Abstract
Aim
The aim of this study was to investigate health literacy in patients with chronic kidney disease in a multidimensional perspective.
Design
A descriptive, cross‐sectional study.
Methods
Patients with chronic kidney disease at stages 3–5 were included in the study between February–August 2017 (N = 187). Health literacy was measured by the Health Literacy Questionnaire (HLQ). Multiple linear regression analysis was performed to identify associations between health literacy and demographic and clinical variables. Hierarchical cluster analysis was performed to identify characteristics of groups with high and low health literacy.
Results
Finding and critical appraise health information were the most challenging dimensions of health literacy. Female gender, lower level of education, greater number of prescribed medications and depressive symptoms were associated with lower health literacy. The group identified with lowest health literacy was further characterized by living alone and presence of comorbidity.
Keywords: chronic kidney disease, clinical variables, demographic variables, health literacy, healthcare providers including nurses
1. INTRODUCTION
Chronic kidney disease (CKD) affects between 11%–13% of the population worldwide, and the incidence of CKD has increased in developed countries due to an ageing population and an increased prevalence of lifestyle‐related diseases such as obesity, type 2 diabetes and hypertension (Eckardt et al., 2013; Hallan et al., 2006; Helsedirektoratet, 2011; Hill et al., 2016). CKD is divided into five stages where stage 5 also is referred to as end‐stage renal disease (K/DOQI, 2002). If left untreated, CKD in earlier stages is more likely to progress to end‐stage renal disease, requiring renal replacement therapy, developing comorbidities such as cardiovascular disease and posing a higher risk of mortality (Helsedirektoratet, 2011; Tangkiatkumjai, Walker, Praditpornsilpa, & Boardman, 2017). In addition to being provided with a complex medication regime, patients with CKD are often recommended a range of lifestyle changes such as a complex dietary regime, fluid control, regular exercise and weight control to reduce these risks (Levey & Coresh, 2012; Levey, Schoolwerth, et al., 2009; Whaley‐Connell, Nistala, & Chaudhary, 2011). To comply with the health recommendations, it is crucial that the patients are able to gain access to, understand and use health information.
Health literacy (HL) is a multidimensional concept defined by the World Health Organization (WHO) as the cognitive and social skills that determine the motivation and ability to gain access to, understand and use information in ways which promote and maintain good health (WHO, 1998). Previous studies indicate that HL in patients with CKD is not optimal (Fraser et al., 2013; Taylor et al., 2017) and that lower levels of HL is associated with worse health outcomes and higher medical costs (Devraj et al., 2015; Fraser et al., 2013; Green et al., 2013; Grubbs, Gregorich, Perez‐Stable, & Hsu, 2009; Ricardo et al., 2014; Taylor et al., 2016). Health literacy (HL) is therefore seen as an essential aspect of the care of patients with CKD (Berkman, Sheridan, Donahue, Halpern, & Crotty, 2011; Fraser et al., 2013; Green et al., 2011).
1.1. Background
Over the last decades, the concept of HL has evolved from being a personal attribute solely depending on personal skills, to a broader concept also including dimensions such as trust and interaction with healthcare providers, social support and accessibility of the healthcare services (Batterham, Beauchamp, & Osbourne, 2017; Van der Heide et al., 2018; Sorensen et al., 2012). ‘The integrated model of HL’ from 2012 describes HL to be a prerequisite for use of health services, health behaviour, active participation in own health situation and equality in health (Sorensen et al., 2012). According to the model, social, environmental, personal and situational factors are determinative for a persons’ HL; hence, demographic and clinical characteristics are essential when exploring HL in patients with CKD.
Furthermore, depressive symptoms are well known to be underrecognized and undertreated in patients with CKD across all stages of the disease (Amira, 2011; Hedayati, Minhajuddin, Toto, Morris, & Rush, 2009) and such symptoms are also associated with low HL (Dodson, Osicka, Huang, McMahon, & Roberts, 2016). Depressive symptoms negatively affect the motivation to manage health issues and may therefore influence a patient's HL (Dodson et al., 2016; Shin et al., 2017).
Until recently, instruments measuring HL have mostly been one‐ or two‐dimensional, focusing on health‐related numeracy and reading skills. Frequently used instruments have been the ‘Rapid Estimate of Adult Literacy in Medicine’, focusing on word recognition (Davis et al., 1991) and the ‘Test Of Functional Health Literacy in Adults’, which tests reading and numeracy skills (Parker, Baker, Williams, & Nurss, 1995). However, having good health‐related numeracy and reading skills does not mean that one can understand the consequences of the choices one makes; in addition, former instruments used for measuring HL have been reported to be suboptimal (Jordan, Osborne, & Buchbinder, 2011). To identify HL challenges beyond reading and numeracy skills, such as a lack of social support, difficulties in engaging with healthcare providers and difficulties in navigating the healthcare system, a multidimensional assessment tool is required. Hence, the aim of this study was to describe multidimensional HL in patients with CKD and to identify possible associations between different dimensions of HL and demographic and clinical variables.
2. THE STUDY
2.1. Design
The present study was a descriptive, single‐centre cross‐sectional study. We used patient‐reported outcome measures (PROMs) and data from the patients’ medical records to assess HL and the associations between HL and both demographics (gender, age, education, income, employment status and living arrangement) and clinical variables (medications, comorbidity, depressive symptoms, stage and duration of CKD).
3. METHODS
3.1. Participants
The study hospital provides healthcare services for approximately 330,000 people in the south‐western part of Norway. According to the renal registry at the hospital, the potential study population consisted of approximately 500 individuals diagnosed with CKD stages 3–5 who were followed in the outpatient clinic and in the in‐hospital dialysis unit at the Nephrology Department. Patients with CKD stages 1 and 2 were not included, as they are mainly followed in the primary healthcare system. According to the research protocol, we estimated that a total of 200 patients (80 with CKD stage 3, 80 patients with stages 4 and 5 not on haemodialysis and 40 haemodialysis patients) were sufficient to answer our research question. It was not possible to perform sample size calculations, as numbers for calculation are not available. The inclusion criteria were age 18 years and older, CKD stages 3–5, written informed consent and ability to read and understand the Norwegian language. Patients with active noncutaneous cancer or unstable cardiovascular disease, patients with a history of a significant vascular incident (myocardial infarction, transient ischaemic attack or cerebral vascular accident) in the last three months and patients who had undergone major surgery in the previous three months were excluded.
3.2. Data collection
Consecutive patients at routine outpatient appointments or who were scheduled for haemodialysis treatment during a six‐month period (from February–August 2017) were included until the prespecified number of patients was reached (Figure 1). However, we were unable to include more than 26 haemodialysis patients because the total number of haemodialysis patients at the time of recruitment was 74, 44 of whom did not fulfil the inclusion criteria. Of the 30 haemodialysis patients eligible for our study, four declined to participate.
