Abstract
Several epidemiological and preclinical studies supported the protective effect of coffee on Alzheimer’s disease (AD). However, it is still unknown whether coffee is specifically related with reduced brain AD pathologies in human. Hence, this study aims to investigate relationships between coffee intake and in vivo AD pathologies, including cerebral beta-amyloid (Aβ) deposition, the neurodegeneration of AD-signature regions, and cerebral white matter hyperintensities (WMH). A total of 411 non-demented older adults were included. Participants underwent comprehensive clinical assessment and multimodal neuroimaging including [11C] Pittsburgh compound B-positron emission tomography (PET), [18F] fluorodeoxyglucose PET, and magnetic resonance imaging scans. Lifetime and current coffee intake were categorized as follows: no coffee or <2 cups/day (reference category) and ≥2 cups/day (higher coffee intake). Lifetime coffee intake of ≥2 cups/day was significantly associated with a lower Aβ positivity compared to coffee intake of <2 cups/day, even after controlling for potential confounders. In contrast, neither lifetime nor current coffee intake was not related to hypometabolism, atrophy of AD-signature region, and WMH volume. The findings suggest that higher lifetime coffee intake may contribute to lowering the risk of AD or related cognitive decline by reducing pathological cerebral amyloid deposition.
Subject terms: Molecular neuroscience, Psychiatric disorders
Introduction
Coffee is one of the most popularly consumed beverages in the world and a high proportion of adults drink coffee daily1. Coffee contains hundreds of bioactive compounds, including caffeine, chlorogenic acid, polyphenols, and small amounts of minerals and vitamins, some of which are known to have positive effects on health2. Many epidemiological studies suggest that coffee has beneficial effects on various medical conditions, including stroke3, heart failure4, cancers5, diabetes6, suicide7, Parkinson’s disease8, and mortality9.
Several epidemiological studies also supported the protective effect of coffee on Alzheimer’s disease (AD)10–12 and cognitive decline13–15. Nevertheless, there is limited information available on the neuropathological evidences that support the protective effects of coffee on AD and related cognitive decline in humans. Although a preclinical study of aged transgenic AD mice reported that caffeine, a major component of coffee, decreases brain beta-amyloid (Aβ) levels16–18, it is still unknown whether coffee is specifically related with reduced brain AD pathologies, including Aβ deposition and regional neurodegenerations in human.
Therefore, we investigate relationships between coffee intake and in vivo AD biomarkers on multimodal brain imaging, including cerebral Aβ deposition, AD-signature region cerebral glucose metabolism (AD-CM), AD-signature region cortical thickness (AD-CT), and cerebral white matter hyperintensities (WMH) in non-demented older adults.
Methods
Participants
This study was part of the Korean Brain Aging Study for Early Diagnosis and Prediction of Alzheimer’s Disease (KBASE), which is an ongoing prospective cohort study that begun in 201419. As of February 2017, 411 individuals [282 cognitively normal (CN) adults, and 129 adults with mild cognitive impairment (MCI)], between 55 and 90 years of age were enrolled in the study.
The CN group consisted of participants with a Clinical Dementia Rating (CDR)20 score of 0 and no diagnosis of MCI or dementia. All participants with MCI met the current consensus criteria for amnestic MCI, including: (1) memory complaints confirmed by an informant; (2) objective memory impairments; (3) preservation of global cognitive function; (4) independence in functional activities; and (5) no dementia. Regarding Criterion 2, the age-, education-, and gender-adjusted z-score was <−1.0 for at least one of four episodic memory tests: Word List Memory, Word List Recall, Word List Recognition, and Constructional Recall tests; these are included in the Korean version of the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD-K) neuropsychological battery21. All MCI individuals had a CDR score of 0.5. The exclusion criteria were as follows: (1) presence of a major psychiatric illness; (2) significant neurological or medical condition or comorbidity that could affect mental functioning; (3) contraindications for an magnetic resonance imaging (MRI) scan (e.g., pacemaker or claustrophobia); (4) illiteracy; (5) the presence of significant visual/hearing difficulties and/or severe communication or behavioral problems that would make clinical examinations or brain scans difficult; (6) pregnant or lactation; (7) use of an investigational drug; and (8) drinking tea extract regularly. The Institutional Review Board of Seoul National University Hospital and the SMG-SNU Boramae Medical Center in South Korea approved the present study, and all subjects provided written informed consent prior to participation. More detailed information on recruitment of the KBASE cohort is described in our previous report19.
Clinical and neuropsychological assessments
All participants were administered standardized clinical assessments by trained board-certified psychiatrists based on the KBASE clinical assessment protocol which incorporated the CERAD-K clinical assessment19, which incorporates the CERAD-K22. All subjects were also given a comprehensive neuropsychological assessment battery, administered by a clinical neuropsychologist or trained psychometrists according to a standardized protocol incorporating the CERAD-K neuropsychological battery21. Details on full assessment battery were described previously19.
