Abstract
Background
Sound knowledge of the abundance and distribution of intermediate host snails is key to understanding schistosomiasis transmission and to inform effective interventions in endemic areas.
Methods
A longitudinal field survey of freshwater snails of biomedical importance was undertaken in the Niger River Valley (NRV) between July 2011 and January 2016, targeting Bulinus spp. and Biomphalaria pfeifferi (intermediate hosts of Schistosoma spp.), and Radix natalensis (intermediate host of Fasciola spp.). Monthly snail collections were carried out in 92 sites, near 20 localities endemic for S. haematobium. All bulinids and Bi. pfeifferi were inspected for infection with Schistosoma spp., and R. natalensis for infection with Fasciola spp.
Results
Bulinus truncatus was the most abundant species found, followed by Bulinus forskalii, R. natalensis and Bi. pfeifferi. High abundance was associated with irrigation canals for all species with highest numbers of Bulinus spp. and R. natalensis. Seasonality in abundance was statistically significant in all species, with greater numbers associated with dry season months in the first half of the year. Both B. truncatus and R. natalensis showed a negative association with some wet season months, particularly August. Prevalences of Schistosoma spp. within snails across the entire study were as follows: Bi. pfeifferi: 3.45% (79/2290); B. truncatus: 0.8% (342/42,500); and B. forskalii: 0.2% (24/11,989). No R. natalensis (n = 2530) were infected. Seasonality of infection was evident for B. truncatus, with highest proportions shedding in the middle of the dry season and lowest in the rainy season, and month being a significant predictor of infection. Bulinus spp. and Bi. pfeifferi showed a significant correlation of snail abundance with the number of snails shedding. In B. truncatus, both prevalence of Schistosoma spp. infection, and abundance of shedding snails were significantly higher in pond habitats than in irrigation canals.
Conclusions
Evidence of seasonality in both overall snail abundance and infection with Schistosoma spp. in B. truncatus, the main intermediate host in the region, has significant implications for monitoring and interrupting transmission of Schistosoma spp. in the NRV. Monthly longitudinal surveys, representing intensive sampling effort have provided the resolution needed to ascertain both temporal and spatial trends in this study. These data can inform planning of interventions and treatment within the region.
Keywords: Schistosomiasis, Freshwater snails, Seasonality, Niger, Bulinus, Biomphalaria, Schistosoma, B. truncatus, B. forskalii, B. pfeifferi, R. natalensis, S. haematobium, S. bovis, S. mansoni
Background
Schistosomiasis is a neglected tropical disease (NTD) affecting over 200 million people worldwide, with an at-risk population estimated at 700 million [1]. It is caused by digenean trematodes of the genus Schistosoma. The complex indirect life-cycle involves an intermediate freshwater snail host and transmission is through water contact. The distribution of schistosomes directly relates to the geographical range of their intermediate snail hosts. This is influenced by factors including climate, altitude, rainfall, water chemistry and aquatic vegetation [2]. Schistosomiasis is present throughout West Africa, including Niger. Urogenital schistosomiasis is endemic in the Niger River Valley (NRV), and is caused by Schistosoma haematobium, the most widespread and prevalent human schistosome species across Africa, which displays often severe pathologies [3–6]. Schistosoma bovis, a pathogen of domestic livestock and some non-domestic artiodactyls [7] is also prevalent in the region [8–11]. Schistosoma haematobium and S. bovis have overlapping distributions across mainland Africa [12], and can infect several different species of the freshwater snail genus Bulinus as their intermediate hosts [13–15]. These two species of schistosome show evidence of hybridization in several West African countries, including Niger, complicating disease control [16–20]. In addition, the NRV has localised areas of intestinal schistosomiasis [21], which appear to be spreading (A. Garba, personal communication). Intestinal schistosomiasis is caused by Schistosoma mansoni, which infects over 83 million people across sub-Saharan Africa, the Middle East, parts of South America and some Caribbean islands [22, 23]; snail species of the genus Biomphalaria act as the intermediate host [24].
Together, the multiple snail and schistosome species result in a complex and persistent pattern of schistosomiasis transmission in Niger, influenced by the country’s geography. The Niger River, which crosses approximately 550 km of western Niger, is the country’s main water supply, critical in a country which is two-thirds desert [25]. The catchment is home to freshwater snail species of biomedical importance, including the pulmonate snails Bulinus truncatus, B. globosus, B. senegalensis, B. forskalii and Biomphalaria pfeifferi, all acting as hosts for Schistosoma spp., and Radix natalensis, a host for Fasciola spp. [24]. Studies have been undertaken in West Africa on how dam building has impacted schistosomiasis distributions by altering available habitat for freshwater snail intermediate hosts [23]. Some recent studies have shed light on abundance and distribution of these snail species at different spatial and temporal scales, both in the NRV and in sub-Saharan Africa as a whole [26–32]. However, substantial knowledge gaps remain. In addition, there is often a mismatch between intermediate host snail abundance and distribution and snail infection, let alone between snail infection levels and human schistosomiasis transmission [33–36]. For example, analysis of a recent outbreak of urogenital schistosomiasis in Corsica found evidence of ongoing transmission but no infected Bulinid snails [37]. It is not clear if this is due to insufficient sampling, the characteristic low levels of infection (and latent infection period) in snails, or if transmission is so patchy that there is little correlation of snail abundance and infection prevalence, and resulting transmission [38]. Schistosomiasis is highly focal, requiring overlap of intermediate and definitive hosts [24], and multiplication of the larval cercaria stage in snails can continue transmission even with very low snail infection prevalence [37]. Recent reviews therefore highlight the crucial importance of snail surveys to aid understanding of transmission, and to improve predictive modelling of future schistosomiasis distributions in relation to climate change and schistosomiasis control [39–41]. Currently there is a focus on longitudinal survey data across years, and critically, seasons, to characterize snail populations with more precision [42]. These surveys require accurate identification of snails and schistosome cercariae to provide high quality data to support treatment program activities and contribute to schistosomiasis knowledge more widely. The Schistosomiasis Consortium for Operational Research and Evaluation programme (SCORE, https://score.uga.edu) has recently undertaken studies to investigate and quantify the factors related to snail-human infection processes within the context of mass drug administration strategies in five African countries, including Niger [43]. Among other study goals, the programme aimed to address the gap in longitudinal abundance and distribution data for intermediate freshwater snail hosts to help inform public health planning for schistosomiasis control within Niger. Here, we report on longitudinal surveys, carried out in the context of the SCORE studies, for several species of freshwater snails of biomedical importance in the Niger River Valley. Specifically, this study conducted surveys to identify Schistosoma spp. transmission sites and to determine the intermediate snail hosts (particularly Bulinus spp.) at these sites and the parameters that influence intermediate snail host abundance and disease transmission potential.
Methods
Study region
The Niger River Valley has a Sahelian ecotone and climate. Highly seasonal, the first half of the year has very low or no rainfall, but flooding is frequent during the rainy season in the second half of the year. The study area transects different (broad) ecological regions, the Bassin des Dallois on the east side of the river, (a relatively more productive zone), the Liptako Sahel on the northwest side and the Plateau Goumantche on the southwest [25]. Other notable features of the study region are extensive rice growing areas supported by irrigation canal systems along the river, and the Kandadji Dam north of Tillaberi, which has been under construction since 2008 [21, 44].
Surveys
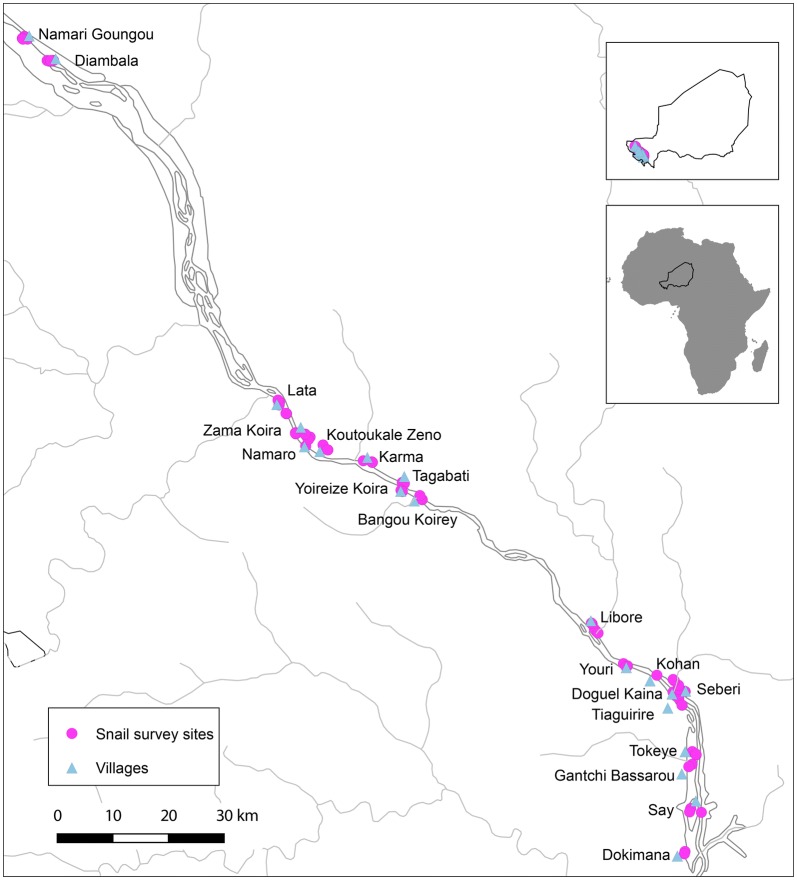
Monthly snail surveys were undertaken between July 2011 and January 2016 in 92 potential transmission sites, near 20 villages associated with human schistosomiasis (Table 1, Additional file 1: Table S1). Sites were surveyed every month (apart from April in 2014 and 2015 due to logistical reasons). Site selection was based on local knowledge of water contact sites and snail presence. Altogether, 16 villages were from the wider SCORE programme and four from an earlier study, CONTRAST [45], including two northern villages, Namari Goungou and Diambala, mixed infection foci with evidence of S. mansoni [44, 46]. Villages here are written as localities as additional villages not included in the study are often close by. Most study sites are within approximately 60 km up or downstream of Niamey, apart from these two northerly localities which are approximately double that distance from Niamey (Fig. 1). The survey covered a range of site types including irrigation canals, both concrete-lined secondary canals, which draw water directly from the river, and the smaller dirt-lined tertiary canals, branching off secondary canals to deliver water to rice paddies; the rice paddies themselves, the river (shallows of the main body of the Niger River), rivulets (small streams), ponds, and spillways (floodplain of tributaries feeding into the river) (Fig. 2). One stream site (at Say) was also surveyed but as it was the only one of its type, was excluded from final analysis. Irrigation canals were the most sampled sites, because of prior knowledge of high Bulinus spp. densities there from the earlier CONTRAST studies. Snail species surveyed were from the genera Bulinus and Biomphalaria (both Planorbidae), and Radix (Lymnaeidae). Species included Bulinus truncatus and B. globosus, which have a degree of morphological overlap [47], B. forskalii and B. senegalensis (again with some morphological overlap), Biomphalaria pfeifferi and Radix natalensis. Sites were examined for snails by two collectors, scooping and examining vegetation for approximately 15-min intervals per site. If abundance was very low, the interval was doubled to 30 min. At each site, GPS was recorded on a handheld Global Positioning System GPS (Garmin eTrex, Taiwan), and water chemistry including pH, total dissolved solids (TDS), and conductivity were recorded during site visits (apart from 2015 due to equipment failure) on a handheld water meter (Thermo Scientific Eutech Multiparameter PCTEST35K, Fisher Scientific UK Ltd., Loughborough, UK). Other relevant parameters such as water temperature, estimated water flow and depth were also recorded. All collected snails were taken back to the laboratory and morphologically identified to the species level, and the number of each species per site was recorded. Weather station data were acquired from the Niamey Weather Station (ID 61052), and downloaded from the USDA Foreign Agricultural Service weather meteorological office (WMO) pecad site in October, 2018 (https://gis.pecad.fas.usda.gov/WmoStationExplorer/).
