Abstract
Background
To assess late toxicity, quality of life and oncological outcome after consolidative whole abdominal radiotherapy (WART) following cytoreductive surgery and carboplatin/paclitaxel chemotherapy in high risk patients with advanced ovarian cancer FIGO stage III using IMRT (Intensity modulated radiation therapy).
Methods
The OVAR-IMRT-02 study is a multi-center single-arm phase-II-trial. Twenty patients with optimally debulked ovarian cancer stage FIGO III with complete remission after chemotherapy were treated with intensity modulated WART. A total dose of 30 Gy in 20 fractions was applied to the entire peritoneal cavity. Primary endpoint was treatment tolerability; secondary objectives were acute and chronic toxicities, quality of life, rates of therapy disruption/abortion, progression-free survival (PFS) and overall survival (OS).
Results
All patients completed treatment and 10/20 patients (50%) reached the final study follow-up of 36 months. Late side effects consisted of °1-°2 lower limb edema (44.5%), with one patient (5.6%) showing °3 edema. Three patients (16.7%) showed elevated gamma-Glutamyltransferase. There were no severe late side effects regarding renal or hepatic function or any gastrointestinal toxicity greater than °2. During WART, mean global health status decreased by 18.1 points (95%-CI: 7.1–29.0), but completely normalized after 6 months. The same trend was observed for the function scale scores. Kaplan-Meier-estimated 1-, 2- and 3-year PFS was 74, 51 and 40%, respectively. 1-, 2- and 3-year OS was 89, 83 and 83%, respectively.
Conclusions
Intensity modulated WART after aggressive surgery and carboplatin/paclitaxel chemotherapy is associated with an acceptable risk of acute and late toxicity and minor impact on long-term quality of life. Together with the promising results for PFS and OS, intensity modulated WART could offer a new therapeutic option for consolidation treatment of patients with advanced ovarian cancer.
Trial registration
The study is registered with ClinicalTrials.gov (NCT01180504). Registered 12 August 2010 – retrospectively registered.
Keywords: Ovarian cancer, Whole abdominal radiotherapy, Consolidation treatment, Toxicity, Quality of life, Oncological outcome
Background
With 295,414 new cases diagnosed in 2018, ovarian cancer accounts for 1.6% of all malignancies and 1.9% of all cancer-associated deaths worldwide [1]. Attempts for a better screening and thus early diagnosis or even prevention of ovarian cancer failed so far. Most women (75%) present with advanced-stage disease (FIGO stage III) or even distant metastases (FIGO stage IV) [2, 3]. Approximately 75–80% of patients with advanced stage ovarian cancer relapse after a median interval of 18–24 months [4], the abdominal cavity being the main location of recurrence. Overall survival (OS) decreases significantly in FIGO stage III and IV with 5-year-OS rates of only 31 and 13%, respectively [2, 3].
First-line treatment of advanced ovarian cancer patients consists of radical cytoreductive surgery (“no residual tumor”) and a platinum- and taxane-based chemotherapy (6 cycles of platinum-based chemotherapy in combination with paclitaxel 175 mg/m2) [5–18]. Simultaneous followed by consolidative administration of bevacizumab for 12–15 months can be considered in stage IIIB-IV patients with high risk for recurrence, accepting reduced quality of life [19–21].
In recent years many trials evaluated potential drugs to improve treatment for advanced ovarian cancer patients, aiming to prolong survival. PARP inhibitors represent one novel therapeutic option which is being investigated in several trials. To date, the greatest benefit has been in the maintenance setting, prolonging the progression-free survival of ovarian cancer patients with a BRCA1/2 mutation [4, 22–25].
Despite new technical developments in the field of radiation therapy in the last decades, consolidative radiotherapy of the peritoneal cavity has not been re-evaluated ever since it has been abandoned from standard treatment many years ago due to its severe side effects. Toxicity of WART using conventional radiation therapy has been widely investigated in the last decades [26–33], showing high toxicity and low therapy compliance rates. On the other hand, promising results for improved Progression-free Survival after WART have been reported. For example, significantly improved disease-free survival has been described for patients receiving surgery and radiation therapy compared with surgery and chemotherapy in a retrospective case-control study [31]. Furthermore, consolidation radiation therapy after resection and chemotherapy was associated with significantly prolonged relapse-free interval and OS. In this study, patients with complete remission after chemotherapy seemed to benefit most from consolidative radiation therapy. However, also in patients with microscopically residual disease, radiation treatment seems to be an efficacious treatment option [34]. Taking all these findings into consideration, WART may play a role as consolidation treatment in patients with advanced ovarian cancer showing macroscopically complete response after surgery and chemotherapy. Nowadays, using new technical developments like intensity modulated radiation therapy (IMRT), it is possible to reduce toxicity by sparing organs at risk (OARs) [35, 36]. The OVAR-IMRT-01 trial already showed the clinical feasibility of WART using IMRT [37]. Considering these promising results, we initiated a phase 2 study for further evaluation of treatment tolerance: the OVAR-IMRT-02 trial [38]. The primary objective of this study was to show a better treatment tolerance of WART using an IMRT technique compared with historical patient collectives receiving conventional radiation therapy. Preliminary data regarding treatment tolerability, acute toxicity and quality of life have already been published in 2017 [39]. Now we report the final results of late toxicities and quality of life, as well as PFS and OS.
