Abstract
Background
Non-invasive ventilation (NIV) is preferred as the initial ventilatory support to treat acute hypercapnic respiratory failure in patients with chronic obstructive pulmonary disease (COPD). High-flow nasal cannula (HFNC) may be an alternative method; however, the effects of HFNC in hypercapnic COPD are not well known. This preliminary study aimed at assessing the physiologic effects of HFNC at different flow rates in hypercapnic COPD and to compare it with NIV.
Methods
A prospective physiologic study enrolled 12 hypercapnic COPD patients who had initially required NIV, and were ventilated with HFNC at flow rates increasing from 10 to 50 L/min for 15 min in each step. The primary outcome was the effort to breathe estimated by a simplified esophageal pressure–time product (sPTPes). The other studied variables were respiratory rate, oxygen saturation (SpO2), and transcutaneous CO2 pressure (PtcCO2).
Results
Before NIV initiation, the median [interquartile range] pH was 7.36 [7.28–7.37] with a PaCO2 of 51 [42–60] mmHg. sPTPes per minute was significantly lower with HFNC at 30 L/min than 10 and 20 L/min (p < 0.001), and did not significantly differ with NIV (median inspiratory/expiratory positive airway pressure of 11 [10–12] and [5–5] cmH2O, respectively). At 50 L/min, sPTPes per minute increased compared to 30 L/min half of the patients. Respiratory rate was lower (p = 0.003) and SpO2 was higher (p = 0.028) with higher flows (30–50 L/min) compared to flow rate of 10 L/min and not different than with NIV. No significant differences in PtcCO2 between NIV and HFNC at different flow rates were observed (p = 0.335).
Conclusions
Applying HFNC at 30 L/min for a short duration reduces inspiratory effort in comparison to 10 and 20 L/min, and resulted in similar effect than NIV delivered at modest levels of pressure support in hypercapnic COPD with mild to moderate exacerbation. Higher flow rates reduce respiratory rate but sometimes increase the effort to breathe. Using HFNC at 30 L/min in hypercapnic COPD patients should be further evaluated. Trial registration Thai Clinical Trials Registry, TCTR20160902001. Registered 31 August 2016, http://www.clinicaltrials.in.th/index.php?tp=regtrials&menu=trialsearch&smenu=fulltext&task=search&task2=view1&id=2008.
Keywords: Chronic obstructive pulmonary disease, Esophageal pressure, High-flow oxygen therapy, Non-invasive ventilation, Respiratory failure
Background
Exacerbation of chronic obstructive pulmonary disease (COPD) is defined as an acute worsening of respiratory symptoms, including increased dyspnea, cough, and sputum production, that results in the requirement for additional treatment and hospitalization [1]. Acute hypercapnic respiratory failure frequently occurs in patients with moderate to severe COPD exacerbation, and this condition generally necessitates an emergency room visit and hospital admission [2, 3]. Non-invasive ventilation (NIV) has been demonstrated to reduce the intubation rate and to improve survival in COPD patients who could require ventilatory support, and it is recommended to use it in hypercapnic COPD patients with respiratory acidosis [4–6]. However, skill of the caregivers is important to the success of this technique. In addition, patient tolerance is often poor due to patient discomfort and adverse effects frequently occur during its use, such as skin damage, air leaks, and claustrophobia [7–9].
High-flow nasal cannula (HFNC) is an oxygen device that delivers gas through a special large-bore nasal cannula. The current system can provide heated and humidified gas with a maximum flow rate of 60 L per minute (L/min) and an adjustable oxygen fraction (FiO2) from 21% to 100% [10]. The main mechanisms of HFNC include generation of a small amount of positive end-expiratory pressure (PEEP) varying from 1 to 7 cmH2O, wash out of nasopharyngeal dead space and provision of heat and humidity to reduce dryness symptom and facilitate secretion clearance [11]. Moreover, HFNC can improve oxygenation, modify breathing pattern, and decrease inspiratory effort. Several studies demonstrated physiological and clinical benefits of HFNC in patients with acute hypoxemic respiratory failure [12, 13] and for prevention of postextubation failure [14, 15]. The washing out of airway dead space by HFNC can increase CO2 clearance and improve alveolar ventilation, both of which could be beneficial in patients with hypercapnia. However, evidence supporting the efficacy of HFNC in hypercapnic respiratory failure is still limited. Furthermore, the maximum effect of HFNC for reducing inspiratory effort in acute hypoxemic respiratory failure occurs when using the highest flow rate, but we have had no data regarding the optimum flow rate of HFNC in patients with acute hypercapnic respiratory failure. Accordingly, the aim of this preliminary study was to investigate the physiologic effects of HFNC at different flow rates in patients with mild to moderate exacerbation, compared to the effect of NIV on inspiratory effort and other physiologic variables in COPD patients with hypercapnia.
