Abstract
Background
We aimed to investigate treatment response, survival profiles, safety profiles, and predictive factors of drug‐eluting beads‐transarterial chemoembolization (DEB‐TACE) with CalliSpheres® Microspheres (CSM) in treating Chinese hepatocellular carcinoma (HCC) patients.
Methods
A total of 66 HCC patients about to receive DEB‐TACE with CSM therapy were consecutively enrolled in this prospective cohort study. Treatment response was recorded. Besides, progression‐free survival (PFS) and overall survival (OS) were also recorded. All adverse events including pain, nausea, vomiting, fever, and liver function damage were recorded during hospitalization.
Results
37.9% of patients achieved complete response (CR) and 81.8% of patients achieved an objective response rate (ORR). For survival, mean PFS and OS were 13.7 (11.7‐15.8) months and 18.8 (95% CI: 16.3‐21.2) months, respectively. Multivariate logistic regression analysis revealed that a number of nodules ≥2 was an independent factor for worse CR; moreover, multivariate Cox's regression analysis disclosed that largest sample size ≥5 cm was an independent factor for shorter PFS, and Child‐Pugh B and BCLC stage B/C were independent predictive factors for unfavorable OS. As to AEs, numbers of patients suffered liver function damage, pain, nausea, vomiting, and fever were 29 (43.9%), 27 (40.9%), 22 (33.3%), 13 (19.7%), and 37 (56.1%), respectively.
Conclusion
Drug‐eluting beads‐transarterial chemoembolization with CSM is an effective and tolerated treatment for Chinese HCC patients, and number of nodules ≥2, largest nodule size ≥5 cm, Child‐Pugh stage B, and BCLC stage B/C correlates with unfavorable prognosis.
Keywords: drug‐eluting beads‐transarterial chemoembolization, hepatocellular carcinoma, predictive factor, safety, treatment response
1. INTRODUCTION
Hepatocellular carcinoma (HCC) is the sixth most common malignant tumor and the third leading cause of cancer‐related death worldwide; meanwhile, there are around 466 100 new cases and 422 100 deaths occurring due to HCC in China according to 2015 cancer statistics.1, 2 Moreover, a substantial percentage of HCC cases are diagnosed at an advanced stage, with portal vein invasion or intrahepatic metastases, with limited time and chance for treatment, and the highly vascular and necrotic tumors in advanced HCC are prone to develop spontaneous rupture with intraperitoneal hemorrhage, which is a fetal complication of HCC with higher incidence in Asian countries than in Western countries and is classified as stage C by the Barcelona Clinic Liver Cancer (BCLC) staging system.3, 4, 5, 6 Although the managements for HCC patients have been improved such as diagnostic examinations, surgical technologies, and neoadjuvant therapies, the 5‐year survival is still less than 20%, which is far from a satisfactory prognosis.7, 8, 9, 10, 11 Moreover, a large number of HCC patients present with poor liver function and are not suitable for the current curative treatments (liver transplantation, surgical resection, and radiofrequency ablation) for HCC. Thus, it is in urgent need of exploring additional and effective treatment for HCC patients.
Transarterial chemoembolization (TACE), which is a minimally invasive treatment, has been widely applied in HCC patients, especially in patients who were not eligible for surgery.1, 12 Conventional TACE, which forms of intra‐arterial chemotherapy using lipiodol and chemotherapeutic agents, has been utilized in last decade, while it results in severe cytotoxic effect combined with ischemia and is gradually replaced by drug‐eluting beads (DEB)‐TACE, which is able to better standardize the treatment procedure and improve the delivery capacity of high dose of chemotherapeutic agents.13, 14, 15 Regarding the beads used in DEB‐TACE, several previous studies applying HepaSphere®, DcBeads®, and some other beads have been reported.16, 17, 18, 19, 20 CalliSpheres® Microspheres (CSM), which is a type of microbeads made of polyvinyl alcohol hydrogel, is the first DEB product developed in China, and it is able to load some positively charged drugs such as adriamycin, pirarubicin, and epirubicin due to its negatively charged functional groups.21, 22, 23, 24 A previous study investigating the pharmacokinetics of DEB‐TACE with CSM in rabbit liver has demonstrated that DEB‐TACE with CSM presents well performance in drug loading, drug releasing, and tolerance, whereas DEB‐TACE with CSM has just been used for treating HCC patients in clinical practice within the past 2 years, and limited information about the treatment efficacy of DEB‐TACE with CSM in HCC patients is reported.24 Hence, this study was conducted to investigate the treatment response, survival profiles, safety profiles, and predictive factors of DEB‐TACE with CSM in treating Chinese HCC patients.
2. MATERIALS AND METHODS
2.1. Patients
Sixty‐six HCC patients who were about to receive DEB‐TACE therapy in The First Affiliated Hospital, Zhejiang University, between January 2016 and January 2018 were consecutively enrolled in this prospective cohort study. The inclusion criteria included the following: (a) diagnosed as primary HCC proven by clinical and pathological findings in accordance with the American Association for the Study of the Liver Diseases (AASLD) guidelines; (b) age ≥ 18 years; (c) at least one measurable lesion according to the modified Response Evaluation Criteria in Solid Tumors (mRECIST) criteria; (d) about to undergo DEB‐TACE therapy; (e) life expectancy more than 12 months. The exclusion criteria were as follows: (a) diagnosed as diffuse HCC; (b) with contraindications to DEB‐TACE therapy; (c) undergoing thrombolysis or anticoagulant therapy; (d) complicated with other malignancies; (e) concurrent ischemic heart disease, heart failure, severe kidney dysfunction, or serious infection; (f) pregnant or lactating women; (g) unlikely to be regularly followed up. This study was approved by the Ethical Committee of The First Affiliated Hospital, Zhejiang University, and the study protocol was carried out according to the Declaration of Helsinki. Written informed consents were obtained from all patients.