Methods
Study design
The OVAR-IMRT-02 study is a multi-center single-arm phase-II-trial (investigator-initiated trial). Between 2010 and 2015, 20 patients with FIGO stage III ovarian cancer were treated with WART using IMRT with a dose of 30 Gy (daily fractions of 1.5 Gy 5 times per week) within a consolidation concept. Patient characteristics have already been described previously [39] and are shown in Table 1. All patients had maximal cytoreductive surgery (including at least total abdominal hysterectomy, bilateral adnexectomy, omentectomy, debulking of tumor masses) with postoperative residual tumor of < 1 cm followed by chemotherapy. Consolidative WART should not start later than 10 weeks after the last cycle of chemotherapy. Performance of IMRT using helical tomotherapy has been described previously [37, 38]. In short, WART was applied as helical IMRT using a tomotherapy device (TomoTherapy, Madison, WI) with 6-MeV photons. Control of positioning accuracy was performed daily with integrated megavoltage computed tomography for tomotherapy (3.5 MV). The clinical target volume included the whole peritoneal cavity extending from diaphragm to Douglas cavity, the liver surface, and the pelvic and para-aortic node regions. The planning target volume (PTV) encompassed an axial margin of 1.5 cm around the clinical target volume (2.5 cm in the cranial direction). Organs at risk (OARs) were kidneys, liver (except the 1-cm outer border), lungs, bones (vertebral bodies and pelvic bones), heart, and spinal cord. A dose of 30.0 Gy was prescribed to the median of the PTV. Inverse radiation dose planning was performed according to general recommendations (International Commission on Radiation Units and Measurements Report 50, 1999). There were no strict dose constraints for OARs used for optimization. The goal of optimization was to deliver a dose distribution in the PTV as homogeneous as possible, in addition to maximal sparing of OARs with a high priority on liver, kidney, and bone marrow. Tolerated maximum doses to OARs were not to exceed the tolerance dose 5/5 for each organ [25, 26]. Dosimetric information about the dose distribution in the target volume (PTV) and the OARs have already been reported previously [39].
Table 1.
Baseline and clinical characteristics of the patients (n = 20)
| Age (years) | 58.4 ± 7.8 |
| Karnofsky Index | |
| 80% | 5 (25%) |
| 90% | 14 (70%) |
| 100% | 1 (5%) |
| Size (cm) | 163.6 ± 3.8 |
| Weight (kg) | 66.7 ± 11.7 |
| Primary tumor localisation | |
| Ovary | 16 (80%) |
| Fallopian tube | 3 (15%) |
| Peritoneal | 1 (5%) |
| Histology | |
| Serous | 13 (65%) |
| Endometrial | 4 (20%) |
| Others | 3 (15%) |
| Tumor grade | |
| G2 | 3 (15.8%) |
| G3 | 16 (84.2%) |
| missing | 1 |
| Resection status | |
| R0 | 17 (85%) |
| R1 | 2 (10%) |
| R2 (< 1 cm) | 1 (5%) |
| Excised lymph nodes | 48.8 ± 25.2 |
| Affected lymph nodes | 3.4 ± 5.7 |
| pN | |
| pN0 | 7 (35%) |
| pN1 | 12 (60%) |
| pN2 | 1 (5%) |
| Staging | |
| Ascites preoperatively | 14 (70%) |
| Ascites postoperatively | 3 (15.8%) |
| Affection of liver surface | 2 (10%) |
| Peritoneal carcinosis | 17 (85%) |
| bowel resection | 4 (20%) |
| Tumor marker CA-125a | |
| Preoperatively | 1625.7 ± 3567.0 |
| 317.0 (89.0, 1369.7) | |
| Postoperatively | 160.7 ± 257.9 |
| 61.9 (21.8, 187.8) | |
| After chemotherapy | 13.3 ± 7.4 |
| 13.4 (7.0, 18.6) | |
| Before start of radiation | 14.5 ± 9.9 |
| 13.4 (6.8, 20.1) | |
| 6 weeks after radiation | 18.2 ± 17.5 |
| 9.5 (5.8, 29.1) | |
aDue to the skewed distribution of tumor marker CA-125 also median, first and third quartiles are presented
Inclusion criteria were: histologically confirmed ovarian cancer stage FIGO III, maximal cytoreductive surgery (including at least total abdominal hysterectomy, bilateral adnexectomy, omentectomy, debulking of tumor masses) with postoperative residual tumor of < 1 cm (R0, R1, R2 with maximal diameter of largest tumor residual of 1 cm), adjuvant chemotherapy with complete remission after chemotherapy, Karnofsky performance score > 60, age > 18 years, and written informed consent.
Patients were followed for 36 months after WART. Follow-up visits were scheduled at 6 weeks and 3, 6, 9, 12, 15, 18, 24, 30, and 36 months after treatment. Each visit included update of case history, documentation of adverse events according to Common Terminology Criteria for Adverse Events (CTCAE) version 3.0, assessment of quality of life using the European Organization for Research and Treatment of Cancer QLQ-C30 questionnaire, gynecologic examination, transvaginal ultrasound, and a blood sample including tumor marker checks (CA- 125). In addition, pelvic-abdominal CT or MRI scans were performed 6, 12, and 24 months after treatment. The primary endpoint was treatment tolerance, defined as the lack of any CTCAE °4 toxicity. Secondary endpoints were rate of therapy disruption, rate of therapy abortion, acute toxicity (< 6 weeks after end of WART), chronic toxicity (> 6 weeks after end of WART), quality of life, PFS and OS. The study was approved by the local Ethics Committee of Heidelberg University. Written informed consent was obtained from each participant before entering the trial.
