Abstract
The aim of the present work was to evaluate the nematicidal potential of Flammulina velutipes and its spent mushroom compost. Additionally, the nematicidal activity of enzymes and metabolites was analyzed. Isolated F. velutipes and its SMC had significant nematicidal effect on Panagrellus sp. larvae. The percentages of reduction in relation to the control group were: 69, 57.5 and 70% for SMC and 56, 24.5 and 26.6% for the isolated fungus, for 24, 48 and 72 h, respectively. The active SMC crude extract showed nematicidal action with reduction percentages of 43 and 57% for 24 and 48 h of incubation, respectively. The boiled crude extract also showed nematicidal action, however, the reduction percentages were lower than those of the active extract. This demonstrated that the nematicidal action was due to enzyme activities and other metabolites. The results demonstrated that SMC, the isolated fungus, the crude extract and the boiled crude extract showed a significant percentage of reduction on Panagrellus sp. larvae. SMC evidenced a higher nematicidal activity than the isolated fungus. In addition, nematophagous activity of F. velutipes was observed for the first time.
Keywords: Flammulina velutipes, Spent mushroom compost, Biological control, Nematicidal, Nematophagous fungus
Introduction
Edible mushrooms have outstanding importance concerning their nutritional composition. It is a rich source of essential amino acids, vitamins and fibers. In addition, it is a source for possible pharmaceutical drugs, considering that certain polysaccharides have antimicrobial and antioxidant activity described (Dong et al. 2017; Jing et al. 2014; Leung et al. 1997). Eight million tons of mushrooms are consumed per year, mainly in Asian countries (Nakamura et al. 2011). Worldwide consumption of edible mushrooms increased from 1 to 4.7 kg in the period from 1997 to 2013 (Royse et al. 2017). The Golden Needle Mushroom or Enokitake (Flammulina velutipes) is the fourth most consumed edible mushroom in the world. It has innumerable nutritional properties, since it is rich in β-glucans, terpenes, mineral elements, unsaturated fatty acids, ascorbic acid, phenolic and other compounds (Rathore et al. 2017). It also has pharmacological properties such as antimicrobial, antioxidant, immunomodulatory and antitumor activities (Jing et al. 2014; Leifa et al. 2001; Leung et al. 1997).
Most of the industrial production of F. velutipes uses lignocellulose rich agricultural residues such as paper waste, hazelnut leaves, wheat straw or sawdust as substrates (Leifa et al. 2001; Yildiz et al. 2002). The waste product of the mushroom industry, known as spent mushroom compost (SMC), contains residual organic and inorganic substances, like nitrogen, potassium and phosphorus, mushroom mycelium as well as a large number of enzymes just as cellulase, hemicellulase, protease and laccase (Zhang and Sun 2014). One kg of fresh mushrooms results in 5 kg of SMC (Finney et al. 2009), so a huge amount of SMC is generated. The amount of SMC discarded per year can be estimated at approximately 210,000 tons (Ishihara et al. 2018). Thus, the elimination of such an amount of waste poses a serious problem for the mushroom industry. Therefore, recycling SMC is beneficial and urgent.
Several possible uses of SMC, derived from the production of many mushroom species, are being studied. SMCs can be used as a substrate for other fungi, animal feed, the promotion of animal health in bioremediation, the manufacturing of packaging and building materials, biofuels and enzymes (Grimm and Wösten 2018). In addition, certain edible mushrooms are known for having nematophagous action and producing nematicidal metabolites (Genier et al. 2015; Soares et al. 2019; Sufiate et al. 2017).
Every year, plant-parasitic nematodes cause serious economic loss, threatening global food security. It is estimated that the losses mount up to more than 215 billion US dollars around the world due to plant pathologies caused by nematodes, and the crop yield losses may be higher than those caused by insect pests or weeds. The reason for the arduous nematode control lies in the insidious nature of the nematode and the difficulty to detect the pathology, as the cause of the plant symptoms can be easily confounded with other pathogens or abiotic factors. As a result, every year about 500 million US dollars are spent on nematode control (Abd-Elgawad and Askary 2018; Hassan et al. 2012).