2.2. DEB‐TACE procedures
Before DEB‐TACE treatment, dexamethasone was administrated to patients for allergy prevention, and ondansetron hydrochloride injection was given to patients to prevent gastrointestinal reactions. The CSM (Jiangsu Hengrui Medicine Co., Ltd.) with diameters of 100‐300 μm were used for embolization in the DEB‐TACE procedures, and the CBs were loaded with Epirubicin (Pfizer) (50 mg, 20 mg/mL solution) and mixed with high concentration contrast agent as 1:1 ratio according to the manufacturer's guidelines. Before initiation of DEB‐TACE procedure, triphasic computerized tomography (CT) or magnetic resonance imaging (MRI) scanning was performed to assess the targeted tumor according to the Milan criteria.25, 26
Drug‐eluting beads‐transarterial chemoembolization procedures were performed in the digital subtraction angiography (DSA) room. Firstly, tumor supplying vessels were identified by the hepatic angiography using segment or subsegment super selective catheterization, and then, the femoral artery was punctured using 2.4F microcatheters (Merit Maestro, Merit Medical System, Inc) with Seldinger technique; subsequently, the CBs mixed with chemoembolization reagent and contrast agent were injected into the tumor supplying vessel through the microcatheter, and the end point for embolization was stasis in the flow of contrast agent in the arterial feeders to the tumor. After the embolization, the microcatheter was pulled out and the wound was bandaged. As for the patients with massive HCC, DEB‐TACE was performed for multiple times. In addition, all patients were admitted for observation for 24 hours with the punctured leg extended for 6‐12 hours following the procedure. After embolization, the analgesic treatment was given to patients with the use of morphine 10 mg (IM) and tramadol 50 mg (po). In addition, omeprazole 40 mg (ivgtt) was given for stomach protection, glutathione 2.4 g (ivgtt) was given for liver protection, and indomethacin suppository 50 mg was given as antipyretic treatment.
2.3. Assessment of treatment response
Patients underwent enhanced CT or MRI examination at 1‐3 months after DEB‐TACE therapy to assess treatment response according to the mRECIST. Complete response (CR) was defined as the disappearance of any intratumoral arterial enhancement in all target lesions; partial response (PR) was defined as at least a 30% decrease in the sum of diameters of viable (enhancement in the arterial phase) target lesions, taking as reference the baseline sum of the diameters of the target lesions; stable disease (SD) was defined as any cases that did not qualify either PR or progressive disease (PD); PD was defined as an increase in at least 20% in the sum of the diameters of the viable (enhancing) target lesions; taking as reference, the smallest sum of the diameters of the viable (enhancing) target lesions recorded since treatment started. Moreover, the objective response rate (ORR) was defined as CR + PR.
2.4. Assessment of survival
All patients were followed up until 2018/3/15, and the median follow‐up duration was 9.2 months (range: 2.1‐24.5 months). Progression‐free survival (PFS) and overall survival (OS) were used to assess survival profiles. PFS was defined as the duration from the time of treatment to the time of disease progression or death, and OS was defined as the duration from the time of treatment to the time of death.
2.5. Assessment of adverse events
All adverse events were recorded in detail during hospitalization, which included pain, nausea, vomiting, fever, and liver function damage, which was defined as liver function indexes elevated after treatment. Furthermore, numeric rating scale (NRS) was used to evaluate pain grade, which was a 10‐point numeric scale, with 0 representing “no pain,” 1‐3 “mild pain,” 4‐6 “moderate pain,” 7‐9 “severe pain,” and 10 “unbearable pain,” and fever was classified as follows: low‐grade fever: 37.3‐38.0°C; moderate‐grade fever: 38.1‐39.0°C; high‐grade fever: 39.1‐41.0°C; ultrahyperpyrexia: >41°C.
2.6. Statistical analysis
SPSS 21.0 statistical software (SPSS Inc) was used for statistical data processing, and GraphPad Prism 6.01 software (GraphPad Software Inc) was used for figures making. Normal distributed continuous variable was presented as mean value ± SD, skewed distributed continuous variable was presented as median (25th‐75th quantiles), and categorized variable was presented as count (percentage). Subgroup analysis of CR was conducted using chi‐square test, and survival analysis was performed using Kaplan‐Meier method and determined by the Log‐rank test. Univariate and multivariate logistic regression models were used to determine the factors predicting CR, and the multivariate logistic regression model analysis was performed using Forward Stepwise (Conditional) method. Univariate and multivariate Cox's proportional hazards regression model analyses were performed to evaluate the prognostic factors, and the multivariate Cox's proportional hazards regression model analysis was performed with the use of Forward Stepwise (Conditional LR) method. P value <0.05 was considered significant, and the significant results were shown in boldface.
3. RESULTS
3.1. Baseline characteristics
A total of 66 HCC patients with a mean age of 59.4 ± 9.9 years were enrolled in this study, including 48 males and 18 females (Table 1). There were 51 (77.3%) de novo patients and 15 (22.7%) relapsed patients. Median number of nodules was 2.0 (1.0‐4.0), and the median largest nodule size was 5.4 (2.6‐9.8) cm. Besides, 59 (89.4%) patients and 7 (10.6%) patients were classified as Child‐Pugh stage A and Child‐Pugh stage B, respectively, and 25 (37.9%), 23 (34.8%), and 18 (27.3%) patients were categorized as BCLC stage A, BCLC stage B, and BCLC stage C, respectively. Numbers of patients with 1 cycle of DEB‐TACE and patients with ≥2 cycles of DEB‐TACE were 41 (62.1%) and 25 (37.9%), respectively. The median AFP level was 98.8 (9.8‐1004.9) μg/L.
Table 1.
Characteristics of HCC patients at baseline
| Characteristics | HCC patients (N = 66) |
|---|---|
| Age (y) | 59.4 ± 9.9 |
| Gender (male/female) | 48/18 |
| De novo or relapsed (n/%) | |
| De novo | 51 (77.3) |
| Relapsed | 15 (22.7) |
| Number of nodules | 2.0 (1.0‐4.0) |
| Largest nodule size (cm) | 5.4 (2.6‐9.8) |
| Child‐Pugh stage (n/%) | |
| A | 59 (89.4) |
| B | 7 (10.6) |
| BCLC stage (n/%) | |
| A | 25 (37.9) |
| B | 23 (34.8) |
| C | 18 (27.3) |
| Cycles of DEB‐TACE (n/%) | |
| 1 cycle | 41 (62.1) |
| ≥2 cycles | 25 (37.9) |
| AFP (μg/L) | 98.8 (9.8‐1004.9) |
Data were presented as mean ± SD, median (25th‐75th quantiles) or count (%).