Table 1.
Snail survey site list, showing totals of sites by site type and locality/village
| District | Locality | Canal 2 | Canal 3 | Pond | Rice | River | Rivulet | Spillway | n |
|---|---|---|---|---|---|---|---|---|---|
| Kollo | Bangou Koirey | 3 | 3 | ||||||
| Tillaberi | Diambala | 3 | 4 | 7 | |||||
| Say | Doguel Kaina | 1 | 1 | 1 | 1 | 1 | 5 | ||
| Say | Dokimana | 2 | 2 | ||||||
| Say | Gantchi Bassarou | 1 | 1 | 2 | |||||
| Kollo | Karma | 1 | 2 | 1 | 1 | 1 | 6 | ||
| Say | Kohan Garantche | 1 | 1 | ||||||
| Say | Koutoukale Zeno | 2 | 2 | 1 | 5 | ||||
| Kollo | Lata Kabia | 3 | 2 | 1 | 1 | 1 | 8 | ||
| Kollo | Libore | 6 | 2 | 2 | 10 | ||||
| Tillaberi | Namari Goungou | 2 | 4 | 6 | |||||
| Kollo | Namaro | 1 | 1 | 2 | 1 | 5 | |||
| Say | Say | 1 | 1 | 1 | 3 | ||||
| Kollo | Seberi | 4 | 2 | 6 | |||||
| Kollo | Tagabati | 2 | 1 | 3 | |||||
| Kollo | Tiaguirire | 1 | 1 | 1 | 3 | ||||
| Kollo | Tokeye | 2 | 1 | 1 | 4 | ||||
| Kollo | Yoreize Koira | 1 | 2 | 1 | 4 | ||||
| Kollo | Youri | 1 | 1 | 1 | 3 | ||||
| Kollo | Zama Koira Tegui | 2 | 2 | 1 | 1 | 6 | |||
| 30 | 26 | 8 | 10 | 8 | 7 | 3 | 92 |
Notes: Canal 2, secondary irrigation canal; Canal 3, tertiary irrigation canal; rice, rice paddy; n, total number of sites for a given locality/village
Fig. 1.
Map of sampling localities showing locations of snail survey sites and villages in the study
Fig. 2.

Examples of some of the site types surveyed. a Rice paddy. b River. c Tertiary irrigation canal. d Pond. e Secondary irrigation canal. f Spillway. Photo credit Amadou Garba
Checking for snail infection status
All snails were individually placed in clean freshwater in a well within a 12-well microtiter plate, exposed to light and checked for the shedding of cercariae at different intervals; first, one to three days after collection, and additionally at two weeks to one month post-collection [2]. Snails were kept in the wells for several hours while shedding was induced, then placed in aquaria for the interim period between shedding attempts. Snails were inspected under a stereo-microscope for shedding of schistosome cercariae, which were identified by using a key [48]. If shedding Schistosoma spp. cercariae, snails were recorded as positive for patent infection (hereafter, referred to as ‘shedding’). The cercariae were individually collected by micro-pipette in 3–5 µl of water and preserved on Whatman FTA cards (GE Healthcare Life Sciences, Buckinghamshire, UK) for any future molecular identification [49]. All snails were preserved in 100% ethanol to allow future molecular analysis. Both the snail voucher specimens and cercariae were archived with their contextual data in the Schistosomiasis Collection at the Natural History Museum, SCAN [50].
Statistical analysis
All data analysis was undertaken in R version 3.5.3 “Great Truth” [51] and R-Studio [52]. All statistical tests were conducted with the significance level α = 0.05 for rejecting null hypotheses. Exploratory data analysis was performed to identify broad scale spatial and/or temporal trends, and test statistical assumptions, following Zuur et al. [53]. This revealed high collinearity between water conductivity and TDS, and we proceeded to include only the former variable in the following models.
Variation in snail counts was analysed using generalized linear mixed effect models (GLMM) in the package glmmTMB [54]. This package efficiently fits negative binomial models that can account for overdispersed count data, while also allowing for arguments to account for zero inflation, if needed [55]. The fit of all constructed models was investigated visually and statistically using a simulation-based approach in the package DHARMa [56]. Models with good fit showed no significant deviation in the QQ plot of simulated residuals, and passed a non-parametric dispersion test (function ‘testDispersion()’). In all count models, a negative binomial distribution showed an improved fit over the Poisson distribution, and was therefore chosen as the family. Zero inflation, which assumes a mix of structural and sampling zero data, was initially considered as initial data exploration revealed a high proportion of zeros in the data, which can indicate zero inflation, or overdispersion relative to the Poisson distribution [55]. However, we did not find sufficient reason to consider zeros as structural, and zero inflation tests in DHARMa (function: ‘testZeroInflation()’) did not show statistical support that data were zero-inflated. We therefore did not include terms for zero inflation in the models. Since sampling sites were not spatially independent and could show variation in intercepts due to varying initial snail abundance, we included sites as a random intercept term for count models, nested in locality to reflect the sampling structure. To account for the temporal pseudoreplication caused by repeatedly measuring over time, we included the collection date as a random intercept term. Sampling duration varied, and was therefore included as an offset in the model. Fixed main effects were included if they were ecologically meaningful potential influences on snail abundances, totals of shedding snails, or prevalence of Schistosoma spp. infection within the snails (proportion of snails shedding Schistosoma spp. as a percentage of the total). We did not conduct stepwise single term deletion on the maximal model, due to the issues associated with model simplification [57, 58]. Models investigating overall Bulinus spp. counts included water temperature, pH, water speed, water depth, water conductivity, precipitation, locality, site type and total number of shedding Bulinus spp. snails as fixed main effects, with interactions between site type and water temperature, pH, conductivity, and precipitation respectively. Models investigating counts on the species level of a given species included precipitation, site type, month, locality and total counts of the remaining species of snails as fixed main effects. Continuous variables were centred and scaled to the mean. Models investigating counts of shedding snails for B. truncatus, B. forskalii and Bi. pfeifferi included site type, month and locality as fixed main effects.
Variation in Schistosoma spp. prevalence in the snail hosts studied was analysed using simple generalized linear models, based on abundance data pooled by month. As here we were modelling proportional data, we chose a binomial distribution, with prevalence specified as the number of shedding snails divided by total snails per locality/timepoint, weighted in the model by the total number of snails. Significance of terms in all models were retrieved using the function ‘Anova.glmmTMB()’ for glmmTMB models, and function ‘Anova()’ from the package car [59] for glmm models. For models of shedding B. forskalii, terms were run separately because of convergence problems owing to small sample size. For some models also, localities with zero abundance were removed. For statistically significant terms, we conducted post-hoc tests using the emmeans package [60].
Results
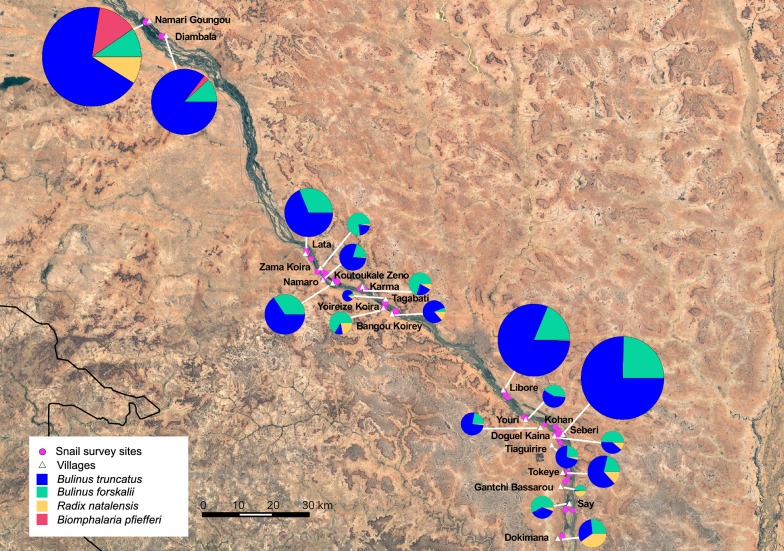
A total of 59,674 snails were found throughout the four and a half year study. Bulinus truncatus was the most abundant (n = 42,500), followed by B. forskalii (n = 11,989), R. natalensis (n = 2530) and Bi. pfeifferi (n = 2290) (Fig. 3, Table 2). Bulinus globosus and B. senegalensis were also present but found in low numbers (n = 290 and n = 76 in total, respectively, Table 2). Prevalences of Schistosoma spp. within snails across the entire study were as follows: Bi. pfeifferi: 3.45% (79/2290); B. truncatus: 0.8% (342/42,500); and B. forskalii: 0.2% (24/11,989). No R. natalensis (n = 2530) were infected.
Fig. 3.
Snail species by locality, pie charts scaled by proportion of total snails found. Data shown: collected field data (final dataset for analysis), not modelled counts. Apart from Koutoukale Zeno village, R. natalensis was found at all localities, although was evident in very low numbers at several localities, e.g. Seberi
Table 2.