Assessment of coffee intake
All participants were systematically assessed by trained nurses to determine coffee intake. Specifically, the amount of coffee intake (cups/day) for each participant were assessed for the past one year (i.e., current) and overall lifetime. Previous epidemiologic studies on the effect of coffee intake10,12,23 showed that there was a clear difference in the risk of overall or AD dementia between “<2 cups/day (no or lower drinker)” and “≥2 cups/day (higher drinker)” group. Based on the findings, we categorized the participant into the two group, and tried to test the hypothesis that there is a difference in AD pathology between the two.
Assessment of potential confounders
Coffee intake may be influenced by various other conditions. Therefore, all participants were systematically evaluated about potential confounders, such as lifetime cognitive activity (LCA), occupational complexity, annual income, vascular risk, depression, smoking, and alcohol intake.
Cognitive activity participation frequency was measured by 39-item structured questionnaires24,25. The details of the measurement of cognitive activity are described in our previous report26. Item scores were averaged to yield separate values for each age period. We then calculated the composite score of LCA to use in the subsequent analysis which was an average of all 4-epoch means. With regard to occupational complexity, we considered only the longest-held occupation and then classified into four levels based on the skill levels described in International Standard Classification of Occupations (http://www.ilo.org/public/english/bureau/stat/isco/). Occupations typically involve simple and routine physical or manual tasks at skill level 1, the performance of tasks, such as operating machinery and electronic equipment; driving vehicles; maintenance and repair of electrical and mechanical equipment; and manipulation, ordering and storage of information at skill level 2, the performance of complex technical and practical tasks that require complex problem solving, reasoning, and decision making in a specialized field at skill level 3, and the performance of tasks that require complex problem-solving, decision-making, and creativity based on an extensive body of theoretical and factual knowledge in a specialized field at skill level 4. Information about occupation was obtained from self-report by the participants and confirmed by reliable informants. Annual income was evaluated and categorized into three groups (below the minimum cost of living (MCL), more than MCL but below twice the MCL, twice the MCL or more (http://www.law.go.kr). The MCL was determined according to the administrative rule published by the Ministry of Health and Welfare, Republic of Korea in November 2012. The MCL was 572,168 Korea Won (KRW) for single-person household and added 286,840 KRW for each additional housemate. The comorbidity rates of vascular risk factors were assessed by interviews of participants and their reliable informants; a vascular risk score (VRS) was calculated based on the number of vascular risk factors present and reported as a percentage27. To acquire accurate information, reliable informants were interviewed, and medical records were reviewed. The Geriatric Depression Scale (GDS)28 was used to measure the severity of depressive symptoms. Smoking status (never/former/smoker) and alcohol intake status (never/former/drinker) were evaluated through nurse interview. Blood samples were also obtained via venipuncture, genomic DNA was extracted from whole blood and apolipoprotein E (APOE) genotyping was performed as described previously29. APOE ε4 (APOE4) positivity was defined as the presence of at least one ε4 allele was present.
Measurement of cerebral Aβ deposition
All participants underwent simultaneous three-dimensional [11C] Pittsburg compound B (PiB)-positron emission tomography (PET) and T1-weighted MRI scans using a 3.0 T Biograph mMR (PET-MR) scanner (Siemens; Washington DC, WC, USA) according to the manufacturer’s guidelines. The details of PiB-PET acquisition and preprocessing were described in our previous report30. An AAL algorithm and a region-combining method31 were applied to determine the regions of interest (ROIs) for characterization of PiB retention levels in the frontal, lateral parietal, posterior cingulate-precuneus, and lateral temporal regions. The standardized uptake value ratio (SUVR) values for each ROI were calculated by dividing the mean value for all voxels within each ROI by the mean cerebellar uptake value on the same image. Each participant was classified as Aβ positive (Aβ+) if the SUVR value was >1.4 in at least one of the four ROIs31,32. Considering the bimodal distribution of our PiB data, only Aβ positivity was used as an outcome variable33,34.
Measurement of AD-CM
All subjects underwent [18F] fluorodeoxyglucose (FDG)-PET imaging using the above-described PET-MR machine. The details of FDG-PET acquisition and preprocessing were described in our previous report30. AD-signature FDG ROIs that are sensitive to the changes associated with AD, such as the angular gyri, posterior cingulate cortex, and inferior temporal gyri32, were determined. AD-CM was defined as the voxel-weighted mean SUVR extracted from the AD-signature FDG ROIs.
Measurement of AD-CT
All T1-weighted images were acquired in the sagittal orientation using the above-described 3.0 T PET-MR machine. MR image acquisition and preprocessing were described in our previous report30. AD-CT was defined as the mean cortical thickness values obtained from AD-signature regions including the entorhinal, inferior temporal, middle temporal, and fusiform gyrus, as described previously32.
Measurement of WMH
All participants underwent MRI scans with fluid attenuated inversion recovery using the abovementioned 3.0 T PET-MR scanner in a validated automatic procedure that has previously been reported35. The details of the volume measurement of cerebral WMH were previously described36.