Statistical analysis
The study was planned using an adaptive two-stage design allowing for a sample size recalculation after the interim analysis. Details on the applied design and sample size recalculation can be found in the article presenting the results on the short-term endpoints [39]. The first part included the confirmatory analysis, where the tested null hypothesis stated that the true rate of patients for whom no CTCAE °4 toxicity occurs during radiation therapy and until 6 weeks after its termination amounts to at most 70%. In this article, results on the secondary endpoints regarding follow-up data are presented. Different types of toxicities occurring 6 weeks after termination of WART are analyzed using absolute and relative frequencies. Quality of life is evaluated for each follow-up visit using mean and standard deviation. Kaplan-Meier method is used to estimate survival rates of PFS and OS. For PFS and OS, time was calculated from start of WART until disease recurrence or death. Statistical analyses were performed using the Statistical Analysis System, version 9.4 (SAS Institute, Cary NC), and figures were prepared in R, version 3.5.0 [40].
The study is registered with ClinicalTrials.gov (NCT01180504).
Results
All patients completed WART according to the study protocol. Results of the primary endpoints have already been published, showing an observed tolerability rate of the study treatment of 95%. Ten of 20 (50%) patients completed the study follow-up of 36 months. Two patients withdrew the consent to participate in the trial. Five patients were lost to follow-up, one after 9 months and two after 12 and 24 months, respectively. Three patients had died in the course of the follow-up with the last available follow-up visit at 6 weeks, 6 and 12 months, respectively.
Late toxicity analysis
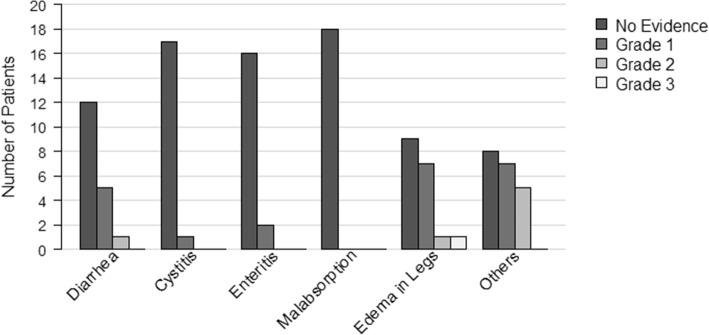
Late toxicities are shown in Fig. 1. Late side effects of intensity modulated WART mostly consisted of lower limb edema. Seven patients (38.9%) developed °1 edema, one patient (5.6%) °2 and °3 edema, respectively. No late gastrointestinal toxicities greater than °2 were observed. Five patients (27.8%) reported °1 and one patient (5.6%) °2 diarrhea, respectively. Two patients (11.1%) showed enteritic symptoms °1. One patient developed cystitis °1 (5.6%). Other side effects mostly consisted of fatigue °1-°2, constipation °1, nausea °1-°2, abdominal pain °1-°2, edema of the abdomen °1 and pruritus °1. For 3 patients, an ileus has been reported during follow-up, which was mainly associated with disease recurrence.
Fig. 1.
Late toxicities of intensity modulated WART. Incidence of late toxicities according to Common Terminology Criteria for Adverse Events version 3.0. (Maximal Common Terminology Criteria for Adverse Events grade later than 6 weeks after end of WART)
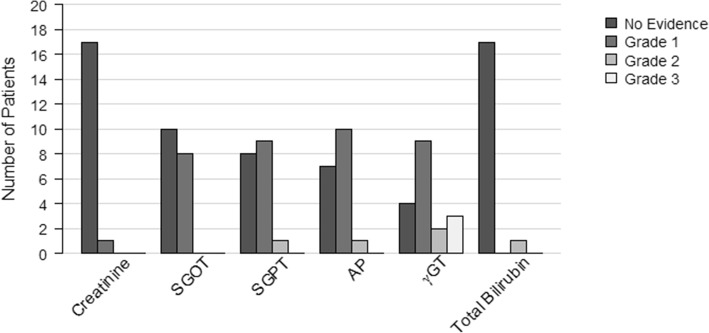
Hematological toxicities are shown in Fig. 2. There were no severe late side effects regarding renal or hepatic function. One patient (5.6%) showed elevated creatinine °1. In total, 12 patients showed slightly elevated liver enzymes with 8 patients (44.4%) showing elevated SGOT (serum glutamic oxaloacetic transaminase) °1, 9 patients (50%) and one patient (5.6%) showing elevated SGPT (serum glutamic pyruvic transaminase) °1 and °2, respectively. Three patients (16.7%) showed elevated gamma-Glutamyltransferase (γGT) °3, which is a parameter for cholestasis. Furthermore, 50%/11.1% of patients showed elevated γGT °1/°2 and 55.6%/5.6% of patients showed elevated alkaline phosphatase (AP) °1/°2, respectively. One patient (5.6%) was observed with isolated elevation of bilirubin °2.
Fig. 2.
