Abstract
Background
Explore markers to predict the clinical outcomes of checkpoint inhibitors have high unmet needs. The following study investigates whether hematologic parameter such as systemic immune‐inflammation index (SII), neutrophil‐to‐lymphocyte ratio (NLR), platelet‐to‐lymphocyte ratio (PLR) is associated with nivolumab efficacy in advanced non‐small‐cell lung cancer (NSCLC).
Methods
Advanced/metastatic NSCLC patients treated with nivolumab monotherapy for second‐line or further‐line treatment at Jilin Cancer Hospital between March 2016 and July 2018 were enrolled in this retrospective study. The optimal cutoff values of SII, NLR, and PLR for predicting efficacy and prognosis were determined according to receiver operating characteristic (ROC) curve and the areas under the ROC curve. Progression‐free survival (PFS) and overall survival (OS) were calculated and compared using Kaplan‐Meier method and log‐rank test. Prognostic values of each variable were evaluated with univariate and multivariate Cox proportional hazard regression (PHR) analyses.
Results
A total of 44 patients with advanced NSCLC were included; the median age was 60 (range: 43‐74). The optimal cutoff value of SII/NLR/PLR predicted PFS and OS was 603.5, 3.07, and 144. Low SII, NLR, and PLR were associated with longer PFS (HR for SII = 0.34, 95%CI 0.15‐0.76, P = 0.006; HR for NLR = 0.46, 95%CI 0.22‐0.99, P = 0.048; HR for PLR = 0.39, 95%CI 0.17‐0.94, P = 0.025) and OS (HR for SII = 0.16, 95%CI 0.05‐0.51, P = 0.005; HR for NLR = 0.20, 95%CI 0.06‐0.62, P = 0.002; HR for PLR = 0.20, 95%CI 0.06‐0.73, P = 0.008). NLR ≤ 3.07, PLR ≤ 144, SII ≤ 603.5 were independently associated with longer PFS and OS.
Conclusion
The SII, NLR, and PLR are promising prognostic predictor for patients with metastatic NSCLC patients.
Keywords: neutrophil‐to‐lymphocyte ratio, nivolumab, non‐small‐cell lung cancer, platelet‐to‐lymphocyte ratio, systemic immune‐inflammation index
1. INTRODUCTION
Non‐small‐cell lung cancer (NSCLC) accounts for approximately 85% of all lung cancers, with an estimated 2 093 876 new lung cancer cases that will occur worldwide in 2018.1 According to National Cancer Center, the incidence and mortality rates of lung cancer in China are very high, and they are increasing year by year.2
Over the recent years, the treatment strategies for advanced and metastatic NSCLC have been dramatically changed. Immune checkpoint inhibitors (ICIs), including programmed death 1 (PD‐1) inhibitors (nivolumab, pembrolizumab) and programmed cell death ligand‐1 (PD‐L1) inhibitors (atezolizumab) monotherapy or combined chemotherapy have become one of the standard treatments for NSCLC patients without treatable driver mutations.3, 4, 5, 6, 7 Nivolumab (a fully human IgG4 PD‐1 antibody) is the first ICIs to be approved for previously treated advanced NSCLC.3, 4 Despite the improved survival benefit with ICIs compared with conventional chemotherapy, but a considerable proportion of NSCLC patients still failed to respond.8, 9, 10, 11, 12 Up to date, PD‐L1 and tumor mutational burden (TMB) were used to screen patients who would potentially benefit from ICIs, but they are not the ideal biomarker due to different test platform, panel, cutoff value and many patients could not provide sufficient tumor tissue for testing. Explore markers to predict the clinical outcomes of checkpoint inhibitors have high unmet needs.13
Inflammation is an important feature of tumor microenvironment and associated with poor prognosis of various types of tumor.14 Hematological inflammatory parameters such as neutrophil, lymphocyte, monocytes, and platelets can reflect the immune status and have important predictive value for the prognosis of tumors.15, 16 Some studies have evaluated the value of some blood cell count indexes, particularly the neutrophil‐to‐lymphocyte ratio (NLR), platelet‐to‐lymphocyte ratio (PLR) and demonstrated these biomarkers have the prognostic role in different tumors include NSCLC.17, 18, 19, 20, 21, 22, 23, 24 Recently, several studies suggested that NLR and PLR also strongly associated with poor clinical outcomes in patients treated with ICIs.25, 26, 27, 28 Systemic immune‐inflammation index (SII) is a novel inflammatory marker which combines NLR and platelet is an independent risk factor for the development of solid cancer.29 Higher SII was independently associated with worse outcomes for metastatic renal cell carcinoma (RCC) patients treated with nivolumab.30 However, rare studies reported whether SII is associated with the prognosis of NSCLC patients treated with ICIs. Consequently, the aim of this retrospective study was to examine the correlation between SII and efficacy in patients with NSCLC treated with ICIs.