Statistical analysis
We first compared demographic variables, other potential confounders [APOE4, clinical diagnosis (CN vs. MCI), LCA score, occupational complexity, annual income status, VRS, GDS score, smoking status, and alcohol intake status] for the relationship between coffee intake and AD biomarkers, and AD imaging biomarkers between lifetime coffee intake categories (<2 cups/day and ≥2 cups/day) by t test or χ2 test as appropriate. In order to explore the relationship between lifetime coffee intake amount and potential confounders, we performed Spearman correlation analyses. To examine the relationships between lifetime (or current) coffee intake category and neuroimaging parameters, multivariate logistic or linear regression analyses were performed as appropriate. In these analyses, “<2 cups/day” category was used as a reference. Three models were tested for controlling the covariates stepwisely. The first model included age, gender, education, APOE4, clinical diagnosis as covariates; the second model included covariates in the first model plus LCA score, occupational complexity, annual income status, VRS, GDS score, smoking status, and alcohol intake status; and third model included covariates in the second model plus the duration of coffee intake and the age of first coffee intake. To reduce false positive error due to multiple testing, we applied Bonferroni correction. Actually, p < 0.00625 (=0.05/8) was used as the threshold for statistical significance for each analysis considering 4 biomarkers and 2 time periods.
For the AD neuroimaging biomarker with significant association with coffee intake in above analyses, additional exploratory analyses were performed. First, to explore whether there are any brain regional specificity in regard of the relationship between lifetime coffee intake and the biomarker, the same analysis was done for each of the four ROI (i.e., the frontal, lateral parietal, posterior cingulate-precuneus, and lateral temporal region). Second, in order to investigate the modulating effects of the potential confounders (i.e., age, gender, education, APOE4, clinical diagnosis, LCA score, occupational complexity, annual income status, VRS, GDS score, smoking status, and alcohol intake status) on the relationships between coffee intake and the biomarker, we performed the same analysis including two-way interaction term between coffee intake and any one of the confounders, as well as coffee intake itself, as an independent variable. We additionally examined the three-way interaction between lifetime coffee intake and any two of age, education, gender, and APOE4 on the relationship between coffee intake and the biomarker. Third, to explore the dose-effect relationship between overall amount of coffee intake and the biomarker, the same analysis including the total amount of lifetime coffee intake (=duration of coffee intake × cups of coffee intake/day) as an independent variable instead of coffee intake category (lower vs. higher) were performed. For similar purpose, we also compared the AD biomarker among four coffee intake categories (i.e., 0 or <1 cups/day, 1≤ and <2 cups/day, 2≤ and <3 cups/day, and 3≤ cups/day) instead of the dichotomous categories by using χ2 test. For these exploratory analyses, p < 0.05 was served as a statistical threshold. All statistical analyses were performed using IBM SPSS Statistics 24 software (IBM Corp., Armonk, NY, USA).
Results
Participant characteristics
The demographic and clinical characteristics of the participants are presented by the categories of lifetime coffee intake in Table 1. Of the 411 participants, 269 were no or lower coffee drinkers (<2 cups/day) and 142 were higher coffee drinkers (≥2 cups/day). There were significant differences of sex, education, duration of coffee intake, age of first coffee intake, LCA score, occupational complexity, smoking status, alcohol drinking status, and Aβ positivity between the two lifetime coffee intake groups. Correlations of lifetime coffee intake amount with potential confounders for the relationship between coffee intake and AD biomarkers were also presented in Supplementary Table 1.
