nasopharyngeal carcinoma. PLoS ONE. 2014;9(4):e94473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kim EY, Yim HW, Park CH, Song KY. C‐reactive protein can be an early predictor of postoperative complications after gastrectomy for gastric cancer. Surg Endosc. 2017;31(1):445‐454. [DOI] [PubMed] [Google Scholar]
- 34. Tennent GA, Brennan SO, Stangou AJ, O'Grady J, Hawkins PN, Pepys MB. Human plasma fibrinogen is synthesized in the liver. Blood. 2007;109(5):1971‐1974. [DOI] [PubMed] [Google Scholar]
- 35. Jain A, Zhang Q, Toh HC. Awakening immunity against cancer: a 2017 primer for clinicians. Chin J Cancer. 2017;36(1):67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zheng S, Shen J, Jiao Y, et al. Platelets and fibrinogen facilitate each other in protecting tumor cells from natural killer cytotoxicity. Cancer Sci. 2009;100(5):859‐865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jensen T, Kierulf P, Sandset P, et al. Fibrinogen and fibrin induce synthesis of proinflammatory cytokines from isolated peripheral blood mononuclear cells. Thromb Haemost. 2007;97(5):822‐829. [DOI] [PubMed] [Google Scholar]
- 38. Perisanidis C, Psyrri A, Cohen EE, et al. Prognostic role of pretreatment plasma fibrinogen in patients with solid tumors: a systematic review and meta‐analysis. Cancer Treat Rev. 2015;41(10):960‐970. [DOI] [PubMed] [Google Scholar]
- 39. Suh B, Park S, Shin DW, et al. Low albumin‐to‐globulin ratio associated with cancer incidence and mortality in generally healthy adults. Ann Oncol. 2014;25(11):2260‐2266. [DOI] [PubMed] [Google Scholar]
- 40. Xu W‐Y, Zhang H‐H, Xiong J‐P, et al. Prognostic significance of the fibrinogen‐to‐albumin ratio in gallbladder cancer patients. World J Gastroenterol. 2018;24(29):3281‐3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Masoomi H, Buchberg B, Dang P, Carmichael JC, Mills S, Stamos MJ. Outcomes of right vs. left colectomy for colon cancer. J Gastrointest Surg. 2011;15(11):2023‐2028. [DOI] [PubMed] [Google Scholar]
- 42. von Einem JC, Heinemann V, von Weikersthal LF, et al. Left‐sided primary tumors are associated with favorable prognosis in patients with KRAS codon 12/13 wild‐type metastatic colorectal cancer treated with cetuximab plus chemotherapy: an analysis of the AIO KRK‐0104 trial. J Cancer Res Clin Oncol. 2014;140(9):1607‐1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Price TJ, Beeke C, Ullah S, et al. Does the primary site of colorectal cancer impact outcomes for patients with metastatic disease? Cancer. 2015;121(6):830‐835. [DOI] [PubMed] [Google Scholar]
- 44. Iacopetta B. Are there two sides to colorectal cancer? Int J Cancer. 2002;101(5):403‐408. [DOI] [PubMed] [Google Scholar]
- 45. Brudvik KW, Kopetz SE, Li L, Conrad C, Aloia TA, Vauthey JN. Meta‐analysis of KRAS mutations and survival after resection of colorectal liver metastases. Br J Surg. 2015;102(10):1175‐1183. [DOI] [PubMed] [Google Scholar]
- 46. Lu JF, Bruno R, Eppler S, Novotny W, Lum B, Gaudreault J. Clinical pharmacokinetics of bevacizumab in patients with solid tumors. Cancer Chemother Pharmacol. 2008;62(5):779‐786. [DOI] [PubMed] [Google Scholar]
- 47. Azzopardi N, Lecomte T, Ternant D, et al. Cetuximab pharmacokinetics influences progression‐free survival of metastatic colorectal cancer patients. Clin Cancer Res. 2011;17(19):6329‐6337. [DOI] [PubMed] [Google Scholar]
- 48. Pant S, Martin LK, Geyer S, et al. Baseline serum albumin is a predictive biomarker for patients with advanced pancreatic cancer treated with bevacizumab: a pooled analysis of 7 prospective trials of gemcitabine‐based therapy with or without bevacizumab. Cancer. 2014;120(12):1780‐1786. [DOI] [PMC free article] [PubMed] [Google Scholar]