Chemical nematicides are very efficient, however, the pressure to use ecofriendly products for pest control management is growing daily. Biological control or biopesticides are alternatives available to substitute these products. Biological control uses nematode antagonists such as nematophagous fungi, while biopesticides are products from plants or microorganisms containing such compounds as enzymes or other metabolites with nematicidal effects (Akhtar and Malik 2000). Nematophagous fungi have shown different strategies to predate nematodes and are able to adhere and penetrate the cuticle of all stages, using the nematode as a nutrient source. However, the fungus is sensitive to abiotic factors, such as humidity. At this point, SMC has great potential as a product for biological control due to its high levels of mycelium, residual enzymes, high humidity and unique microbiota that may contain other nematode antagonists (Aslam 2013; Siddiqui and Mahmood 1996).
Thus, the aim of the present work is to evaluate the nematicidal potential of F. velutipes, its spent mushroom compost and metabolites.
Materials and methods
Organisms
The spent mushroom compost (SMC) from the production of F. velutipes species was obtained from the Urakami Group, Mogi das Cruzes, São Paulo-Brazil. A few grams of the SMC were added to a Petri dish containing Potato Dextrose Agar (PDA) medium and chloramphenicol (0.5 g/l). After 2 weeks, the fungus F. velutipes was isolated in another Petri dish containing PDA medium and identified by comparison with what is described in the literature (Redhead and Petersen 1999). In addition, the crude extract was obtained from the SMC with water, following the methodology described by Nakajima et al. (2018).
Free-living nematodes of the genus Panagrellus were used in the experiment. These organisms are being kept in Petri dishes in an oatmeal-based culture medium, in the Laboratory of Pathology of Invertebrates, Institute of Tropical Pathology and Public Health (IPTSP) of the Federal University of Goiás, Brazil. Panagrellus are free-living nematodes used worldwide as a model for studies in many laboratories, including for tests with nematophagous fungi (Gomes et al. 2000).
Enzyme assay
Proteolytic activity of the extract obtained from the SMC was measured (Soares et al. 2013). The volumes of the solutions were: 100 µl of crude extract, 400 µl of citrate–phosphate buffer 100 mM, pH 6.0 and 500 µl of casein 1%. The reaction medium was incubated for 60 min at 37 °C after which the reaction was stopped by adding 1 ml of trichloroacetic acid (TCA) 10%. After 10 min, the reaction medium was centrifuged at 10,000g for 5 min and the supernatant was collected for the determination of absorbance in a spectrophotometer at 280 nm. A standard tyrosine curve was constructed for the quantification of the enzymatic activity. One protease unit was defined as the amount of enzyme required to release 1.0 μm tyrosine per minute under the assay conditions.
Experimental assays
Flammulina velutipes
Two groups were formed in Petri dishes of 4.5 cm in diameter containing 2% water-agar (WA2%), one treated group and one control group, with 8 replicates for each group. In the treated group, after the growth (5 days) of the fungus F. velutipes in WA2% medium, approximately 1000 larvae Panagrellus sp. were added to the middle of the plate. The control group contained only 1000 larvae of Panagrellus sp. (without fungus). Both groups of plates were incubated in the dark, at 28 °C. For 3 days, every 24 h, 10 random fields in each plate of the treated and control groups were observed under an optical microscope at 10 × objective, counting the number of intact larvae (Sufiate et al. 2017). Photomicrographs were performed to prove the activity of nematode destruction and the possible production of toxin microbeads. The experiment was repeated three times.
Spent mushroom compost (SMC)
Analogously to the F. velutipes assay, a Petri dish assay using the spent mushroom compost was performed. Two groups were formed in Petri dishes of 4.5 cm in diameter containing WA2%, one treated group and one control group, with 8 replicates for each group. In the treated group, approximately 0.1 g of the SMC of F. velutipes was inoculated in the middle of Petri dishes containing medium WA2% and, after 5 days, approximately 1000 larvae Panagrellus sp. were added to the middle of the plate. The control group contained only the 1000 larvae of Panagrellus sp. (without SMC). Both groups of plates were incubated in the dark, at 28 °C. The analysis was performed as previously described. The experiment was repeated three times.
Crude extract
Nematicidal activity of the extract obtained from the SMC was evaluated on the model nematodes Panagrellus sp. Three groups were formed in sterile microtubes, two treated groups and one control group. Eight replicates were performed for each group. In one treated group, about 50 larvae of Panagrellus sp. were put into sterile microtubes containing the crude extract of F. velutipes SMC. In another treated group, about 50 larvae of Panagrellus sp. were put into sterile microtubes containing the boiled extract with denatured enzymes (without protease activity). The control group contained approximately 50 larvae of Panagrellus sp. in the presence of distilled water. The microtubes were incubated at 28 °C, in the dark, for 24, 48 and 72 h. After this period, the number of intact larvae in each microtube was counted with an optical microscope at 10 × objective (Sufiate et al. 2017). The experiment was repeated three times.