Figure 1.
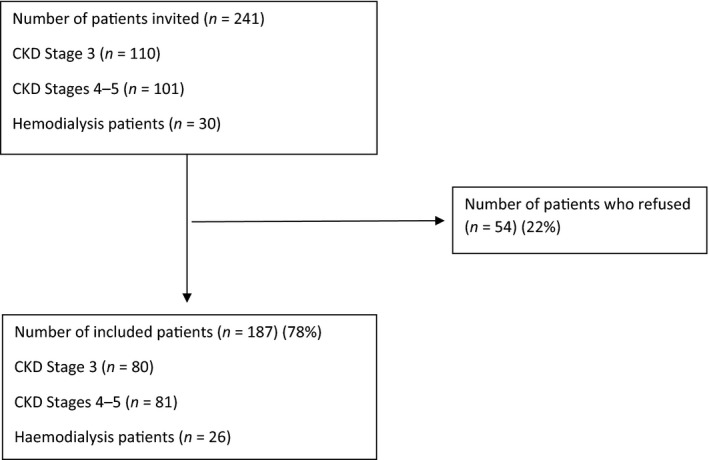
Flow diagram of the study participants. CKD, chronic kidney disease
3.3. Instruments
HL was assessed using the Health Literacy Questionnaire (HLQ), which is a multidimensional validated questionnaire that contains 44 items across nine independent scales. Each of the nine scales consists of 4–6 items and provides independent information about different dimensions of HL (measures using one scale per dimension). The questionnaire is divided into two main parts. In the first part (HLQ scales 1–5), the respondents have four options to indicate how strongly they disagree or agree with a set of statements (1 = strongly disagree, 2 = disagree, 3 = agree and 4 = strongly agree). In the second part (HLQ scales 6–9), the respondents have five options to indicate how difficult or easy different tasks are (1 = cannot do, 2 = usually difficult, 3 = sometimes difficult, 4 = usually easy and 5 = always easy). The questionnaire does not provide a total score or cut‐off value, but higher numbers indicate better HL (Osborne, Batterham, Elsworth, Hawkins, & Buchbinder, 2013).
Depressive symptoms were assessed by the Beck Depression Inventory Short Form (BDI‐SF), which has been used to assess depressive symptoms in patients with CKD across different stages of the disease (Andrade et al., 2010). The questionnaire contains 13 items concerning guilt, pessimism, suicidal thoughts and other depressive symptoms. The maximum possible score is 39 and indicates severe depression. The cut‐off score to detect clinical depression in a medical context is commonly set to 13–14 (Furlanetto, Mendlowicz, & Bueno, 2005). Renal function, renal diagnosis, number of prescribed medications, comorbidities and duration with known CKD expressed in months were extracted from the patients’ medical records.
Renal function was estimated using the CKD‐EPI creatinine equation to find the estimated glomerular filtration rate (eGFR; Levey, Stevens, et al., 2009). Renal diagnoses were classified as vascular/hypertensive, diabetic nephropathy, glomerulonephritis, polycystic kidney disease, other diseases or unknown.
The number of comorbidities was expressed according to the Davies Comorbidity Index (DCI). The DCI was originally developed to predict the risk of hospitalization and mortality in patients with CKD based on the presence or absence of seven different comorbidities: active cancer, ischaemic heart disease, peripheral vascular disease, left ventricular dysfunction, diabetes mellitus, systemic collagen vascular disease and other significant pathology (e.g., asthma, cirrhosis and chronic obstructive lung disease). DCI is scored as follows: 0 = no comorbidity, 1 = one or two comorbidities and 2 = three or more comorbidities (Davies, Russell, Bryan, Phillips, & Russell, 1995). Demographic data, including gender, age, level of education, level of household income, living arrangement, employment status and clinical data, are listed in Table 1.
Table 1.
Patient characteristics for the overall cohort and the different HL clusters
| Total group (N = 187) | Low‐level (N = 27) | Mid‐level (N = 106) | High‐level (N = 52) | |
|---|---|---|---|---|
| Age in years, mean ± SD | 67 ± 13 | 69 ± 11 | 67 ± 13 | 66 ± 13 |
| Female gender, N (%) | 65 (35) | 16 (59) | 33 (30) | 15 (29) |
| Education level, N (%) | ||||
| Low = ≤higher secondary school | 113 (60) | 22 (81) | 62 (59) | 28 (53) |
| High = >higher secondary school | 73 (40) | 5 (19) | 43 (41) | 24 (46) |
| Household income in NOK, N (%) | ||||
| Low = ≤300,000 | 37 (20) | 9 (33) | 21 (20) | 7 (13) |
| Average => 300,000 | 147 (80) | 18 (67) | 83 (80) | 44 (85) |
| Living alone, N (%) | 49 (26) | 11 (40) | 28 (27) | 9 (13) |
| DCI score, N (%) | ||||
| 0 | 66 (35) | 8 (30) | 34 (32) | 24 (46) |
| 1 | 88 (47) | 13 (48) | 50 (47) | 25 (48) |
| 2 | 33 (18) | 6 (22) | 22 (21) | 3 (6) |
| BDI‐SF, median (range) | 2 (0–29) | 3 (0–29) | 2 (0–25) | 0 (0–19) |
| Medications, mean (SD) | 7.5 ± 3.7 | 9.11 ± 3.24 | 7.71 ± 3.80 | 6.12 ± 3.22 |
| Renal diagnosis, N (%) | ||||
| Hypertensive nephropathy | 62 (33) | 7 (26) | 31 (29) | 22 (42) |
| Glomerulonephritis | 40 (22) | 6 (22) | 22 (21) | 12 (23) |
| Diabetic nephropathy | 23 (12) | 5 (19) | 14 (13) | 4 (7) |
| Polycystic kidney disease | 14 (7) | 3 (11) | 6 (6) | 5 (10) |
| Other | 30 (16) | 4 (15) | 18 (17) | 8 (15) |
| Unknown | 17 (10) | 2 (7) | 15 (14) | 7 (2) |
| Time CKD in months, median (range) | 46 (1–515) | 81 (1–270) | 41 (1–516) | 50 (2–278) |
| Employment, N (%) | 36 (19) | 3 (11) | 12 (23) | 21 (20) |
Abbreviations: BDI‐SF, Beck Depression Inventory Short Form; CKD, chronic kidney disease; DCI, Davies Comorbidity Index, (DCI = 0 means no co‐morbid condition, DCI = 1 means 1–2 co‐morbid conditions and DCI = 2 means ≥ 3 co‐morbid conditions), NOK, Norwegian kroner.