Late hematological toxicities of intensity modulated WART. Incidence of late hematologic toxicities according to Common Terminology Criteria for Adverse Events version 3.0. (Maximal Common Terminology Criteria for Adverse Events grade later than 6 weeks after end of WART). Abbreviations: AP = alkaline phosphatase; γGT = gamma-Glutamyltranferase; S-GOT = serum glutamic oxaloacetic transaminase; S-GPT = serum glutamic pyruvic transaminase
Analysis of long-term quality of life
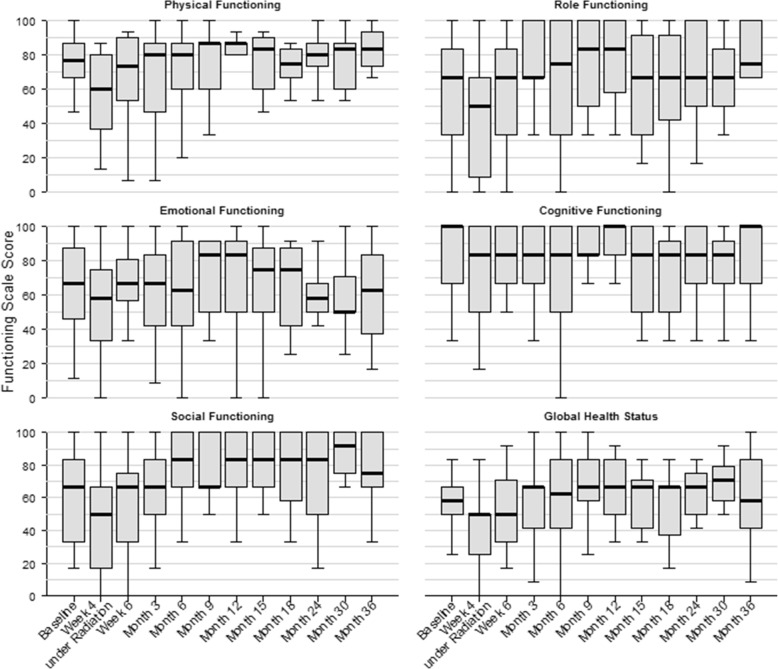
Baseline function scale scores were available for all 20 patients; baseline global health status scores were available for 19 patients. Data for week 4 during and 6 weeks after WART and month 3, 6, 9, 12, 15, 18, 24, 30 and 36 were available for 19, 19, 18, 18, 17, 15, 12, 11, 9, 8 and 8 patients, respectively. Box plots of function scale scores and global health status scores at baseline and all follow-up visits are shown in Fig. 3. Patients presented with a mean global health status score of 57.9 +/− 15.6 at baseline. We could already show that the mean global health status score decreased by 18.1 points (95% CI 7.1–29.0) 4 weeks after starting WART [39]. However, 6 months after WART the score had normalized completely with a mean score of 57.9 +/− 26.3. The score even increased above baseline level during further follow-up with a maximum mean score of 69.8 +/− 14.7 after 30 months. Physical functioning score at baseline was 74.1 +/− 19.2. The lowest score could be observed 4 weeks after starting WART, with continuous increase back to baseline after 9 months with a mean score of 76.1 +/− 19.2, which even increased further with a maximum mean score after 36 months of 83.3 +/− 11.7. Role functioning score at baseline was 58.3 +/− 33.1. The lowest score could be observed 4 weeks after starting WART, with continuous increase back to normal already 6 weeks after WART with a mean score of 60 +/− 33.5. The maximum mean score of 75.6 +/− 25.1 was observed after 12 months. Emotional functioning score at baseline was 63.9 +/− 26.4, which decreased to a minimum mean score of 55.7 +/− 29.4 4 weeks after starting WART and showed an increase back to baseline level 6 weeks after WART with a mean score of 65.8 +/− 22.9. The maximum score could be observed after 9 months with a mean score of 71.6 +/− 22.3. Cognitive functioning score at baseline was 84.2 +/− 21.3. The lowest score was observed after 6 months with a mean score of 71.3 +/− 33.7, which increased nearly back to baseline after 36 months with a mean score of 83.3 +/− 25.2. Social functioning score at baseline was 61.7 +/− 26.5, which decreased to a minimum score of 43.9 +/− 36.1 4 weeks after starting WART, with continuous increase back to baseline after 3 months. The maximum mean score of 80.6 +/− 19.9 was observed after 15 months.
Fig. 3.
Analysis of quality of life. Function scale scores and global health status scores at baseline, week 4 during radiation therapy, 6 weeks and month 3, 6, 9, 12, 15, 18, 24, 30 and 36 after radiation therapy
Analysis of oncological outcome
Eleven patients (55%) experienced disease recurrence, all of them intraperitoneally. 8 (72.7%) of all first recurrences were localized inside the peritoneal cavity only, 3 (27.3%) were localized inside as well as outside. Different patterns of intraperitoneal recurrence were observed: 2 (18.2%) patients presented with malignant ascites only, 5 (45.5%) with a new macroscopic tumor lesion and 3 (27.3%) with a combination of both. One patient (9.1%) developed a new macroscopic tumor lesion together with malignant pleural effusion. All in all, 7 patients showed up with distant metastases during follow-up. Details of recurrence patterns and salvage treatment are listed in Table 2. Partial remission after salvage therapy of first recurrence could be achieved in 5 cases, 3 patients showed progressive disease and 4 patients received further second line therapies because of progression in the course of the follow-up.
Table 2.
Patterns of recurrence and salvage treatment
| Site of recurrence | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Distant metastases | Salvage treatment | |||||||||||||
| Pat. | Number of recurrence | Peritoneal carcinomatosis | LR | HEP | PUL | pleural | MED | SPL | CER | CTX | ITX | Targeted TX | OP | others |
| 1 | 1 | x | x | x | ||||||||||
| 2 | 1 | x | x | x | x | x | ||||||||
| 3 | 1 | x | x | |||||||||||
| 3 | 2 | x | x | x | ||||||||||
| 5 | 1 | x | x | x | x | |||||||||
| 7 | 1 | x | ||||||||||||
| 8 | 1 | x | x | |||||||||||
| 8 | 2 | x | x | x | ||||||||||
| 11 | 1 | x | ||||||||||||
| 11 | 2 | x | x | x | x | |||||||||
| 11 | 3 | x | x | x | x | x | x | |||||||
| 11 | 4 | x | x | x | x | x | ||||||||
| 11 | 5 | x | x | x | x | x | ||||||||
| 13 | 1 | |||||||||||||
| 16 | 1 | x | x | x | x | |||||||||
| 18 | 1 | x | x | x | ||||||||||
| 19 | 1 | x | x | x | ||||||||||
| 19 | 2 | x | x | x | x | x | ||||||||
Pat patient, LR local recurrence, HEP hepatic, PUL pulmonal, MED mediastinal, SPL splenic, CER cerebral, CTX chemotherapy, ITX immunotherapy, TX therapy, OP operation
Median time to recurrence was 25.3 months. Estimated 1-, 2- and 3-year-PFS was 74, 51 and 40%, respectively (Fig. 4). Three patients died during follow-up after 5, 8.3 and 16.9 months, respectively. Two of them died because of disease progression, 1 patient died because of operative complications. Ten patients (50%) were known to be alive at the end of the study, 7 patients were censored in the course of the trial. Estimated 1-, 2- and 3-year-OS was 89, 83 and 83%, respectively (Fig. 5).