Table 1.
Participant characteristicsa
| Characteristic | Coffee intake amount, lifetime | t or χ2 | p Value | ||
|---|---|---|---|---|---|
| <2 cups/day | ≥2 cups/day | Total | |||
| n | 269 | 142 | 411 | ||
| Age, y | 71.06 (7.73) | 69.67 (8.43) | 70.58 (8.00) | 1.675 | 0.095 |
| Female, no. (%) | 175 (65.06) | 57 (40.04) | 232 (56.45) | 23.467 | <0.001 |
| Education, y | 10.56 (4.90) | 12.27 (4.49) | 11.15 (4.82) | −3.574 | <0.001 |
| MMSE | 25.26 (3.41) | 25.96 (3.34) | 25.50 (3.40) | −2.007 | 0.045 |
| APOE4 positivity, no. (%) | 61 (22.76) | 35 (24.65) | 96 (23.41) | 0.184 | 0.668 |
| Clinical diagnosis, CN, no. (%) | 183 (68.03) | 99 (69.72) | 282 (68.61) | 0.123 | 0.726 |
| Duration of coffee intake, y | 27.61 (19.06) | 34.12 (15.06) | 25.93 (18.73) | −6.784 | <0.001 |
| Age of first coffee intake, y | 41.17 (17.94) | 34.03 (14.54) | 38.31 (17.01) | 3.940 | <0.001 |
| Cognitive activity | |||||
| Childhood score | 2.00 (0.64) | 2.06 (0.58) | 2.02 (0.62) | −0.892 | 0.373 |
| Adulthood score | 2.29 (0.90) | 2.46 (0.84) | 2.35 (0.86) | −1.800 | 0.073 |
| Midlife score | 2.24 (0.84) | 2.44 (0.79) | 2.31 (0.83) | −2.338 | 0.020 |
| Current score | 2.37 (0.69) | 2.52 (0.71) | 2.42 (0.70) | −1.934 | 0.054 |
| Lifetime composite score | 2.23 (0.67) | 2.37 (0.59) | 2.27 (0.64) | −2.113 | 0.035 |
| Occupational complexity, no. (%) | 11.571 | 0.021 | |||
| None | 59 (22.01) | 16 (11.27) | 75 (18.29) | ||
| Skill level 1 | 20 (7.46) | 9 (6.34) | 29 (7.07) | ||
| Skill level 2 | 88 (32.84) | 44 (30.99) | 132 (30.19) | ||
| Skill level 3 | 28 (10.45) | 26 (18.31) | 54 (13.17) | ||
| Skill level 4 | 73 (27.24) | 47 (33.10) | 120 (29.27) | ||
| Annual income, no. (%) | 2.530 | 0.282 | |||
| <MCL | 19 (7.06) | 16 (11.27) | 35 (8.52) | ||
| ≥MCL, <2 × MCL | 124 (46.10) | 58 (40.85) | 182 (44.28) | ||
| ≥2 × MCL | 126 (46.84) | 68 (47.89) | 194 (47.20) | ||
| VRS | 18.77 (15.76) | 16.31 (17.47) | 17.92 (16.39) | 0.148 | 0.148 |
| GDS score | 6.65 (5.95) | 6.47 (6.76) | 6.59 (6.24) | 0.270 | 0.787 |
| Smoking status, no. (%) | 27.087 | <0.001 | |||
| Never | 206 (76.58) | 73 (51.41) | 279 (67.88) | ||
| Former | 54 (20.07) | 58 (40.85) | 112 (27.25) | ||
| Smoker | 9 (3.35) | 11 (7.75) | 20 (4.87) | ||
| Alcohol drink status, no. (%) | 12.651 | 0.002 | |||
| Never | 161 (59.85) | 63 (44.37) | 224 (54.50) | ||
| Former | 25 (9.29) | 28 (19.72) | 53 (12.90) | ||
| Drinker | 83 (30.86) | 51 (35.92) | 134 (32.60) | ||
| Cerebral Aβ deposition | |||||
| Aβ positivity, no. (%) | 73 (27.14) | 25 (17.61) | 98 (23.84) | 4.650 | 0.031 |
| Neurodegeneration | |||||
| AD-CM, SUVR | 1.40 (0.13) | 1.39 (0.12) | 1.39 (0.13) | 0.462 | 0.645 |
| AD-CT, mm | 2.81 (0.22) | 2.80 (0.23) | 2.81 (0.22) | 0.262 | 0.794 |
| WMH volume, cm3 | 5.89 (5.56) | 6.02 (5.03) | 5.94 (5.37) | −0.217 | 0.828 |
APOE4 apolipoprotein ε4, CN cognitive normal, MCL minimum cost of living, VRS vascular risk score, GDS Geriatric depression scale, Aβ beta-amyloid, AD Alzheimer’s disease, AD-CM Alzheimer’s disease signature cerebral glucose metabolism, AD-CT Alzheimer’s disease signature cortical thickness, SUVR standardized uptake value ratio, WMH white matter hyperintensities
aUnless otherwise indicated, data are expressed as mean (standard deviation)
Difference of Aβ positivity between high and low coffee intakes
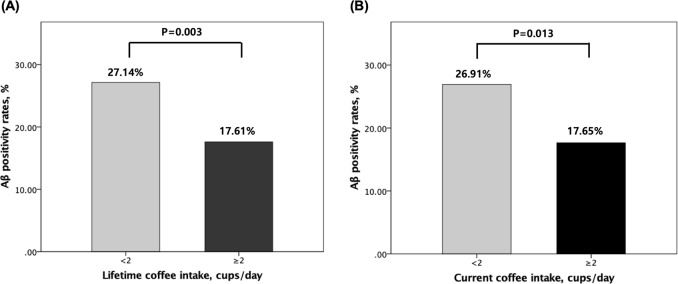
The association between coffee intake and Aβ positivity presented in Table 2 and Fig. 1. Lifetime coffee intake of ≥2 cups/day showed significantly lower Aβ positivity compared to coffee intake of <2 cups/day, regardless of the models. To explore whether there are any brain regional specificity in regard of the relationship between lifetime coffee intake and Aβ positivity, the difference of Aβ positivity between high and low lifetime coffee intakes was tested for each of the four ROI (i.e., the frontal, lateral parietal, posterior cingulate-precuneus, and lateral temporal region). Lifetime coffee intake of ≥2 cups/day showed lower Aβ positivity in all four regions (Table 3). In contrast to lifetime coffee intake, current coffee intake was not related to Aβ positivity regardless of the covariates.
Table 2.