Statistical analysis
Efficient Panagrellus sp. larvae destruction in relation to the control group was evaluated for the three experimental trials (F. velutipes, SMC and crude extract). Data were statistically interpreted by analysis of variance at significance levels of 1 and 5% of probability and by Tukey test at 1% level of probability (Ayres et al. 2003). Subsequently, the average reduction percentage of the larvae was calculated:
Results and discussion
Nematophagous behavior was first described by Drechsler (1941). In relation to predatory activity, those fungi have several attack mechanisms on nematodes (Soares et al. 2018). In the present study, the nematophagous effect of the edible fungus F. velutipes was demonstrated for the first time.
Isolated F. velutipes and its SMC had significant nematicidal effect (p < 0.01) on the larvae of Panagrellus sp. SMC had greater action on the nematodes, presenting a significant difference (p < 0.01) when compared to the group treated only with the isolated fungus F. velutipes, in the time intervals of 48 and 72 h. However, after 24 h, there was no significant difference between the groups treated with SMC and the isolated fungus. The percentages of reduction in relation to the control group were: 69, 57.5 and 70% for SMC and 56, 24.5 and 26.6% for the isolated fungus, for 24, 48 and 72 h, respectively (Fig. 1a).
Fig. 1.
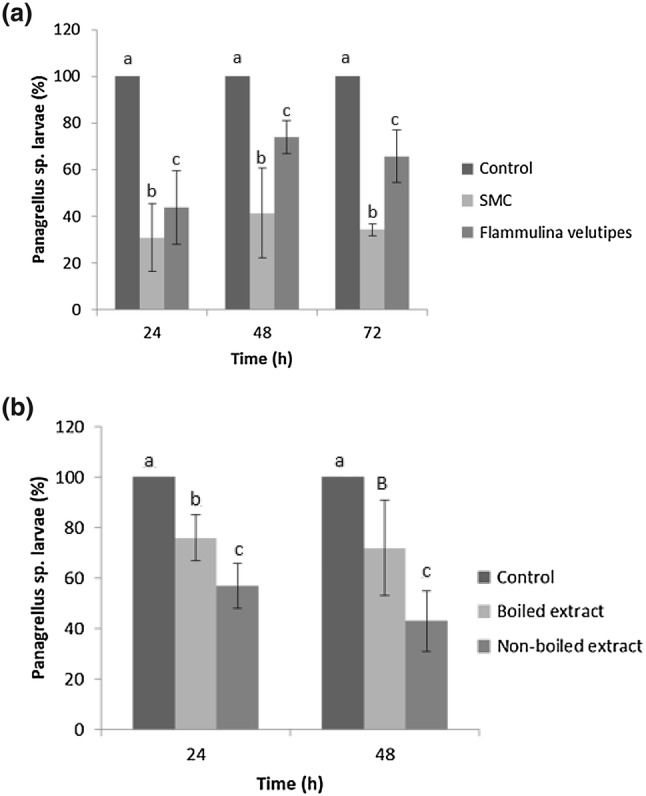
a Averages, standard error and percentage reduction of Panagrellus sp. larvae in water-agar (2% WA) during treatment with the edible fungus Flammulina velutipes, its spent mushroom compost (SMC) and the control group without fungus. Averages followed by equal letters in the same column do not differ significantly from each other by Tukey test at 1% probability level. b Averages, standard error and percentage reduction of Panagrellus sp. larvae during treatment with the crude extract obtained from the spent mushroom compost (SMC) of the edible fungus Flammulina velutipes. Averages followed by equal letters (lower case) in the same column do not differ significantly from each other by Tukey test at 1% probability level. The capital letter denotes difference (p < 0.05)
SMCs have a very diverse microbial population, besides the cultivated fungus itself. Several populations of bacteria, archaebacteria and fungi have already been detected in SMCs. Bacteria of several genera have been found, from mesophilic to thermophilic bacteria, e.g. Paenibacillus spp., Bacillus spp., Pseudomonas spp., Streptomyces spp., Thermornonospora spp., Microbacterium spp., Stenotrophomonas spp. Likewise, several fungal genera have already been isolated from SMCs, e.g., Aspergillus spp., Penicillium spp., Mucor spp., Nigrosporaspp., Oidiodendron spp. (Kleyn and Wetzler 1981; Ribas et al. 2009; Watabe et al. 2004). Probably, these other microorganisms also had effect on nematodes, explaining the higher nematicidal activity of the SMC compared to the isolated fungus. This also suggests that the application of SMC has a more promising potential than the isolated fungus for use in the control of these organisms.