Methods
Subjects and study design
This prospective physiologic study was conducted in the Respiratory Intensive Care Unit and the Respiratory Ward of the Division of Respiratory Diseases and Tuberculosis, Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand during September 2016–May 2017. This study was approved by the Siriraj Institutional Review Board (SIRB) (COA no. 455/2559[EC4]), and was registered in the Thai Clinical Trials Registry (TCTR) (reg. no. 20160902001). Written informed consent to participate was obtained from each subject or their relatives.
We enrolled patients who had a known diagnosis of COPD with post-bronchodilator forced expiratory volume at 1 s/forced vital capacity (FEV1/FVC) < 70% [1], who were 40–85 years old and who had presented an exacerbation that initially required NIV based on at least two of the following criteria [16]:
Respiratory rate > 24 breaths/min.
Use of respiratory accessory muscles or paradoxical motion of the abdomen.
Acute respiratory acidosis (arterial pH ≤ 7.35 and/or PaCO2 ≥ 45 mmHg).
After the initial management and stabilization with NIV, patients were considered for the study. Patients were not enrolled in the study if they had any of the following exclusion criteria: arterial pH < 7.25, hemodynamic instability, persistent hypoxemia despite supplemental oxygen therapy, diminished consciousness or uncooperative, active hemoptysis, pneumothorax, and/or contraindication for esophageal balloon catheter insertion, such as recent upper airway/esophageal surgery or active upper gastrointestinal bleeding.
Device description
The HFNC device (Airvo-2™; Fisher & Paykel Healthcare, Auckland, New Zealand) consisted of a flow generator (up to 60 L/min), an air–oxygen blender that allows for adjustment of FiO2 from 21 to 100%, and an auto-fill MR 290 heated chamber. The gas mixture at 34–37 °C was delivered via a single-limb heated breathing tube to the patient via the Optiflow™ nasal cannula (Fisher & Paykel, Auckland, New Zealand). The dedicated NIV machine (BIPAP Vivo 40; Breas Medical AB, Mölnlycke, Sweden or Respironics V60; Philips Healthcare, Best, the Netherlands) was applied via an oronasal mask and was connected to an active humidification system (VH2000, VADI Medical Technology, Taoyuan, Taiwan). An appropriately sized oronasal mask was chosen to minimize leaks and to optimize patient comfort. The NIV settings, including inspiratory positive airway pressure, expiratory positive airway pressure, respiratory rate, and FiO2, were clinically adjusted by an attending physician and not modified during the study period.
Study protocol
Subjects meeting all of the eligibility criteria and none of the exclusion criteria were enrolled. An esophageal balloon catheter (Cooper Surgical, Inc., Trumbull, Connecticut, USA) was inserted through the nose and positioned in the lower one-third of the esophagus. The balloon was filled with 0.5 mL of air and connected to a pressure transducer (BIOPAC Systems, Goleta, California, USA). To confirm the position of the esophageal catheter, the presence of cardiac oscillations was checked and gentle pressure on the abdomen was applied to verify the absence of gastric pressure fluctuations. Esophageal pressure (Pes) was recorded using an MP150 Data Acquisition System and AcqKnowledge Data Acquisition and Analysis Software (both BIOPAC Systems, Goleta, California, USA).
At inclusion, subjects were ventilated with NIV using their clinical settings for 15 min, after which they were switched to HFNC starting at a flow rate of 10, with subsequent progressive increases to 20, 30, 40, and 50 L/min. The NIV step and all 5 HFNC steps were applied for 15 min each including a recording period of 5 min at the end (Fig. 1). During HFNC, the FiO2 was adjusted to achieve oxygen saturation measured by pulse oximetry (SpO2) of at least 92%, and then this adjustment was kept constant until the end of the study protocol. To enhance the maximum effect of HFNC, we encouraged patients to breathe with their mouth closed as often as possible. After completing the study, the type and settings of respiratory support were decided by the attending physician.
Fig. 1.
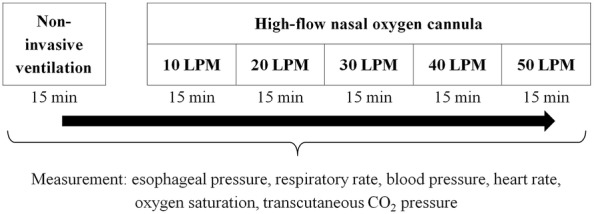
Study protocol
Data collection
Baseline demographic and clinical data, included age, gender, body mass index, co-morbidity, and most recent pulmonary function test were collected. Acute Physiologic and Chronic Health Evaluation (APACHE) II score and arterial blood gas were evaluated at the time of enrollment during NIV session. SpO2 and transcutaneous CO2 pressure (PtcCO2) were continuously recorded throughout the study using a SenTec Digital Monitoring System (SenTec, Therwil, Switzerland). Other physiologic variables, including respiratory rate, blood pressure, and heart rate, were recorded immediately after starting the protocol and at the end of each step.