Fig. 4.
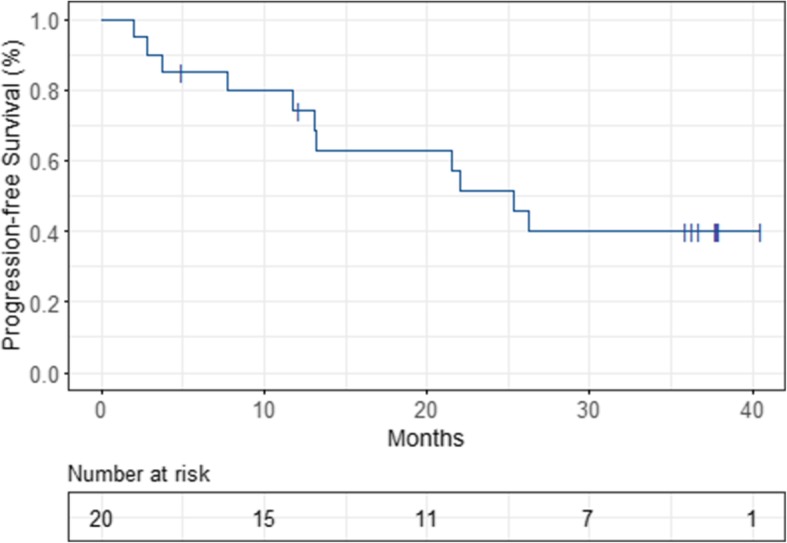
Kaplan-Meier-estimated Progression-free Survival (PFS)
Fig. 5.
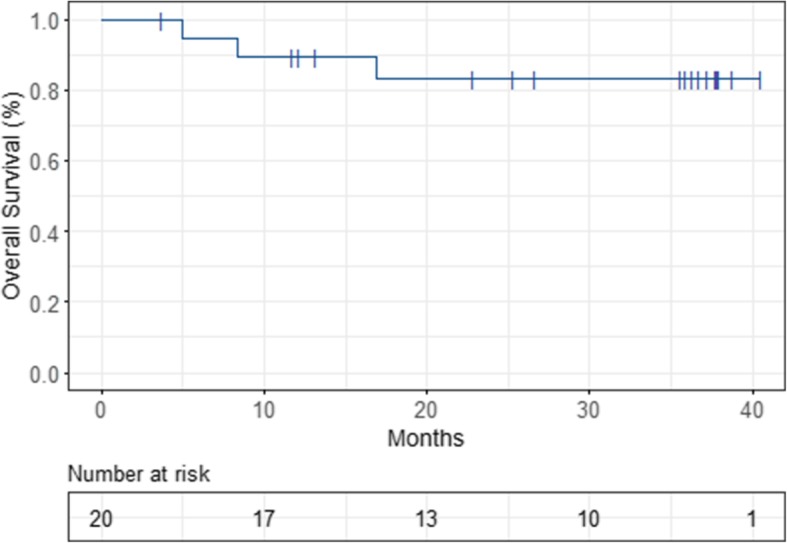
Kaplan-Meier-estimated Overall Survival (OS)
Discussion
These data confirm the previously reported excellent tolerability rate of intensity modulated WART with no clinically relevant severe acute or late side effects. The clinical feasibility of intensity modulated WART has already been shown in the OVAR-IMRT-01 trial. The use of IMRT resulted in excellent coverage of the whole peritoneal cavity and effective sparing of OARs [37]. Furthermore, the OVAR-IMRT-02 trial showed excellent treatment tolerability of intensity modulated WART with an observed tolerability rate of 95%. Treatment could be completed in all cases without any toxicity-related treatment interruption [39]. Only 1 acute CTCAE °4 toxicity was observed (thrombocytopenia). No acute nor late gastrointestinal, hepatic or renal toxicities greater than °2 have been observed [39]. Late side effects of intensity modulated WART mostly consisted of lower limb edema °1-°2, with only one patient showing °3 edema. Laboratory signs of °3 cholestasis with elevated γGT could be observed in 3 patients, without any clinical relevance. Analysis of patient-reported quality of life showed decreased quality of life during WART but also a very quick recovery after treatment completion within 6 months after WART. The score even increased above baseline level with a maximum score after 30 months. Function scale scores showed similar characteristics, showing full recovery between 6 weeks and 9 months after completion of WART and showing even higher scores compared to baseline during follow-up. However, these findings have to be interpreted with caution. As patients with progression and death aren’t represented in the scores at later time points of the follow-up, the scores might be estimated too high. Only the cognitive function scale score increased only slowly and reached baseline levels at the end of study at 36 months.