Snail survey species data broken down by locality, site type and month
| n | Bul.r | Bul | Bul+ | BT.r | BT | BT+ | BT.p | BF.r | BF | BF+ | BF.p | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Locality | ||||||||||||
| Bangou Koirey | 55 | 8.2 | 453 | 10 | 7.4 | 406 | 10 | 2.46 | 0.6 | 31 | 0 | 0.00 |
| Diambala | 250 | 30.1 | 7529 | 32 | 26.0 | 6506 | 32 | 0.49 | 3.7 | 914 | 0 | 0.00 |
| Doguel Kaina | 134 | 5.3 | 704 | 6 | 2.2 | 296 | 6 | 2.03 | 3.0 | 397 | 0 | 0.00 |
| Dokimana | 50 | 12.1 | 606 | 14 | 5.8 | 290 | 13 | 4.48 | 6.3 | 316 | 1 | 0.32 |
| Gantchi Bassarou | 59 | 1.3 | 75 | 5 | 0.1 | 3 | 0 | 0.00 | 1.2 | 72 | 5 | 6.94 |
| Karma | 87 | 6.2 | 542 | 2 | 1.5 | 128 | 2 | 1.56 | 4.7 | 409 | 0 | 0.00 |
| Kohan Garantche | 35 | 7.3 | 254 | 0 | 5.1 | 179 | 0 | 0.00 | 2.1 | 75 | 0 | 0.00 |
| Koutoukale Zeno | 130 | 20.8 | 2708 | 5 | 13.8 | 1789 | 5 | 0.28 | 6.7 | 867 | 0 | 0.00 |
| Lata Kabia | 179 | 22.6 | 4046 | 10 | 15.6 | 2787 | 9 | 0.32 | 7.0 | 1259 | 1 | 0.08 |
| Libore | 270 | 30.8 | 8320 | 150 | 24.8 | 6694 | 143 | 2.14 | 5.8 | 1565 | 7 | 0.45 |
| Namari Goungou | 233 | 54.1 | 12,594 | 11 | 47.2 | 10,987 | 9 | 0.08 | 6.6 | 1530 | 1 | 0.07 |
| Namaro | 99 | 10.2 | 1009 | 3 | 8.1 | 798 | 2 | 0.25 | 2.1 | 211 | 1 | 0.47 |
| Say | 94 | 7.3 | 683 | 1 | 2.7 | 258 | 1 | 0.39 | 4.3 | 402 | 0 | 0.00 |
| Seberi | 255 | 43.6 | 11,123 | 46 | 35.0 | 8929 | 43 | 0.48 | 8.6 | 2194 | 3 | 0.14 |
| Tagabati | 52 | 3.0 | 155 | 0 | 2.7 | 142 | 0 | 0.00 | 0.3 | 13 | 0 | 0.00 |
| Tiaguirire | 69 | 9.7 | 670 | 0 | 7.2 | 496 | 0 | 0.00 | 2.4 | 163 | 0 | 0.00 |
| Tokoye | 112 | 13.1 | 1470 | 60 | 10.0 | 1117 | 57 | 5.10 | 3.2 | 354 | 3 | 0.85 |
| Yoreize Koira | 102 | 4.3 | 437 | 0 | 0.6 | 66 | 0 | 0.00 | 3.6 | 371 | 0 | 0.00 |
| Youri | 99 | 8.6 | 850 | 3 | 5.0 | 497 | 2 | 0.40 | 3.6 | 352 | 1 | 0.28 |
| Zama Koira Tegui | 132 | 4.7 | 626 | 9 | 1.0 | 132 | 8 | 6.06 | 3.7 | 494 | 1 | 0.20 |
| Total | 2496 | 22 | 54,854 | 367 | 17.0 | 42,500 | 342 | 0.80 | 4.8 | 11,989 | 24 | 0.20 |
| Site type | ||||||||||||
| Canal 2 | 924 | 30.4 | 28,094 | 137 | 25.3 | 23,362 | 131 | 0.56 | 5.0 | 4590 | 5 | 0.11 |
| Canal 3 | 715 | 28.5 | 20,349 | 114 | 22.0 | 15,715 | 112 | 0.71 | 6.3 | 4518 | 2 | 0.04 |
| Pond | 209 | 11.6 | 2422 | 81 | 6.4 | 1347 | 71 | 5.27 | 4.9 | 1024 | 10 | 0.98 |
| Rice | 215 | 7.7 | 1659 | 14 | 2.7 | 571 | 7 | 1.23 | 5.0 | 1078 | 7 | 0.65 |
| River | 208 | 5.3 | 1105 | 4 | 3.9 | 815 | 4 | 0.49 | 1.4 | 284 | 0 | 0.00 |
| Rivulet | 141 | 5.0 | 705 | 16 | 4.4 | 624 | 16 | 2.56 | 0.5 | 64 | 0 | 0.00 |
| Spillway | 84 | 6.2 | 520 | 1 | 0.8 | 66 | 1 | 1.52 | 5.1 | 431 | 0 | 0.00 |
| Total | 2496 | 22 | 54,854 | 367 | 17.0 | 42,500 | 342 | 0.80 | 4.8 | 11,989 | 24 | 0.20 |
| Month | ||||||||||||
| January | 232 | 17.0 | 3951 | 17 | 14.7 | 3405 | 16 | 0.47 | 2.3 | 536 | 1 | 0.19 |
| February | 224 | 27.5 | 6157 | 66 | 21.9 | 4895 | 64 | 1.31 | 5.6 | 1261 | 2 | 0.16 |
| March | 216 | 47.4 | 10,242 | 109 | 38.6 | 8330 | 100 | 1.20 | 8.9 | 1913 | 9 | 0.47 |
| April | 41 | 56.2 | 2305 | 20 | 45.9 | 1882 | 20 | 1.06 | 10.3 | 423 | 0 | 0.00 |
| May | 185 | 30.6 | 5652 | 21 | 24.4 | 4522 | 20 | 0.44 | 6.1 | 1130 | 1 | 0.09 |
| June | 173 | 32.3 | 5592 | 52 | 26.0 | 4502 | 51 | 1.13 | 5.2 | 895 | 1 | 0.11 |
| July | 227 | 17.1 | 3887 | 22 | 14.2 | 3221 | 18 | 0.56 | 2.6 | 595 | 4 | 0.67 |
| August | 197 | 12.0 | 2355 | 4 | 7.8 | 1543 | 3 | 0.19 | 4.0 | 797 | 1 | 0.13 |
| September | 215 | 17.6 | 3782 | 8 | 9.4 | 2026 | 6 | 0.30 | 7.9 | 1704 | 2 | 0.12 |
| October | 279 | 12.7 | 3551 | 18 | 8.7 | 2433 | 16 | 0.66 | 4.0 | 1118 | 1 | 0.09 |
| November | 232 | 15.7 | 3648 | 11 | 12.1 | 2810 | 11 | 0.39 | 3.6 | 838 | 0 | 0.00 |
| December | 275 | 13.6 | 3732 | 19 | 10.7 | 2931 | 17 | 0.58 | 2.8 | 779 | 2 | 0.26 |
| Total | 2496 | 22 | 54,854 | 367 | 17.0 | 42,500 | 342 | 0.80 | 4.8 | 11,989 | 24 | 0.20 |
| BG | BS | BP.r | BP | BP+ | BP.p | RN.r | RN | |
|---|---|---|---|---|---|---|---|---|
| Locality | ||||||||
| Bangou Koirey | 16 | 0 | 0.0 | 0 | 0 | 1.3 | 70 | |
| Diambala | 109 | 0 | 0.8 | 210 | 3 | 1.43 | 0.0 | 7 |
| Doguel Kaina | 11 | 0 | 0.0 | 0 | 0 | 0.6 | 78 | |
| Dokimana | 0 | 0 | 0.0 | 0 | 0 | 7.3 | 365 | |
| Gantchi Bassarou | 0 | 0 | 0.0 | 0 | 0 | 1.1 | 63 | |
| Karma | 5 | 0 | 0.0 | 0 | 0 | 0.6 | 51 | |
| Kohan Garantche | 0 | 0 | 0.0 | 0 | 0 | 0.1 | 3 | |
| Koutoukale Zeno | 0 | 52 | 0.0 | 0 | 0 | 0.0 | 0 | |
| Lata Kabia | 0 | 0 | 0.0 | 0 | 0 | 0.0 | 6 | |
| Libore | 61 | 0 | 0.0 | 0 | 0 | 0.1 | 32 | |
| Namari Goungou | 77 | 0 | 8.9 | 2080 | 76 | 3.65 | 6.1 | 1432 |
| Namaro | 0 | 0 | 0.0 | 0 | 0 | 0.1 | 12 | |
| Say | 0 | 23 | 0.0 | 0 | 0 | 0.0 | 1 | |
| Seberi | 0 | 0 | 0.0 | 0 | 0 | 0.0 | 1 | |
| Tagabati | 0 | 0 | 0.0 | 0 | 0 | 0.3 | 15 | |
| Tiaguirire | 11 | 0 | 0.0 | 0 | 0 | 0.3 | 23 | |
| Tokoye | 0 | 0 | 0.0 | 0 | 0 | 2.0 | 225 | |
| Yoreize Koira | 0 | 0 | 0.0 | 0 | 0 | 1.2 | 124 | |
| Youri | 0 | 1 | 0.0 | 0 | 0 | 0.1 | 6 | |
| Zama Koira Tegui | 0 | 0 | 0.0 | 0 | 0 | 0.1 | 16 | |
| Total | 290 | 76 | 0.9 | 2290 | 79 | 3.45 | 1.0 | 2530 |
| Site type | ||||||||
| Canal 2 | 142 | 0 | 2.0 | 1819 | 46 | 2.53 | 0.1 | 53 |
| Canal 3 | 116 | 0 | 0.7 | 471 | 33 | 7.01 | 2.2 | 1541 |
| Pond | 0 | 52 | 0.0 | 0 | 0 | 2.9 | 610 | |
| Rice | 10 | 0 | 0.0 | 0 | 0 | 0.8 | 168 | |
| River | 5 | 1 | 0.0 | 0 | 0 | 0.3 | 69 | |
| Rivulet | 17 | 0 | 0.0 | 0 | 0 | 0.6 | 88 | |
| Spillway | 0 | 23 | 0.0 | 0 | 0 | 0.0 | 1 | |
| Total | 290 | 76 | 0.9 | 2290 | 79 | 3.45 | 1.0 | 2530 |
| Month | ||||||||
| January | 10 | 0 | 1.1 | 262 | 10 | 3.82 | 0.6 | 134 |
| February | 0 | 1 | 2.9 | 656 | 13 | 1.98 | 2.5 | 554 |
| March | 0 | 0 | 3.0 | 646 | 10 | 1.55 | 4.0 | 870 |
| April | 0 | 0 | 2.0 | 80 | 5 | 6.25 | 9.0 | 368 |
| May | 0 | 0 | 0.8 | 154 | 5 | 3.25 | 0.8 | 156 |
| June | 195 | 0 | 0.6 | 102 | 9 | 8.82 | 0.8 | 137 |
| July | 0 | 71 | 0.1 | 15 | 2 | 13.33 | 0.3 | 74 |
| August | 11 | 4 | 0.1 | 16 | 0 | 0.00 | 0.1 | 23 |
| September | 52 | 0 | 0.4 | 78 | 2 | 2.56 | 0.1 | 15 |
| October | 0 | 0 | 0.1 | 25 | 2 | 8.00 | 0.1 | 20 |
| November | 0 | 0 | 0.9 | 206 | 17 | 8.25 | 0.1 | 25 |
| December | 22 | 0 | 0.2 | 50 | 4 | 8.00 | 0.6 | 154 |
| Total | 290 | 76 | 0.9 | 2290 | 79 | 3.45 | 1.0 | 2530 |
Abbreviations: n, total count i.e. total number of site visits per category (e.g. 84 records in total from site visits to spillway sites); Bul.r, total count divided by n; Bul, Bulinus spp. raw count; Bul+, Bulinus spp. total shedding i.e. positive for Schistosoma spp. infection; BT.r, B. truncatus count divided by n; BT, B. truncatus raw count, BT+, B. truncatus total positive for Schistosoma spp. infection; BT.p, B. truncatus prevalence; BF.r, B. forskalii count divided by n; BF, B. forskalii raw count; BF+, B. forskalii total positive for Schistosoma spp. infection; BF.p, B. forskalii prevalence; BG, B. globosus; BS, B. senegalensis; BP.r, Bi. pfeifferi total count divided by n; BP, Bi. pfeifferi raw count; BP+, Bi. pfeifferi positive for Schistosoma spp. infection; BP.p, Bi. pfeifferi prevalence; RN, R. natalensis; RN.r, total count divided by n
Effect of locality on snail abundance
Differences in abundances across localities was evident in all species surveyed (Fig. 3). Bulinus truncatus, most abundant at the majority of localities, showed an almost bimodal distribution of either very low abundances or relatively much higher numbers at a few localities: Namari Goungou, Seberi and Diambala (Fig. 4a). Locality was a highly significant predictor of abundance in B. truncatus (χ2 = 107, df = 18, P < 0.001, Table 3). Bulinus forskalii was found in lower numbers than B. truncatus, with a less variable distribution, but the differences across localities were still significant (χ2 = 34, df = 19, P < 0.001, Table 3). Seven localities had higher abundances of B. forskalii than B. truncatus, all with low numbers overall (Fig. 4a, Table 2). Locality was also a significant predictor of abundance in R. natalensis (χ2 = 46.4, df = 19, P < 0.001, Table 4). This species had low abundances overall, and was found in very low numbers at several sites and absent altogether from Koutoukale Zeno (Fig. 3). Biomphalaria pfeifferi was found only at Namari Goungou and Diambala, with higher abundance at the former; however, this difference was not significant (P = 0.09).