From Pes, we used the recorded waveforms during the last 2 min of each step to calculate Pes swing and esophageal pressure–time product (PTPes). The average value of Pes swing (cmH2O), PTPes per minute (cmH2O × s × min−1), and PTPes per breath (cmH2O × s) as an index of inspiratory effort were calculated using a dedicated software program (Sistema Respiratorio, Barcelona, Spain). Since measurement of chest wall elastance in non-intubated patients is not possible, and determining the phase of inspiration without airflow signal is also very difficult, we modified the calculation of PTPes per breath by integrating the area under the Pes signal from the onset of negative deflection to the return of Pes to baseline. This technique was used and reported in a previous study [17]; we refer to it as a “simplified PTPes” (sPTPes). sPTPes per minute was obtained by multiplying sPTPes per breath by respiratory rate.
Outcomes
The primary outcome was inspiratory effort as evaluated by sPTPes per minute during NIV and during HFNC at different flow rates. Other physiologic variables included sPTPes per breath, Pes swing, respiratory rate, SpO2, PtcCO2, blood pressure, and heart rate.
Statistical analysis
There was no previous study evaluating patient inspiratory effort during HFNC in COPD patients with acute exacerbation. We performed a pilot exploratory study by enrolling 12 patients in this study. Normality of data distribution was assessed by the Shapiro–Wilk test. Normally distributed variables are expressed as mean ± standard deviation, and were analyzed by repeated measures analysis of variance (ANOVA) followed by a post hoc pairwise comparison with Bonferroni adjustment. Non-normally distributed variables are expressed as median and interquartile range, and were compared by Friedman’s two-way ANOVA by ranks with a Dunn’s test post hoc pairwise comparison with Bonferroni correction. Categorical variables are expressed as frequency and percentage. Data were analyzed using PASW Statistics version 18 (SPSS, Inc., Chicago, Illinois, USA). A two-sided p < 0.05 was considered as statistically significant.
Results
Twelve patients were included, with a mean age of 74 ± 11 years. Post-bronchodilator FEV1 and FEV1/FVC were 34% [IQR 31–47] of predicted and 45% [IQR 35–55], respectively. Patients were enrolled a median of 17 h [IQR 5–68] after initiation of NIV. Other baseline characteristics are shown in Table 1.
Table 1.
Baseline patient demographic and clinical characteristics (n = 12)
| Variables | N = 12 |
|---|---|
| Age, years | 74.0 ± 11.0 |
| Male gender, n (%) | 9 (75%) |
| Body mass index, kg/m2 | 22 ± 3 |
| Long-term oxygen therapy, n (%) | 3 (25%) |
| Comorbidity, n (%) | |
| Diabetes mellitus | 2 (17%) |
| Hypertension | 8 (67%) |
| Coronary artery disease | 3 (25%) |
| Chronic kidney disease | 4 (33%) |
| Others | 2 (17%) |
| Post-bronchodilator FEV1/FVC, % | 45 [35–55] |
| Post-bronchodilator FEV1, % predicted | 34 [31–47] |
| APACHE II at enrollment | 12 ± 4 |
| Arterial blood gas at enrollment | |
| pH | 7.36 [7.28–7.37] |
| PaCO2, mmHg | 51 [42–60] |
| PaO2, mmHg | 139 [80–233] |
| Time from initiation of NIV to enrollment, hours | 17 [5–68] |
| Non-invasive ventilation setting at enrollment | |
| Inspiratory positive airway pressure, cmH2O | 11 [10–12] |
| Expiratory positive airway pressure, cmH2O | 5 [5] |
| Tidal volume, mL | 425 [370–514] |
| Oxygen flow rate, liters per minute | 4 [3–5] |
Continuous variables were presented as mean ± SD or median [interquartile range] and categorical variables were presented as absolute value (%)
APACHE acute physiologic and chronic health evaluation, FEV1 forced expiratory volume at 1 s, FVC forced vital capacity, PaCO2 arterial partial pressure of carbon dioxide, PaO2 arterial partial pressure of oxygen
Inspiratory effort and respiratory rate during HFNC and NIV
Changes in sPTPes per breath and per minute between HFNC at different flow rates and NIV are shown in Table 2. Changes in sPTPes per breath were relatively modest and non-significant. sPTPes per minute was significantly lower with HFNC at a flow rate of 30 L/min compared to flow rates of 10 L/min and 20 L/min (187 ± 84, 220 ± 100 cmH2O × s × min−1 and 211 ± 90 cmH2O × s × min−1, respectively; p < 0.01). sPTPes per minute was not different between HFNC at a flow rate of 30 L/min and NIV (187 ± 84 vs. 183 ± 84 cmH2O × s × min−1; p = 0.839). In half of the subjects, sPTPes per minute increased progressively when increasing the flow rate of HFNC to 50 L/min (Fig. 2); however, the overall increase was not statistically significant (30 L/min vs. 50 L/min = 187 ± 84 vs. 201 ± 86 cmH2O × s×min−1; p = 0.269).
Table 2.