2. MATERIALS AND METHODS
2.1. Study population
Patients with advanced or metastatic NSCLC treated with nivolumab monotherapy for second‐line or further‐line treatment at the Jilin Cancer Hospital between March 2016 and July 2018 were enrolled in this study. Data were analyzed by professional statisticians; treatment records were evaluated by clinical experienced doctors; all information were extracted in accordance with uniform requirements. The electronic medical records of patients were reviewed, and all patients had complete blood parameters collected on the date of initial clinic visit or within 7 days prior to starting nivolumab. The last follow‐up was on November 9, 2018.
This study was approved by Jilin Cancer Hospital ethic committee. In addition, all patients have signed the informed consent before receiving the nivolumab treatment.
2.2. Determination of efficacy, immune‐related adverse reactions and SII, NLR, PLR
Low‐dose computed tomography (LDCT)/magnetic resonance imaging (MRI) scan examinations were performed every 6 weeks. Responses to treatment were evaluated based on Response Evaluation Criteria of Solid Tumor (RECIST) ver.1.1,31 and they were categorized as progressive disease (PD), stable disease (SD), partial remission (PR), and complete remission (CR) according to the therapeutic effect evaluated in the medical record. Toxicity assessment was performed according to Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. Immune‐related adverse events (irAEs) were defined as adverse events with a potential immunologic basis that required frequent monitoring and potential intervention with immune suppression or endocrine therapy.32, 33 IrAEs were determined and graded independently by two experienced physicians and re‐evaluated according to the course record. The onset time and end time of irAE were recorded according to management of immunotherapy‐related toxicities NCCN 2018 Version 1. A third higher level physician examined the information above. All disagreements were resolved by discussion between three doctors until the consensus was reached.
SII = platelet count × neutrophil count/lymphocyte count. The NLR was defined as the absolute number of neutrophils divided by the absolute number of lymphocytes, the PLR as the absolute number of platelets by the absolute number of lymphocytes.
2.3. Statistical analyses
Overall survival (OS) was defined as the interval from treatment initiation until death. Patients who were still alive were censored at the final follow‐up. Progression‐free survival (PFS) was defined as the interval from treatment initiation until disease progression or death. Patients still manifested disease control were censored at the final follow‐up. ORR was defined as the percentage of the best overall remission confirmed by the investigator, that is, PR + CR accounted for the proportion of enrolled patients. DCR was defined as the proportion of patients whose RECIST, CR or PR or SD lasted longer than 24 weeks.
Descriptive analysis was used for all variables. Counting variables were presented as percentages. The optimal cutoff values of SII, NLR, and PLR for predicting efficacy and prognosis were determined according to receiver operating characteristic (ROC) curve and the areas under the ROC curve (AUC). Patients were divided into high SII/NLR/PLR groups and low SII/NLR/PLR groups based on cutoff values. PFS and OS were calculated and compared using the Kaplan‐Meier method and the log‐rank test. The prognostic values of each variable were evaluated with univariate and multivariate Cox proportional hazard regression (PHR) analyses. Reverse Kaplan‐Meier method was used to compute the median follow‐up time. P < 0.05 was considered statistically significant. All analyses were statistically analyzed using R 3.4.3 and SPSS24.0 (Chicago, Illinois, USA).
3. RESULTS
3.1. Patients
A total of 44 patients with advanced NSCLC treated with nivolumab (3 mg/kg, every 2 weeks) were enrolled in this study. The median age at diagnosis was 60 (range: 43‐74) years with 33 (75%) men. PD‐1/PD‐L1 status of all patients was unknown. Baseline characteristics of patients are presented listed in Table 1.
Table 1.
Baseline characteristics of patients
| Characteristics | Overall (n = 44) |
|---|---|
| Age at diagnosis, median (range) | 60 (43‐74) |
| Sex | |
| Male | 33 |
| Female | 11 |
| ECOG PS | |
| 1 | 44 |
| Smoking history | |
| Never | 15 |
| Current | 8 |
| Former | 21 |
| Histology | |
| Squamous | 13 |
| Adenocarcinoma | 31 |
| Radiotherapy history | |
| Yes | 12 |
| No | 32 |
| Stage | |
| IIIB | 9 |
| IV | 35 |
| CNS metastasis | |
| Yes | 2 |
| No | 42 |
| Pulmonary metastasis | |
| Yes | 20 |
| No | 24 |
| Liver metastasis | |
| Yes | 7 |
| No | 37 |
| Bone metastases | |
| Yes | 11 |
| No | 33 |
| Adrenal metastases | |
| Yes | 2 |
| No | 42 |
| EGFR mutation status | |
| Positive | 5 |
| Negative | 28 |
| Not examined | 11 |
| ALK fusion status | |
| Positive | 3 |
| Negative | 21 |
| Not examined | 20 |
Abbreviations: ALK, anaplastic lymphoma kinase; CNS, central nervous system; ECOG PS, Eastern Cooperative Oncology Group performance status; EGFR, epidermal growth factor receptor.