Results of multiple logistic regression analyses for assessing the relationships of stratified coffee intake with Aβ positivity in non-demented individuals
| Coffee intake | Aβ positivity | |
|---|---|---|
| OR (95% CI) | p Value | |
| Model 1 a | ||
| Lifetime | ||
| <2 cup/day | Reference | |
| ≥2 cup/day | 0.401 (0.208 to 0.772) | 0.006* |
| Current | ||
| <2 cup/day | Reference | |
| ≥2 cup/day | 0.453 (0.236 to 0.869) | 0.017 |
| Model 2 b | ||
| Lifetime | ||
| <2 cup/day | Reference | |
| ≥2 cup/day | 0.386 (0.197 to 0.757) | 0.006* |
| Current | ||
| <2 cup/day | Reference | |
| ≥2 cup/day | 0.443 (0.227 to 0.862) | 0.017 |
| Model 3 c | ||
| Lifetime | ||
| <2 cup/day | Reference | |
| ≥2 cup/day | 0.334 (0.162 to 0.689) | 0.003* |
| Current | ||
| <2 cup/day | Reference | |
| ≥2 cup/day | 0.402 (0.197 to 0.822) | 0.013 |
Aβ beta-amyloid, OR odds ratio, CI confidence interval, APOE4 apolipoprotein ε4, LCA lifetime cognitive activity, VRS vascular risk score, GDS geriatric depression scale
a Adjusted for age, gender, education, apolipoprotein ε4, and clinical diagnosis
b Adjusted for covariates in Model 1 plus, LCA score, occupational complexity, and annual income status, VRS, GDS score, smoking status, and alcohol status
c Adjusted for covariates in Model 2 plus, duration of coffee intake and age of first coffee intake
*Statistically significant (p < 0.00625)
Fig. 1.
Aβ positivity rates according to the categories of (a) lifetime or (b) current coffee intake: comparison of Aβ positivity rates between 0 or <2 cups/day and ≥2 cups/day in non-demented older adults. Multivariate logistic regression analyses were performed after controlling for age, gender, education, apolipoprotein ε4, clinical diagnosis, LCA score, occupational complexity, annual income status, VRS, GDS score, smoking status, alcohol intake status, duration of coffee intake and age of first coffee intake. Aβ beta-amyloid, LCA lifetime cognitive activity, VRS vascular risk score, GDS geriatric depression scale. The English in this document has been checked by at least two professional editors, both native speakers of English. For a certificate, please see: http://www.textcheck.com/certificate/1uduau
Table 3.
Results of multiple logistic regression analyses for assessing the relationship between stratified lifetime coffee intake and subregional Aβ positivity in non-demented individuals
| Lifetime coffee intake | Frontal region | PC-PRC region | Lt. parietal region | Lt. temporal region | ||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | |
| Model 1 a | ||||||||
| <2 cup/day | Reference | Reference | Reference | Reference | ||||
| ≥2 cup/day | 0.400 (0.197 to 0.813) | 0.011 | 0.417 (0.218 to 0.798) | 0.008 | 0.393 (0.197 to 0.783) | 0.008 | 0.500 (0.252 to 0.993) | 0.048 |
| Model 2 b | ||||||||
| <2 cup/day | Reference | Reference | Reference | Reference | ||||
| ≥2 cup/day | 0.381 (0.183 to 0.793) | 0.010 | 0.402 (0.206 to 0.783) | 0.007 | 0.370 (0.181 to 0.757) | 0.007 | 0.475 (0.234 to 0.966) | 0.040 |
| Model 3 c | ||||||||
| <2 cup/day | Reference | Reference | Reference | Reference | ||||
| ≥2 cup/day | 0.324 (0.149 to 0.707) | 0.005 | 0.349 (0.170 to 0.713) | 0.004 | 0.285 (0.132 to 0.617) | 0.001 | 0.400 (0.188 to 0.851) | 0.017 |
Aβ beta-amyloid, PC-PRC posterior cingulate-precuneus, OR odds ratio, CI confidence interval, APOE4 apolipoprotein ε4, LCA lifetime cognitive activity, VRS vascular risk score, GDS geriatric depression scale
aAdjusted for age, gender, education, apolipoprotein ε4, and clinical diagnosis
bAdjusted for covariates in Model 1 plus, LCA score, occupational complexity, annual income status, VRS, GDS score, smoking status, and alcohol status
cAdjusted for covariates in Model 2 plus, duration of coffee intake and age of first coffee intake
Moderating effect of potential confounders on the relationship between lifetime coffee intake and Aβ positivity
Any two-way interaction between lifetime coffee intake and each of age, gender, gender, APOE4, clinical diagnosis, LCA score, occupational complexity, annual income status, VRS, GDS score, smoking status, and alcohol intake status was not significant, indicating that the potential confounders do not moderate the relationship between lifetime coffee intake and Aβ positivity (Supplementary Table 2). We additionally examined the three-way interaction between lifetime coffee intake and any two of age, gender, education, and APOE4 on the relationship between coffee intake and Aβ positivity, but did not find any significant finding.