Abbreviations: AFP, alpha‐fetoprotein; BCLC, Barcelona Clinic Liver Cancer; DEB‐TACE, drug‐eluting bead transarterial chemoembolization; HCC, hepatocellular carcinoma.
3.2. Treatment response of patients and nodules
There were 66 patients and 162 nodules available for treatment response. Regarding the treatment response assessed by patients, CR, PR, ORR, SD, and PD rates were 37.9%, 43.9%, 81.8%, 10.6%, and 7.6%, respectively (Figure 1). As to the treatment response assessed by nodules, CR, PR, ORR, SD, and PD rates were 42.0%, 41.4%, 83.4%, 11.7%, and 4.9%, respectively.
Figure 1.
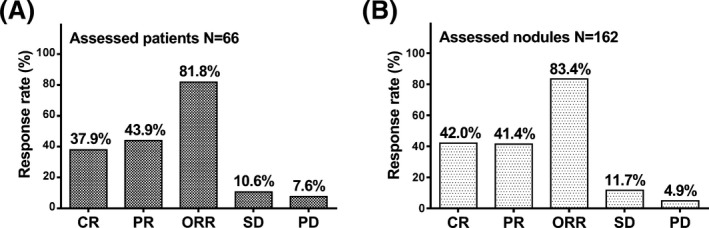
Treatment efficacy assessed by patients and modules. CR, PR, ORR, SD, and PD rates in the 66 patients who were available for treatment response were 37.9%, 43.9%, 81.8%, 10.6%, and 7.6%, respectively (A). CR, PR, ORR, SD, and PD rates assessed by nodules were 42.0%, 41.4%, 83.4%, 11.7%, and 4.9%, respectively (B). CR, Complete response; PR, partial response; ORR, objective response rate; SD, stable disease; PD, progressive disease. P < 0.05 was considered significant
3.3. Subgroup analysis of CR
All patients were divided into different groups based on baseline characteristics to evaluate the correlation of CR achievement with baseline characteristics in HCC patients treated by DEB‐TACE with CSM (Table 2); besides, number of nodules ≥2 (P < 0.001), largest nodule size ≥5 cm (P = 0.030), BCLC stage B/C (P = 0.004), and AFP ≥98.8 (μg/L) (P = 0.017) were associated with worse CR, whereas no correlation of CR with other factors was found, including age (P = 0.994), gender (P = 0.917), de novo or relapsed disease (P = 0.847), Child‐Pugh stage (P = 0.774), or cycles of DEB‐TACE (P = 0.781).
Table 2.
Subgroup analysis of CR achievement
| Characteristics | CR patients (N = 25) | Non‐CR patients (N = 41) | P value |
|---|---|---|---|
| Age (n/%) | |||
| ≥60 y | 14 (37.8) | 23 (62.2) | 0.994 |
| <60 y | 11 (37.9) | 18 (62.1) | |
| Gender (n/%) | |||
| Male | 18 (37.5) | 30 (62.5) | 0.917 |
| Female | 7 (38.9) | 11 (61.1) | |
| De novo or relapsed (n/%) | |||
| De novo | 6 (40.0) | 9 (60.0) | 0.847 |
| Relapsed | 19 (37.3) | 32 (62.7) | |
| Number of nodules (n/%) | |||
| ≥2 nodules | 10 (22.7) | 34 (77.3) | <0.001 |
| 1 nodule | 15 (68.2) | 7 (31.8) | |
| Largest nodule size (n/%) | |||
| ≥5 cm | 9 (25.7) | 26 (74.3) | 0.030 |
| <5 cm | 16 (51.6) | 15 (48.4) | |
| Child‐Pugh stage (n/%) | |||
| B | 3 (42.9) | 4 (57.1) | 0.774 |
| A | 22 (37.3) | 37 (62.7) | |
| BCLC stage (n/%) | |||
| B/C | 10 (24.4) | 31 (75.6) | 0.004 |
| A | 15 (60.0) | 10 (40.0) | |
| Cycles of DEB‐TACE (n/%) | |||
| ≥2 cycles | 10 (40.0) | 15 (60.0) | 0.781 |
| 1 cycle | 15 (36.6) | 26 (63.4) | |
| AFP (n/%) | |||
| ≥98.8 (μg/L) | 8 (24.2) | 25 (75.8) | 0.017 |
| <98.8 (μg/L) | 17 (53.1) | 15 (46.9) | |
Data were presented as count (%). The comparison was determined by chi‐square test. P value <0.05 was considered significant, and the significant results were shown in boldface.
Abbreviations: AFP, alpha‐fetoprotein; BCLC, Barcelona Clinic Liver Cancer; CR, complete response; DEB‐TACE, drug‐eluting bead transarterial chemoembolization.
3.4. Analysis of factors affecting CR
Univariate logistic regression analysis disclosed that number of nodules (≥2 vs 1) (P = 0.001), largest nodule size (≥5 cm vs <5 cm) (P = 0.033), BCLC stage (B/C vs A) (P = 0.005), and AFP (≥98.8 μg/L vs <98.8 μg/L) (P = 0.019) predicted worse CR in HCC patients (Table 3). Multivariate logistic regression was conducted to further assess independent factors predicting CR, and a number of nodules (≥2 vs 1) was verified to be an independent factor predicting the absence of CR in HCC patients (P = 0.001).
Table 3.