3.2. Treatment response and survival
The median follow‐up time was 6.9 m (range: 0.6‐28.5). The median PFS was 4.8 m (95%CI: 3.7‐NA), median OS was 13.4 m (95%CI: 10.5‐NA). ORR was 31.8%, DCR was 65.9%. No patients achieved complete response, 31.8% achieved partial response, 34.1% had SD, and 29.5% had PD. 27 (61.4%) patients experienced disease progression and 20 (45.5%) patients died at the time of follow‐up date.
3.3. The association between hematological inflammatory parameters and PFS/OS
According to the ROC curve, the optimal cutoff value of SII predicted PFS and OS was 603.5, the sensitivity of this point was 0.89, the specificity was 0.67, and the AUC was 0.83. Patients were divided into two groups according to the optimum cutoff value of SII, 22 patients in low SII group (SII ≤ 603.5) and 22 patients in high SII group (SII ˃ 603.5). Patients in low SII group before treatment had longer OS and PFS compared with high SII group (median OS: 8.9 m [5.3‐12.0] vs 19.8 m [17.9‐NA], P = 0.005; HR and 95%CI: 0.16 [0.05‐0.51]; Median PFS: 2.4 m [1.4‐5.6] vs 6.9 m [3.7‐NA], P = 0.006, HR and 95%CI: 0.34 [0.15‐0.76]; Figure 1A,B).
Figure 1.
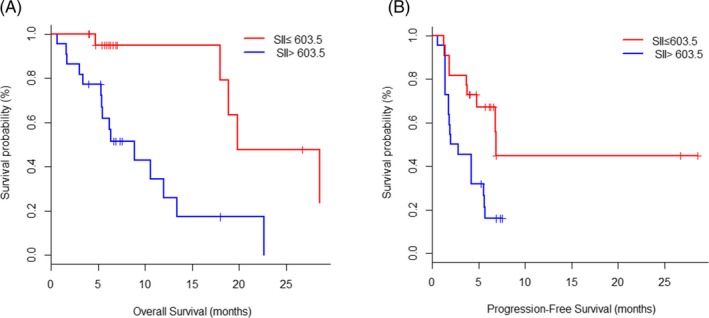
Kaplan‐Meier plots of overall survival (A) and progression‐free survival (B) according to systemic immune‐inflammation index (SII) at baseline
The optimal cutoff value of NLR predicted PFS and OS was 3.07, the sensitivity of this point was 0.81, the specificity was 0.73, and the AUC was 0.84. Patients were divided into two groups according to the optimum cutoff value of NLR, 24 patients in low NLR group (NLR ≤ 3.07) and 20 patients in high NLR group (NLR ˃ 3.07). Patients in low NLR group before treatment had longer OS and PFS compared with high NLR group (median OS: 8.9 m [3.4‐13.4] vs 19.8 m [17.9‐NA], P = 0.002; HR and 95%CI: 0.20 [0.06‐0.62]; Median PFS: 3.9 m [1.4‐5.6] vs 6.7 m [2.7‐NA], P = 0.048; HR and 95%CI: 0.46 [0.22‐0.99]; Figure 2A,B).
Figure 2.