Prior studies have shown that WART can be curative in certain groups of patients [41, 42]. Our study showed an estimated 1-, 2- and 3-year-PFS of 74, 51 and 40%, respectively. Median time to recurrence was 25.3 months, which is very good compared to other trials. For example, the ICON7 trial reported a median PFS of 19.9 months after administration of chemotherapy + bevacizumab, which even dropped to 16 months in the high-risk collective [21]. 3-year-OS of our cohort was also relatively good with 83%. Informative value of this statistical analysis is very limited due to the small sample size of 20 patients, of which only 10 patients (50%) completed the study follow-up of 36 months and of which only 3 patients experienced death.
One promising development within the scope of establishing better treatment strategies for advanced ovarian cancer patients was the introduction of bevacizumab into treatment [19, 20]. In 2011, a phase 3 study reported significantly prolonged median PFS by the use of bevacizumab during and up to 10 months after carboplatin/paclitaxel chemotherapy in patients with advanced ovarian cancer [20]. In the updated analysis in 2015, PFS of the entire population was no longer statistically significant and there was no impact on OS [21]. Furthermore, the addition of bevacizumab was associated with side effects like hypertension (22.9%) and gastrointestinal wall disruption (2.6%) requiring medical therapy [20]. PARP inhibitors represent another novel therapeutic option, especially for BRCA-mutated ovarian cancer patients. The SOLO-1 trial investigated olaparib as maintenance at the completion of first-line chemotherapy in FIGO stage III–IV, BRCA-mutated ovarian cancer patients, showing a substantial PFS-benefit [24]. Serious adverse events occurred in 21% with anemia (7%) being the most common event. There are also trials evaluating combined treatment with both bevacizumab and olaparib, for example the ongoing PAOLA-1 trial.
Despite all these achievements, the main reason for progression and death is still recurrence inside the peritoneal cavity. This fact suggests that more locally aggressive treatment regimens are necessary to improve PFS and OS rates of patients with advanced ovarian cancer being at high risk for recurrence. In that context, intensity modulated WART is a promising therapeutic option for consolidation treatment. Further investigations are necessary, evaluating the potential of WART, also in combination with any of the above mentioned substances.
Conclusions
Intensity modulated WART is a promising treatment option for all advanced-stage ovarian cancer patients with excellent treatment tolerance, acceptable acute and late toxicities and only minor impact on long-term quality of life. Further randomized trials are necessary to further evaluate the promising PFS and OS rates observed in the OVAR-IMRT-02 trial. Additionally, the combination of WART with PARP inhibitors and bevacizumab should be evaluated.
Acknowledgements
We thank Deutsche Krebshilfe for supporting this work.
We acknowledge financial support by Deutsche Forschungsgemeinschaft within the funding program Open Access Publishing, by the Baden-Württemberg Ministry of Science, Research and the Arts and by Ruprecht-Karls-Universität Heidelberg.
Abbreviations
- CT
Computed tomography
- CTCAE
Common Terminology Criteria for Adverse Events
- FIGO
Fédération Internationale de Gynécologie et d‘Obstétrique
- Gy
Gray
- IMRT
Intensity Modulated Radiation Therapy
- MeV
Mega electron volt
- MRI
Magnetic resonance imaging
- OAR
Organ at risk
- OS
Overall survival
- PARP
Poly ADP ribose polymerase
- PFS
Progression-free survival
- PTV
Planning target volume
- SGOT
Serum glutamic oxaloacetic transaminase
- SGPT
Serum glutamic pyruvic transaminase
- WART
Whole abdominal radiotherapy
- γGT
gamma-Glutamyltransferase
Authors’ contributions
All authors contributed substantially to the work reported. NR, NA and KL have made substantial contributions to conception and design as well as acquisition of data. LS was substantially involved in data acquisition and patient management. SK, NR, KH and KS were mainly responsible for performing WART and for data acquisition. AS and CS were also involved in conducting the trial, data acquisition and providing resources. LB and MK were responsible for data curation, analyzed the data and performed statistical analyses. NA was mainly responsible for data curation, interpretation and writing the manuscript. Resources and supervision were provided by JD and KL. JD, KL, LB, MK have been involved in revising the manuscript critically for important intellectual content. All authors read and approved the final manuscript.
Funding
This work was supported by Deutsche Krebshilfe (reference number: 109092).