Fig. 4.
a Bulinus truncatus and B. forskalii modelled mean abundance by locality, a significant predictor of abundance for both species: B. truncatus (χ2 = 107, df = 18, P < 0.001); B. forskalii (χ2 = 34, df = 19, P < 0.001). b B. truncatus and B. forskalii modelled mean abundance by site type, a significant predictor of abundance for both species: B. truncatus (χ2 = 33.2, df = 6, P < 0.001); B. forskalii (χ2 = 27.8, df = 6, P < 0.001). c B. truncatus and B. forskalii modelled mean abundance by month, a significant predictor of abundance for both species: B. truncatus (χ2 = 85.4, df = 11, P < 0.001); B. forskalii (χ2 = 32.4, df = 11, P < 0.001. Bk Bangou Koirey, Di Diambala, Dk Doguel Kaina, Do Dokimana, Gb Gantchi Bassarou, Ka Karma, Kg Kohan Garantche, Kz Koutoukale Zeno, Li Libore, Lk Lata Kabia, Na Namaro, Ng Namari Goungou, Sa Say, Se Seberi, i Tagabati, Ti Tiaguirire, To Tokoye, I Yoreize Koira, Yo Youri, Zk Zama Koira Tegui, canal.2 secondary irrigation canal, canal.3 tertiary irrigation canal; rice rice paddy
Table 3.
Summary statistics of all glmmTMB models for B. truncatus and B. forskalii abundance, reporting χ2 df and P-values, all negative binomial apart from binomial for prevalence models
| Term | B. truncatus | BT positive | BT prevalence | B. forskalii | BF positive | BF prevalence | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| χ 2 | df | P | χ 2 | df | P | χ 2 | df | P | χ 2 | df | P | χ 2 | df | P | χ 2 | df | P | |
| Precipitation | 0.4 | 1 | 0.548 | 0.6 | 1 | 0.427 | ||||||||||||
| Site type | 33.2 | 6 | < 0.0001 | 15 | 6 | 0.02 | 92.9 | 6 | < 0.0001 | 27.8 | 6 | < 0.0001 | 4.3 | 7 | 0.742 | 9.5 | 7 | 0.219 |
| Locality | 107 | 18 | < 0.0001 | 41.7 | 19 | 0.002 | 139.4 | 13 | < 0.0001 | 34 | 19 | 0.018 | 2 | 19 | 1 | 13.5 | 9 | 0.139 |
| Month | 85.4 | 11 | < 0.0001 | 62.3 | 11 | 0 | 18.9 | 11 | 0.063 | 32.4 | 11 | 0.001 | 0.1 | 1 | 0.781 | 29.4 | 11 | 0.002 |
| BT positive total | 33.8 | 1 | < 0.0001 | |||||||||||||||
| BF positive total | 2.4 | 1 | 0.119 | |||||||||||||||
Key: Headers: names for given model, BT/BF positive: model of abundance of shedding snails for B. truncatus, B. forskalii and Bi. pfeifferi, respectively; BT/BF prevalence: model of Schistosoma spp. prevalence (proportion of snails shedding) in the given species; precipitation, WMO weather station precipitation data
Table 4.
Summary statistics of all glmmTMB models for B. pfeifferi and R. natalensis, reporting χ2, df and P-values, all negative binomial apart from binomial for prevalence model
| Term | Bi. pfeifferi | BP positive | BP prevalence | R. natalensis | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| χ 2 | df | P | χ 2 | df | P | χ 2 | df | P | χ 2 | df | P | |
| Precipitation | 0.4 | 1 | 0.527 | 0.1 | 1 | 0.787 | ||||||
| Site type | 3.1 | 1 | 0.079 | 0.5 | 1 | 0.486 | 7.1 | 1 | 0.008 | 15.7 | 6 | 0.016 |
| Locality | 3 | 1 | 0.086 | 8.2 | 1 | 0.004 | 5.1 | 1 | 0.023 | 46.4 | 19 | < 0.0001 |
| Month | 20.9 | 11 | 0.035 | 1 | 1 | 0.315 | 24.3 | 9 | 0.004 | 122 | 11 | < 0.0001 |
| BP positive total | 19.1 | 1 | < 0.0001 | |||||||||
Key: BP positive: model of abundance of shedding snails for Bi. pfeifferi; BP prevalence: model of Schistosoma spp. prevalence
Effect of site type on snail abundance
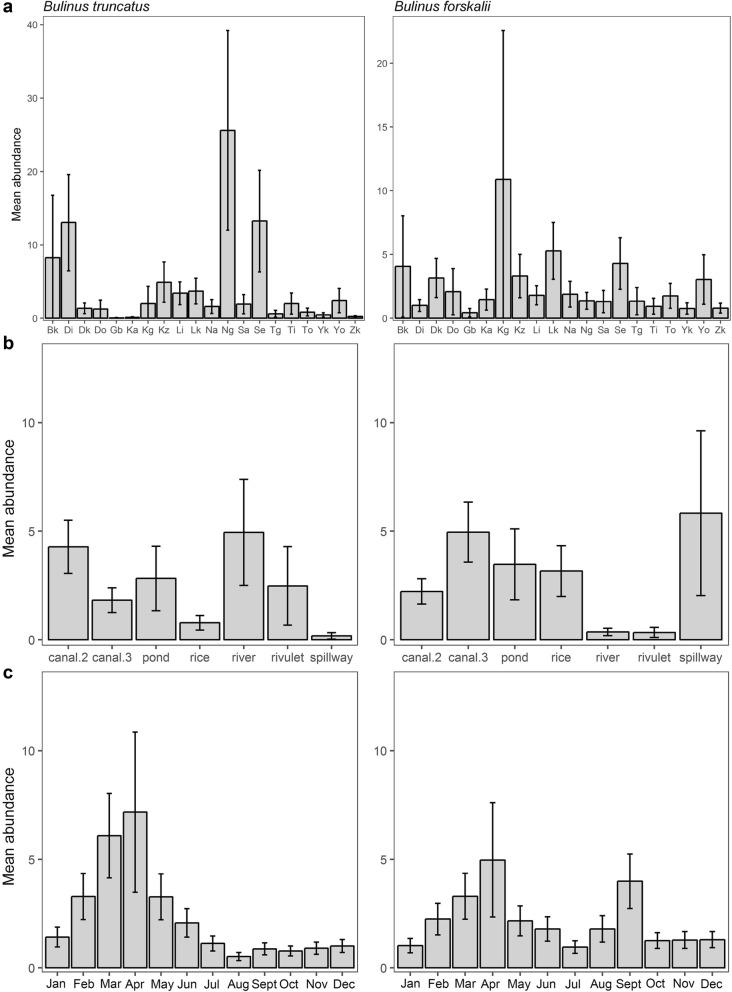
Bulinus truncatus was significantly more abundant in secondary than tertiary canals, and had lowest abundance in spillways and rice paddies. Site type was a significant predictor of abundance in B. truncatus (χ2 = 33.2, df = 6, P < 0.001, Fig. 4b, Additional file 1: Figure S1). In contrast, B. forskalii was more abundant in tertiary than secondary canals, and also had high abundance in pond, rice paddy and spillway sites (although the latter show very large standard errors due to large variation). River and rivulet sites had very low abundances. These differences were also significant (χ2 = 27.8, df = 6, P < 0.001). Radix natalensis were mostly found in tertiary canals, then ponds, with very low numbers in secondary canals, contrasting with both Bulinus species and Bi. pfeifferi. Site type was also a significant predictor of abundance (χ2 = 15.7, df = 6, P = 0.016). Biomphalaria pfeifferi was only found in tertiary and secondary canals, particularly the latter, but the difference was not significant (P = 0.08).
Effect of month on snail abundance
All species surveyed showed significant seasonality in abundance, particularly B. truncatus and R. natalensis. B. truncatus abundance had a significant positive association with dry season months February to May, and a significant negative association with the wet season month of August (Fig. 4c, Additional file 1: Table S2; note that large standard errors are evident for April because surveys were not undertaken for this month in 2014 and 2015). Month was a significant predictor of B. truncatus abundance (χ2 = 85.4, df = 11, P < 0.001). For the first half of the year, B. forskalii showed similar patterns to B. truncatus; however, later in the year there was an additional peak in abundance (Fig. 4c). Bulinus forskalii abundance showed a significant positive association with February through to May, and also the wet season month of September (Fig. 4c, Additional file 1: Table S3). Month was a significant predictor of B. forskalii abundance (χ2 = 32.4, df = 11, P = 0.001). Radix natalensis abundance showed significant positive associations with February through April, and a significant negative association for July to October (Additional file 1: Table S4). Radix natalensis had marked significant seasonal variation overall (χ2 = 121.7, df = 11, P < 0.001). In Bi. pfeifferi, although counts were more variable by month than either for R. natalensis or Bulinus spp., month was also a significant predictor of abundance (χ2 = 20.9, df = 11, P = 0.035, Additional file 1: Table S5).
Effect of water variables on snail abundance
Although significant seasonality on abundance in all species surveyed appeared correlated with precipitation, the effect of precipitation on snail abundance was not significant for either Bulinus spp. pooled or testing any species separately. However, there was a marginally significant interaction of precipitation and site type for Bulinus spp. abundance (χ2 = 18.7, df = 6, P = 0.05, Table 5), with a significant positive association for pond and rice paddy sites and negative for rivulets (Additional file 1: Table S6). A significant interaction of water temperature and site type with Bulinus spp. abundance was also evident (χ2 = 31.1, df = 6, P < 0.001), with significant negative associations for river and rivulet sites, and a significant positive association for spillways (i.e. higher temperatures in river and rivulet sites were associated with higher numbers of Bulinus, and vice versa for spillway; Additional file 1: Figure S2). An interaction of conductivity and site type with Bulinus abundance was significant (χ2 = 21, df = 6, P = 0.002), with ponds showing a significant positive association, and rivulets a negative association (Additional file 1: Table S6). Considering single terms, water speed had a significant negative association with Bulinus spp. abundance (χ2 = 14.6, df = 1, P < 0.001, Additional file 1: Figure S3), as did water depth (χ2 = 6.7, df = 1, P = 0.01). All water variable data are summarised in Additional file 1: Table S7.
Table 5.
Summary statistics of glmmTMB (negative binomial) model for Bulinus spp., reporting χ2, df and P-values
| χ 2 | df | P | |
|---|---|---|---|
| Water temperature | 0.4 | 1 | 0.552 |
| pH | 0.6 | 1 | 0.445 |
| Water speed (m/s) | 14.6 | 1 | 0.000 |
| Water depth (cm) | 6.7 | 1 | 0.010 |
| Conductivity | 5.6 | 1 | 0.018 |
| Precipitation | 1.3 | 1 | 0.252 |
| Site type | 9.9 | 6 | 0.131 |
| Bulinus positive tot | 30.6 | 1 | < 0.0001 |
| Month | 45.4 | 11 | < 0.0001 |
| Locality | 92.7 | 19 | 0.000 |
| Water temp × site type | 31.1 | 6 | 0.000 |
| pH × site type | 12.1 | 6 | 0.060 |
| Conductivity × site type | 21 | 6 | 0.002 |
| Precipitation × site type | 18.7 | 6 | 0.005 |
Key: Terms: Water temperature × site type, interaction of water temperature and site type; pH × site type, interaction of water temperature and site type; precipitation × site type, interaction of WMO weather station recorded precipitation and site type
Infection and prevalence
Overall, numbers of snails shedding Schistosoma spp. cercariae were very low for B. forskalii with a prevalence of Schistosoma spp. infection in the snail (proportion of snails that were shedding cercariae) of 0.2% (24/11,989 in total over the study period, overall range by locality 0–6.9%), also low in B. truncatus (0.8%, 342/42,500, range by locality 0–6.1%) and relatively high in Bi. pfeifferi (3.4%, 79/2290, range for Namari Goungou and Diambala respectively, 1.4–3.7%, Table 2). All R. natalensis were negative for Fasciola spp. infection. In the glmmTMB of Bulinus spp., the total snail abundance showed a significant positive association with the abundance/total number of infected snails shedding cercariae (χ2 = 30.6, df = 1, P < 0.001). In testing separately by species, for B. truncatus total abundance, the total number of shedding snails was also significant (χ2 = 33.8, df = 1, P < 0.001), and similarly for Bi. pfeifferi (χ2 = 19.1, df = 1, P < 0.001), but not for B. forskalii.