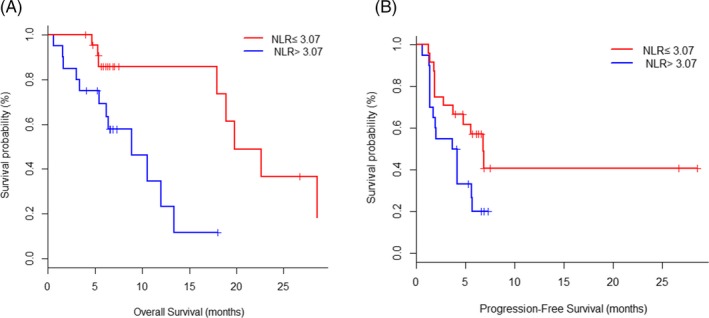
Kaplan‐Meier plots of overall survival (A) and progression‐free survival (B) according to neutrophil‐to‐lymphocyte ratio (NLR) at baseline
The optimal cutoff value of PLR predicted PFS and OS was 144, the sensitivity of this point was 0.67, the specificity was 0.65, and the AUC was 0.67. Patients were divided into two groups according to the optimum cutoff value of PLR, 18 patients in low PLR group (PLR ≤ 144) and 26 patients in high PLR group (PLR˃144). Patients in low PLR group before treatment had longer OS and PFS compared with high PLR group (median OS: 10.5 m [6.2‐17.9] vs 28.5 m [19.8‐NA], P = 0.008; HR and 95%CI: 0.20 [0.06‐0.73]; Median PFS: 3.9 m [1.7‐5.6] vs 6.9 m [3.7‐NA], P = 0.025; HR and 95%CI: 0.39 [0.17‐0.94]; Figure 3A,B).
Figure 3.
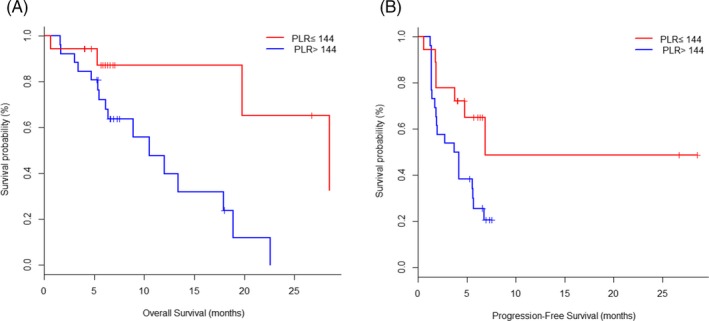
Kaplan‐Meier plots of overall survival (A) and progression‐free survival (B) according to platelet‐to‐lymphocyte ratio (PLR) at baseline
3.4. The association between hematological inflammatory parameters and irAEs
A total of 15 (34.1%) patients developed irAEs. Grade and duration of irAEs for different treatment regimens are shown in Table 2.
Table 2.
Summary of irAE (N = 44)
| irAE | Nivolumab |
Total N (%) |
|
|---|---|---|---|
|
Grade1‐2 N (%) |
Grade ≥ 3 N (%) |
||
| Skin | |||
| Rash | 2 (4.5) | 0 | 2 (4.5) |
| Dermatitis | 1 (2.3) | 0 | 1 (2.3) |
| Pneumonia | 1 (2.3) | 1 (2.3) | 2 (4.5) |
| Endocrine | |||
| Hyperthyroidism | 5 (11.4) | 0 | 5 (11.4) |
| Hypothyroidism | 6 (13.6) | 0 | 6 (13.6) |
| Pancreatic toxicity | |||
| Lipase increase | 2 (4.5) | 0 | 2 (4.5) |
| Amylase increase | 2 (4.5) | 0 | 2 (4.5) |
| Liver toxicity | |||
| ALT increase | 2 (4.5) | 0 | 2 (4.5) |
| AST increase | 2 (4.5) | 0 | 2 (4.5) |
| GGT increase | 2 (4.5) | 0 | 2 (4.5) |
| Others | |||
| Thirsty | 1 (2.3) | 0 | 1 (2.3) |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, gamma‐glutamyl transpeptidase; irAEs, immune‐related adverse events.
The most common irAEs were hypothyroidism (n = 6, 13.6%) followed by hyperthyroidism (n = 5, 11.4%). Grade 3 or higher irAE was observed in 1 case (2.3%), which was pneumonia. irAE leading to discontinuation were reported in 2 cases, with 1 case of grade 2 pneumonia, and 1 case of grade 2 AST elevation. No irAE‐related deaths occurred.
The relationship between SII/NLR/PLR and irAE was also analyzed, but we found there were no statistically significant. (For SII and irAE: P = 0.738; For NLR and irAE: P = 0.665; For PLR and irAE: P = 0.814).
3.5. Univariate and multivariate analyses
Univariate and multivariate analyses of PFS and OS were performed using COX regression model, and factors considered included age, gender, smoking status, pathological typing, disease stage, pretreatment NLR level, pretreatment PLR level, and pretreatment SII level. In univariate analysis, we found that NLR ≤ 3.07 before treatment, PLR ≤ 144 before treatment, and SII ≤ 603.5 before treatment were associated with longer PFS and OS (Tables 3 and 4).
Table 3.