3.4. Data analysis
SPSS package 25 and Excel 98 (pivot table in the cluster analysis) were used in the statistical analysis, and p ≤ .05 was considered statistically significant. Categorical data are presented as frequencies, and percentages and continuous data are presented as the mean and standard deviations (SDs) if normally distributed and as the median and range otherwise. Student's t test and ANOVA were used to test differences between normally distributed samples, and Mann–Whitney and Kruskal–Wallis tests were used to test for samples that were not distributed normally. Stepwise backward multiple linear regression analysis was performed to identify associations between HLQ scales as the dependent variables and the following independent variables: gender, age, level of education, level of household income, living situation, number of prescribed medications, presence of comorbidity, depressive symptoms, stage of CKD and duration of known CKD. We used the BDI‐SF total score as a continuous variable for depressive symptoms and the DCI scores as a dichotomous variable indicating the presence or absence of comorbidity. Independent variables were included in the model if the univariate analysis resulted in p < .2, and then, the variables were stepwise excluded from the model in a backward manner if p > .05. Hierarchical cluster analysis (Ward's minimum variance method) was used on standardized scores (z‐scores) for each HLQ scale to identify patients with similar HLQ profiles (Ward, 1963). For the total data set, there were less than 5% data missing, and no correction was performed.
3.5. Ethical considerations
The study was approved by the Data Protection Officer at the study hospital (ID number 2017/1). All participants signed a written, informed consent form.
4. RESULTS
4.1. Patient characteristics
A total of 241 patients (110 in CKD stage 3, 101 in CKD stages 4 and 5 not in dialysis and 30 patients receiving maintenance haemodialysis) fulfilled the inclusion criteria and were consecutively invited to participate; 22% of the invited patients declined (Figure 1). In all, 187 patients were included, 35% of whom were female, with a mean (SD) age of 67 years (13) (Table 1). Patients who refused to participate were not significantly different from our sample in terms of age and gender (mean (SD) age of 65 years (14), 33% female). Of the 187 patients, 39% had higher education and 20% of the patients had a household income characterized as low in Norway (Statistics Norway, 2016). Sixty‐five per cent of the patients had comorbidities, and the main renal diagnosis was hypertensive nephropathy (Table 1). The patients were mainly Caucasian with a Norwegian cultural background. Two non‐Caucasians with a non‐Norwegian cultural background were also included.
4.2. HLQ scores
The highest HLQ scores of the questionnaire were obtained for the scales feel understood and supported by healthcare providers (mean 3.19, 95% CI 3.12–3.27) in part one and ability to actively engage with healthcare providers (mean 3.83, 95% CI 3.74–3.93) in part two. The lowest scores were obtained for the scales appraise health information (mean 2.56, 95% CI 2.48–2.63) in part one and ability to find good health information (mean 3.42, 95% CI 3.33–3.51) in part two (Table 2). Males scored significantly higher than females for the scales ability to actively engage with healthcare providers, ability to navigate the healthcare system, ability to find good health information and ability to understand health information well enough to know what to do. Females scored significantly higher than males for the scales actively managing health.
Table 2.
Health literacy questionnaire scale scores for overall cohort (N = 187)
| Mean | 95% CI | |
|---|---|---|
| Part 1. HLQ scale scores, possible range of scores 1–4 | ||
|
1. Feeling understood and supported by healthcare providers Number of items = 4 |
3.19 | 3.12–3.27 |
|
2. Having sufficient information to manage health Number of items = 4 |
2.92 | 2.85–3.01 |
|
3. Actively managing health Number of items = 5 |
2.94 | 2.87–3.02 |
|
4. Social support for health Number of items = 5 |
3.02 | 2.94–3.10 |
|
5. Appraisal of health information Number of items = 5 |
2.56 | 2.48–2.63 |
| Part 2. HLQ scale scores, possible range of scores 1–5 | ||
|
6. Ability to actively engage with healthcare providers Number of items = 5 |
3.83 | 3.74–3.93 |
|
7. Ability to navigate the healthcare system Number of items = 6 |
3.51 | 3.42–3.60 |
|
8. Ability to find good health information Number of items = 5 |
3.42 | 3.33–3.51 |
|
9. Ability to read and understand health information well enough to know what to do Number of items = 5 |
3.71 | 3.63–3.80 |
HLQ scores in part 1 indicate the following responses: 1 = strongly disagree, 2 = disagree, 3 = agree, 4 = strongly agree. HLQ scores in part 2 indicate the following responses: 1 = cannot do, 2 = usually difficult, 3 = sometimes difficult, 4 = usually easy and 5 = always easy.
Abbreviations: CI, confidence interval. HLQ, Health Literacy Questionnaire.
4.3. Associations between demographic and clinical variable HLQ scales
After we performed the stepwise backward multiple linear regression analysis, gender, level of education, living situation, number of prescribed medications, depressive symptoms and duration of CKD were the remaining independent variables that possibly explained the different HLQ scores (Table 3). The duration of CKD showed a weak negative association with the HLQ scale item social support and will not be further discussed.
Table 3.