Simplified esophageal pressure–time product, respiratory variables, and hemodynamic variables during non-invasive ventilation and high-flow nasal cannula at different flow rates
| Variables | NIV | HFNC-10 | HFNC-20 | HFNC-30 | HFNC-40 | HFNC-50 | p |
|---|---|---|---|---|---|---|---|
| sPTPes per breath (cmH2O × s) | 9 ± 4 | 11 ± 5 | 11 ± 6 | 10 ± 6 | 10 ± 5 | 10 ± 4 | 0.090 |
| sPTPes per minute (cmH2O × s × min−1) | 183 ± 84 | 220 ± 100 | 211 ± 90 | 187 ± 84*,** | 189 ± 87 | 201 ± 86 | < 0.001 |
| RR (breaths/min) | 25 [20–28] | 24 [20–29] | 23 [18–28] | 21 [18–27]* | 21 [18–27]* | 21 [18–26]* | 0.003 |
| SpO2 (%) | 96 ± 2 | 95 ± 3 | 96 ± 3* | 97 ± 3*,** | 97 ± 2*,** | 98 ± 2*,**, γ | 0.028 |
| PtcCO2 (mmHg) | 42 ± 7 | 41 ± 7 | 41 ± 8 | 41 ± 7 | 41 ± 7 | 41 ± 7 | 0.335 |
| MAP (mmHg) | 99 ± 22 | 94 ± 15 | 92 ± 9 | 91 ± 12 | 89 ± 12 | 91 ± 14 | 0.179 |
| HR (beats/min) | 96 ± 16 | 97 ± 15 | 94 ± 13 | 93 ± 14 | 94 ± 16 | 95 ± 17 | 0.174 |
| Oxygen flow (L/min) or Set FiO2 | 4 [3–5] | 0.35 [0.30–0.40] | 0.35 [0.30–0.40] | 0.35 [0.30–0.40] | 0.35 [0.30–0.40] | 0.35 [0.30–0.40] | – |
Continuous variables were presented as mean ± SD or median [interquartile range] and categorical variables were presented as absolute value (%)
HFNC high-flow nasal cannula, HR heart rate, MAP mean arterial pressure, NIV non-invasive ventilation, sPTPes, simplified esophageal pressure–time product, PtcCO2, transcutaneous carbon dioxide pressure, RR respiratory rate, SpO2, oxygen saturation
* Indicates p < 0.05 compared with HNFC-10, ** Indicates p < 0.05 compared with HFNC-20, γIndicates p < 0.05 compared with NIV
Fig. 2.
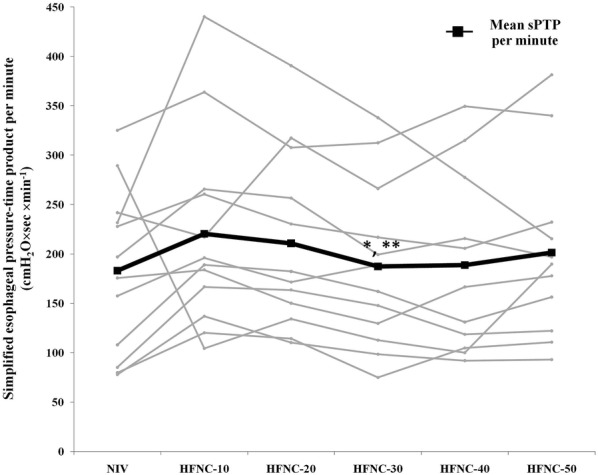
Individual data and mean value of simplified esophageal pressure–time product (sPTPes) per minute during non-invasive ventilation (NIV) and high-flow nasal cannula (HFNC) at different flow rates. *Indicates p < 0.05 in comparison to HFNC at a flow rate of 10 L/min, **Indicates p < 0.05 in comparison to HFNC at a flow rate of 20 L/min
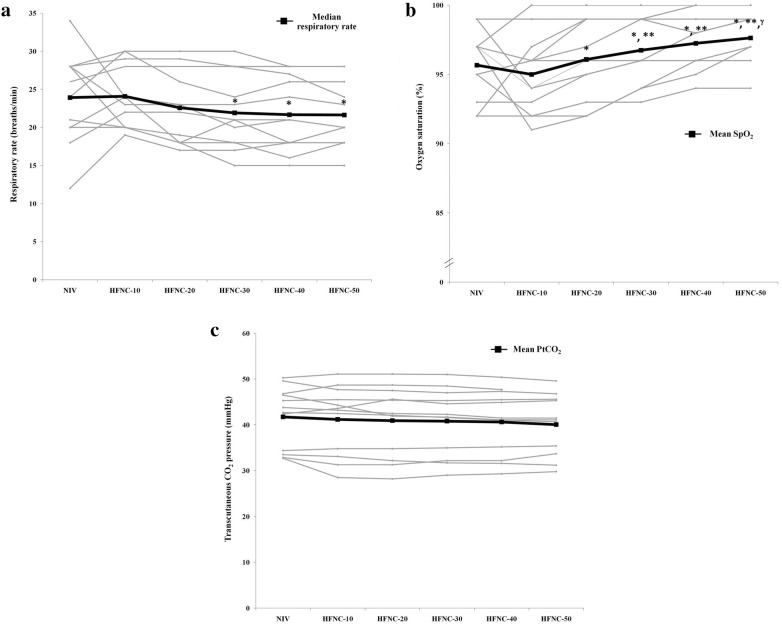
HFNC at flow rate of 30, 40, and 50 L/min, respiratory rate was significantly lower compared to HFNC at a flow rate of 10 L/min (p = 0.003). There was no significant difference in respiratory rate between HFNC at flow rates of 30–50 L/min and NIV (Fig. 3 and Table 2).