Effect of locality on prevalence of Schistosoma spp. infection in snails and on abundance of shedding snails
Locality was a significant predictor for both proportion of B. truncatus that were shedding (χ2 = 139.4, df = 13, P < 0.001), and for total abundance of shedding B. truncatus (χ2 = 41.7, df = 19, P = 0.002), with a positive significant association for Libore only (Fig. 5a, Additional file 1: Table S8). In B. forskalii, there was no significant association of locality with either prevalence of Schistosoma spp. infection or abundance/total of shedding snails. Some localities without any shedding B. truncatus or B. forskalii overlapped (Tiaguirie, Tagabati and Yoireize Koira, all with low abundance); similarly some localities with high Schistosoma spp. infection prevalence for B. truncatus overlapped with high prevalence localities for B. forskalii (Tokeye, Libore and Dokimana). For Bi. pfeifferi, both Schistosoma spp. infection prevalence (χ2 = 5.1, df = 1, P = 0.023) and abundance of shedding snails (χ2 = 8.2, df = 1, P = 0.004) were significantly higher in Namari Goungou than in Diambala.
Fig. 5.
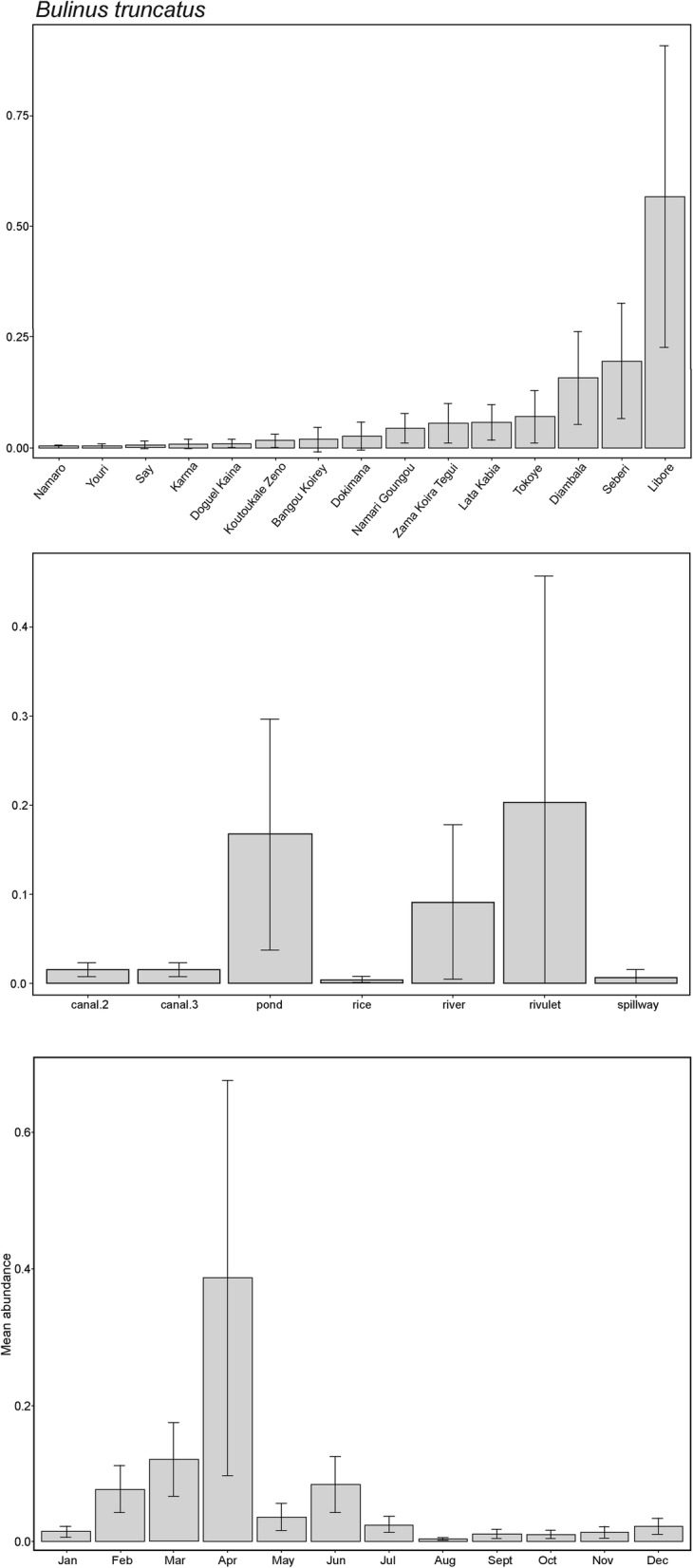
a Bulinus truncatus modelled abundance of shedding snails by locality (χ2 = 41.7, df = 19, P < 0.002). b B. truncatus modelled abundance of shedding snails by site type (χ2 = 15, df = 6, P < 0.02). c B. truncatus modelled abundance of shedding snails by month (χ2 = 62.3, df = 11, P < 0.001).
Effect of site type on prevalence of Schistosoma spp. infection in snails and on abundance of shedding snails
Site type was a significant predictor for Schistosoma spp. infection prevalence in B. truncatus (χ2 = 92.9, df = 6, P < 0.001). Site type and abundance of shedding B. truncatus also showed a significant association (χ2 = 15, df = 6, P = 0.02, Fig. 5b), and a significant positive association for ponds (Additional file 1: Table S8). There was no significant association of site type and either Schistosoma spp. infection prevalence or abundance of shedding B. forskalii. Ponds had the highest proportion of snails shedding Schistosoma spp. for both B. truncatus and B. forskalii, and irrigation canals the lowest for B. truncatus, also very low for B. forskalii (Table 2). The prevalence of Schistosoma spp. infection in Bi. pfeifferi was significantly higher in tertiary canals (7%) than in secondary canals (2.5%, χ2 = 7.1, df = 1, P = 0.008), but not for total shedding.
Effect of month on prevalence of Schistosoma spp. infection in snails and on abundance of shedding snails
Month was a significant predictor of Schistosoma spp. infection prevalence in B. truncatus (χ2 = 18.9, df = 11, P = 0.06); and of abundance of shedding B. truncatus (χ2 = 62.3, df = 11, P < 0.001, Fig. 5c), with a significant positive association of shedding B. truncatus and months February to April, and June, and a significant negative association for August (Additional file 1: Table S8). Prevalence of Schistosoma spp. infection in B. forskalii was more variable by month, with no shedding snails collected in April or November in any year. Month was a significant predictor of prevalence of Schistosoma spp. infection in B. forskalii (χ2 = 29.4, df = 11, P = 0.002), but not of abundance of shedding B. forskalii. In Bi. pfeifferi, the number of infected snails found month by month was also variable. Month was a significant predictor of prevalence of Schistosoma spp. infection (χ2 = 24.3, df = 9, P = 0.004), but not for abundance of shedding snails. For August, the month with the highest precipitation (Additional file 1: Table S7), prevalence was low for all snails surveyed.
Discussion
We found significant evidence of seasonality affecting the abundance of freshwater snails in this study, with higher numbers found in the dry season, and reductions in the wet season (Fig. 4c). While this finding was particularly marked for B. truncatus and R. natalensis, it was evident across all snail species surveyed. This suggests the observation is due to abiotic factors, likely working in concert; for example, snail displacement in wet months as water levels rise and flow increases, and rain creating turbidity, intensifying impact on snails already being washed away. Rain may also affect cumulative impacts through sudden temperature reduction causing thermal shock in snails, reducing egg-laying success, and dampening post-rain recruitment as overall numbers will be reduced [13]. The changing seasonal environment may also affect experimental sampling. Snails may be more difficult to find in turbid water and when dislodged from vegetation; the search-area may increase as water levels rise, and snails may also accumulate in highly localised areas such as eddies which may be missed. Water speed and precipitation both showed a significant negative association with Bulinus spp. abundance, and it is well established that Bulinus spp. for example prefer low flow environments [13]. Seasonal patterns could also be influenced by density-dependence in the dry season, or where numbers rise to a tipping point, where they start to decline because of factors like food limitation, or potential aestivation [61]. Findings of greater snail abundance in the dry season are consistent with the published data for Bulinus spp. in the NRV [62] and in similar environments such as Burkina Faso and Mali [61, 63], but also in very different climates, including Kenya and Lake Victoria [26, 34], although the latter showed differences between ephemeral and permanent sites.
Schistosoma spp. infections in B. truncatus were also impacted by seasonality (Fig. 4b, Additional file 1: Table S8). Month was a significant predictor of both prevalence of Schistosoma spp. infection and overall abundance of shedding B. truncatus, with positive associations of shedding snail counts with the dry season and negative with the wet season. Previous work in the NRV has found higher infection in B. truncatus in dry season months [62], and here we have statistically confirmed this trend through longitudinal analysis. In a parallel study (Pennance et al. unpublished data), the majority of the shedding snails from this survey were confirmed using molecular markers as infected with the cattle schistosome S. bovis, with fewer shedding the human-infecting S. haematobium or S. haematobium group hybrids. As our study has characterized abundance and distribution of B. truncatus that are compatible hosts for Schistosoma spp., these data provide a proxy of animal and human schistosomiasis transmission risk. The findings of a higher abundance of B. truncatus and higher numbers shedding in the dry season have clear implications for monitoring of transmission for Schistosoma spp., and could for example, contribute to an evidence-based snail control programme to tackle interruption of transmission in the region.
We also found a statistically significant correlation between the overall abundance and the total number of infected snails for both B. truncatus and Bi. pfeifferi. This relationship was not evident for B. forskalii, but this may be a sampling effect as infection rates were much lower, consistent with published findings [24], including for the NRV [8, 10]. There is often little evidence of a relationship between snail abundance and the number of infected snails [34, 36]. Here however, monthly sampling has allowed sound resolution of the rates of infection.
Infection in Bi. pfeifferi, while variable by month and year, was comparatively high overall, consistent with previous reports in the NRV [21, 44], and in the Niger River catchment in neighbouring Mali [63–65]. It is not clear why this is the case. Biomphalaria pfeifferi has a restricted distribution in the NRV, consistent with its recent establishment [46]. It is present downstream of Kandadji Dam, currently under construction. Dam building has previously contributed to the spread of S. mansoni as large open water bodies are favourable habitats for Biomphalaria spp. [23]; therefore, this dam could also facilitate an increase in S. mansoni through the NRV [44]. Moreover Bi. pfeifferi could potentially spread further through the NRV regardless of the dam, thereby increasing the risk of S. mansoni transmission, and has in fact recently appeared in several more villages (A. Garba, personal communication).