Relationships between Health Literacy Questionnaire scales and demographic and clinical variables
| Independent variables | 1. Healthcare provider support | 2. Have sufficient health information | 3. Actively managing health | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Uni | MA | MaB | Uni | MA | MaB | Uni | MA | MaB | |
| p | p | p (beta) | p | p | p (beta) | p | p | p (beta) | |
| Female gender | .78 | .85 | .89 | .84 | .02 | .15 | .02 (.19) | ||
| Age | .22 | .81 | .16 | .30 | .16 | .91 | |||
| Education | .25 | .49 | .23 | .44 | .80 | .23 | |||
| Low income | .06 | .18 | .16 | .81 | .79 | .64 | |||
| Living alone | .11 | .15 | .99 | .78 | .65 | .90 | |||
| Medications | .10 | .63 | .04 | .46 | .22 | .14 | |||
| Comorbidity | .27 | .10 | .24 | .17 | .03 | .82 | |||
| BDI‐SF | .38 | .20 | .01 | .03 | .01 (−.02) | .79 | .98 | ||
| CKD stage | .52 | .85 | .38 | .17 | .38 | .43 | |||
| CKD duration | .21 | .20 | .34 | .23 | .46 | .52 | |||
| Adjusted R 2 | .03 | .03 | |||||||
| 4. Social support | 5. Critical appraisal | 6. Actively engage with healthcare providers | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Uni | MA | MaB | Uni | MA | MaB | Uni | MA | MaB | |
| p | p | p (beta) | p | p | p (beta) | p | p | p (beta) | |
| Female | .66 | .82 | .38 | .82 | .03 | .06 | .04 (−.21) | ||
| Age | .22 | .18 | .40 | .99 | .01 | .80 | |||
| Education | .08 | .41 | .32 | .31 | .16 | .44 | |||
| Low income | .39 | .69 | .44 | .83 | .53 | .90 | |||
| Living alone | .01 | .01 | .01 (−.23) | .27 | .61 | .44 | .90 | ||
| Medication | .78 | .28 | .05 | .32 | .04 (−.02) | .03 | .85 | ||
| Comorbidity | .14 | .51 | .30 | .82 | .14 | .09 | |||
| BDI‐SF | <.01 | <.01 | <.01 (−.03) | .15 | .28 | .05 | .17 | .02 (−.02) | |
| CKD stage | .42 | .55 | .21 | .24 | .94 | .96 | |||
| CKD duration | .03 | .05 | .02 (−.001) | .57 | .97 | .56 | .89 | ||
| Adjusted R 2 | .10 | .02 | .05 | ||||||
| 7. Navigating healthcare system | 8. Find good health information | 9. Understand health information | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Uni | MA | MaB | Uni | MA | MaB | Uni | MA | MaB | |
| p | p | p (beta) | p | p | p (beta) | p | p | p (beta) | |
| Female | <.01 | .04 | .03 (−.21) | <.01 | <.01 | <.01 (−.36) | .02 | .21 | .02 (−.22) |
| Age | .99 | .68 | .04 | .10 | .19 | .42 | |||
| Education | .01 | .12 | <.01 | .02 | .02 (.23) | <.01 | .05 | .02 (.22) | |
| Low income | .08 | .91 | .04 | .69 | .13 | .55 | |||
| Living alone | .11 | .45 | .02 | .57 | .12 | .77 | |||
| Medications | <.01 | .14 | <.01 (−.04) | <.01 | .22 | <.01 (−.04) | <.01 | .09 | <.01 (−.04) |
| Comorbidity | .08 | .40 | .02 | .50 | .03 | .65 | |||
| BDI‐SF | <.01 | .06 | .03 (−.02) | .02 | .15 | .01 | .06 | ||
| CKD stage | .84 | .71 | .68 | .10 | .95 | .55 | |||
| CKD duration | .84 | .71 | .88 | .72 | .97 | .55 | |||
| Adjusted R 2 | .11 | .16 | .12 | ||||||
Abbreviations: Adjusted R 2, adjusted R squared; BDI‐SF, Beck Depression Inventory Short Form; CKD stage, stage of chronic kidney disease; CKD duration, duration of chronic kidney disease in months; Comorbidity, presence of comorbidity (no/yes); Education, higher education (no/yes); MA, multiple regression including all independent variables; MaB, multiple regression analysis after stepwise backward elimination; Medications, number of prescribed medications; beta, unstandardized coefficient; Uni, univariate analysis.
4.4. Clustering HL in CKD patients
To characterize patients with different HL profiles, we divided the total group of patients into smaller groups using Ward's method for hierarchical clustering. Patients with similar HLQ profiles were clustered in three different groups with low, medium and high HL. Twenty‐seven (14%) patients were in the group with overall low HL scores (low‐level group) and 52 (28%) patients were in the group with overall high HL scores (high‐level group). The remaining 106 (57%) patients were clustered in the mid‐level group (Figure 2).
Figure 2.
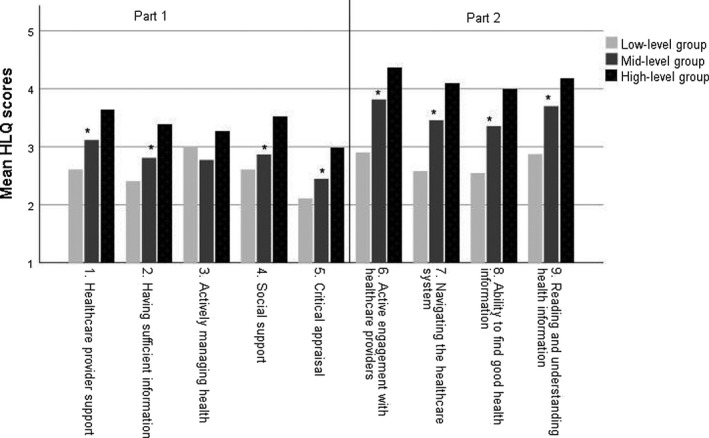
Mean HLQ scale scores for patients in different clusters of HL. HLQ, Health Literacy Questionnaire. Part 1: scales 1–5, Part 2: scales 6–9. HLQ scores in Part 1 were denoted as follows: 1 = strongly disagree, 2 = disagree, 3 = agree and 4 = strongly agree. HLQ scores in part 2 were denoted as follows: 1 = cannot do, 2 = usually difficult, 3 = sometimes difficult, 4 = usually easy and 5 = always easy. *p‐Value < .01
4.5. Characteristics of the different clusters
Of the 27 patients in the low‐level group, 59% were female, 70% had comorbidities, 19% had higher education levels and 40% lived alone (Table 1). The mid‐level group was characterized as follows: 30% were female, 68% had comorbidities, 41% had higher education and 27% lived alone. The 52 patients in the high‐level group were characterized as follows: 29% were female, 54% had comorbidities, 47% had higher education and 13% lived alone.
5. DISCUSSION
By using a multidimensional tool, we identified HL strengths and weaknesses in a Norwegian CKD population. Based on our results, cooperation between healthcare providers and patients seems to be the least problematic dimensions of HL, while finding and apprising health information seems most challenging for this population. Female gender, lower level of education, medication burden and depressive symptoms are variables associated with low HL. The cluster analysis confirmed the results from the regression analysis and revealed that living alone and having comorbidity also might be unfavourable for the CKD patients’ HL.
In general, few published studies have evaluated the HL of patients with CKD by using a multidimensional tool. In an Australian study including patients in haemodialysis and in a Canadian study including renal transplant patients, higher scores were found in almost all HLQ scales compared with results from our current study which included patients with CKD across different stages of the disease (Demian, Shapiro, & Thornton, 2016; Dodson et al., 2016). This difference might be explained by the fact that patients on haemodialysis and renal transplant patients usually have more frequent contact with healthcare providers than patients with CKD followed in an outpatient clinic. Frequent contact with the healthcare system might provide patients with more opportunities to discuss health challenges and obtain relevant health information, which may explain the higher HLQ scores in these patient groups. Furthermore, renal transplant patients are, as a group, highly selected and often highly motivated towards information gathering and learning in terms of caring for their new graft (Urstad, Wahl, Andersen, Øyen, & Fagermoen, 2012). Earlier research shows that patients with low HL are less likely to receive access to renal transplantation than patients with higher HL (Grubbs et al., 2009). Despite previous studies reporting better HLQ scores in haemodialysis and renal transplant patients than in the group of unselected CKD patients in our study, the HLQ profiles were similar. That the HLQ profiles were similar indicates that the patients have the same HL challenges irrespective of the disease stage. In general, finding good health information and critical appraising health information appears to be the most challenging dimensions of HL for patients with CKD. Challenges in finding and appraising health information may reflect the overload of health information accessible by the Internet and social media, which may confuse patients (Klerings, Weinhandl, & Thaler, 2015). To reduce confusion due to information overload, strategies for filtering out irrelevant information should be developed and healthcare providers should be able to inform patients where to find and how to interpret relevant information (Klerings et al., 2015).