Factors affecting CR by logistic regression model analysis
| Parameters | Logistic regression | |||
|---|---|---|---|---|
| P value | OR | 95% CI | ||
| Lower | Higher | |||
| Univariate logistic regression | ||||
| Age (≥60 y vs <60 y) | 0.994 | 0.996 | 0.366 | 2.713 |
| Gender (male vs female) | 0.917 | 0.943 | 0.310 | 2.870 |
| Relapsed vs de novo | 0.847 | 1.123 | 0.345 | 3.649 |
| Number of nodules (≥2 vs 1) | 0.001 | 0.137 | 0.044 | 0.430 |
| Largest nodule size (≥5 cm vs <5 cm) | 0.033 | 0.325 | 0.115 | 0.913 |
| Child‐Pugh stage (B vs A) | 0.774 | 1.261 | 0.258 | 6.168 |
| BCLC stage (B/C vs A) | 0.005 | 0.215 | 0.074 | 0.628 |
| Cycles of DEB‐TACE (≥2 vs 1) | 0.782 | 1.156 | 0.416 | 3.210 |
| AFP (≥98.8 μg/L vs <98.8 μg/L) | 0.019 | 0.282 | 0.098 | 0.812 |
| Multivariate logistic regression | ||||
| Number of nodules (≥2 vs 1) | 0.001 | 0.141 | 0.045 | 0.443 |
Data were presented as P value, OR (odds ratio), and 95% CI (confidence interval). Factors affecting CR were determined by univariate and multivariate logistic regression model analyses, and the multivariate logistic regression model analysis was performed using Forward Stepwise (Conditional) method. P value <0.05 was considered significant, and the significant results were shown in boldface.
Abbreviations: AFP, alpha‐fetoprotein; BCLC, Barcelona Clinic Liver Cancer; CR, complete response; DEB‐TACE, drug‐eluting bead transarterial chemoembolization.
3.5. Survival profiles
Mean PFS and mean OS in HCC patients were 13.7 (95% CI: 11.7‐15.8) months and 18.8 (95% CI: 16.3‐21.2) months, respectively (Figure 2).
Figure 2.
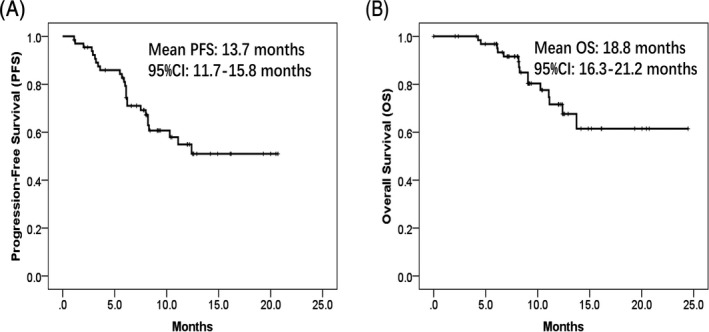
PFS and OS in HCC patients. HCC patients treated by DEB‐TACE with CSM showed mean PFS of 13.7 mo (95% CI: 11.7‐15.8 mo) (A) and mean OS of 18.8 mo (95% CI: 16.3‐21.2 mo) (B). Survival analysis was performed using Kaplan‐Meier method and determined by the Log‐rank test. PFS, progression‐free survival; OS, overall survival; HCC, hepatocellular carcinoma; DEB‐TACE, drug‐eluting beads‐transarterial chemoembolization; CSM, CalliSpheres® Microspheres
3.6. Subgroup analysis of PFS and OS
To further investigate the correlation between baseline characteristics and survival profiles, all patients were classified into different groups, and we found the largest sample size ≥5 cm (P = 0.026) (Figure 3E) was associated with shorter PFS, and number of nodules ≥2 (P = 0.082) (Figure 3D) and BCLC stage B/C (P = 0.068) (Figure 3G) were numerically associated with poor PFS but without statistical analysis, while no correlation of PFS with age (P = 0.662) (Figure 3A), gender (P = 0.723) (Figure 3B), relapsed or de novo (P = 0.870) (Figure 3C), Child‐Pugh stage (P = 0.649) (Figure 3F), cycles of DEB‐TACE (P = 0.177) (Figure 3H), or AFP level (P = 0.193) (Figure 3I) was found. As to OS, a number of nodules ≥2 (P = 0.026) (Figure 4D), largest nodule size ≥5 cm (P = 0.033) (Figure 4E), Child‐Pugh B (P = 0.045) (Figure 4F), and BCLC stage B/C (P = 0.037) (Figure 4G) were correlated with decreased OS in HCC patients, while cycles of DEB‐TACE ≥2 (P = 0.026) (Figure 4H) were correlated with prolonged OS. However, no correlation of OS with age (P = 0.177) (Figure 4A), gender (P = 0.202) (Figure 4B), relapsed or de novo (P = 0.510) (Figure 4C), or AFP level (P = 0.258) (Figure 4I) was observed.
Figure 3.
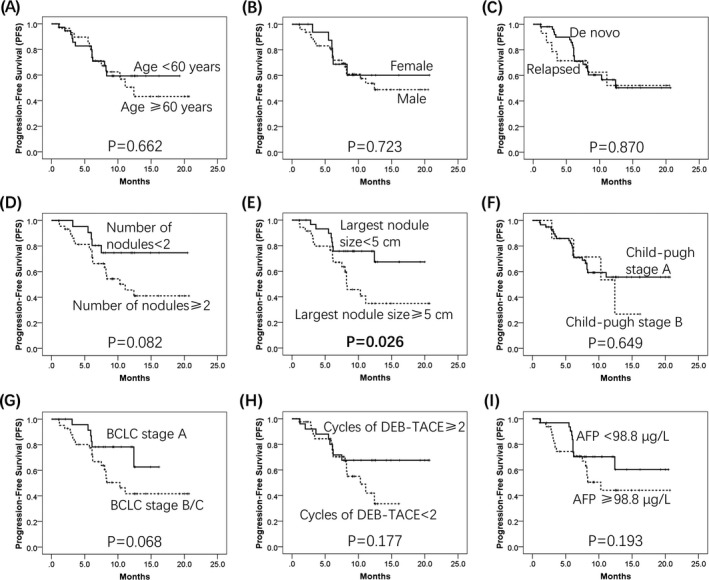
PFS analysis in subgroups. Largest sample size ≥5 cm was correlated with decreased PFS in HCC patients (E). Number of nodules ≥2 and BCLC stage B/C were numerically associated with shorter PFS (D, G). No correlation of PFS with age (A), gender (B), relapsed/de novo (C), Child‐Pugh stage (F), cycles of DEB‐TACE (H), and AFP level (I) was observed. Survival analysis was performed using Kaplan‐Meier method and determined by the Log‐rank test. PFS, progression‐free survival; HCC, hepatocellular carcinoma; BCLC, Barcelona Clinic Liver Cancer; DEB‐TACE, drug‐eluting beads‐transarterial chemoembolization; AFP, alpha‐fetoprotein
Figure 4.