Univariate and multivariate analysis of PFS
| Characteristics | HR for PFS (95% CI) | |||
|---|---|---|---|---|
| Univariate | P | Multivariate | P | |
|
Age at diagnosis (>65 vs ≤65) |
0.59 (0.18‐1.97) | 0.361 | 0.50 (0.13‐1.84) | 0.293 |
|
Gender (female vs male) |
1.06 (0.45‐2.52) | 0.892 | 0.98 (0.37‐2.56) | 0.974 |
|
Smoking history (ever vs never) |
0.82 (0.37‐1.79) | 0.620 | 0.63 (0.27‐1.50) | 0.298 |
| Histology (squamous vs adenocarcinoma) | 1.56 (0.71‐3.40) | 0.278 | 1.23 (0.51‐2.98) | 0.640 |
| Stage (IIIB vs IV) | 0.94 (0.38‐2.32) | 0.888 | 1.29 (0.48‐3.49) | 0.615 |
| NLR ≤ 3.07 vs >3.07 | 0.46 (0.22‐0.99) | 0.048 | 0.38 (0.17‐0.90) | 0.027 |
| PLR ≤ 144 vs >144 | 0.39 (0.17‐0.94) | 0.025 | 0.33 (0.13‐0.85) | 0.021 |
| SII ≤ 603.5 vs >603.5 | 0.34 (0.15‐0.76) | 0.006 | 0.23 (0.09‐0.60) | 0.003 |
Table 4.
Univariate and multivariate analysis of OS
| Characteristics | HR for OS (95% CI) | |||
|---|---|---|---|---|
| Univariate | P | Multivariate | P | |
|
Age at diagnosis (>65 vs ≤65) |
0.80 (0.23‐2.79) | 0.716 | 0.53 (0.12‐2.30) | 0.395 |
|
Gender (female vs male) |
0.56 (0.19‐1.70) | 0.284 | 0.49 (0.15‐1.64) | 0.249 |
|
Smoking history (ever vs never) |
1.22 (0.47‐3.21) | 0.677 | 0.91 (0.32‐2.54) | 0.851 |
| Histology (squamous vs adenocarcinoma) | 1.37 (0.55‐3.44) | 0.506 | 1.04 (0.36‐3.01) | 0.949 |
| Stage (IIIB vs IV) | 0.83 (0.31‐2.21) | 0.711 | 0.85 (0.28‐2.59) | 0.775 |
| NLR ≤ 3.07 vs >3.07 | 0.20 (0.06‐0.62) | 0.002 | 0.18 (0.05‐0.60) | 0.005 |
| PLR ≤ 144 vs >144 | 0.20 (0.06‐0.73) | 0.008 | 0.13 (0.03‐0.60) | 0.009 |
| SII ≤ 603.5 vs >603.5 | 0.16 (0.05‐0.51) | 0.005 | 0.13 (0.03‐0.47) | 0.002 |
In multivariate analysis, in order to avoid the multicollinearity among NLR, PLR, and SII, we established three independent COX regression models, respectively, and only one of the three indicators was included in each test. The results revealed that NLR ≤ 3.07, PLR ≤ 144, SII ≤ 603.5 were independently associated with longer PFS and OS (Tables 3 and 4).
4. DISCUSSION
ICIs have become one of the important treatment strategies for NSCLC. Inflammatory cells have important effects on tumor development and systemic inflammation markers can be of use in determining prognosis.34 In this study, we found that SIIs, NLR, PLR were significantly associated with the prognosis of metastatic NSCLC patients treated with nivolumab for second‐line or further‐line treatment.
Inflammation is regarded as an important factor in tumor progression and is one of the hallmarks of cancer.35 In addition, inflammation can supply the tumor microenvironment with bioactive molecules and the products of inflammatory processes can be considered as potential biomarkers.36, 37, 38 Numerous studies have elucidated in hematological markers, the NLR and PLR can reflect inflammation and host immune reaction, high pretreatment NLR and/or PLR level are potential prognostic predictor for poor PFS and OS in RCC,26 melanoma,39 gastric cancer40 and NSCLC patients received ICIs,27, 41, 42 some meta‐analysis43, 44 results also demonstrated this conclusion. The results of our analysis further confirm that pretreatment NLR and PLR are the prognostic factors for NSCLC, low NLR and PLR is associated with better outcomes for ICIs. Previous studies reported the cutoff value of NSCLC patients treated with immunotherapy was 2.8‐5 and 169‐262, respectively. The cutoff values selected in our study were NLR = 3.07, PLR = 144, which is close to the value in previous studies.