Generally, patients in the low‐level HL group had low scores on all HLQ scales. The low‐level group is characterized by low education, the presence of comorbidities, a high medication burden and the presence of depressive symptoms. The patients in the low‐level HL group may not understand their health situation or the health consequences of the choices they make. The motivation for active self‐management in patients with CKD is also most likely influenced by the patients’ understanding of the risks and benefits related to the different treatments, which may be difficult for these patients. Ideally, healthcare providers should be able to identify patients with HL challenges, but earlier research indicates that they often fail at identifying these patients (Bass, Wilson, Griffith, & Barnett, 2002; Dickens, Lambert, Cromwell, & Piano, 2013; Goggins, Wallston, Mion, Cawthon, & Kripalani, 2016). Integrating HL training into the education of healthcare professionals and screening a patient's HL are solutions suggested to increase the ability to identify vulnerable patients with low HL (Bass et al., 2002; Dickens et al., 2013; Health Literacy: Report of the Council on Scientific Affairs, 1999). When healthcare providers recognize patients with low HL, alternative methods for information dissemination, such as the teach‐back method, might be useful to ensure that crucial information is understood (Ha Dinh, Bonner, Clark, Ramsbotham, & Hines, 2016). In addition, measuring multidimensional HL in patients with comorbidities and a high medication burden may help healthcare providers identify HL needs in an individual patient and respond to them. From a long‐term perspective, creating HL‐responsive organizations by integrating how to respond to different HL needs into the education of healthcare professionals might improve the HL for the individual patient (Batterham, Hawkins, Collins, Buchbinder, & Osborne, 2016).
According to previous research, the level of education is a strong predictor of HL (Friis, Lasgaard, Osborne, & Maindal, 2016; Van der Heide et al., 2013; Maindal et al., 2016; Paasche‐Orlow & Wolf, 2007; Sorensen et al., 2015). In our study, higher education was associated with a better ability to find good health information and to understand health information. Some examples of finding and evaluating health information include the ability to compare information from different sources and to be critical of new information given. Highly educated individuals are more likely able to understand, interpret and evaluate the information given than are individuals with a lower education. Our findings indicate that the current information available to patients may be too complicated or not adapted to patients with lower education levels, which underlines the importance of individualized and facilitated information and follow‐up.
Our study indicates that male patients with CKD are more confident than females in their abilities to navigate the healthcare system, engage with healthcare providers and find and understand health information. Other studies have also found gender differences in HL, but the differences are not consistent across the literature. A Slovakian HLQ study including 360 adults found that females had fewer difficulties understanding health information than males (Kolarcik et al., 2017), while an Australian study including 814 health consumers found that males had fewer difficulties engaging with healthcare providers than females (Beauchamp et al., 2015). However, in our CKD population, more males than females had higher education, which may contribute to our findings that males scored higher than women in four out of the nine HLQ scales. In contrast to other studies that have found associations between older age and lower HL (Jessup, Osborne, Beauchamp, Bourne, & Buchbinder, 2017; Sorensen et al., 2015), we found no such association in our study. An explanation for the lack of association between age and HL may be that the age spread in our study sample was narrow, with most patients aged 60–80 years. The narrow age spread limits the possibility of identifying such associations. However, our study population reflects the typical age spread for CKD and a larger study population is probably necessary to identify any potential association between age and HL.
Patients living alone experienced less social support for health in our study, which is in accordance with findings in previous works (Beauchamp et al., 2015; Sorensen et al., 2015). Living with someone may be favourable for discussing health issues and obtaining mental and physical support, resulting in better HL (Lee, Arozullah, & Cho, 2004). Patients who live alone or need more social support can benefit from referrals to relevant patient organizations because patient organizations often arrange social gatherings that may create an arena for receiving social support from peers. In addition, patient organizations offer classes and conferences to educate patients on disease‐specific topics as well as legal rights relevant to patients living with CKD, which may be useful for most patients regardless of social support.
To the best of our knowledge, this is the first HLQ study investigating the association between HL and the number of prescribed medications in patients with CKD. We found that a higher number of prescribed medications were associated with a reduced ability to find and appraise health information, to navigate the healthcare system and to understand health information well enough to know what to do. An explanation for the negative association between pill burden and lower HL may be that a heavy pill burden causes unpleasant side effects and is a marker of comorbidity, which makes the health situation more complicated. According to previous studies that use less complex tools to evaluate HL, patients with low HL are more likely to misunderstand medical prescriptions and take drugs improperly than patients with high HL (Davis et al., 2006; Wolf, Davis, Tilson, Bass, & Parker, 2006). A heavy pill burden may not in itself lead to low HL, but it is likely that the pill burden is linked to comorbidity. Patients with comorbidities must visit more specialists, which demand more navigation in the healthcare system. Additionally, the level of medical instructions and information might be complicated, which may explain why these patients find it difficult to understand health information. The different specialties involved in patients with comorbidities should aim to cooperate regarding the medical treatment regimens so the patient does not need to be the messenger between the different departments.
Having more depressive symptoms was negatively associated with the CKD patients’ experience of having sufficient health information and social support for health, the ability to engage with healthcare providers and the ability to navigate the healthcare system. The negative association between depressive symptoms and HL in general is in accordance with the findings in an Australian HLQ study that included 100 dialysis patients. The level of HL in the Australian study was strongly associated with the level of depressive and anxiety symptoms (Dodson et al., 2016), but the specific dimensions of HL were not reported. Another study including 702 patients with type 2 diabetes showed impaired self‐management and problem‐solving in depressed patients compared with nondepressed patients (Shin et al., 2017), indicating that depression is an important factor in self‐management. The results from the diabetes study correspond with our findings that more depressive symptoms were associated with reduced abilities to actively engage with healthcare providers and to navigate the healthcare system. We also found that depressive symptoms were more prevalent in the low‐level HL group than in the middle and high‐level groups, but whether depressive symptoms are the cause or a result of low HL needs to be explored. Healthcare providers should be aware of depressive symptoms in patients with low HL because they can be more vulnerable about HL and the self‐management of CKD.