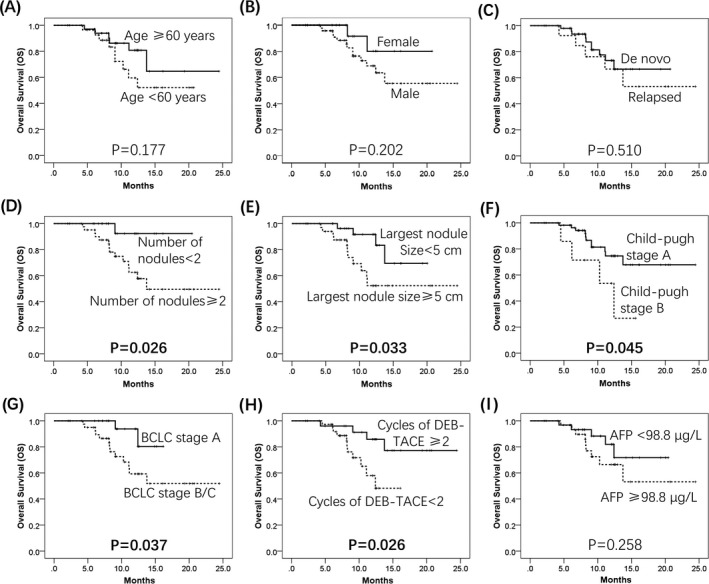
OS analysis in subgroups. Numbers of nodules ≥2 (D), largest nodule size ≥5 cm (E), Child‐Pugh B (F), and BCLC stage B/C (G) were associated with unfavorable OS in HCC patients treated by DEB‐TACE with CSM. Cycles of DEB‐TACE ≥2 were correlated with longer OS (H). No correlation of age (A), gender (B), relapsed/de novo (C), and AFP level (I) was observed. Survival analysis was performed using the Kaplan‐Meier method and determined by the Log‐rank test. OS, overall survival; BCLC, Barcelona Clinic Liver Cancer; HCC, hepatocellular carcinoma; CSM, CalliSpheres® Microspheres; DEB‐TACE, drug‐eluting beads‐transarterial chemoembolization; AFP, alpha‐fetoprotein
3.7. Analysis of factors affecting PFS and OS
Largest nodule size (≥5 cm vs <5 cm) (P = 0.033) predicted poor PFS according to univariate Cox's regression analysis, which was further verified as an independent predictive factor for shorter PFS in multivariate Cox's regression analysis in HCC patients (Table 4) (P = 0.040). As to factors affecting OS, univariate Cox's regression disclosed that largest nodule size (≥5 cm vs <5 cm) (P = 0.045) and Child‐Pugh stage (B vs A) (P = 0.030) predicted worse OS, while cycles of DEB‐TACE (≥2 vs 1) (P = 0.036) predicted better OS in HCC patients (Table 5). Moreover, multivariate Cox's regression was performed, and Child‐Pugh stage (B vs A) (P = 0.011) and BCLC stage (B/C vs A) (P = 0.044) were independent factors predicting unfavorable OS in HCC patients.
Table 4.
Cox's proportional hazards regression model analysis of factors affecting PFS
| Parameters | Cox's regression | |||
|---|---|---|---|---|
| P value | HR | 95% CI | ||
| Lower | Higher | |||
| Univariate Cox's regression | ||||
| Age (≥60 y vs <60 y) | 0.663 | 0.843 | 0.391 | 1.819 |
| Gender (male vs female) | 0.725 | 1.178 | 0.473 | 2.938 |
| Relapsed vs de novo | 0.870 | 1.079 | 0.431 | 2.702 |
| Number of nodules (≥2 vs 1) | 0.092 | 2.312 | 0.871 | 6.138 |
| Largest nodule size (≥5 cm vs <5 cm) | 0.033 | 2.490 | 1.078 | 5.751 |
| Child‐Pugh stage (B vs A) | 0.601 | 1.329 | 0.457 | 3.866 |
| BCLC stage (B/C vs A) | 0.077 | 2.280 | 0.915 | 5.681 |
| Cycles of DEB‐TACE (≥2 vs 1) | 0.185 | 0.566 | 0.244 | 1.312 |
| AFP (≥98.8 μg/L vs <98.8 μg/L) | 0.200 | 1.688 | 0.757 | 3.763 |
| Multivariate Cox's regression | ||||
| Largest nodule size (≥5 cm vs <5 cm) | 0.040 | 2.420 | 1.040 | 5.629 |
Data were presented as P value, HR (hazards ratio), and 95% CI (confidence interval). Factors affecting PFS were determined by univariate and multivariate Cox's proportional hazards regression model analyses, and the multivariate Cox's proportional hazards regression model analysis was performed using Forward Stepwise (Conditional LR) method. P value <0.05 was considered significant, and the significant results were shown in boldface.
Abbreviations: AFP, alpha‐fetoprotein; BCLC, Barcelona Clinic Liver Cancer; DEB‐TACE, drug‐eluting bead transarterial chemoembolization; PFS, progression‐free survival.
Table 5.
Cox's proportional hazards regression model analysis of factors affecting OS
| Parameters | Cox's regression | |||
|---|---|---|---|---|
| P value | HR | 95% CI | ||
| Lower | Higher | |||
| Univariate Cox's regression | ||||
| Age (≥60 y vs <60 y) | 0.187 | 0.498 | 0.177 | 1.403 |
| Gender (male vs female) | 0.220 | 2.542 | 0.573 | 11.272 |
| Relapsed vs de novo | 0.513 | 1.438 | 0.484 | 4.271 |
| Number of nodules (≥2 vs 1) | 0.057 | 7.164 | 0.942 | 54.510 |
| Largest nodule size (≥5 cm vs <5 cm) | 0.045 | 3.242 | 1.029 | 10.215 |
| Child‐Pugh stage (B vs A) | 0.030 | 3.623 | 1.130 | 11.618 |
| BCLC stage (B/C vs A) | 0.056 | 4.279 | 0.964 | 18.997 |
| Cycles of DEB‐TACE (≥2 vs 1) | 0.036 | 0.273 | 0.081 | 0.918 |
| AFP (≥98.8 μg/L vs <98.8 μg/L) | 0.266 | 1.862 | 0.622 | 5.569 |
| Multivariate Cox's regression | ||||
| Child‐Pugh stage (B vs A) | 0.011 | 4.803 | 1.426 | 16.180 |
| BCLC stage (B/C vs A) | 0.044 | 4.752 | 1.044 | 21.621 |
Data were presented as P value, HR (hazards ratio), and 95% CI (confidence interval). Factors affecting OS were determined by univariate and multivariate Cox's proportional hazards regression model analyses, and the multivariate Cox's proportional hazards regression model analysis was performed using Forward Stepwise (Conditional LR) method. P value <0.05 was considered significant.