Although NLR and PLR can help evaluate the prognosis of ICI treatment, however, these two indexes only integrate two cell types. SII is a new composite measure of the neutrophil, lymphocyte, and platelet counts in the peripheral blood and significantly associated with prognosis in metastatic NSCLC. SII also has been confirmed to be more promising than NLR or PLR.45, 46, 47, 48 De Giorgi et al30 found that SII is one of the critical prognostic factors for OS in patients with RCC treated with nivolumab. Lower ORR and DCR were associated with higher values of SII at baseline and SII ≥ 1375 can independently predicted OS. Our results also confirm that in patients with metastatic NSCLC, low SII have longer PFS and OS after nivolumab treatment. But Putzu et al27 showed that SII at 6 weeks was significantly correlated only with PFS, but SII at baseline was not. This conclusion is in contrast with our finding. Further analysis of the reasons may be due to racial differences or different analysis methods. In our study, we performed ROC analysis to determine cutoffs value of SII, but Putzu et al used the median. Therefore, we suggest that cutoff values of SII at baseline may be more prognostic than median. We calculated the best cutoff value of SII to be 603.5, further verification is needed in future studies. Compared with PD‐L1 and TMB, these three hematological parameters are the most cost‐effective and easily obtained in clinical practice.
To our knowledge, there are no data on the correlation between baseline SII and the efficacy of nivolumab in NSCLC patients. We have confirmed this association in NSCLC for the first time. Although some investigators proposed that immune‐modified RECIST (imRECIST) criteria may better identify patients with survival benefit than RECIST criteria.49 But we have used RECIST v1.1 to reflected efficacy and survival benefit due to the key clinical studies of nivolumab such as Checkmate017 and Checkmate 057 are both used this evaluation method. In addition, challenges remain for advancing the broad utility of imRECIST because this conclusion was derived from post hoc and need to be further validated. Furthermore, our study had several limitations mainly due small cohort size and retrospective design, which may need further verified by prospective study with adequate sample sizes.
5. CONCLUSIONS
Our study demonstrated that at baseline, SII, NLR, and PLR are an independent prognostic predictor in advanced NSCLC patients with the efficacy of nivolumab. These results also offer potential predictive biomarkers and cutoff values to be explored further. In the future, these hematologic parameters could also be used to help stratify patients in randomized studies of ICIs.
ETHICAL APPROVAL
For this type of study, formal consent is not required.
ACKNOWLEDGMENTS
The authors thank all patients who participated in this study.
Liu J, Li S, Zhang S, et al. Systemic immune‐inflammation index, neutrophil‐to‐lymphocyte ratio, platelet‐to‐lymphocyte ratio can predict clinical outcomes in patients with metastatic non‐small‐cell lung cancer treated with nivolumab. J Clin Lab Anal. 2019;33:e22964 10.1002/jcla.22964
Jingjing Liu and Shuang Li equally contributed to this study.
Funding information
This study was funded by Science and Technology Development Project of Jilin Provincial Department of Science and Technology Commission (Grant Number 20170622005JC).
REFERENCES
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394‐424. [DOI] [PubMed] [Google Scholar]
- 2. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA: Cancer J Clin. 2016;66(2):115‐132. [DOI] [PubMed] [Google Scholar]
- 3. Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous‐cell non‐small‐cell lung cancer. N Engl J Med. 2015;373(2):123‐135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Borghaei H, Paz‐Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non‐small‐cell lung cancer. N Engl J Med. 2015;373(17):1627‐1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non‐small‐cell lung cancer. N Engl J Med. 2015;372(21):2018‐2028. [DOI] [PubMed] [Google Scholar]
- 6. Herbst RS, Baas P, Kim D‐W, et al. Pembrolizumab versus docetaxel for previously treated, PD‐L1‐positive, advanced non‐small‐cell lung cancer (KEYNOTE‐010): a randomised controlled trial. Lancet (London, England). 2016;387(10027):1540‐1550. [DOI] [PubMed] [Google Scholar]