5.1. Limitations
Most patients were Caucasians with a Norwegian cultural background. Perception of illness might vary by culture and might affect approaches to health care, and future research should therefore aim to include patients with CKD of other ethnicities and with different cultural backgrounds. Due to the modest participation from non‐Caucasians, the regression models were not corrected for race. Furthermore, the cross‐sectional design of this study limits the possibility of identifying causation.
6. CONCLUSION
This study provides extended knowledge about HL in patients with CKD. The multidimensional perspective put us in a better position to identify vulnerable patients and to develop target interventions that may reduce health inequalities in this patient group. When designing and implementing HL interventions for patients with CKD, extra focus should be placed on providing patients with strategies to access relevant health information and enabling them to critically appraise the information they access. Furthermore, special attention should be given to vulnerable patients characterized by a complex health situation, presence of depressive symptoms, low education levels and low social support.
CONFLICT OF INTEREST
There is no conflict of interest to report.
AUTHOR CONTRIBUTIONS
Une Elisabeth Stømer have made substantial contributions to conception and design, analysing and interpreting data and been involved in drafting the article. Lasse Gunnar Gøransson, Astrid Klopstad Wahl and Kristin Hjorthaug Urstad have made substantial contributions to conception and design and been involved in drafting the article and revising it critically for important intellectual content. All of the authors have given final approval for the final version to be published and are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
ACKNOWLEDGEMENTS
The authors are grateful to the patients who participated in this study and to the Nephrology Department staff at Stavanger University Hospital for their cooperation during the data collection period. We also thank biostatistician Anastasia Ushakova at Stavanger University Hospital and Professor Jan Terje Kvaløy at the University of Stavanger for statistical advice.
Stømer UE, Gøransson LG, Wahl AK, Urstad KH. A cross‐sectional study of health literacy in patients with chronic kidney disease: Associations with demographic and clinical variables. Nursing Open. 2019;6:1481–1490. 10.1002/nop2.350
REFERENCES
- Amira, O. (2011). Prevalence of symptoms of depression among patients with chronic kidney disease. Nigerian Journal of Clinical Practice, 14(4), 460–463. 10.4103/1119-3077.91756 [DOI] [PubMed] [Google Scholar]
- Andrade, C. P. , Cruz, M. C. , Urrutia, M. , Pereira, O. , Draibe, S. A. , Nogueira‐Martins, L. A. , & Sesso, R. (2010). Evaluation of depressive symptoms in patients with chronic renal failure. Journal of Nephrology, 23(2), 168–174. [PubMed] [Google Scholar]
- Bass, P. F. , Wilson, J. F. , Griffith, C. H. , & Barnett, D. R. (2002). Residents' ability to identify patients with poor literacy skills. Academic Medicine, 77(10), 1039–1041. 10.1097/00001888-200210000-00021 [DOI] [PubMed] [Google Scholar]
- Batterham, R. W. , Beauchamp, A. , & Osbourne, R. H. (2017). The international encyclopedia of public health (2nd ed.). Oxford, UK: Academic Press. [Google Scholar]
- Batterham, R. W. , Hawkins, M. , Collins, P. A. , Buchbinder, R. , & Osborne, R. H. (2016). Health literacy: Applying current concepts to improve health services and reduce health inequalities. Public Health, 132, 3–12. 10.1016/j.puhe.2016.01.001 [DOI] [PubMed] [Google Scholar]
- Beauchamp, A. , Buchbinder, R. , Dodson, S. , Batterham, R. W. , Elsworth, G. R. , McPhee, C. , … Osborne, R. H. (2015). Distribution of health literacy strengths and weaknesses across socio‐demographic groups: A cross‐sectional survey using the Health Literacy Questionnaire (HLQ). BMC Public Health, 15, 678 10.1186/s12889-015-2056-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkman, N. D. , Sheridan, S. L. , Donahue, K. E. , Halpern, D. J. , & Crotty, K. (2011). Low health literacy and health outcomes: An updated systematic review. Annals of Internal Medicine, 155(2), 97–107. 10.7326/0003-4819-155-2-201107190-00005 [DOI] [PubMed] [Google Scholar]
- Davies, S. J. , Russell, L. , Bryan, J. , Phillips, L. , & Russell, G. I. (1995). Comorbidity, urea kinetics and appetite in continuous ambulatory peritoneal dialysis patients: Their interrelationship and prediction of survival. American Journal of Kidney Diseases, 26(2), 353–361. 10.1016/0272-6386(95)90657-6 [DOI] [PubMed] [Google Scholar]
- Davis, T. C. , Crouch, M. A. , Long, S. W. , Jackson, R. H. , Bates, P. , George, R. B. , & Bairnsfather, L. E. (1991). Rapid assessment of literacy levels of adult primary care patients. Family Medicine, 23(6), 433–435. [PubMed] [Google Scholar]
- Davis, T. C. , Wolf, M. S. , Bass, P. F. , Middlebrooks, M. , Kennen, E. , Baker, D. W. , … Parker, R. M. (2006). Low literacy impairs comprehension of prescription drug warning labels. Journal of General Internal Medicine, 21(8), 847–851. 10.1111/j.1525-1497.2006.00529.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demian, M. N. , Shapiro, R. J. , & Thornton, W. L. (2016). An observational study of health literacy and medication adherence in adult kidney transplant recipients. Clinical Kidney Journal, 9(6), 858–865. 10.1093/ckj/sfw076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devraj, R. , Borrego, M. , Vilay, A. M. , Gordon, E. J. , Pailden, J. , & Horowitz, B. (2015). Relationship between health literacy and kidney function. Nephrology (Carlton), 20(5), 360–367. 10.1111/nep.12425 [DOI] [PubMed] [Google Scholar]
- Dickens, C. , Lambert, B. L. , Cromwell, T. , & Piano, M. R. (2013). Nurse overestimation of patients' health literacy. Journal of Health Communication, 18, 62–69. 10.1080/10810730.2013.825670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodson, S. , Osicka, T. , Huang, L. , McMahon, L. P. , & Roberts, M. A. (2016). Multifaceted assessment of health literacy in people receiving dialysis: Associations with psychological stress and quality of life. Journal of Health Communication, 21, 91–98. 10.1080/10810730.2016.1179370 [DOI] [PubMed] [Google Scholar]