Fig. 3.
Individual data of a respiratory rate, b oxygen saturation (SpO2), and c transcutaneous CO2 pressure (PtcCO2) during non-invasive ventilation (NIV) and high-flow nasal cannula (HFNC) at different flow rates. *Indicates p < 0.05 compared with HFNC at a flow rate of 10 L/min, **Indicates p < 0.05 compared with HFNC at a flow rate of 20 L/min, γIndicates p < 0.05 compared with NIV
Other physiological variables
With constant FiO2, HFNC at a higher flow rate resulted in significantly increased SpO2 compared to that of HFNC at a flow rate of 10 L/min (Table 2). Furthermore, SpO2 was significantly higher in HFNC at a flow rate of 50 L/min compared to NIV (98 ± 2% vs. 96 ± 2%; p = 0.024). No significant difference in PtcCO2 between HFNC at any flow rate and NIV was found (p = 0.335). The individual data of SpO2 and PtcCO2 are shown in Fig. 3.
No significant difference in mean arterial pressure or heart rate was observed between NIV and HFNC at any flow rate (Table 2).
Adverse events
No adverse events were observed during either HFNC or NIV in this study. All patients tolerated both study interventions until the end of the study.
Discussion
In this preliminary, short-term physiological study, we evaluated the effects of HFNC at different flow rates and compared to NIV in patients with mild to moderate COPD exacerbation who had initially been managed and stabilized with NIV. The primary outcome demonstrated that HFNC at a flow rate of 30 L/min significantly reduced inspiratory effort as assessed by sPTPes per minute compared to HFNC at a flow rate of 10 L/min; at this flow rate, HFNC was comparable to NIV for reducing sPTPes per minute. Although we did not observe a significant difference in sPTPes per breath between HFNC at flow rates of 30 and 10 L/min, decreased sPTPes per minute when increasing the flow rate may be explained by a significant reduction in respiratory rate. Interestingly, we observed a trend of increasing sPTPes per minute, when increasing the HFNC flow rate from 30 to 50 L/min at least in some patients. The effect of increasing the flow rate of HFNC on inspiratory effort in our study was different from the effect observed and reported in previous studies. Two studies in patients with acute hypoxemic respiratory failure by Mauri, et al. found that PTPes per minute progressively decreased [18], and that patients were more comfortable [19] when the HFNC flow rate was uptitrated from 30 to 60 L/min. A study by Delorme and colleagues [20] in 12 patients recovering from acute respiratory failure also reported significant decrease in PTPes per minute when increasing the HFNC flow rate from 20 to 60 L/min. However, they did not find any significant difference in PTPes per minute in the subgroup of patients with hypercapnic respiratory failure. We have no clear explanation why an increase in the HFNC flow rate from 30 to 50 L/min in our study led to increased inspiratory effort in half of COPD patients with exacerbation; however, patient discomfort, worsening dynamic hyperinflation or increased resistance to breathe could explain an observed increased inspiratory effort with HFNC at a flow rate of 50 L/min. A study comparing HFNC at 30 L/min with conventional oxygen therapy in patients with stable COPD found significant increases in end-expiratory lung volume with HFNC [21]. The increase in end-expiratory lung volume with HFNC may have aggravated dynamic hyperinflation and effort to breathe in some COPD patients in our study.
Several studies have demonstrated that HFNC improved work of breathing and breathing pattern in patients with acute hypoxemic respiratory failure when compared to conventional oxygen therapy [22], and it was not found to be inferior to NIV [17]; however, no previous study has investigated the effects of HFNC on inspiratory effort in COPD patients with exacerbation. Somewhat similar to our study, Sklar and colleagues [23] conducted a study that compared HFNC and NIV in 15 patients with cystic fibrosis who developed acute hypercapnia. They found that HFNC was not inferior to NIV with respect to diaphragmatic work as assessed by diaphragm thickening fraction measured by ultrasound. Other short-term physiological studies of HFNC in patients with stable COPD found that HFNC significantly reduced respiratory rate and PtcCO2 in comparison to low-flow oxygen therapy [21, 24]. In contrast to our study, Pisani et al. [25] demonstrated that diaphragm pressure–time product was significantly lower with NIV than with HFNC at flow rate of 20 and 30 L/min in patients with stable COPD. This difference, however, may be explained by longer duration of the intervention and different NIV settings. Several mechanisms of HFNC that influence reduced inspiratory effort have been proposed, including higher flow rate of gas matching the patient’s demand [10, 26], washing out anatomic dead space that results in reduction of ineffective ventilation [27–29], and the effect of external PEEP to overcome intrinsic PEEP caused by dynamic airway collapse in COPD with acute exacerbation [30].