Another key finding is that both prevalence of Schistosoma spp. in B. truncatus and abundance of shedding B. truncatus were significantly higher in ponds (Fig. 5a), suggesting a higher transmission potential in these habitats. Most of the Bulinus spp. infections were S. bovis (Pennance et al. unpublished data), this may be due to greater water contact by cattle in ponds versus other kinds of water contact sites, such as irrigation canals. Wood et al. [66] found a significant correlation of site area and risk of S. haematobium infection in Senegal, attributed to more contiguous snail habitat in larger sites. Potentially a similar effect is occurring in the present study in ponds versus canals for example. Ponds had the highest average temperatures of 28.5 °C (Additional file 1: Table S7), close to the optimal temperature for schistosome development [41, 67], potentially facilitating successful infection of intermediate host snails. The average temperatures in irrigation canals in contrast is optimal for Bulinus spp. (26–27 °C [68]), which may contribute to high abundances of snails in these environments but lower levels of infection. However, snails may also move deeper into a water body with an increase in temperature; therefore, the relationship between snail microhabitat, temperature and infection may be complex. We also found relatively high (although variable) prevalence of Schistosoma spp. infection in B. truncatus in rivulets, and for Bi. pfeifferi, tertiary canals contained proportionally more infected snails, which being dirt-lined are closer to a natural habitat than secondary canals. This presents a picture of higher infection in (more) natural habitats. Variables such as amount of aquatic vegetation may affect the abundance of infected snails, observed as a key factor determining snail presence in other studies [64–66, 69]. This may also occur at the locality level, e.g. Libore is a densely populated area downstream of Niamey, a region previously recorded as hyper-endemic [70] and had significantly highest numbers of infected B. truncatus (Fig. 5a). Further Libore was one of several localities with high prevalence across years for both B. truncatus and B. forskalii. An association of high densities of schistosome-infected snails and high levels of human water contact has previously been observed [64, 70], ascribed to water contamination producing greater growth of aquatic vegetation, favourable for snails. Overall, as shedding B. truncatus were found at all site types surveyed, this indicates a range of habitats in the NRV play a role in transmission. Diverse environs and sub-habitats here may result in increased risk of infection of Schistosoma spp. in the NRV.
Other spatial trends were evident in the data. High snail abundance in irrigation canals was evident across all species (Table 2). Consistent with this, localities with extensive irrigation, such as Namari Goungou and Seberi, had particularly high snail abundance. Irrigation may represent denser snail habitat, as snails can inhabit both the rice paddies and their adjoining network of canals. Sampling bias may have contributed to the high abundances found in the canals as it may be easier to find snails present in a canal than in a pond, although rivulets, a more similar geography to canals, had low abundances of snails. Differences in site type associations by species were evident; for example, B. truncatus was more abundant in secondary than tertiary canals, and rare in rice paddy sites and spillways, whereas B. forskalii showed the opposite trends, more abundant in tertiary than secondary canals and common in rice paddies (Fig 5b). This is consistent with knowledge of their general habitat associations, B. forskalii being more likely to occur in open habitats such as rice paddies (F. Allan, unpublished observations). Patchiness in the distribution of snails was also evident; snail abundance varied by locality, and some snail species were not present at some localities. Many factors come into play here, snail abundance (and Schistosoma spp. prevalence in snails) depend on multiple factors acting in various combinations [71]. Snail modelling has previously looked at snail populations as homogenous [72] but this study, like others shows the need to account for spatial and temporal heterogeneity [61]. Future monitoring of freshwater snails and schistosomes in NRV will need to take into account both seasonality and variable snail distribution [38, 61].
Several limitations to our study and directions for additional work require discussion. A key limitation was that site selection and sampling targeted sites with known high abundances of snails. This was accounted for as far as possible in statistical analysis, but can make comparative analysis problematic, and may have introduced bias. As the survey did not perform density sampling [39, 66], we cannot draw strong conclusions on relative abundances of the different species surveyed. Sampling bias therefore may be substantially affecting relative counts, for example B. forskalii, being much smaller than B. truncatus, may be missed (as may small juvenile snails and hatchlings of all species). Further, in a parallel study 5 B. truncatus from a subset of 137 from our survey were re-identified as B. globosus using cox1 and ITS molecular markers ([47], Pennance et al. unpublished data). Due to similar morphology of the two species, field identification can be challenging. Therefore, some snails identified as B. truncatus may be B. globosus, and the latter species may also contribute to transmission of Schistosoma spp. in the region. However, numbers of B. truncatus overall were much higher than B. globosus. Also, B. truncatus was substantially more abundant than B. forskalii, and the species primarily involved in Schistosoma spp. transmission. B. truncatus therefore is the main intermediate host in the region. Regarding monitoring of schistosome infections, dissection of snails or use of molecular markers to check for prepatent infections could provide a more accurate and less time-intensive alternative to our method of additional shedding attempts on snails several weeks after collection. Other factors could significantly affect the abundance and distribution of the snail species surveyed that were not measured, such as submerged vegetation [64–66, 69, 73–75]. Some site types might have had more vegetation present, resulting in higher snail abundances, but this would require further analysis with remote sensing data and spatial statistical models [76, 77]. Some localities (Tokeye, Libore, and Dokimana) had high infection prevalence (across multiple years) for both B. truncatus and B. forskalii, and additional spatial analysis is needed to understand why. In terms of other additional work, hydrological analysis could enhance our understanding of schistosomiasis transmission in the NRV, for example, to model potential accumulation of snails at particular microhabitats or up and down-stream dispersal post-flooding [78–80]. Geomorphology like circumscription of sites in ponds for example combined with localised water flow patterns could influence retention levels for cercariae [38, 66], requiring further investigation. A multidisciplinary approach would see great advances in accurate mapping of schistosomiasis transmission.
Conclusions
Whether affecting the human population or our livestock, schistosomiasis transmission is dependent on the presence of compatible snail intermediate hosts at water-contact sites. An intensive, longitudinal approach to snail sampling has provided this study with the resolution to reveal significant seasonal and spatial variation in snail infection and abundance, which could be used in an evidence-based intervention strategy to control schistosomiasis in the Niger River Valley. The impact of season on B. truncatus is a key finding with these snails acting as the main intermediate host for species of the Schistosoma haematobium group in the region. Within the dry season B. truncatus was more abundant and showed higher levels of schistosome infections. These results could inform timing of praziquantel administration among the human population and support any other behavioral or snail control interventions. As monitoring of snails is often overlooked, these data show how crucial local-scale snail surveys are to fully understanding transmission dynamics and mapping schistosomiasis risk in a given region and contribute to any future attempts at adjunct interventions through control of snail populations.
Supplementary information
Additional file 1: Table S1. Snail survey sites (coordinates in decimal, WGS84). Figure S1. Predicted counts by site type, B. truncatus. Figure S2. Estimated slope of Bulinus spp. with temperature by site type. Here a 1 degree increase in temperature equals decrease in abundance in rivulet and river, rice paddies, ponds and irrigation canals no real change although in latter—v slight increase in abundance with temp, spillway and stream (I site)—increase in abundance with temperature. Figure S3. Estimated slope of Bulinus spp. with water speed. Table S2. B. truncatus glmmTMB negative binomial model summary output. Table S3. B. forskalii glmmTMB negative binomial model summary output. Table S4. Radix natalensis glmmTMB negative binomial model summary output. Table S5. Biomphalaria pfeifferi glmmTMB negative binomial model summary output. Table S6. Bulinus spp glmmTMB negative binomial model summary output. Table S7. Water chemistry and physical variable data, including USDA (WMO) weather station data, A: averaged by month, and B: by site type (final year of ground collected data missing due to equipment failure). Table S8. Shedding/infected B. truncatus glmmTMB negative binomial model summary output.
Acknowledgements
We wish to thank Omar Barkire, Salem Boukary, Amadou Djibril, Omar Garba, Ali Maiga, Bachir Madougou, Mohamed Ousmane, Samira Souley, Tankari Dan Kountche, (RISEAL) for collection of snails and field data, Natalie Cooper (NHM) for advice on statistical analysis, Chiho Ikebe, Alina Hancock, Ellie Clark, Imogen Lindsley and Ruth Clements for assistance with curation and maintenance of the snail and FTA collections at NHM, SCORE for funding and the SCORE secretariat including Dan Colley, Jennifer Castlemann, Nupur Kittur, Carl Campbell, Sue Binder, and Charlie King.
Abbreviations
- P
P-value
- df
degrees of freedom
- χ2
Chi-square value
- n
total count
- BT
Bulinus truncatus
- BF
Bulinus forskalii
- BP
Biomphalaria pfeifferi
- RN
Radix natalensis
- NRV
Niger River Valley
- Canal 2
secondary irrigation canal
- Canal 3
tertiary irrigation canal
- Rice
rice paddy site
- WMO
USDA Foreign Agricultural Service weather meteorological office
Authors’ contributions
The study design was designed by ADG and DR, field surveys and field data collection were done by AAH, ADG and RL, project administration by ADG, AAH, AG, and DR, curating of collections at NHM by MR and FA. MR undertook curating of data, MR and JW designed and undertook data analysis. MR produced first draft, FA, JW, AG, TP, AAH, AE, RL, BW and DR contributed to subsequent drafts. MR and FA led the writing process. All authors read and approved the final manuscript.
Funding
This work has been funded by the University of Georgia Research Foundation, Inc., which was funded by the Bill & Melinda Gates for the SCORE project through the following sub-awards (RR374-053/5054146; RR374-053/4785426). The Bill and Melinda Gates Foundation provided funding for open access publication (OPP50816). MR and FA are generally supported by the Wellcome Trust Grant (104958/Z/14/Z), at the Natural History Museum, London.
Availability of data and materials
The complete dataset, metadata and scripts for data analysis are available at GitHub (https://github.com/howlerMoonkey/Niger_snail_survey_data_analysis). Collection data for voucher snail specimens and schistosome cercariae are available at the NHM data portal (http://data.nhm.ac.uk) and the SCAN website (http://scan.myspecies.info).
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Muriel Rabone, Email: m.rabone@nhm.ac.uk.
Joris Hendrik Wiethase, Email: j.wiethase@gmail.com.
Fiona Allan, Email: f.allan@nhm.ac.uk.
Anouk Nathalie Gouvras, Email: anouk.gouvras@eliminateschisto.org.
Tom Pennance, Email: t.pennance@nhm.ac.uk.
Amina Amadou Hamidou, Email: aminamadou@yahoo.fr.
Bonnie Lee Webster, Email: b.webster@nhm.ac.uk.
Rabiou Labbo, Email: rabiou@cermes.org.
Aidan Mark Emery, Email: a.emery@nhm.ac.uk.
Amadou Djirmay Garba, Email: garbamadou@yahoo.fr.
David Rollinson, Email: d.rollinson@nhm.ac.uk.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s13071-019-3745-8.