- 7. Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non‐small‐cell lung cancer (OAK): a phase 3, open‐label, multicentre randomised controlled trial. Lancet (London, England). 2017;389(10066):255‐265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Langer CJ, Gadgeel SM, Borghaei H, et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non‐squamous non‐small‐cell lung cancer: a randomised, phase 2 cohort of the open‐label KEYNOTE‐021 study. Lancet Oncol. 2016;17(11):1497‐1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gandhi L, Rodríguez‐Abreu D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non‐small‐cell lung cancer. N Engl J Med. 2018;378(22):2078‐2092. [DOI] [PubMed] [Google Scholar]
- 10. Paz‐Ares L, Luft A, Vicente D, et al. Pembrolizumab plus chemotherapy for squamous non‐small‐cell lung cancer. N Engl J Med. 2018;379(21):2040‐2051. [DOI] [PubMed] [Google Scholar]
- 11. Hellmann MD, Ciuleanu T‐E, Pluzanski A, et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med. 2018;378(22):2093‐2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for first‐line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378(24):2288‐2301. [DOI] [PubMed] [Google Scholar]
- 13. Havel JJ, Chowell D, Chan TA. The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nat Rev Cancer. 2019;19(3):133‐150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer‐related inflammation. Nature. 2008;454(7203):436‐444. [DOI] [PubMed] [Google Scholar]
- 15. Peng F, Hu D, Lin X, et al. The monocyte to red blood cell count ratio is a strong predictor of postoperative survival in colorectal cancer patients: the Fujian prospective investigation of cancer (FIESTA) study. J Cancer. 2017;8(6):967‐975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zou ZY, Liu HL, Ning N, Li SY, Du XH, Li R. Clinical significance of pre‐operative neutrophil lymphocyte ratio and platelet lymphocyte ratio as prognostic factors for patients with colorectal cancer. Oncol Lett. 2016;11(3):2241‐2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yodying H, Matsuda A, Miyashita M, et al. Prognostic significance of neutrophil‐to‐lymphocyte ratio and platelet‐to‐lymphocyte ratio in oncologic outcomes of esophageal cancer: a systematic review and meta‐analysis. Ann Surg Oncol. 2016;23(2):646‐654. [DOI] [PubMed] [Google Scholar]
- 18. Mei Z, Shi LU, Wang BO, et al. Prognostic role of pretreatment blood neutrophil‐to‐lymphocyte ratio in advanced cancer survivors: a systematic review and meta‐analysis of 66 cohort studies. Cancer Treat Rev. 2017;58:1‐13. [DOI] [PubMed] [Google Scholar]
- 19. Yang JJ, Hu ZG, Shi WX, Deng T, He SQ, Yuan SG. Prognostic significance of neutrophil to lymphocyte ratio in pancreatic cancer: a meta‐analysis. World J Gastroenterol. 2015;21(9):2807‐2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Feng JF, Huang Y, Chen QX. Preoperative platelet lymphocyte ratio (PLR) is superior to neutrophil lymphocyte ratio (NLR) as a predictive factor in patients with esophageal squamous cell carcinoma. World J Surg Oncol. 2014;12:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Templeton AJ, Ace O, McNamara MG, et al. Prognostic role of platelet to lymphocyte ratio in solid tumors: a systematic review and meta‐analysis. Cancer Epidemiol Biomarkers Prev. 2014;23(7):1204‐1212. [DOI] [PubMed] [Google Scholar]
- 22. Stotz M, Gerger A, Eisner F, et al. Increased neutrophil‐lymphocyte ratio is a poor prognostic factor in patients with primary operable and inoperable pancreatic cancer. Br J Cancer. 2013;109(2):416‐421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Guo W, Cai S, Zhang F, et al. Systemic immune‐inflammation index (SII) is useful to predict survival outcomes in patients with surgically resected non‐small cell lung cancer. Thoracic Cancer. 2019;10(4):761‐768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yilmaz U, Ozdemir O, Batum O, Ermin S. The prognostic role of neutrophil‐lymphocyte ratio and platelet‐lymphocyte ratio in patients with stage III non‐small cell lung cancer treated with concurrent chemoradiotherapy. Indian J Cancer. 2018;55(3):276‐281. [DOI] [PubMed] [Google Scholar]
- 25. Amaral SR, Casal Moura M, Carvalho J, Chaves A, Jesus E, Sousa G. 6PPrognostic significance of neutrophil‐to‐lymphocyte ratio (NLR) and platelet‐to‐lymphocyte ratio (PLR) in non‐small cell lung cancer (NSCLC) treated with immune checkpoint inhibitors. Ann Oncol. 2019;30(Suppl. 1):mdz027.004. [Google Scholar]