Dose–effect relationship between lifetime coffee intake and Aβ positivity
To explore the dose-effect relationship between lifetime coffee intake amount and Aβ positivity further, we compared Aβ positivity rates according to four lifetime coffee intake strata, i.e., 0 or <1 cups/day, 1≤ and <2 cups/day, 2≤ and <3 cups/day, and 3≤ cups/day by using χ2 test. As shown in Supplementary Fig. 1, there was a significant trend of association between lifetime coffee intake strata and Aβ positivity (p = 0.048). Multiple logistic regression analysis also demonstrated that there was a trend toward significance on dose–effect association between the total amount of lifetime coffee intake (=duration of coffee intake × cups of coffee intake/day) and Aβ positivity [OR (95% CI) = 0.991 (0.982–1.001), p = 0.067]. As the amount increased, so Aβ-positivity rate decreased (Supplementary Table 3).
Association of coffee intake with cerebral tau deposition, AD-CM, AD-CT, and WMH
In contrast to the results for Aβ positivity, neither lifetime nor current coffee intake was related with any of AD-CM, AD-CT, and WMH (Table 4).
Table 4.
Results of multiple linear model analyses for assessing the relationship between stratified coffee intake and AD-CM, AD-CT, or WMH volume in non-demented individuals
| Coffee intake | AD-CM | AD-CT | WMH | |||
|---|---|---|---|---|---|---|
| B (95% CI) | p Value | B (95% CI) | p value | B (95% CI) | p Value | |
| Model 1 a | ||||||
| Lifetime | ||||||
| <2 cup/day | Reference | Reference | Reference | |||
| ≥2 cup/day | −0.007 (−0.034 to 0.021) | 0.633 | 0.002 (−0.037 to 0.042) | 0.910 | 0.237 (−0.990 to 1.464) | 0.704 |
| Current | ||||||
| <2 cup/day | Reference | Reference | Reference | |||
| ≥2 cup/day | −0.009 (−0.037 to 0.019) | 0.540 | −0.001 (−0.041 to 0.040) | 0.980 | 0.620 (−0.626 to 1.867) | 0.328 |
| Model 2 b | ||||||
| Lifetime | ||||||
| <2 cup/day | Reference | Reference | Reference | |||
| ≥2 cup/day | −0.008 (−0.035 to 0.020) | 0.580 | 0.003 (−0.037 to 0.042) | 0.888 | 0.282 (−0.961 to 1.526) | 0.655 |
| Current | ||||||
| <2 cup/day | Reference | Reference | Reference | |||
| ≥2 cup/day | −0.013 (−0.040 to 0.015) | 0.369 | −0.002 (−0.041 to 0.038) | 0.935 | 0.674 (−0.591 to 1.939) | 0.295 |
| Model 3 c | ||||||
| Lifetime | ||||||
| <2 cup/day | Reference | Reference | Reference | |||
| ≥2 cup/day | 0.006 (−0.022 to 0.033) | 0.678 | 0.008 (−0.033 to 0.048) | 0.707 | 0.181 (−1.124 to 1.486) | 0.785 |
| Current | ||||||
| <2 cup/day | Reference | Reference | Reference | |||
| ≥2 cup/day | 0.001 (−0.027 to 0.029) | 0.951 | 0.004 (−0.038 to 0.045) | 0.864 | 0.612 (−0.714 to 1.938) | 0.365 |
Aβ beta-amyloid, AD-CM Alzheimer’s disease signature cerebral glucose metabolism, AD-CT Alzheimer’s disease signature cortical thickness, WMH white matter hyperintensities, CI confidence interval, LCA lifetime cognitive activity, GDS geriatric depression scale, APOE4 apolipoprotein ε4
aAdjusted for age, gender, education, APOE4, and clinical diagnosis
bAdjusted for covariates in Model 1 plus, LCA score, occupational complexity, annual income status, vascular risk score, GDS score, smoking status, and alcohol status
cAdjusted for covariates in Model 2 plus, duration of coffee intake and age of first coffee intake
Discussion
The present study found that a lifetime coffee intake of ≥2 cups/day (higher coffee intake) was associated with lower cerebral Aβ positivity rate in non-demented older adults when compared to the coffee intake of <2 cups/day. We did not find any association of coffee intake with regional neurodegeneration and WMH. This is the first study to investigate the association between higher coffee intake and in vivo AD pathologies in human.
The present finding of the relationship between higher coffee intake and a decreased rate of pathological Aβ deposition is in line with results from previous studies using animal models, which indicated that higher caffeine, one of the major ingredients of coffee, intake exerts a protective effect via molecular Aβ-related mechanisms16–18,37,38. For example, Arendash et al.18 suggested that caffeine protects AD mice against cognitive impairment and reduces brain Aβ production by deactivating the positive-feedback loop from the γ- to β-secretase cleavages on the Aβ protein precursor. The same group also reported that high caffeine intake improves cognitive performance of aged AD mice, but not of aged wild-type mice, with reduced brain Aβ levels, suggesting that the cognitive enhancing effect of caffeine in AD mice is mediated by a decrease in Aβ concentration16. Furthermore, Cao et al. reported that caffeine suppresses Aβ levels in the plasma and brain of AD mice17 and also suggested that caffeine and other components in coffee may synergize to protect against cognitive decline in AD mice38. Moreover, Li et al.37 indicated that caffeine suppresses Aβ protein precursor internalization and Aβ generation via adenosine A3 receptor-mediated actions. The present finding also provides a neuropathological explanation for the relationship between higher coffee intake and reduced risk of AD dementia observed in several clinical and epidemiological studies10–12. Those studies reported higher coffee drinkers had 31–65% decrease in the risk of AD dementia, which is quite comparable to about 65% decrease of Aβ positivity rate in higher coffee drinkers (27.14%) compared to lower coffee drinkers (17.61%). Furthermore, the relationship between higher coffee intake and lower Aβ positivity was prominent for lifetime coffee intake than for current coffee intake. This suggests that the protective effects of higher coffee intake against Aβ pathology involve the chronic effects associated with prolonged exposure rather than an acute or short-term effect.