There are several studies involving the SMC of F. velutipes, from studies concerning methane production (Luo et al. 2018) to antioxidative activities (Bao et al. 2010). However, this is the first report on the use of isolated F. velutipes and its SMC in the control of nematodes.
Mushrooms belong to a toxin-producing group of nematophagous fungi. These fungi produce certain toxins that paralyze and/or kill nematodes (Soares et al. 2018). Besides that, enzymes are important actors in the process of nematode infection and digestion by nematophagous mushrooms (Genier et al. 2015). However, the production of nematicidal toxins and enzymes by F. velutipes is still unknown. As described by Degenkolb and Vilcinskas (2016), nematophagous basidiomycetes produce a wide variety of metabolites, including toxins, with nematicidal activity. The production of toxins by nematophagous fungi has been studied for a long time and described for many species (Cairol et al. 1989; Nordbring-Hertz et al. 2011). This way, isolated F. velutipes and its SMC are also a source of many of these biomolecules, thus holding a great biotechnological potential for biological nematode control (Soares et al. 2013, 2019). In this sense, we sought to observe whether the fungus produced any such toxins and enzymes that have destructive nematicidal action, and therefore we analyzed nematicidal activity of the crude extract of the isolated fungus.
Flammulina velutipes SMC crude extract was a poor source of proteases (0.05 U/ml). On the other hand, the active (non-boiled) crude extract obtained from the SMC showed a significant (p < 0.01) nematicidal action on the larvae of Panagrellus sp., with reduction percentages of 43 and 57% for 24 and 48 h of incubation, respectively, compared to the water control group. The boiled crude extract also showed a significant (p < 0.01) nematicidal action on the larvae of Panagrellus sp. However, the reduction percentages were lower than those of the active extract (Fig. 1b). As pointed out by Sufiate et al. (2017), boiling the extract denatures the enzymes. Thus, in the present study, we can suggest that the nematicidal action was due not only to enzyme activities, but also due to the presence of other metabolites. This becomes clear by comparing the data of the active extract treated group with the boiled extract treated group, which showed a significant difference (p < 0.01).
Previous studies have demonstrated that Hypsizygus marmoreus SMC is a rich source of proteases and has nematicidal activity against Panagrellus sp., with a percentage reduction of 52%, after 24 h (Soares et al. 2019). These data corroborate the present study, since the SMC of F. velutipes has also shown to contain proteases (despite the low activity) and having nematicidal action with similar percentage reduction after 24 h of assay (43%).
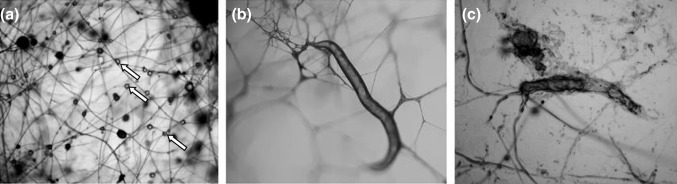
In addition, we could observe the production of metabolite containing drops (Fig. 2a), presence of adhesive hyphae (Fig. 2b) and nematode destruction (Fig. 2c). Despite the absence of tests to assure the drop content and based solely on the test results with crude extract, we suggest that these drops contain metabolites that cause nematode immobilization (Degenkolb and Vilcinskas 2016; Lopez-Llorca et al. 2008). Moreover, the presence of adhesive hyphae has already been described for other edible mushrooms, such as Pleurotus ostreatus, as shown by Thorn and Barron (1984), what confirms that the F. velutipes hyphae shown in Fig. 1b are adhesive hyphae.
Fig. 2.
Nematophagous action of the fungus Flammulina velutipes on Panagrellus sp. larvae: a production of drops containing toxic metabolites; b formation of adhesive hyphae. White Arrows: possible toxin drops
Furthermore, the evidence of F. velutipes SMC nematicidal activity contributes to integrated nematode management (INM) programs. Commercial bio-nematicides based on nematophagous fungi and bacteria found a substantial space on the marketplace, especially after the ban of several chemical nematicides. However, bioproducts are more sensible to abiotic factor than chemical products. In face of that challenge, the use of SMC can be an efficient strategy in the search for bio-nematicide development, mainly considering that SMC presented higher nematicidal activity.