Abbreviations: AFP, alpha‐fetoprotein; BCLC, Barcelona Clinic Liver Cancer; DEB‐TACE, drug‐eluting bead transarterial chemoembolization; OS, overall survival.
3.8. Adverse events
The occurrences of liver function damage, pain, nausea, vomiting, and fever were 29 (43.9%), 27 (40.9%), 22 (33.3%), 13 (19.7%), and 37 (56.1%) in HCC patients, respectively (Table 6). Among cases with pain, there were 15 (22.7%), 7 (10.6%), and 5 (7.6%) cases classified as mild pain, moderate pain, and severe pain, respectively. In addition, fever grade was categorized as low‐grade fever, moderate‐grade fever and high‐grade fever, which were observed in 21 (31.8%), 6 (9.1%), and 10 (15.2%) cases, respectively.
Table 6.
Adverse events
| Events | Patients (n/%) |
|---|---|
| Liver function damage | 29 (43.9) |
| Pain (n/%) | 27 (40.9) |
| Pain grade (NRS) (n/%) | |
| Mild pain | 15 (22.7) |
| Moderate pain | 7 (10.6) |
| Severe pain | 5 (7.6) |
| Nausea (n/%) | 22 (33.3) |
| Vomiting (n/%) | 13 (19.7) |
| Fever (n/%) | 37 (56.1) |
| Fever grade (n/%) | |
| Low‐grade fever | 21 (31.8) |
| Moderate‐grade fever | 6 (9.1) |
| High‐grade fever | 10 (15.2) |
Data were presented as count (%).
Abbreviation: NRS, numeric rating scale.
4. DISCUSSION
In this study, we found that: (a) DEB‐TACE with CSM achieved CR and ORR of 7.9% and 81.8% in patients, and the mean PFS and OS were 13.7 (95% CI: 11.7‐15.8) months and 18.8 (95% CI: 16.3‐21.2) months, respectively; (b) number of nodules (≥2) was an independent factor for CR absence, and largest nodule size (≥5 cm), increased Child‐Pugh stage, and higher BCLC stage were independent factors for predicting poor survival profiles; (c) adverse events including liver function damage, pain, nausea, vomiting, and fever were observed in HCC patients after treated by DEB‐TACE with CSM.
Drug‐eluting beads‐transarterial chemoembolization is designed with drug‐loaded microspheres, and it is able to realize a favorable pharmacokinetic profile: The release of cytotoxic agents is in a sustained manner, which enhances the intratumoral concentration of loaded drugs and reduces the systemic drug‐related toxicity.27, 28, 29 Some previous studies are carried out to explore the treatment response of DEB‐TACE in liver cancer patients.16, 17, 18, 19 For instance, a study reveals that DEB‐TACE with DcBeads® achieves CR of 22% and ORR of 89% in 34 Irish HCC patients.19 For DEB‐TACE using CSM, a cohort study enrolling 38 Chinese liver cancer patients shows that DEB‐TACE with CSM obtains CR rate of 26.3% and ORR rate of 86.8%.18 Another study discloses CR of 16.2% and ORR of 75.8% in liver cancer patients treated by DEB‐TACE with CSM.22 Considering that CSM is the first drug‐eluting beads product in China, more comprehensive studies investigating the efficacy of DEB‐TACE with CSM in Chinese patients are needed. In this present study, we found that DEB‐TACE with CSM achieved CR rate of 37.9% and ORR rate of 81.8% in HCC patients, and CR in our study was numerically higher compared with the previous studies, which might due to: (a) the beads used in the DEB‐TACE procedures varied among studies, which might result in different treatment responses, such as, in a previous study they used DcBeads® in the DEB‐TACE procedure19; (b) the criteria that used to assess treatment responses were different among studies, which led to differentiate CR rates; for example, a previous study used mRECIST criteria and European Association for the Study of the Liver (EASL) criteria, while only mRECIST criteria was used in our study19; (c) histological types of liver cancers were inconsistent among studies, and thereby, different CR rates were observed; for instance, a previous study enrolled HCC patients, primary intrahepatic cholangiocarcinoma patients, and secondary liver cancer patients, while only HCC patients were included in our study18, 22; (d) skills of surgeons might be different among these studies, thereby leading to different CR rates. As to survival profiles, there are some previous studies investigate OS in liver cancer patients treated by DEB‐TACE; for example, median OS treated by DEB‐TACE with DcBeads® in a study is 369 days (95% CI: 310‐589 days), and another study shows that mean OS in liver cancer patients treated by DEB‐TACE with CSM is 384 days (95% CI 373‐395 days).17, 22 In our study, we observed that mean PFS and OS in 66 HCC patients treated by DEB‐TACE with CSM were 13.7 (95% CI: 11.7‐15.8) months and 18.8 (95% CI: 16.3‐21.2) months, respectively. OS in our study was numerically better compared with the previous studies, and the reasons might be the following: (a) The follow‐up durations that might affect OS data were different among studies; for example, the median follow‐up duration in a previous studies was shorter than that in our study, and thus, decreased OS was observed in the previous study22; (b) the beads were different among these studies, and thus, OS might be different17; (c) baseline characteristics of patients vary among studies; for instance, age in a previous study (85.5% patients >60 years) was older than that in our study (mean age of 59.4 ± 9.9 years), thereby leading to reduced OS in the previous study compared to ours17; (d) surgical skills were critical to OS in patients treated by DEB‐TACE; thus, inconsistent surgical skills among studies might result in different OS.17, 22 These results revealed that DEB‐TACE with CSM achieved satisfactory CR, ORR, and survival profiles in Chinese HCC patients.