In the present study, we did not find any association of coffee intake with regional neurodegeneration and WMH. Although no previous study investigated the relationship between coffee intake and brain metabolism, the Honolulu-Asia Aging Study showed that coffee intake was not associated with generalized brain atrophy and microvascular ischemic lesions39, similarly to our findings. In addition, the Health Professional Follow-up Study also showed that chronic coffee or caffeine intake is not associated with a risk of cerebrovascular or cardiovascular disease40. Although some previous reports indicated an association between coffee intake and cerebrovascular risk, they examined the acute effect of coffee intake, but not the chronic effect of long-term coffee intake41,42. Such a null association between coffee intake and AD-related neurodegeneration or vascular changes indicates that chronic coffee intake has no direct effects on neurodegenerative or cerebrovascular changes through Aβ-independent mechanisms. Given the significant association between higher coffee intake and lower Aβ positivity, the negative finding for AD-related regional neurodegeneration appears related to the long-time delay between pathological Aβ accumulation and Aβ-dependent neurodegeneration43,44.
The present study had several limitations that should be considered. First, because this was a cross-sectional study, it is difficult to infer causal relationships from the findings. However, the significant relationship between lifetime coffee intake and amyloid pathology supports the possible causal nature of the relationship. Second, underestimates of coffee intake or retrospective recall bias may have affected the results of lifetime coffee intake in older individuals. However, coffee intake is less prone to misreporting because coffee intake is a long-term habitual behavior. Evaluation for coffee intake is known to be performed with the highest validity and reproducibility45. In addition, the current finding between coffee intake and amyloid was significant even after controlling the effect of clinical diagnosis on cognitive status, and the reported frequency of coffee intake was not related with the proportion of MCI (Table 1). Finally, it is unclear which ingredient(s) in coffee acts on Aβ pathology. Although caffeine is among hundreds of bioactive compounds in coffee46, it is the most widely studied ingredient against Aβ pathology16–18. Other bioactive compounds include chlorogenic acid, polyphenols, small amount of minerals, and vitamin B3, which have also been investigated47–49. However, it remains controversial whether a single ingredient in coffee is effective against Aβ pathology or whether a combination of ingredients is effective. Therefore, further investigations are needed to clarify which ingredient(s) in coffee are important for reducing Aβ pathology. The comparison between coffee with and without caffeine may give us a clue on the specific effect of caffeine.
In conclusion, the findings of present study suggest that higher lifetime coffee intake is likely to contribute to lowering the risk of AD or related cognitive decline by reducing pathological cerebral amyloid deposition.
Supplementary information
Supplementary Table1, Supplementary Table 2, Supplementary Table 3
Acknowledgements
We thank the KBASE study participants and their caregivers. This study was supported by a grant from the Ministry of Science, ICT, and Future Planning, Republic of Korea (Grant no. NRF-2014M3C7A1046042) and a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (Grant nos. HI18C0630 and HI19C0149). The funding source had no role in the study design, data collection, data analysis, data interpretation, writing of the paper, or decision to submit it for publication.
Authors' contributions
J.W.K. and D.Y.L. conceived and designed the study. M.S.B., D.Y., J.H.L., S.Y.J., G.J., H.N., B.K.S., J.Y.L., Y.K.K., S.A.S., C.-H.S. and D.Y.L. were involved in acquisition, or analysis and interpretation of the data and helped to draft the paper. J.W.K., M.S.B., D.Y., J.H.L. and D.Y.L. were major contributors in writing the paer and critically revising the paper for intellectual content. D.Y.L. served as principal investigator and supervised the study. All authors read and approved the final paper.
Availability of data and materials
The datasets generated and analyzed during the present study are not publicly available, owing to ethics considerations and privacy restrictions. Data may be obtained from the corresponding author after approval by the Institutional Review Board of the Seoul National University Hospital, South Korea has been sought.
Ethics approval and consent to participate
The study protocol was approved by the Institutional Review Boards of Seoul National University Hospital and SNU-SMG Boramae Center, Seoul, South Korea, and the study was conducted in accordance with the recommendations of the current version of the Declaration of Helsinki. All subjects provided written informed consent.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
The coinvestigators of the KBASE Research Group are listed in elsewhere (http://kbase.kr).
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information accompanies this paper at (10.1038/s41398-019-0604-5).