References
- 1.Vos T, Abajobir AA, Abate KH, Abbafati C, Abbas KM, Murray CJL, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study. Lancet. 2017;390:1211–1259. doi: 10.1016/S0140-6736(17)32154-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sturrock RF. The intermediate hosts and host-parasite relationships. In: Jordan P, Webbe G, Sturrock RF, editors. Human schistosomiasis. Wallingford: CAB International; 1993. pp. 33–85. [Google Scholar]
- 3.Bustinduy A, King C, Scott J, Appleton S, Sousa-Figueiredo J, Betson M, Stothard J. HIV and schistosomiasis co-infection in African children. Lancet Infect Dis. 2014;14:640–649. doi: 10.1016/S1473-3099(14)70001-5. [DOI] [PubMed] [Google Scholar]
- 4.Christinet V, Lazdins-Helds J, Stothard J, Reinhard-Rupp J. Female genital schistosomiasis (FGS): from case reports to a call for concerted action against this neglected gynaecological disease. Int J Parasitol. 2016;46:395–404. doi: 10.1016/j.ijpara.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 5.Hotez PJ, Harrison W, Fenwick A, Bustinduy AL, Ducker C, Sabina Mbabazi P, et al. Female genital schistosomiasis and HIV/AIDS: reversing the neglect of girls and women. PLoS Negl Trop Dis. 2019;13:e0007025. doi: 10.1371/journal.pntd.0007025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kayuni S, Lampiao F, Makaula P, Juziwelo L, Lacourse EJ, Reinhard-Rupp J, et al. A systematic review with epidemiological update of male genital schistosomiasis (MGS): a call for integrated case management across the health system in sub-Saharan Africa. Parasite Epidemiol Control. 2019;4:e00077. doi: 10.1016/j.parepi.2018.e00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Standley CJ, Dobson AP, Stothard JR. Out of animals and back again: schistosomiasis as a zoonosis in Africa. In: Rokni MB, editor. schistosomiasis. Rijeka-Shanghai: InTech; 2012. pp. 209–230. [Google Scholar]
- 8.Labbo R, Djibrilla A, Zamankaa H, Garba A, Chippaux J-PB. forskalii: a new potential intermediate host for Schistosoma haematobium in Niger. Trans R Soc Trop Med Hyg. 2007;101:847–848. doi: 10.1016/j.trstmh.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 9.Labbo R, Ernould JC, Djibrila A, Bangre M, Labbo R. Epidémiologie de la schistosomose à Schistosoma bovis: détermination des charges parasitaires, de la période de transmission et des sites de contamination a partir des enquêtes d’abattoir—document CERMES No. 01/01 Niamey, Niger; 2001. p. 13.
- 10.Bremond P, Vera C, Labbo R, Sellin E, Jourdane J, Sellin B. Variability in the compatibility between Schistosoma haematobium, S. bovis, S. curassoni and bulinid snails in Niger: implications on interactions between parasites. In: VIIIth international congress of parasitology (ICOPA VIII), 10–14 October 1994, Izmir, Turkey (Abstracts, vol. 1); 1994. p. 207.
- 11.Bremond P, Sellin B, Sellin E, Nameoua B, Labbo R, et al. Evidence for the introgression of the human parasite Schistosoma haematobium by genes from Schistosoma bovis, Niger. C R Acad Sci III. 1993;316:667–670. [PubMed] [Google Scholar]
- 12.Moné H, Mouahid G, Morand S. The distribution of Schistosoma bovis Sonsino, 1876 in relation to intermediate host mollusc-parasite relationships. Adv Parasitol. 1999;44:99–138. doi: 10.1016/S0065-308X(08)60231-6. [DOI] [PubMed] [Google Scholar]
- 13.Rollinson D, Stothard JR, Southgate VR. Interactions between intermediate snail hosts of the genus Bulinus and schistosomes of the Schistosoma haematobium group. Parasitology. 2001;123:S245–S260. doi: 10.1017/S0031182001008046. [DOI] [PubMed] [Google Scholar]
- 14.Southgate VR, Knowles RJ. Observations on Schistosoma bovis Sonsino, 1976. J Nat Hist. 1975;9:273–314. doi: 10.1080/00222937500770201. [DOI] [Google Scholar]
- 15.Southgate VR, Knowles RJ. The intermediate hosts of Schistosoma bovis in western Kenya. Trans R Soc Trop Med Hyg. 1975;69:356–357. doi: 10.1016/0035-9203(75)90132-7. [DOI] [PubMed] [Google Scholar]
- 16.Tian-Bi Y-N, Webster B, Konan CK, Allan F, Diakité NR, Ouattara M, et al. Molecular characterization and distribution of Schistosoma cercariae collected from naturally infected bulinid snails in northern and central Côte d’Ivoire. Parasites Vectors. 2019;12:117. doi: 10.1186/s13071-019-3381-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leger E, Garba A, Hamidou AA, Webster BL, Pennance T, Rollinson D, Webster JP. Introgressed animal schistosomes Schistosoma curassoni and S. bovis naturally infecting humans. Emerg Infect Dis. 2016;22:2212–2214. doi: 10.3201/eid2212.160644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Webster BL, Diaw OT, Seye M, Webster JP, Rollinson D. Introgressive hybridization of Schistosoma haematobium group species in Senegal: species barrier break down between ruminant and human schistosomes. PLoS Negl Trop Dis. 2013;7:e2110. doi: 10.1371/journal.pntd.0002110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huyse T, Webster B, Geldof S, Stothard J, Diaw O, Polman K, Rollinson D. Bidirectional introgressive hybridization between a cattle and human schistosome species. PloS Pathog. 2009;5:e1000571. doi: 10.1371/journal.ppat.1000571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rollinson D, Southgate VR, Vercruysse J, Moore PJ. Observations on natural and experimental interactions between Schistosoma bovis and S. curassoni from West Africa. Acta Trop. 1990;47:101–114. doi: 10.1016/0001-706X(90)90072-8. [DOI] [PubMed] [Google Scholar]
- 21.Garba A, Barkiré N, Djibo A, Lamine MS, Sofo B, Gouvras AN, et al. Schistosomiasis in infants and preschool-aged children: infection in a single Schistosoma haematobium and a mixed S. haematobium–S. mansoni foci of Niger. Acta Trop. 2010;115:212–219. doi: 10.1016/j.actatropica.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 22.Chitsulo LD, Engels A, Montresor A, Savioli L. The global status of schistosomiasis and its control. Acta Trop. 2000;77:41–51. doi: 10.1016/S0001-706X(00)00122-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steinmann P, Keiser J, Bos R, Tanner M, Utzinger J. Schistosomiasis and water resources development: systematic review, meta-analysis, and estimates of people at risk. Lancet Infect Dis. 2006;6:411–425. doi: 10.1016/S1473-3099(06)70521-7. [DOI] [PubMed] [Google Scholar]
- 24.Brown DS. Freshwater snails of Africa and their medical importance. 2. London: Taylor and Francis; 1994. [Google Scholar]
- 25.Comité Permanent Inter-états de Lutte contre la Sécheresse dans le Sahel (CILSS). Landscapes of West Africa—a window on a changing world. Ouagadougou, Burkina Faso: CILSS; 2016. p. 219. 10.5066/f7n014qz.
- 26.Gouvras AN, Allan F, Kinung’hi S, Rabone M, Emery A, Angelo T, et al. Longitudinal survey on the distribution of Biomphalaria sudanica and B. choanomophala in Mwanza region, on the shores of Lake Victoria, Tanzania: implications for schistosomiasis transmission and control. Parasites Vectors. 2017;10:316. doi: 10.1186/s13071-017-2252-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Allan F, Sousa-Figueiredo JC, Emery A, Rossely P, Mirante C, Rollinson D, et al. Mapping freshwater snails in north-western Angola: distribution, identity and molecular diversity of medically important taxa. Parasites Vectors. 2017;10:460. doi: 10.1186/s13071-017-2395-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Senghor B, Diaw OT, Doucoure S, Seye M, Talla I, Diallo A, Tidiane Bâ B, Sokhna C. Study of the snail intermediate hosts of urogenital schistosomiasis in Niakhar, region of Fatick, West central Senegal. Parasites Vectors. 2015;8:410. doi: 10.1186/s13071-015-1030-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Labbo R, Boulanger D, Bremond P, Chippaux J-P. Infestation expérimentale de caprins par Schistosoma bovis et S. curassoni: effets pathogènes comparés. Parasite. 2007;14:77–82. doi: 10.1051/parasite/2007141077. [DOI] [PubMed] [Google Scholar]
- 30.Mutuku MW, Laidemitt MR, Beechler BR, Mwangi IN, Otiato FO, Agola EL. A search for snail-related answers to explain differences in response of Schistosoma mansoni to praziquantel treatment among responding and persistent hotspot villages along the Kenyan shore of Lake Victoria. Am J Trop Med Hyg. 2019;101:65–77. doi: 10.4269/ajtmh.19-0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stensgaard AS, Jørgensen A, Kabatereine NB, Rahbek C, Kristensen TK. Modeling freshwater snail habitat suitability and areas of potential snail-borne disease transmission in Uganda. Geospat Health. 2006;1:93–104. doi: 10.4081/gh.2006.284. [DOI] [PubMed] [Google Scholar]
- 32.Moser W, Greter H, Schindler C, Allan F, Ngandolo BNR, Moto DD, Utzinger J, Zinsstag J. The spatial and seasonal distribution of B. truncatus, B. forskalii and Bi. pfeifferi, the intermediate host snails of schistosomiasis, in N’Djamena, Chad. Geospat Health. 2014;9:109–118. doi: 10.4081/gh.2014.9. [DOI] [PubMed] [Google Scholar]
- 33.Diakité NR, N’Zi KG, Ouattara M, Coulibaly JT, Saric J, Yao PK, Hattendorf J, Utzinger J, N’Goran EK. Association of riverine prawns and intermediate host snails and correlation with human schistosomiasis in two river systems in south-eastern Côte d’Ivoire. Parasitology. 2018;145:1792–1800. doi: 10.1017/S003118201800135X. [DOI] [PubMed] [Google Scholar]
- 34.Muhoho ND, Katsumata T, Kimura E, Migwi DK, Mutua WR, Kiliku FM, Habe S, Aoki Y. Cercarial density in the river of an endemic area of Schistosomiasis haematobium in Kenya. Am J Trop Med Hyg. 1997;57:162–167. doi: 10.4269/ajtmh.1997.57.162. [DOI] [PubMed] [Google Scholar]
- 35.Gryseels B. The epidemiology of schistosomiasis in Burundi and its consequences for control. Trans R Soc Trop Med Hyg. 1991;85:626–633. doi: 10.1016/0035-9203(91)90371-5. [DOI] [PubMed] [Google Scholar]
- 36.Klumpp, R. A study of the transmission of Schistosoma haematobium in Volta Lake, Ghana. Ph.D. thesis, London School of Hygiene & Tropical Medicine; 1983. 10.17037/pubs.00682402.