The development of efficient formulations for these bioproducts are another challenge, and the use of this industrial residue would reduce the formulation costs (Abd-Elgawad and Askary 2018). This would also aggregate value to an industrial residue and generate a sustainable product as has been suggested for other edible mushrooms SMCs (Grujié et al. 2015; Lau et al. 2003). This work thus focused on evaluating whether F. velutipes and its SMC had nematicidal activity due to attack mechanisms or enzyme and metabolite activity and therefore, no comparison to any chemical nematicides was made.
Conclusions
This is the first report of the nematophagous action of the fungus F. velutipes, an edible mushroom very popular in the culinary world. We demonstrated for the first time that its SMC equally shows nematicidal activity, even higher (p < 0.01) than the isolated fungus. In addition, this is the first study demonstrating nematicidal activity of the crude extract from F. velutipes SMC. Moreover, we demonstrated that the extract from the SMC contains proteases that probably act in the process of nematode destruction.
Further research is necessary to clarify the nematicidal metabolites produced by this edible mushroom and its SMC. Additionally, more studies can result in a product based on F. velutipes SMC for biocontrol of nematodes, contributing to INM programs.
Acknowledgements
The authors thank the National Council for Scientific and Technological Development (CNPq), the Coordination for the Improvement of Higher Education (CAPES) and Foundation for Supporting Research and Innovation in Espirito Santo (FAPES) for financial support.
Author contributions
All authors prepared the manuscript. FEFS and FRB analyzed the data. All the experiments were designed by FEFS. All the experiments were executed by JMF and DNC.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Abd-Elgawad MMM, Askary TH. Fungal and bacterial nematicides in integrated nematode management strategies. Egypt J Biol Pest Control. 2018;28:74. doi: 10.1186/s41938-018-0080-x. [DOI] [Google Scholar]
- Akhtar M, Malik A. Roles of organic soil amendments and soil organisms in biological control of plant parasitic nematodes: a review. Bioresour Technol. 2000;74:35–47. doi: 10.1016/S0960-8524(99)00154-6. [DOI] [Google Scholar]
- Aslam SS. Organic management of root knot nematodes in tomato with spent mushroom compost. Sarhad J Agric. 2013;29:63–69. [Google Scholar]
- Ayres M, Ayres JRM, Ayres DL, Santos AS (2003) Aplicações estatísticas nas áreas de ciências biológicas. Belém: Sociedade civil mamirauá: Brasília CNPq, p 290
- Bao HN, Ochiai Y, Ohshima T. Antioxidative activities of hydrophilic extracts prepared from the fruiting body and spent culture medium of Flammulinavelutipes. Bioresour Technol. 2010;101:6248–6255. doi: 10.1016/j.biortech.2010.03.026. [DOI] [PubMed] [Google Scholar]
- Cairol J, Djian C, Pijarowski L. Study of the nematocidal properties of the culture filtrate of the nematophagous fungus Paecilomyces lilacinus. Nematology. 1989;12:331–336. [Google Scholar]
- Degenkolb T, Vilcinskas A. Metabolites from nematophagous fungi and nematicidal natural products from fungi as alternatives for biological control. Part II: metabolites from nematophagous basidiomycetes and non = nematophagous fungi. Appl Microbiol Biotechnol. 2016;100:3813–3824. doi: 10.1007/s00253-015-7234-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y, Cheng S, Qi G, Yang Z, Yin S, Chen G. Antimicrobial and antioxidant activities of Flammulina velutipes polisacchrides and polysaccharide-iron(III) complex. Carbohydr Polym. 2017;161:26–32. doi: 10.1016/j.carbpol.2016.12.069. [DOI] [PubMed] [Google Scholar]