In this study, higher flow rate of HFNC improved oxygenation compared to NIV. However, we did not find a significant reduction in PtcCO2 with HFNC. Reduction of anatomic dead space and subsequent CO2 washout is the mechanism that has been proposed to explain the decrease in PtcCO2 [28]. A study in 30 patients with stable COPD who had an indication for long-term oxygen therapy by Fraser et al. [21] found that HFNC at a flow rate of 30 LPM significantly improved oxygenation and reduced PtcCO2 when compared to conventional oxygen therapy. A study by Braunlich et al. [31] compared HFNC, nasal CPAP, and nasal NIV in 67 hospitalized patients with COPD and found that increasing the flow rate of HFNC from 20 to 30 L/min enhanced CO2 clearance and lowered PaCO2. They did not find any difference between HFNC at a flow rate of 30 LPM and NIV in terms of reduction in PaCO2. Conversely, a randomized crossover study in 24 hospitalized patients with COPD exacerbation by Pilcher and colleagues [32] did not find significant difference in PtcCO2 when compared between HFNC at a flow rate of 35 L/min and standard nasal prongs. A study by Atwood et al. compared HFNC at a flow rate of 35 L/min with low-flow oxygen therapy in 32 stable COPD patients. They found that HFNC markedly reduced respiratory rate with no significant changes in tidal volume or PaCO2, which suggested more efficient ventilation with HFNC. Thus, the lack of significant change in PtcCO2 in our study might be explained by the reduction in respiratory rate, and we assume that alveolar ventilation was improved when increasing the flow rate of HFNC.
Limitations
This study has some limitations. First, this preliminary study had a small number of enrolled subjects. Second, the study interventions were not randomized then an effect of the treatment duration on many physiologic variables may not be ruled out. Third, we did not measure delivered FiO2 during NIV, tidal volume during HFNC, and level of intrinsic PEEP due to device-related limitations. Forth, the time spent on each step was relatively short and may not have been long enough to detect significant differences in physiologic effects between HFNC and NIV, in particular change in PtcCO2. Fifth, the level of pressure support during NIV in our study was low compared to the levels reported in previous studies in COPD patients with exacerbation, and most patients were stabilized before enrollment in the study. This factor may influence a bias toward HFNC in terms of lowering inspiratory effort. Last, the level of patient discomfort was not assessed in our study, and this factor could limit the efficacy of these techniques. Thus, another physiological study with longer duration of the treatment and also a larger randomized controlled study of HFNC in COPD patients with acute hypercapnic respiratory failure are needed to confirm our results and to further elucidate the efficacy of HFNC in this patient population.
Conclusions
After a short duration of HFNC at 30 L/min, inspiratory effort as determined by sPTPes per minute decreases similar to the reduction effectuated by NIV delivered at modest levels of pressure support in hypercapnic COPD patients with mild to moderate exacerbation in comparison to HFNC at 10 and 20 L/min. Higher flow rates reduce respiratory rate and improves oxygenation but sometimes increase the effort to breathe. However, higher HFNC flow rate do not provide significant change in PaCO2. Our results suggest that HFNC at 30 L/min might be optimal in many hypercapnic COPD patients with mild to moderate exacerbation and should be tested in the future study.
Acknowledgements
The authors gratefully acknowledge the patients who generously agreed to participate in this study. We would also like to thank Mr. Suthipol Udompunthurak (Clinical Epidemiology Unit, Research Department, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand) and Miss Khemajira Karaketklang (Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand) for their assistance with statistical analysis.
Abbreviations
- APACHE
Acute Physiologic and Chronic Health Evaluation
- COPD
chronic obstructive pulmonary disease
- FEV1
forced expiratory volume at 1 s
- FiO2
oxygen fraction
- FVC
forced vital capacity
- HFNC
high-flow nasal cannula
- LPM
liters per minute
- NIV
non-invasive ventilation
- PaCO2
arterial partial pressure of carbon dioxide
- PaO2
arterial partial pressure of oxygen
- PEEP
positive end-expiratory pressure
- Pes
esophageal pressure
- PtcCO2
transcutaneous carbon dioxide pressure
- sPTPes
simplified esophageal pressure–time product
- SpO2
oxygen saturation by pulse oximetry
Authors’ contributions
NR, PP, NP, JT and LB conceived and designed the study. NR, PP, and NP collected the data. NR, PP, JT and LB analyzed and interpreted the data. NR, PP and LB prepared the first draft of the manuscript. All authors read and final approved the final manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Availability of data and materials
The datasets supporting the conclusions of this article are included within the article.
Ethics approval and consent to participate
This study was approved by the Siriraj Institutional Review Board (SIRB) (COA no. 455/2559[EC4]). Informed consent was obtained from patients or their relatives prior to enrollment.
Consent for publication
Not applicable.