- 37.Woolhouse ME, Chandiwana SK. Spatial and temporal heterogeneity in the population dynamics of Bulinus globosus and Biomphalaria pfeifferi and in the epidemiology of their infection with schistosomes. Parasitology. 1989;98:21–34. doi: 10.1017/S0031182000059655. [DOI] [PubMed] [Google Scholar]
- 38.Ramalli L, Mulero S, Noël H, Chiappini JD, Vincent J, et al. Persistence of schistosomal transmission linked to the Cavu river in southern Corsica since 2013. Eurosurveillance. 2018;23:4. doi: 10.2807/1560-7917.ES.2018.23.4.18-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sokolow S, Wood C, Jones I, Lafferty K, Kuris A, Hsieh M, De Leo G. To reduce the global burden of human schistosomiasis, use “old fashioned” snail control. Trends Parasitol. 2017;34:23–40. doi: 10.1016/j.pt.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.King CH, Sutherland LJ, Bertsch D. Systematic review and meta-analysis of the impact of chemical-based mollusciciding for control of Schistosoma mansoni and S. haematobium transmission. PLoS Negl Trop Dis. 2015;9:e0004290. doi: 10.1371/journal.pntd.0004290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stensgaard AS, Vounatsou P, Sengupta M, Utzinger J. Schistosomes, snails and climate change: current trends and future expectations. Acta Trop. 2019;190:257–268. doi: 10.1016/j.actatropica.2018.09.013. [DOI] [PubMed] [Google Scholar]
- 42.Gurarie D, King CH, Yoon N, Alsallaq R, Wang X. Seasonal dynamics of snail populations in coastal Kenya: model calibration and snail control. Adv Water Resour. 2016;108:397–405. doi: 10.1016/j.advwatres.2016.11.008. [DOI] [Google Scholar]
- 43.Ezeamama A, He CL, Shen Y, Yin X-P, Binder SC, Campbell C, et al. Gaining and sustaining schistosomiasis control: study protocol and baseline data prior to different treatment strategies in five African countries. BMC Infect Dis. 2016;16:229. doi: 10.1186/s12879-016-1575-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Garba A, Labbo R, Tohon Z, Sidiki A, Djibrilla A. Emergence of Schistosoma mansoni in the Niger River valley, Niger. Trans R Soc Trop Med Hyg. 2004;98:296–298. doi: 10.1016/S0035-9203(03)00070-1. [DOI] [PubMed] [Google Scholar]
- 45.Utzinger J, Brattig NW, Kristensen TK. Schistosomiasis research in Africa: how the CONTRAST alliance made it happen. Acta Trop. 2013;128:182–195. doi: 10.1016/j.actatropica.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 46.Labbo R, Garba A, Louboutin-Croc JP, Ernould JC, Sellin B, Chippaux J-P, Stothard JR. The spread of Biomphalaria pfeifferi in the Niger River valley, Niger. Ann Trop Med Parasitol. 2003;97:209–212. doi: 10.1179/000349803235001507. [DOI] [PubMed] [Google Scholar]
- 47.Kane RA, Stothard JR, Emery A, Rollinson D. Molecular characterization of freshwater snails in the genus Bulinus: a role for barcodes? Parasites Vectors. 2008;1:15. doi: 10.1186/1756-3305-1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Frandsen F, Christensen NO. An introductory guide to the identification of cercariae from African freshwater snails with special reference to cercariae of trematode species of medical and veterinary importance. Acta Trop. 1984;41:181–202. [PubMed] [Google Scholar]
- 49.Gower CM, Shrivastava J, Lamberton PHL, Rollinson D, Webster BL, Emery A, et al. Development and application of an ethically and epidemiologically advantageous assay for the multi-locus microsatellite analysis of Schistosoma mansoni. Parasitology. 2007;134:523–536. doi: 10.1017/S0031182006001685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Emery A, Allan F, Rabone M, Rollinson D. Schistosomiasis collection at NHM (SCAN) Parasites Vectors. 2012;5:185. doi: 10.1186/1756-3305-5-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.R Core Team. R: a language and environment for statistical computing. In: R version 3.5.3 (2019-03-11) “Great Truth” edn. Vienna: R Foundation for Statistical Computing; 2019. https://www.R-project.org/.
- 52.Team R. RStudio: integrated development for R. In: 0.99.903 edn. Boston: RStudio, Inc.; 2015.
- 53.Zuur A, Ieno EN, Elphick CS. A protocol for data exploration to avoid common statistical problems. Methods Ecol Evol. 2010;1:3–14. doi: 10.1111/j.2041-210X.2009.00001.x. [DOI] [Google Scholar]
- 54.Brooks M, Kristensen K, van Benthem K, Magnusson A, Berg C, Nielsen A, et al. glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J. 2017;9:378–400. doi: 10.32614/RJ-2017-066. [DOI] [Google Scholar]
- 55.Blasco-Moreno A, Pérez-Casany M, Puig P, Morante M, Castells E. What does a zero mean? Understanding false, random and structural zeros in ecology. Methods Ecol Evol. 2019;10:949–959. doi: 10.1111/2041-210X.13185. [DOI] [Google Scholar]
- 56.Hartig F. DHARMa: residual diagnostics for hierarchical (multi-level/mixed) regression models. R package, version 0.2.4. 2019. http://florianhartig.github.io/DHARMa/.
- 57.Burnham KP, Anderson DR, Huyvaert KP. AIC model selection and multimodel inference in behavioral ecology: some background, observations, and comparisons. Behav Ecol Sociobiol. 2011;65:23–35. doi: 10.1007/s00265-010-1029-6. [DOI] [Google Scholar]
- 58.Mundry R. Issues in information theory-based statistical inference—a commentary from a frequentist’s perspective. Behav Ecol Sociobiol. 2011;65:57–68. doi: 10.1007/s00265-010-1040-y. [DOI] [Google Scholar]
- 59.Fox J, Weisberg S. Car, R package; 2011. https://cran.r-project.org/web/packages/car/index.html.
- 60.Lenth R, Singmann H, Love J, Beurkner P, Herve M. emmeans 2019-04-21; 2019. https://CRAN.R-project.org/package=emmeans.
- 61.Perez-Saez J, Mande T, Ceperley N, Bertuzzo E, Mari L, Gatto M, Rinaldo A. Hydrology and density feedbacks control the ecology of intermediate hosts of schistosomiasis across habitats in seasonal climates. Proc Natl Acad Sci USA. 2016;113:6427–6432. doi: 10.1073/pnas.1602251113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Labbo R, Ernould JC, Djibrilla A, Garba A, Chippaux J-P. Focalisation de la transmission de Schistosoma haematobium au sein des périmètres irrigués de la vallée du Niger: importance des facteurs malacologiques. Rev Epidemiol Sante Publique. 2008;56:3–9. doi: 10.1016/j.respe.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 63.Dabo A, Diarra AZ, Machault V, Touré O, Niambélé DS, Kanté A, et al. Urban schistosomiasis and associated determinant factors among school children in Bamako, Mali, West Africa. Infect Dis Poverty. 2015;4:4. doi: 10.1186/2049-9957-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Madsen H, Coulibaly G, Furu P. Distribution of freshwater snails in the river Niger basin in Mali with special reference to the intermediate hosts of schistosomes. Hydrobiologia. 1987;146:77–88. doi: 10.1007/BF00007580. [DOI] [Google Scholar]
- 65.Madsen H. Ecological studies on the intermediate host snails and the relevance to schistosomiasis control. Mem Inst Oswaldo Cruz. 1992;87:249–253. doi: 10.1590/S0074-02761992000800039. [DOI] [PubMed] [Google Scholar]
- 66.Wood CL, Sokolow S, Jones I, Chamberlin A, Lafferty KD, Kuris AM, et al. Precision mapping of snail habitat provides a powerful indicator of human schistosomiasis transmission. Proc Natl Acad Sci USA; 2019 (in press). [DOI] [PMC free article] [PubMed]
- 67.Appleton CC. Review of literature on abiotic factors influencing the distribution and life cycles of bilharzia intermediate host snails. Malacol Rev. 1978;11:1–25. [Google Scholar]
- 68.Shiff CJ. Studies on Bulinus (Phyopsis) in Rhodesia. The influence of temperature on the intrinsic rate of natural increase. Ann Trop Med Parasitol. 1964;58:94–105. doi: 10.1080/00034983.1964.11686219. [DOI] [PubMed] [Google Scholar]
- 69.Klumpp R, Chu K. Ecological studies of Bulinus rohlfsi, the intermediate host of Schistosoma haematobium in the Volta Lake. Bull World Health Organ. 1977;55:715–730. [PMC free article] [PubMed] [Google Scholar]
- 70.Ernould JC, Kaman AK, Labbo R, Couret D, Chippaux J-P. Recent urban growth and urinary schistosomiasis in Niamey, Niger. Trop Med Int Health. 2000;5:431–437. doi: 10.1046/j.1365-3156.2000.00577.x. [DOI] [PubMed] [Google Scholar]
- 71.Utzinger J, Tanner M. Microhabitat preferences of Biomphalaria pfeifferi and R. natalensis in a natural and a man-made habitat in southeastern Tanzania. Mem Inst Oswaldo Cruz. 2000;95:287–294. doi: 10.1590/S0074-02762000000300002. [DOI] [PubMed] [Google Scholar]
- 72.Gurarie D, Lo N, Ndeffo-Mbah M, Durham D, King C. The human-snail transmission environment shapes long term schistosomiasis control outcomes: implications for improving the accuracy of predictive modeling. PLoS Negl Trop Dis. 2018;12:e0006514. doi: 10.1371/journal.pntd.0006514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Coulibaly G, Diallo M, Madsen H, Dabo A, Traoré M, Keita S. Comparison of schistosome transmission in a single- and a double-cropped area in the rice irrigation scheme, ‘Office du Niger’, Mali. Acta Trop. 2004;91:15–25. doi: 10.1016/j.actatropica.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 74.Scott D, Senker K, England E. Epidemiology of human Schistosoma haematobium infection around Volta Lake, Ghana, 1973–75. Bull World Health Organ. 1982;60:89–100. [PMC free article] [PubMed] [Google Scholar]
- 75.Thomas J, Tait A. Control of the snail hosts of schistosomiasis by environmental manipulation: a field and laboratory appraisal in the Ibadan Area, Nigeria. Philos Trans R Soc Lond B Biol Sci. 1984;305:201–253. doi: 10.1098/rstb.1984.0056. [DOI] [PubMed] [Google Scholar]
- 76.Kulinkina AV, Walz Y, Koch M, Biritwum N-K, Utzinger J, Naumova EN. Improving spatial prediction of Schistosoma haematobium prevalence in southern Ghana through new remote sensors and local water access profiles. PLoS Negl Trop Dis. 2018;12:e0006517. doi: 10.1371/journal.pntd.0006517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Walz Y, Wegmann M, Dech S, Vounatsou P, Poda J-N, N’Goran EK, Utzinger J, Raso G. Modeling and validation of environmental suitability for schistosomiasis transmission using remote sensing. PLoS Negl Trop Dis. 2015;9:e0004217. doi: 10.1371/journal.pntd.0004217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Perez-Saez J, Mande T, Larsen J, Ceperley N, Rinaldo A. Classification and prediction of river network ephemerality and its relevance for waterborne disease epidemiology. Adv Water Resour. 2017;110:263–278. doi: 10.1016/j.advwatres.2017.10.003. [DOI] [Google Scholar]
- 79.Ciddio M, Mari L, Gatto M, Rinaldo A, Casagrandi R. The temporal patterns of disease severity and prevalence in schistosomiasis. Chaos. 2015;25:036405. doi: 10.1063/1.4908202. [DOI] [PubMed] [Google Scholar]
- 80.Clennon JA, King CH, Muchiri EM, Kitron U. Hydrological modelling of snail dispersal patterns in Msambweni, Kenya and potential resurgence of Schistosoma haematobium transmission. Parasitology. 2007;134:683–693. doi: 10.1017/S0031182006001594. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Snail survey sites (coordinates in decimal, WGS84). Figure S1. Predicted counts by site type, B. truncatus. Figure S2. Estimated slope of Bulinus spp. with temperature by site type. Here a 1 degree increase in temperature equals decrease in abundance in rivulet and river, rice paddies, ponds and irrigation canals no real change although in latter—v slight increase in abundance with temp, spillway and stream (I site)—increase in abundance with temperature. Figure S3. Estimated slope of Bulinus spp. with water speed. Table S2. B. truncatus glmmTMB negative binomial model summary output. Table S3. B. forskalii glmmTMB negative binomial model summary output. Table S4. Radix natalensis glmmTMB negative binomial model summary output. Table S5. Biomphalaria pfeifferi glmmTMB negative binomial model summary output. Table S6. Bulinus spp glmmTMB negative binomial model summary output. Table S7. Water chemistry and physical variable data, including USDA (WMO) weather station data, A: averaged by month, and B: by site type (final year of ground collected data missing due to equipment failure). Table S8. Shedding/infected B. truncatus glmmTMB negative binomial model summary output.
Data Availability Statement
The complete dataset, metadata and scripts for data analysis are available at GitHub (https://github.com/howlerMoonkey/Niger_snail_survey_data_analysis). Collection data for voucher snail specimens and schistosome cercariae are available at the NHM data portal (http://data.nhm.ac.uk) and the SCAN website (http://scan.myspecies.info).