- 26. Bilen MA, Dutcher G, Liu Y, et al. Association between pretreatment neutrophil‐to‐lymphocyte ratio and outcome of patients with metastatic renal‐cell carcinoma treated with nivolumab. Clin Genitourin Cancer. 2018;16(3):e563‐e575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Putzu C, Cortinovis DL, Colonese F, et al. Blood cell count indexes as predictors of outcomes in advanced non‐small‐cell lung cancer patients treated with Nivolumab. Cancer Immunol Immunother. 2018;67(9):1349‐1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ho WJ, Yarchoan M, Hopkins A, Mehra R, Grossman S, Kang H. Association between pretreatment lymphocyte count and response to PD1 inhibitors in head and neck squamous cell carcinomas. J ImmunoTherapy Cancer. 2018;6(1):84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fest J, Ruiter R, Mulder M, et al. The systemic immune‐inflammation index is associated with an increased risk of incident cancer‐a population‐based cohort study. Int J Cancer. 2019. 10.1002/ijc.32303. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. De Giorgi U, Procopio G, Giannarelli D, et al. Association of systemic inflammation index and body mass index with survival in patients with renal cell cancer treated with nivolumab. Clin Cancer Res. 2019. 10.1158/1078-0432.CCR-18-3661. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 31. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer (Oxford, England: 1990). 2009;45(2):228‐247. [DOI] [PubMed] [Google Scholar]
- 32. Weber JS, Hodi FS, Wolchok JD, et al. Safety profile of nivolumab monotherapy: a pooled analysis of patients with advanced melanoma. J Clin Oncol. 2017;35(7):785‐792. [DOI] [PubMed] [Google Scholar]
- 33. Sato K, Akamatsu H, Murakami E, et al. Correlation between immune‐related adverse events and efficacy in non‐small cell lung cancer treated with nivolumab. Lung Cancer (Amsterdam, Netherlands). 2018;115:71‐74. [DOI] [PubMed] [Google Scholar]
- 34. Anastasiou D. Tumour microenvironment factors shaping the cancer metabolism landscape. Br J Cancer. 2017;116(3):277‐286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhu H, Cao X. NLR members in inflammation‐associated carcinogenesis. Cell Mol Immunol. 2017;14(5):403‐405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860‐867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883‐899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5(4):263‐274. [DOI] [PubMed] [Google Scholar]
- 39. Capone M, Giannarelli D, Mallardo D, et al. Baseline neutrophil‐to‐lymphocyte ratio (NLR) and derived NLR could predict overall survival in patients with advanced melanoma treated with nivolumab. J ImmunoTherapy Cancer. 2018;6(1):74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ogata T, Satake H, Ogata M, et al. Neutrophil‐to‐lymphocyte ratio as a predictive or prognostic factor for gastric cancer treated with nivolumab: a multicenter retrospective study. Oncotarget. 2018;9(77):34520‐34527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Takeda T, Takeuchi M, Saitoh M, Takeda S. Neutrophil‐to‐lymphocyte ratio after four weeks of nivolumab administration as a predictive marker in patients with pretreated non‐small‐cell lung cancer. Thoracic Cancer. 2018;9(10):1291‐1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sekine K, Kanda S, Goto Y, et al. Change in the lymphocyte‐to‐monocyte ratio is an early surrogate marker of the efficacy of nivolumab monotherapy in advanced non‐small‐cell lung cancer. Lung Cancer (Amsterdam, Netherlands). 2018;124:179‐188. [DOI] [PubMed] [Google Scholar]
- 43. Tan Q, Liu S, Liang C, Han X, Shi Y. Pretreatment hematological markers predict clinical outcome in cancer patients receiving immune checkpoint inhibitors: a meta‐analysis. Thoracic Cancer. 2018;9(10):1220‐1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sacdalan DB, Lucero JA, Sacdalan DL. Prognostic utility of baseline neutrophil‐to‐lymphocyte ratio in patients receiving immune checkpoint inhibitors: a review and meta‐analysis. OncoTargets Therapy. 2018;11:955‐965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chen J‐H, Zhai E‐T, Yuan Y‐J, et al. Systemic immune‐inflammation index for predicting prognosis of colorectal cancer. World J Gastroenterol. 2017;23(34):6261‐6272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fu H, Zheng J, Cai J, et al. Systemic immune‐inflammation index (SII) is useful to predict survival outcomes in patients after liver transplantation for hepatocellular carcinoma within Hangzhou criteria. Cell Physiol Biochem. 2018;47(1):293‐301. [DOI] [PubMed] [Google Scholar]
- 47. Wang L, Wang C, Wang J, Huang X, Cheng Y. A novel systemic immune‐inflammation index predicts survival and quality of life of patients after curative resection for esophageal squamous cell carcinoma. J Cancer Res Clin Oncol. 2017;143(10):2077‐2086. [DOI] [PubMed] [Google Scholar]
- 48. Hu B, Yang X‐R, Xu Y, et al. Systemic immune‐inflammation index predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin Cancer Res. 2014;20(23):6212‐6222. [DOI] [PubMed] [Google Scholar]
- 49. Hodi FS, Ballinger M, Lyons B, et al. Immune‐modified response evaluation criteria in solid tumors (imRECIST): refining guidelines to assess the clinical benefit of cancer immunotherapy. J Clin Oncol. 2018;36(9):850‐858. [DOI] [PubMed] [Google Scholar]