- Eckardt, K. U. , Coresh, J. , Devuyst, O. , Johnson, R. J. , Kottgen, A. , Levey, A. S. , & Levin, A. (2013). Evolving importance of kidney disease: From subspecialty to global health burden. The Lancet, 382(9887), 158–169. 10.1016/S0140-6736(13)60439-0 [DOI] [PubMed] [Google Scholar]
- Fraser, S. D. , Roderick, P. J. , Casey, M. , Taal, M. W. , Yuen, H. M. , & Nutbeam, D. (2013). Prevalence and associations of limited health literacy in chronic kidney disease: A systematic review. Nephrology Dialysis Transplantation, 28(1), 129–137. 10.1093/ndt/gfs371 [DOI] [PubMed] [Google Scholar]
- Friis, K. , Lasgaard, M. , Osborne, R. H. , & Maindal, H. T. (2016). Gaps in understanding health and engagement with healthcare providers across common long‐term conditions: A population survey of health literacy in 29,473 Danish citizens. British Medical Journal Open, 6(1), e009627 10.1136/bmjopen-2015-009627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furlanetto, L. M. , Mendlowicz, M. V. , & Bueno, J. R. (2005). The validity of the beck depression inventory‐short form as a screening and diagnostic instrument for moderate and severe depression in medical inpatients. Journal of Affective Disorders, 86(1), 87–91. 10.1016/j.jad.2004.12.011 [DOI] [PubMed] [Google Scholar]
- Goggins, K. , Wallston, K. A. , Mion, L. , Cawthon, C. , & Kripalani, S. (2016). What patient characteristics influence nurses' assessment of health literacy? Journal of Health Communication, 21, 105–108. 10.1080/10810730.2016.1193919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green, J. A. , Mor, M. K. , Shields, A. M. , Sevick, M. A. , Arnold, R. M. , Palevsky, P. M. , … Weisbord, S. D. (2013). Associations of health literacy with dialysis adherence and health resource utilization in patients receiving maintenance hemodialysis. American Journal of Kidney Diseases, 62(1), 73–80. 10.1053/j.ajkd.2012.12.014 [DOI] [PubMed] [Google Scholar]
- Green, J. A. , Mor, M. K. , Shields, A. M. , Sevick, M. A. , Palevsky, P. M. , Fine, M. J. , … Weisbord, S. D. (2011). Prevalence and demographic and clinical associations of health literacy in patients on maintenance hemodialysis. Clinical Journal of the American Society of Nephrology, 6(6), 1354–1360. 10.2215/CJN.09761110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubbs, V. , Gregorich, S. E. , Perez‐Stable, E. J. , & Hsu, C. Y. (2009). Health literacy and access to kidney transplantation. Clinical Journal of the American Society of Nephrology, 4(1), 195–200. 10.2215/CJN.03290708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha Dinh, T. T. , Bonner, A. , Clark, R. , Ramsbotham, J. , & Hines, S. (2016). The effectiveness of the teach‐back method on adherence and self‐management in health education for people with chronic disease: A systematic review. The JBI Database of Systematic Reviews and Implementation Reports, 14(1), 210–247. 10.11124/jbisrir-2016-2296 [DOI] [PubMed] [Google Scholar]
- Hallan, S. I. , Coresh, J. , Astor, B. C. , Åsberg, A. , Powe, N. R. , Romundstad, S. , … Holmen, J. (2006). International comparison of the relationship of chronic kidney disease prevalence and ESRD risk. Journal of the American Society of Nephrology, 17(8), 2275–2284. 10.1681/ASN.2005121273 [DOI] [PubMed] [Google Scholar]
- Health Literacy: Report of the Council on Scientific Affairs (1999). Health literacy: Report of the council on scientific affairs. Ad hoc committee on health literacy for the council on scientific affairs, American Medical Association. JAMA, 281(6), 552–557. 10.1001/jama.281.6.552 [DOI] [PubMed] [Google Scholar]
- Hedayati, S. S. , Minhajuddin, A. T. , Toto, R. D. , Morris, D. W. , & Rush, A. J. (2009). Prevalence of major depressive episode in CKD. American Journal of Kidney Diseases, 54(3), 424–432. 10.1053/j.ajkd.2009.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helsedirektoratet (2011). Handlingsplan for forebygging og behandling av kronisk nyresykdom (2011–2015). http://www.nephro.no/foreningsnytt/Handlingsplan_forebygging_behandling_kronisk_nyresykdom.pdf [Google Scholar]
- Hill, N. R. , Fatoba, S. T. , Oke, J. L. , Hirst, J. A. , O'Callaghan, C. A. , Lasserson, D. S. , & Hobbs, F. D. (2016). Global prevalence of chronic kidney disease – A systematic review and meta‐analysis. PLoS ONE, 11(7), e0158765 10.1371/journal.pone.0158765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessup, R. L. , Osborne, R. H. , Beauchamp, A. , Bourne, A. , & Buchbinder, R. (2017). Health literacy of recently hospitalised patients: A cross‐sectional survey using the Health Literacy Questionnaire (HLQ). BMC Health Service Research, 17(1), 52 10.1186/s12913-016-1973-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan, J. E. , Osborne, R. H. , , & Buchbinder, R. (2011). Critical appraisal of health literacy indices revealed variable underlying constructs, narrow content and psychometric weaknesses. J Clin Epidemiol, 64(4), 366–379. 10.1016/j.jclinepi.2010.04.005 [DOI] [PubMed] [Google Scholar]
- K/DOQI (2002). K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification and stratification. American Journal of Kidney Diseases, 46, 4 10.1053/j.ajkd.2005.07.028 [DOI] [PubMed] [Google Scholar]
- Klerings, I. , Weinhandl, A. S. , & Thaler, K. J. (2015). Information overload in healthcare: Too much of a good thing? Zeitschrift für Evidenz, Fortbildung und Qualität im Gesundheitswesen, 109(4–5), 285–290. 10.1016/j.zefq.2015.06.005 [DOI] [PubMed] [Google Scholar]
- Kolarcik, P. , Cepova, E. , Geckova, A. M. , Elsworth, G. R. , Batterham, R. W. , & Osborne, R. H. (2017). Structural properties and psychometric improvements of the Health Literacy Questionnaire in a Slovak population. International Journal of Public Health, 62(5), 591–604. 10.1007/s00038-017-0945-x [DOI] [PubMed] [Google Scholar]
- Lee, S. Y. , Arozullah, A. M. , & Cho, Y. I. (2004). Health literacy, social support and health: A research agenda. Social Science and Medicine, 58(7), 1309–1321. 10.1016/S0277-9536(03)00329-0 [DOI] [PubMed] [Google Scholar]
- Levey, A. S. , & Coresh, J. (2012). Chronic kidney disease. The Lancet, 379(9811), 165–180. 10.1016/S0140-6736(11)60178-5 [DOI] [PubMed] [Google Scholar]