Availability of data and materials
The datasets generated and/or analysed during the current study are not publicly available due personal data protection reasons of the study participants but are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
The study was approved by the local Ethics Committee of Heidelberg University. Written informed consent was obtained from each participant before entering the trial.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Daly MB, Ozols RF. Symptoms of ovarian cancer--where to set the bar? JAMA. 2004;291:2755–2756. doi: 10.1001/jama.291.22.2755. [DOI] [PubMed] [Google Scholar]
- 3.Bast RC, Brewer M, Zou C, et al. Prevention and early detection of ovarian cancer: mission impossible? Recent Results Cancer Res. 2007;174:91–100. doi: 10.1007/978-3-540-37696-5_9. [DOI] [PubMed] [Google Scholar]
- 4.Lorusso D, Tripodi E, Maltese G, et al. Spotlight on olaparib in the treatment of BRCA-mutated ovarian cancer: design, development and place in therapy. Drug Des Devel Ther. 2018;12:1501–1509. doi: 10.2147/DDDT.S124447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ozols RF. Chemotherapy for ovarian cancer. Semin Oncol. 1999;26:34–40. [PubMed] [Google Scholar]
- 6.du Bois A, Lück H-J, Meier W, et al. A randomized clinical trial of cisplatin/paclitaxel versus carboplatin/paclitaxel as first-line treatment of ovarian cancer. J Natl Cancer Inst. 2003;95:1320–1329. doi: 10.1093/jnci/djg036. [DOI] [PubMed] [Google Scholar]
- 7.Aabo K, Adams M, Adnitt P, et al. Chemotherapy in advanced ovarian cancer: four systematic meta-analyses of individual patient data from 37 randomized trials. Advanced ovarian Cancer Trialists’ Group. Br J Cancer. 1998;78:1479–1487. doi: 10.1038/bjc.1998.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.du Bois A, Neijt JP, Thigpen JT. First line chemotherapy with carboplatin plus paclitaxel in advanced ovarian cancer--a new standard of care? Ann Oncol. 1999;10(Suppl 1):35–41. doi: 10.1023/A:1008355317514. [DOI] [PubMed] [Google Scholar]
- 9.Ozols RF, Bundy BN, Greer BE, et al. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a gynecologic oncology group study. J Clin Oncol. 2003;21:3194–3200. doi: 10.1200/JCO.2003.02.153. [DOI] [PubMed] [Google Scholar]
- 10.West RJ, Zweig SF. Meta-analysis of chemotherapy regimens for ovarian carcinoma: a reassessment of cisplatin, cyclophosphamide and doxorubicin versus cisplatin and cyclophosphamide. Eur J Gynaecol Oncol. 1997;18:343–348. [PubMed] [Google Scholar]
- 11.Piccart MJ, Bertelsen K, James K, et al. Randomized intergroup trial of cisplatin-paclitaxel versus cisplatin-cyclophosphamide in women with advanced epithelial ovarian cancer: three-year results. J Natl Cancer Inst. 2000;92:699–708. doi: 10.1093/jnci/92.9.699. [DOI] [PubMed] [Google Scholar]
- 12.Neijt JP, Engelholm SA, Tuxen MK, et al. Exploratory phase III study of paclitaxel and cisplatin versus paclitaxel and carboplatin in advanced ovarian cancer. J Clin Oncol. 2000;18:3084–3092. doi: 10.1200/JCO.2000.18.17.3084. [DOI] [PubMed] [Google Scholar]
- 13.Muggia FM, Braly PS, Brady MF, et al. Phase III randomized study of cisplatin versus paclitaxel versus cisplatin and paclitaxel in patients with suboptimal stage III or IV ovarian Cancer: a gynecologic oncology group study. J Clin Oncol. 2000;18:106. doi: 10.1200/JCO.2000.18.1.106. [DOI] [PubMed] [Google Scholar]
- 14.McGuire WP, Hoskins WJ, Brady MF, et al. Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian Cancer. N Engl J Med. 1996;334:1–6. doi: 10.1056/NEJM199601043340101. [DOI] [PubMed] [Google Scholar]
- 15.International Collaborative Ovarian Neoplasm Group Paclitaxel plus carboplatin versus standard chemotherapy with either single-agent carboplatin or cyclophosphamide, doxorubicin, and cisplatin in women with ovarian cancer: the ICON3 randomised trial. Lancet. 2002;360:505–515. doi: 10.1016/S0140-6736(02)09738-6. [DOI] [PubMed] [Google Scholar]
- 16.Kehoe S, Hook J, Nankivell M, et al. Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): an open-label, randomised, controlled, non-inferiority trial. Lancet. 2015;386:249–257. doi: 10.1016/S0140-6736(14)62223-6. [DOI] [PubMed] [Google Scholar]
- 17.Vergote I, Tropé CG, Amant F, et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian Cancer. N Engl J Med. 2010;363:943–953. doi: 10.1056/NEJMoa0908806. [DOI] [PubMed] [Google Scholar]
- 18.Pölcher M, Mahner S, Ortmann O, et al. Neoadjuvant chemotherapy with carboplatin and docetaxel in advanced ovarian cancer--a prospective multicenter phase II trial (PRIMOVAR) Oncol Rep. 2009;22:605–613. doi: 10.3892/or_00000479. [DOI] [PubMed] [Google Scholar]
- 19.Perren TJ, Swart AM, Pfisterer J, et al. A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med. 2011;365:2484–2496. doi: 10.1056/NEJMoa1103799. [DOI] [PubMed] [Google Scholar]
- 20.Burger RA, Brady MF, Bookman MA, et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med. 2011;365:2473–2483. doi: 10.1056/NEJMoa1104390. [DOI] [PubMed] [Google Scholar]
- 21.Oza AM, Cook AD, Pfisterer J, et al. Standard chemotherapy with or without bevacizumab for women with newly diagnosed ovarian cancer (ICON7): overall survival results of a phase 3 randomised trial. Lancet Oncol. 2015;16:928–936. doi: 10.1016/S1470-2045(15)00086-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ledermann JA. PARP inhibitors in ovarian cancer. Ann Oncol. 2016;27(Suppl 1):i40–i44. doi: 10.1093/annonc/mdw094. [DOI] [PubMed] [Google Scholar]