- Drechsler C. Some hyphomycetes parasitic on free-living terricolous nematodes. Phytopathology. 1941;31:773–801. [Google Scholar]
- Finney KN, Ryu C, Sharifi VN, Swithenbank J. The reuse of spent mushroom compost and coal tailings for energy recovery: comparison of thermal treatment technologies. Bioresour Technol. 2009;100:310–315. doi: 10.1016/j.biortech.2008.05.054. [DOI] [PubMed] [Google Scholar]
- Genier HLA, Soares FEF, Queiroz JH, Gouveia AS, Araújo JV, Braga FR, Pinheiro IR, Kasuya MCM. Activity of the fungus Pleurotus ostreatus and of its proteases on Panagrellus sp. larvae. Afr J Biotechnol. 2015;14:1496–1503. doi: 10.5897/AJB2015.14447. [DOI] [Google Scholar]
- Gomes APS, Ramos ML, Vasconcellos RS, Jensen JR, Vieira-Bressan MCR, Araujo JV. In vitro activity of brazilian strains of the predatory fungi Artrhobotrys spp. on free-living nematodes and infective larvae of Haemonchus placei. Mem Inst Oswaldo Cruz. 2000;95:873–876. doi: 10.1590/S0074-02762000000600023. [DOI] [PubMed] [Google Scholar]
- Grimm D, Wösten HA. Mushroom cultivation in the circular economy. Appl Microbiol Biotechnol. 2018;102:7795–7803. doi: 10.1007/s00253-018-9226-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grujié M, Dojnov B, Potočnikc I, Duduk B, Vujčić Z. Spent mushroom compost as substrate for the production of industrially important hydrolytic enzymes by fungi Trichoderma spp. and Aspergillus niger in solid state fermentation. Int Biodeterior Biodegrad. 2015;104:290–298. doi: 10.1016/j.ibiod.2015.04.029. [DOI] [Google Scholar]
- Hassan MA, Pham TH, Shi H, Zeng J. Nematodes threats to global food security. Acta Agric Scand B. 2012;63:420–425. doi: 10.1080/09064710.2013.794858. [DOI] [Google Scholar]
- Ishihara A, Goto N, Kikkawa M, Ube N, Ushijima S, Ueno M, Ueno K, Osaki-Oka K. Identification of antifungal compounds in the spent mushroom substrate of Lentinula edodes. J Pest Sci. 2018;43:108–113. doi: 10.1584/jpestics.D17-094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing P, Zhao SJ, Lu MM, Cai Z, Pang J, Song LH. Multiple-fingerprint analysis for investigating quality control of Flammulina velutipes fruiting body polysaccharides. J Agric Food Chem. 2014;62:12128–12133. doi: 10.1021/jf504349r. [DOI] [PubMed] [Google Scholar]
- Kleyn JG, Wetzler TF. The microbiology of spent mushroom compost and its dust. Can J Microbiol. 1981;27:748–753. doi: 10.1139/m81-116. [DOI] [PubMed] [Google Scholar]
- Lau KL, Tsang YY, Chiu SW. Use of spent mushroom compost to bioremediate PAH-contaminated samples. Chemosphere. 2003;52:1539–1546. doi: 10.1016/S0045-6535(03)00493-4. [DOI] [PubMed] [Google Scholar]
- Leifa F, Pandey A, Soccol CR. Production of Flammulina velutipes on coffee husk and coffee spent-ground. Braz Arch Biol Technol. 2001;44:205–212. doi: 10.1590/S1516-89132001000200015. [DOI] [Google Scholar]
- Leung MYK, Fung KP, Choy YM. The isolation and characterization of an immunomodulatory and anti-tumor polysaccharide preparation from Flammulina velutipes. Immunopharmacol. 1997;35:255–263. doi: 10.1016/S0162-3109(96)00157-9. [DOI] [PubMed] [Google Scholar]
- Lopez-Llorca LV, Maciá-Vicente JG, Jansson HB. Mode of action and interactions of nematophagous fungi. In: Ciancio A, Mukerji KG, editors. Integrated management and biocontrol of vegetable and grain crops nematodes. Integrated management of plant pests and diseases. Dordrecht: Springer; 2008. pp. 52–76. [Google Scholar]
- Luo X, Yuan X, Wang S, Sun F, Hou Z, Hu Q, Zhai L, Cui Z, Zou Y. Methane production and characteristics of the microbial community in the co-digestion of spent mushroom substrate with dairy manure. Bioresour Technol. 2018;250:611–620. doi: 10.1016/j.biortech.2017.11.088. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Iketani A, Shioi Y. A survey of proteases in edible mushrooms with synthetic peptides as substrates. Mycoscience. 2011;52:234–241. doi: 10.1007/S10267-010-0089-9. [DOI] [Google Scholar]