Competing interests
Laurent Brochard’s laboratory has received equipment or research grants from Covidien (PAV), Air liquide (CPR), Philips (sleep), Sentec (tcPCO2), Fisher&Paykel (high-flow therapy), General Electric (lung volume). The other authors have no potential conflict of interest relevant to this study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Nuttapol Rittayamai, Phone: +66-2419-7757, Email: nuttapol.rit@mahidol.ac.th.
Prapinpa Phuangchoei, Email: drdeenee@gmail.com.
Jamsak Tscheikuna, Email: tscheikuna@gmail.com.
Nattakarn Praphruetkit, Email: nattakarn107@yahoo.com.
Laurent Brochard, Email: BrochardL@smh.ca.
References
- 1.Vogelmeier CF, Criner GJ, Martinez FJ, Anzueto A, Barnes PJ, Bourbeau J, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report: GOLD Executive Summary. Eur Respir J. 2017;195:557–582. doi: 10.1164/rccm.201701-0218PP. [DOI] [PubMed] [Google Scholar]
- 2.Wedzicha JA, Seemungal TAR. COPD exacerbations: defining their cause and prevention. Lancet. 2007;370:786–796. doi: 10.1016/S0140-6736(07)61382-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seemungal TA, Donaldson GC, Paul EA, Bestall JC, Jeffries DJ, Wedzicha JA. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157:1418–1422. doi: 10.1164/ajrccm.157.5.9709032. [DOI] [PubMed] [Google Scholar]
- 4.Rochwerg B, Brochard L, Elliott MW, Hess D, Hill NS, Nava S, et al. Official ERS/ATS clinical practice guidelines: noninvasive ventilation for acute respiratory failure. Eur Respir J. 2017 doi: 10.1183/13993003.02426-2016. [DOI] [PubMed] [Google Scholar]
- 5.Brochard L, Mancebo J, Wysocki M, Lofaso F, Conti G, Rauss A, et al. Noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease. N Engl J Med. 1995;333:817–822. doi: 10.1056/NEJM199509283331301. [DOI] [PubMed] [Google Scholar]
- 6.Chandra D, Stamm JA, Taylor B, Ramos RM, Satterwhite L, Krishnan JA, et al. Outcomes of noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease in the United States, 1998–2008. Am J Respir Crit Care Med. 2012;185:152–159. doi: 10.1164/rccm.201106-1094OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mehta S, Hill NS. Noninvasive ventilation. Am J Respir Crit Care Med. 2001;163:540–577. doi: 10.1164/ajrccm.163.2.9906116. [DOI] [PubMed] [Google Scholar]
- 8.Schönhofer B, Sortor-Leger S. Equipment needs for noninvasive mechanical ventilation. Eur Respir J. 2002;20:1029–1036. doi: 10.1183/09031936.02.00404202. [DOI] [PubMed] [Google Scholar]
- 9.Nava S, Navalesi P, Gregoretti C. Interfaces and humidification for noninvasive mechanical ventilation. Respir Care. 2009;54:71–84. [PubMed] [Google Scholar]
- 10.Papazian L, Corley A, Hess D, Fraser JF, Frat J-P, Guitton C, et al. Use of high-flow nasal cannula oxygenation in ICU adults: a narrative review. Intensive Care Med. 2016;42:1336–1349. doi: 10.1007/s00134-016-4277-8. [DOI] [PubMed] [Google Scholar]
- 11.Ricard J-D. High flow nasal oxygen in acute respiratory failure. Minerva Anestesiol. 2012;78:836–841. [PubMed] [Google Scholar]
- 12.Frat J-P, Thille AW, Mercat A, Girault C, Ragot S, Perbet S, et al. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Engl J Med. 2015;372:2185–2196. doi: 10.1056/NEJMoa1503326. [DOI] [PubMed] [Google Scholar]
- 13.Stéphan F, Barrucand B, Petit P, Rézaiguia-Delclaux S, Médard A, Delannoy B, et al. High-flow nasal oxygen vs noninvasive positive airway pressure in hypoxemic patients after cardiothoracic surgery: a randomized clinical trial. JAMA. 2015;313:2331–2339. doi: 10.1001/jama.2015.5213. [DOI] [PubMed] [Google Scholar]
- 14.Hernández G, Vaquero C, González P, Subira C, Frutos-Vivar F, Rialp G, et al. Effect of postextubation high-flow nasal cannula vs conventional oxygen therapy on reintubation in low-risk patients: a randomized clinical trial. JAMA. 2016;315:1354–1361. doi: 10.1001/jama.2016.2711. [DOI] [PubMed] [Google Scholar]
- 15.Hernández G, Vaquero C, Colinas L, Cuena R, González P, Canabal A, et al. Effect of postextubation high-flow nasal cannula vs noninvasive ventilation on reintubation and postextubation respiratory failure in high-risk patients: a randomized clinical trial. JAMA. 2016;316:1565–1574. doi: 10.1001/jama.2016.14194. [DOI] [PubMed] [Google Scholar]
- 16.Global Strategy for Diagnosis, Management, and Prevention of COPD—2016. Glob. Initiat. Chronic Obstr. Lung Dis. – GOLD. https://goldcopd.org/global-strategy-diagnosis-management-prevention-copd-2016/. Accessed Feb 1 2019.