- Levey, A. S. , Schoolwerth, A. C. , Burrows, N. R. , Williams, D. E. , Stith, K. R. , McClellan, W. , & Centers for Disease Control and Prevention Expert Panel (2009). Comprehensive public health strategies for preventing the development, progression and complications of CKD: Report of an expert panel convened by the Centers for Disease Control and Prevention. American Journal of Kidney Diseases, 53(3), 522–535. 10.1053/j.ajkd.2008.11.019 [DOI] [PubMed] [Google Scholar]
- Levey, A. S. , Stevens, L. A. , Schmid, C. H. , Zhang, Y. L. , Castro, A. F. , Feldman, H. I. , … CKD‐EPI (Chronic Kidney Disease Epidemiology Collaboration) (2009). A new equation to estimate glomerular filtration rate. Annals of Internal Medicine, 150(9), 604–612. 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maindal, H. T. , Kayser, L. , Norgaard, O. , Bo, A. , Elsworth, G. R. , & Osborne, R. H. (2016). Cultural adaptation and validation of the Health Literacy Questionnaire (HLQ): Robust nine‐dimension Danish language confirmatory factor model. SpringerPlus, 5(1), 1232 10.1186/s40064-016-2887-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne, R. H. , Batterham, R. W. , Elsworth, G. R. , Hawkins, M. , & Buchbinder, R. (2013). The grounded psychometric development and initial validation of the Health Literacy Questionnaire (HLQ). BMC Public Health, 13, 658 10.1186/1471-2458-13-658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paasche‐Orlow, M. K. , & Wolf, M. S. (2007). The causal pathways linking health literacy to health outcomes. American Journal of Health Behavior, 31, S19–26. 10.5555/ajhb.2007.31.supp.S19 [DOI] [PubMed] [Google Scholar]
- Parker, R. M. , Baker, D. W. , Williams, M. V. , & Nurss, J. R. (1995). The test of functional health literacy in adults: A new instrument for measuring patients' literacy skills. Journal of General Internal Medicine, 10(10), 537–541. 10.1007/BF02640361 [DOI] [PubMed] [Google Scholar]
- Ricardo, A. C. , Yang, W. , Lora, C. M. , Gordon, E. J. , Diamantidis, C. J. , Ford, V. , … Lash, J. P. (2014). Limited health literacy is associated with low glomerular filtration in the chronic renal insufficiency cohort (CRIC) study. Clinical Nephrology, 81(1), 30–37. 10.5414/CN108062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin, N. , Hill‐Briggs, F. , Langan, S. , Payne, J. L. , Lyketsos, C. , & Golden, S. H. (2017). The association of minor and major depression with health problem‐solving and diabetes self‐care activities in a clinic‐based population of adults with type 2 diabetes mellitus. Journal of Diabetes and its Complications, 31(5), 880–885. 10.1016/j.jdiacomp.2017.01.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen, K. , Pelikan, J. M. , Rothlin, F. , Ganahl, K. , Slonska, Z. , Doyle, G. … (Hls‐Eu) Consortium (2015). Health literacy in Europe: Comparative results of the European health literacy survey (HLS‐EU). European Journal of Public Health, 25(6), 1053–1058. 10.1093/eurpub/ckv043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen, K. , Van den Broucke, S. , Fullam, J. , Doyle, G. , Pelikan, J. , Slonska, Z. … (HLS‐EU) Consortium Health Literacy Project European (2012). Health literacy and public health: A systematic review and integration of definitions and models. BMC Public Health, 12, 80 10.1186/1471-2458-12-80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Statistics Norway (2016). Median income, Norway. https://www.ssb.no/289084/medianinntekt-etter-skatt-etter-hushaldstype-og-alder-til-hovudinntektstakaren.kr-sa-190 [Google Scholar]
- Tangkiatkumjai, M. , Walker, D. M. , Praditpornsilpa, K. , & Boardman, H. (2017). Association between medication adherence and clinical outcomes in patients with chronic kidney disease: A prospective cohort study. Clinical and Experimental Nephrology, 21(3), 504–512. 10.1007/s10157-016-1312-6 [DOI] [PubMed] [Google Scholar]
- Taylor, D. M. , Bradley, J. A. , Bradley, C. , Draper, H. , Johnson, R. , Metcalfe, W. , … ATTOM Investigators (2016). Limited health literacy in advanced kidney disease. Kidney International, 90(3), 685–695. 10.1016/j.kint.2016.05.033 [DOI] [PubMed] [Google Scholar]
- Taylor, D. M. , Fraser, S. , Dudley, C. , Oniscu, G. C. , Tomson, C. , Ravanan, R. , & Roderick, P. (2017). Health literacy and patient outcomes in chronic kidney disease: A systematic review. Nephrology Dialysis Transplantation, 33, 1545–1558. 10.1093/ndt/gfx293 [DOI] [PubMed] [Google Scholar]
- Urstad, K. H. , Wahl, A. K. , Andersen, M. H. , Øyen, O. , & Fagermoen, M. S. (2012). Renal recipients' educational experiences in the early post‐operative phase–a qualitative study. Scandinavian Journal of Caring Sciences, 26(4), 635–642. 10.1111/j.1471-6712.2012.00972.x [DOI] [PubMed] [Google Scholar]
- Van der Heide, I. , Poureslami, I. , Mitic, W. , Shum, J. , Rootman, I. , & FitzGerald, J. M. (2018). Health literacy in chronic disease management: A matter of interaction. Journal of Clinical Epidemiology, 102, 134–138. 10.1016/j.jclinepi.2018.05.010 [DOI] [PubMed] [Google Scholar]
- Van der Heide, I. , Wang, J. , Droomers, M. , Spreeuwenberg, P. , Rademakers, J. , & Uiters, E. (2013). The relationship between health, education and health literacy: Results from the Dutch adult literacy and life skills survey. Journal of Health Communication, 18, 172–184. 10.1080/10810730.2013.825668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward, J. (1963). Application of a hierarchial grouping procedure to a problem of grouping profiles. Educational and Psychological Measurement, 23, 69–81. [Google Scholar]
- Whaley‐Connell, A. , Nistala, R. , & Chaudhary, K. (2011). The importance of early identification of chronic kidney disease. Missouri Medicine, 108(1), 25–28. [PMC free article] [PubMed] [Google Scholar]
- WHO (1998). The health promotion glossary. http://www.who.int/healthpromotion/about/HPR%20Glossary%201998.pdf?ua=1 [Google Scholar]
- Wolf, M. S. , Davis, T. C. , Tilson, H. H. , Bass, P. F. , & Parker, R. M. (2006). Misunderstanding of prescription drug warning labels among patients with low literacy. American Journal of Health‐System Pharmacy, 63(11), 1048–1055. 10.2146/ajhp050469 [DOI] [PubMed] [Google Scholar]