- Nakajima VM, Soares FEF, Queiroz JH. Screening and decolorizing potential of enzymes from spent mushroom composts of six different mushrooms. Biocatal Agr Biotechnol. 2018;13:58–61. doi: 10.1016/j.bcab.2017.11.011. [DOI] [Google Scholar]
- Nordbring-Hertz B, Jansson H, Tunlind A. Nematophagous fungi. Encycl Life Sci. 2011 doi: 10.1002/9780470015902.a0000374.pub3. [DOI] [Google Scholar]
- Rathore H, Prasad S, Sharma S. Mushroom nutraceuticals for improved nutrition and better human health: a review. Pharmanutrition. 2017;5:35–46. doi: 10.1016/j.phanu.2017.02.001. [DOI] [Google Scholar]
- Redhead SA, Petersen RH. New species, varieties and combinations in the genus Flammulina. Mycotaxon. 1999;71:285–294. [Google Scholar]
- Ribas LCC, Mendonça MM, Camelini CM, Soares CHL. Use of spent mushroom substrates from Agaricus subrufescens (syn. A. blazei, A. brasiliensis) and Lentinula edodes productions in the enrichment of a soil-based potting media for lettuce (Lactuca sativa) cultivation: growth promotion and soil bioremediation. Bioresour Technol. 2009;100:4750–4757. doi: 10.1016/j.biortech.2008.10.059. [DOI] [PubMed] [Google Scholar]
- Royse DJ, Baars J, Tan Q. Current overview of mushroom production in the world. In: Zied DC, Pardo-Giminez A, editors. Edibleand medicinal mushrooms: technology and applications. Hoboken: Wiley; 2017. pp. 5–13. [Google Scholar]
- Siddiqui ZA, Mahmood I. Biological control of plant parasitic nematodes by fungi: a review. Bioresour Technol. 1996;58:229–239. doi: 10.1016/S0960-8524(96)00122-8. [DOI] [Google Scholar]
- Soares FEF, Braga FR, Araújo JV, Geniêr HLA, Gouveia AS, Queiroz JH. Nematicidal activity of three novel extracellular proteases of the nematophagous fungus Monacrosporium sinense. Parasitol Res. 2013;112:1557–1565. doi: 10.1007/s00436-013-3304-8. [DOI] [PubMed] [Google Scholar]
- Soares FEF, Sufiate BL, Queiroz JH. Nematophagous fungi: far beyond the endoparasite, predator and ovicidal groups. ANRES. 2018;52:1–8. doi: 10.1016/j.anres.2018.05.010. [DOI] [Google Scholar]
- Soares FEF, Nakajima VM, Sufiate BL, Satiro LAS, Gomes EH, Fróes FV, Sena FP, Braga FB, Queiroz JH. Proteolytic and nematicidal potential of the compost colonized by Hypsizygus marmoreus. Exp Parasitol. 2019;197:16–19. doi: 10.1016/j.exppara.2018.12.006. [DOI] [PubMed] [Google Scholar]
- Sufiate BL, Soares FEF, Moreira SS, Gouveia AS, Monteiro TSA, Freitas LG, Queiroz JH. Nematicidal action of Pleurotus eryngii metabolites. Biocatal Agric Biotechnol. 2017;12:216–219. doi: 10.1016/j.bcab.2017.10.009. [DOI] [Google Scholar]
- Thorn RG, Barron GL. Carnivorous mushrooms. Science. 1984;224:76–78. doi: 10.1126/science.224.4644.76. [DOI] [PubMed] [Google Scholar]
- Watabe M, Rao JR, Xu J, Millar BC, Ward RF, Moore JE. Identification of novel eubacteria from spent mushroom compost (SMC) waste by DNA sequence typing: ecological considerations of disposal on agricultural land. Waste Manag. 2004;24:81–86. doi: 10.1016/j.wasman.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Yildiz S, Yildiz ÜC, Gezer ED, Temiz A. Some lignocellulosic wastes used as raw material in cultivation of the Pleurotus ostreatus culture mushroom. Process Biochem. 2002;38:301–306. doi: 10.1016/S0032-9592(02)00040-7. [DOI] [Google Scholar]
- Zhang L, Sun X. Changes in physical, chemical, and microbiological properties during the two stage co-composition of green waste with spent mushroom compost and biochar. Bioresour Technol. 2014;171:274–284. doi: 10.1016/j.biortech.2014.08.079. [DOI] [PubMed] [Google Scholar]