As to factors affecting treatment outcomes by DEB‐TACE, a previous study discloses that a number of nodules >3 and higher BCLC stage is associated with decreased ORR achievements in HCC patients treated by DEB‐TACE with CSM.21 A study reveals that higher Child‐Pugh stage, raised ECOG stage and larger number of nodules are correlated with worse OS in 154 HCC patients received DEB‐TACE with DcBeads®.17 In addition, a study enrolling 38 liver cancer patients treated by DEB‐TACE with CSM discloses that higher Child‐Pugh stage, increased BCLC stage, and largest nodule size ≥5.7 cm are associated with poor OS.18 In line with these previous studies, we found that a number of nodules (≥2) was an independent factor predicting CR absence, and largest nodule size (≥5 cm), increased Child‐Pugh stage, and higher BCLC stage were independent predictive factors for poor survival profiles. The explanations for these results were that: (a) for number of nodules (≥2) or larger nodule size, it exists more possibility for vascular invasion and satellite nodules; thus, it was hard to perform complete chemoembolization in patients with a number of nodules (≥2) or larger nodule size; therefore, number of nodules (≥2) and largest nodule size (≥5 cm) predicted unsatisfactory CR or survival profiles in HCC patients30, 31, 32; (b) increased Child‐Pugh stage was associated with worse liver function, which decreased the tolerance to DEB‐TACE surgical treatment, thereby leading to poor survival profiles; (c) for elevated BCLC stage, it meant worse behavioral states, advanced tumor status, and severer liver function, which might be related to poor physical condition, difficult embolization, and less tolerance to treatment as described above; thus, higher BCLC stage predicted unfavorable survival profiles in HCC patients.33
After being treated by DEB‐TACE in liver cancer patients, there are several common AEs reported, including pain, nausea, and fever. An interesting study displays that pain, fever, nausea, vomiting, and liver dysfunction occur in 63.3%, 34.7%, 18.4%, 28.6%, and 55.1% liver cancer patients treated by DEB‐TACE with CSM.18 Besides, AEs including pain (96%), vomiting (16%), and fever (77%) are also reported in another study that treats HCC patients by DEB‐TACE with CSM.21 In this present study, the incidences of liver function damage, pain, nausea, vomiting, and fever were 29 (43.9%), 27 (40.9), 22 (33.3%), 13 (19.7%), and 37 (56.1%), respectively. Compared with the previous studies, fewer incidences of pain and fever were observed in our study, which might result from that we performed preventions for pain, allergy, and gastrointestinal reactions before DEB‐TACE procedures; therefore, AEs such as pain and fever might be milder in our study.
There were some limitations in our study: (a) Sample size in our study (N = 66) was relatively small, and thus, the statistical efficacy might be relatively low, while the sample size in our study was already higher than that in previous studies; moreover, considering DEB‐TACE with CSM had just been used for treating HCC patients within the past 2 years, and the sample size was limited; (b) the median follow‐up duration of 9.2 months (range: 2.1‐24.5 months) in our study was relatively short, so long‐term efficacy of DEB‐TACE with CSM in HCC patients was not investigated; (c) as a prospective study, we did not intervene the treatment and assignment of patients, and thus, some confounding factors might exist in this study, while their influences have been eliminated by our univariate and multivariate analysis.
In conclusion, DEB‐TACE with CSM is an effective and tolerated treatment for Chinese HCC patients, and number of nodules ≥2, largest nodule size ≥5 cm, Child‐Pugh stage B, and BCLC stage B/C correlates with unfavorable prognosis.
CONFLICTS OF INTEREST
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article
ACKNOWLEDGMENTS
None.
Zhang X, Lin X, Qiu H, Peng Z. An investigation of efficacy, safety, and prognostic factors of drug‐eluting beads‐transarterial chemoembolization operation with CalliSpheres® Microspheres in treating Chinese hepatocellular carcinoma patients. J Clin Lab Anal. 2019;33:e22975 10.1002/jcla.22975
Contributor Information
Xin Zhang, Email: zx_zhangxin0921@163.com.
Xiao Lin, Email: lin1987xiao@outlook.com.