- 17.Vargas F, Saint-Leger M, Boyer A, Bui NH, Hilbert G. Physiologic effects of high-flow nasal cannula oxygen in critical care subjects. Respir Care. 2015;60:1369–1376. doi: 10.4187/respcare.03814. [DOI] [PubMed] [Google Scholar]
- 18.Mauri T, Alban L, Turrini C, Cambiaghi B, Carlesso E, Taccone P, et al. Optimum support by high-flow nasal cannula in acute hypoxemic respiratory failure: effects of increasing flow rates. Intensive Care Med. 2017;43:1453–1463. doi: 10.1007/s00134-017-4890-1. [DOI] [PubMed] [Google Scholar]
- 19.Mauri T, Galazzi A, Binda F, Masciopinto L, Corcione N, Carlesso E, et al. Impact of flow and temperature on patient comfort during respiratory support by high-flow nasal cannula. Crit Care. 2018;22:120. doi: 10.1186/s13054-018-2039-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Delorme M, Bouchard P-A, Simon M, Simard S, Lellouche F. Effects of high-flow nasal cannula on the work of breathing in patients recovering from acute respiratory failure. Crit Care Med. 2017;45:1981–1988. doi: 10.1097/CCM.0000000000002693. [DOI] [PubMed] [Google Scholar]
- 21.Fraser JF, Spooner AJ, Dunster KR, Anstey CM, Corley A. Nasal high flow oxygen therapy in patients with COPD reduces respiratory rate and tissue carbon dioxide while increasing tidal and end-expiratory lung volumes: a randomised crossover trial. Thorax. 2016;71:759–761. doi: 10.1136/thoraxjnl-2015-207962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mauri T, Turrini C, Eronia N, Grasselli G, Volta CA, Bellani G, et al. Physiologic effects of high-flow nasal cannula in acute hypoxemic respiratory failure. Am J Respir Crit Care Med. 2017;195:1207–1215. doi: 10.1164/rccm.201605-0916OC. [DOI] [PubMed] [Google Scholar]
- 23.Sklar MC, Dres M, Rittayamai N, West B, Grieco DL, Telias I, et al. High-flow nasal oxygen versus noninvasive ventilation in adult patients with cystic fibrosis: a randomized crossover physiological study. Ann Intensive Care. 2018;8:85. doi: 10.1186/s13613-018-0432-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McKinstry S, Pilcher J, Bardsley G, Berry J, Van de Hei S, Braithwaite I, et al. Nasal high flow therapy and PtCO2 in stable COPD: a randomized controlled cross-over trial. Respirology. 2018;23:378–384. doi: 10.1111/resp.13185. [DOI] [PubMed] [Google Scholar]
- 25.Pisani L, Fasano L, Corcione N, Comellini V, Musti MA, Brandao M, et al. Change in pulmonary mechanics and the effect on breathing pattern of high flow oxygen therapy in stable hypercapnic COPD. Thorax. 2017;72:373–375. doi: 10.1136/thoraxjnl-2016-209673. [DOI] [PubMed] [Google Scholar]
- 26.Parke R, McGuinness S, Eccleston M. Nasal high-flow therapy delivers low level positive airway pressure. Br J Anaesth. 2009;103:886–890. doi: 10.1093/bja/aep280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hernández G, Roca O, Colinas L. High-flow nasal cannula support therapy: new insights and improving performance. Crit Care. 2017;21:62. doi: 10.1186/s13054-017-1640-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Möller W, Celik G, Feng S, Bartenstein P, Meyer G, Oliver E, et al. Nasal high flow clears anatomical dead space in upper airway models. J Appl Physiol. 2015;118:1525–1532. doi: 10.1152/japplphysiol.00934.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spoletini G, Cortegiani A, Gregoretti C. Physiopathological rationale of using high-flow nasal therapy in the acute and chronic setting: a narrative review. Trends Anaesth Crit Care. 2019. http://www.sciencedirect.com/science/article/pii/S221084401930005X. Accessed Mar 22 2019.
- 30.Pisani L, Vega ML. Use of nasal high flow in stable COPD: rationale and physiology. J Chronic Obstruct Pulmon Dis. 2017;14:346–350. doi: 10.1080/15412555.2017.1315715. [DOI] [PubMed] [Google Scholar]
- 31.Bräunlich J, Köhler M, Wirtz H. Nasal highflow improves ventilation in patients with COPD. Int J Chronic Obstruct Pulm Dis. 2016;11:1077–1085. doi: 10.2147/COPD.S104616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pilcher J, Eastlake L, Richards M, Power S, Cripps T, Bibby S, et al. Physiological effects of titrated oxygen via nasal high-flow cannulae in COPD exacerbations: a randomized controlled cross-over trial. Respirology. 2017;22:1149–1155. doi: 10.1111/resp.13050. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets supporting the conclusions of this article are included within the article.