- 23.Mirza MR, Pignata S, Ledermann JA. Latest clinical evidence and further development of PARP inhibitors in ovarian cancer. Ann Oncol. 2018;29:1366–1376. doi: 10.1093/annonc/mdy174. [DOI] [PubMed] [Google Scholar]
- 24.Moore K, Colombo N, Scambia G, et al. Maintenance Olaparib in patients with newly diagnosed advanced ovarian Cancer. N Engl J Med. 2018;379:2495–2505. doi: 10.1056/NEJMoa1810858. [DOI] [PubMed] [Google Scholar]
- 25.Pujade-Lauraine E, Ledermann JA, Selle F, et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18:1274–1284. doi: 10.1016/S1470-2045(17)30469-2. [DOI] [PubMed] [Google Scholar]
- 26.Petit T, Velten M, d’Hombres A, et al. Long-term survival of 106 stage III ovarian cancer patients with minimal residual disease after second-look laparotomy and consolidation radiotherapy. Gynecol Oncol. 2007;104:104–108. doi: 10.1016/j.ygyno.2006.07.034. [DOI] [PubMed] [Google Scholar]
- 27.Wong R, Milosevic M, Sturgeon J, et al. Treatment of early epithelial ovarian cancer with chemotherapy and abdominopelvic radiotherapy: results of a prospective treatment protocol. Int J Radiat Oncol Biol Phys. 1999;45:657–665. doi: 10.1016/S0360-3016(99)00227-8. [DOI] [PubMed] [Google Scholar]
- 28.Whelan TJ, Dembo AJ, Bush RS, et al. Complications of whole abdominal and pelvic radiotherapy following chemotherapy for advanced ovarian cancer. Int J Radiat Oncol Biol Phys. 1992;22:853–858. doi: 10.1016/0360-3016(92)90779-H. [DOI] [PubMed] [Google Scholar]
- 29.Ledermann JA, Dembo AJ, Sturgeon JF, et al. Outcome of patients with unfavorable optimally cytoreduced ovarian cancer treated with chemotherapy and whole abdominal radiation. Gynecol Oncol. 1991;41:30–35. doi: 10.1016/0090-8258(91)90250-9. [DOI] [PubMed] [Google Scholar]
- 30.Sorbe B, Swedish-Norgewian Ovarian Cancer Study Group Consolidation treatment of advanced (FIGO stage III) ovarian carcinoma in complete surgical remission after induction chemotherapy: a randomized, controlled, clinical trial comparing whole abdominal radiotherapy, chemotherapy, and no further treatment. Int J Gynecol Cancer. 2003;13:278–286. doi: 10.1046/j.1525-1438.2003.13193.x. [DOI] [PubMed] [Google Scholar]
- 31.Einhorn N, Lundell M, Nilsson B, et al. Is there place for radiotherapy in the treatment of advanced ovarian cancer? Radiother Oncol. 1999;53:213–218. doi: 10.1016/S0167-8140(99)00144-9. [DOI] [PubMed] [Google Scholar]
- 32.Fyles AW, Dembo AJ, Bush RS, et al. Analysis of complications in patients treated with abdomino-pelvic radiation therapy for ovarian carcinoma. Int J Radiat Oncol Biol Phys. 1992;22:847–851. doi: 10.1016/0360-3016(92)90778-G. [DOI] [PubMed] [Google Scholar]
- 33.Dinniwell R, Lock M, Pintilie M, et al. Consolidative abdominopelvic radiotherapy after surgery and carboplatin/paclitaxel chemotherapy for epithelial ovarian cancer. Int J Radiat Oncol Biol Phys. 2005;62:104–110. doi: 10.1016/j.ijrobp.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 34.Pickel H, Lahousen M, Petru E, et al. Consolidation radiotherapy after carboplatin-based chemotherapy in radically operated advanced ovarian Cancer. Gynecol Oncol. 1999;72:215–219. doi: 10.1006/gyno.1998.5184. [DOI] [PubMed] [Google Scholar]
- 35.Mundt AJ, Lujan AE, Rotmensch J, et al. Intensity-modulated whole pelvic radiotherapy in women with gynecologic malignancies. Int J Radiat Oncol Biol Phys. 2002;52:1330–1337. doi: 10.1016/S0360-3016(01)02785-7. [DOI] [PubMed] [Google Scholar]
- 36.Hurkmans CW, Cho BCJ, Damen E, et al. Reduction of cardiac and lung complication probabilities after breast irradiation using conformal radiotherapy with or without intensity modulation. Radiother Oncol. 2002;62:163–171. doi: 10.1016/S0167-8140(01)00473-X. [DOI] [PubMed] [Google Scholar]
- 37.Rochet N, Sterzing F, Jensen AD, et al. Intensity-modulated whole abdominal radiotherapy after surgery and carboplatin/Taxane chemotherapy for advanced ovarian Cancer: phase I study. Int J Radiat Oncol Biol Phys. 2010;76:1382–1389. doi: 10.1016/j.ijrobp.2009.03.061. [DOI] [PubMed] [Google Scholar]
- 38.Rochet N, Kieser M, Sterzing F, et al. Phase II study evaluating consolidation whole abdominal intensity-modulated radiotherapy (IMRT) in patients with advanced ovarian cancer stage FIGO III - the OVAR-IMRT-02 study. BMC Cancer. 2011;11:41. doi: 10.1186/1471-2407-11-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arians N, Kieser M, Benner L, et al. Adjuvant intensity modulated whole-abdominal radiation therapy for high-risk patients with ovarian Cancer (International Federation of Gynecology and Obstetrics Stage III): first results of a prospective phase 2 study. Int J Radiat Oncol Biol Phys. 2017;99:912–920. doi: 10.1016/j.ijrobp.2017.06.2465. [DOI] [PubMed] [Google Scholar]
- 40.R Core team . A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2018. [Google Scholar]
- 41.Cannistra SA, Bast RC, Berek JS, et al. Progress in the management of gynecologic cancer: consensus summary statement. J Clin Oncol. 2003;21:129s–132s. doi: 10.1200/JCO.2003.04.003. [DOI] [PubMed] [Google Scholar]
- 42.Lambert HE, Gregory WM, Nelstrop AE, et al. Long-term survival in 463 women treated with platinum analogs for advanced epithelial carcinoma of the ovary: life expectancy compared to women of an age-matched normal population. Int J Gynecol Cancer. 2004;14:772–778. doi: 10.1136/ijgc-00009577-200409000-00006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analysed during the current study are not publicly available due personal data protection reasons of the study participants but are available from the corresponding author on reasonable request.