REFERENCES
- 1. Ni JY, Xu LF, Wang WD, Sun HL, Chen YT. Conventional transarterial chemoembolization vs microsphere embolization in hepatocellular carcinoma: a meta‐analysis. World J Gastroenterol. 2014;20(45):17206‐17217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115‐132. [DOI] [PubMed] [Google Scholar]
- 3. Yang H, Chen K, Wei Y, et al. Treatment of spontaneous ruptured hepatocellular carcinoma: a single‐center study. Pak J Med Sci. 2014;30(3):472‐476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hulin A, Stocco J, Bouattour M. Clinical pharmacokinetics and pharmacodynamics of transarterial chemoembolization and targeted therapies in hepatocellular carcinoma. Clin Pharmacokinet. 2019. 10.1007/s40262-019-00740-w [DOI] [PubMed] [Google Scholar]
- 5. Chedid AD, Klein PW, Tiburi MF, Villwock MM, Bassani LE, Chedid MF. Spontaneous rupture of hepatocellular carcinoma with haemoperitoneum: a rare condition in Western countries. HPB (Oxford). 2001;3(3):227‐230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhuang BW, Li W, Xie XH, Hu HT, Lu MD, Xie XY. Sorafenib versus hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma: a systematic review and meta‐analysis. Jpn J Clin Oncol. 2019. [DOI] [PubMed] [Google Scholar]
- 7. Page AJ, Cosgrove DC, Philosophe B, Pawlik TM. Hepatocellular carcinoma: diagnosis, management, and prognosis. Surg Oncol Clin N Am. 2014;23(2):289‐311. [DOI] [PubMed] [Google Scholar]
- 8. Kennedy AS, Sangro B. Nonsurgical treatment for localized hepatocellular carcinoma. Curr Oncol Rep. 2014;16(3):373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Asham EH, Kaseb A, Ghobrial RM. Management of hepatocellular carcinoma. Surgical Clin North Am. 2013;93(6):1423‐1450. [DOI] [PubMed] [Google Scholar]
- 10. Zamboni CG, Kozielski KL, Vaughan HJ, et al. Polymeric nanoparticles as cancer‐specific DNA delivery vectors to human hepatocellular carcinoma. J Control Release. 2017;263:18‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. El‐Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365(12):1118‐1127. [DOI] [PubMed] [Google Scholar]
- 12. Zou JH, Zhang L, Ren ZG, Ye SL. Efficacy and safety of cTACE versus DEB‐TACE in patients with hepatocellular carcinoma: a meta‐analysis. J Dig Dis. 2016;17(8):510‐517. [DOI] [PubMed] [Google Scholar]
- 13. Raoul J‐L, Sangro B, Forner A, et al. Evolving strategies for the management of intermediate‐stage hepatocellular carcinoma: available evidence and expert opinion on the use of transarterial chemoembolization. Cancer Treat Rev. 2011;37(3):212‐220. [DOI] [PubMed] [Google Scholar]
- 14. Facciorusso A, Di Maso M, Muscatiello N. Drug‐eluting beads versus conventional chemoembolization for the treatment of unresectable hepatocellular carcinoma: a meta‐analysis. Dig Liver Dis. 2016;48(6):571‐577. [DOI] [PubMed] [Google Scholar]
- 15. Woo HY, Heo J. Transarterial chemoembolization using drug eluting beads for the treatment of hepatocellular carcinoma: now and future. Clin Mol Hepatol. 2015;21(4):344‐348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ni J‐Y, Sun H‐L, Chen Y‐T, et al. Drug‐eluting bead transarterial chemoembolization in the treatment for unresectable soft tissue sarcoma refractory to systemic chemotherapy: a preliminary evaluation of efficacy and safety. J Cancer Res Clin Oncol. 2018;144(1):157‐163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kloeckner R, Weinmann A, Prinz F, et al. Conventional transarterial chemoembolization versus drug‐eluting bead transarterial chemoembolization for the treatment of hepatocellular carcinoma. BMC Cancer. 2015;15:465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wu X, Chen R, Zheng W, Hu H. Comprehensive analysis of factors affecting clinical response and short‐term survival to drug‐eluting bead transarterial chemoembolization for treatment in patients with liver cancer. Technol Cancer Res Treat. 2018;17:1533033818759878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dorcaratto D, Udupa V, Hogan NM, et al. Does neoadjuvant doxorubicin drug‐eluting bead transarterial chemoembolization improve survival in patients undergoing liver transplant for hepatocellular carcinoma? Diagn Interv Radiol. 2017;23(6):441‐447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Melchiorre F, Patella F, Pescatori L, et al. DEB‐TACE: a standard review. Future Oncol. 2018;14(28):2969‐2984. [DOI] [PubMed] [Google Scholar]
- 21. Zhou GH, Han J, Sun JH, et al. Efficacy and safety profile of drug‐eluting beads transarterial chemoembolization by CalliSpheres(R) beads in Chinese hepatocellular carcinoma patients. BMC Cancer. 2018;18(1):644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhang X, Zhou J, Zhu DD, et al. CalliSpheres(R) drug‐eluting beads (DEB) transarterial chemoembolization (TACE) is equally efficient and safe in liver cancer patients with different times of previous conventional TACE treatments: a result from CTILC study. Clin Transl Oncol. 2019;21(2):167‐177. [DOI] [PubMed] [Google Scholar]
- 23. Wu B, Zhou J, Ling G, Zhu D, Long Q. CalliSpheres drug‐eluting beads versus lipiodol transarterial chemoembolization in the treatment of hepatocellular carcinoma: a short‐term efficacy and safety study. World J Surg Oncol. 2018;16(1):69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhang S, Huang C, Li Z, et al. Comparison of pharmacokinetics and drug release in tissues after transarterial chemoembolization with doxorubicin using diverse lipiodol emulsions and CalliSpheres Beads in rabbit livers. Drug Delivery. 2017;24(1):1011‐1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yao FY. Liver transplantation for hepatocellular carcinoma: beyond the Milan criteria. Am J Transplantation. 2008;8(10):1982‐1989. [DOI] [PubMed] [Google Scholar]
- 26. Lencioni R, de Baere T, Burrel M, et al. Transcatheter treatment of hepatocellular carcinoma with Doxorubicin‐loaded DC Bead (DEBDOX): technical recommendations. Cardiovasc Intervent Radiol. 2012;35(5):980‐985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nishikawa H, Kita R, Kimura T, Osaki Y. Transcatheter arterial embolic therapies for hepatocellular carcinoma: a literature review. Anticancer Res. 2014;34(12):6877‐6886. [PubMed] [Google Scholar]
- 28. Lencioni R, Petruzzi P, Crocetti L. Chemoembolization of hepatocellular carcinoma. Semin Intervent Radiol. 2013;30(1):3‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lencioni R, Crocetti L. Local‐regional treatment of hepatocellular carcinoma. Radiology. 2012;262(1):43‐58. [DOI] [PubMed] [Google Scholar]
- 30. Hu H, Chen GF, Yuan W, Wang JH, Zhai B. Microwave ablation with chemoembolization for large hepatocellular carcinoma in patients with cirrhosis. Int J Hyperth. 2018;34(8):1351‐1358. [DOI] [PubMed] [Google Scholar]
- 31. Bruix J, Llovet JM. Prognostic prediction and treatment strategy in hepatocellular carcinoma. Hepatology. 2002;35(3):519‐524. [DOI] [PubMed] [Google Scholar]
- 32. European Association for the Study of the L , European Organisation for R, Treatment of C . EASL‐EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56(4):908‐943. [DOI] [PubMed] [Google Scholar]
- 33. Lin SC, Shih SC, Kao CR, Chou SY. Transcatheter arterial embolization treatment in patients with hepatocellular carcinoma and risk of pulmonary metastasis. World J Gastroenterol. 2003;9(6):1208‐1211. [DOI] [PMC free article] [PubMed] [Google Scholar]