References
- 1.Loftfield E, et al. Coffee drinking is widespread in the United States, but usual intake varies by key demographic and lifestyle factors. J. Nutr. 2016;146:1762–1768. doi: 10.3945/jn.116.233940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spiller MA. The chemical components of coffee. Prog. Clin. Biol. Res. 1984;158:91–147. [PubMed] [Google Scholar]
- 3.Larsson SC, Virtamo J, Wolk A. Coffee consumption and risk of stroke in women. Stroke. 2011;42:908–912. doi: 10.1161/STROKEAHA.110.603787. [DOI] [PubMed] [Google Scholar]
- 4.Mostofsky E, Rice MS, Levitan EB, Mittleman MA. Habitual coffee consumption and risk of heart failure: a dose-response meta-analysis. Circ. Heart Fail. 2012;5:401–405. doi: 10.1161/CIRCHEARTFAILURE.112.967299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang A, et al. Coffee and cancer risk: a meta-analysis of prospective observational studies. Sci. Rep. 2016;6:33711. doi: 10.1038/srep33711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akash MS, Rehman K, Chen S. Effects of coffee on type 2 diabetes mellitus. Nutrition. 2014;30:755–763. doi: 10.1016/j.nut.2013.11.020. [DOI] [PubMed] [Google Scholar]
- 7.Lucas M, et al. Coffee, caffeine, and risk of completed suicide: results from three prospective cohorts of American adults. World J. Biol. Psychiatry. 2014;15:377–386. doi: 10.3109/15622975.2013.795243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ross GW, et al. Association of coffee and caffeine intake with the risk of Parkinson disease. J. Am. Med. Assoc. 2000;283:2674–2679. doi: 10.1001/jama.283.20.2674. [DOI] [PubMed] [Google Scholar]
- 9.Ding M, et al. Association of Coffee Consumption With Total and Cause-Specific Mortality in 3 Large Prospective Cohorts. Circulation. 2015;132:2305–2315. doi: 10.1161/CIRCULATIONAHA.115.017341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eskelinen MH, Kivipelto M. Caffeine as a protective factor in dementia and Alzheimer’s disease. J. Alzheimer Dis. 2010;20(Suppl 1):S167–S174. doi: 10.3233/JAD-2010-1404. [DOI] [PubMed] [Google Scholar]
- 11.Lindsay J, et al. Risk factors for Alzheimer's disease: a prospective analysis from the Canadian Study of Health and Aging. Am. J. Epidemiol. 2002;156:445–453. doi: 10.1093/aje/kwf074. [DOI] [PubMed] [Google Scholar]
- 12.Eskelinen MH, Ngandu T, Tuomilehto J, Soininen H, Kivipelto M. Midlife coffee and tea drinking and the risk of late-life dementia: a population-based CAIDE study. J. Alzheimer Dis. 2009;16:85–91. doi: 10.3233/JAD-2009-0920. [DOI] [PubMed] [Google Scholar]
- 13.Arab L, et al. Gender differences in tea, coffee, and cognitive decline in the elderly: the Cardiovascular Health Study. J. Alzheimer Dis. 2011;27:553–566. doi: 10.3233/JAD-2011-110431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Gelder BM, et al. Coffee consumption is inversely associated with cognitive decline in elderly European men: the FINE Study. Eur. J. Clin. Nutr. 2007;61:226–232. doi: 10.1038/sj.ejcn.1602495. [DOI] [PubMed] [Google Scholar]
- 15.Vercambre MN, Berr C, Ritchie K, Kang JH. Caffeine and cognitive decline in elderly women at high vascular risk. J. Alzheimer Dis. 2013;35:413–421. doi: 10.3233/JAD-122371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arendash GW, et al. Caffeine reverses cognitive impairment and decreases brain amyloid-beta levels in aged Alzheimer's disease mice. J. Alzheimer Dis. 2009;17:661–680. doi: 10.3233/JAD-2009-1087. [DOI] [PubMed] [Google Scholar]
- 17.Cao C, et al. Caffeine suppresses amyloid-beta levels in plasma and brain of Alzheimer's disease transgenic mice. J. Alzheimer Dis. 2009;17:681–697. doi: 10.3233/JAD-2009-1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arendash GW, et al. Caffeine protects Alzheimer's mice against cognitive impairment and reduces brain beta-amyloid production. Neuroscience. 2006;142:941–952. doi: 10.1016/j.neuroscience.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 19.Byun MS, et al. Korean Brain Aging Study for the Early Diagnosis and Prediction of Alzheimer’s Disease: methodology and baseline sample characteristics. Psychiatry Investig. 2017;14:851–863. doi: 10.4306/pi.2017.14.6.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/WNL.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 21.Lee DY, et al. A normative study of the CERAD neuropsychological assessment battery in the Korean elderly. J. Int. Neuropsychological Soc. 2004;10:72–81. doi: 10.1017/S1355617704101094. [DOI] [PubMed] [Google Scholar]
- 22.Lee JH, et al. Development of the Korean version of the Consortium to Establish a Registry for Alzheimer's Disease Assessment Packet (CERAD-K): clinical and neuropsychological assessment batteries. J. Gerontol. Ser. B Psychol. Sci. Soc. Sci. 2002;57:P47–P53. doi: 10.1093/geronb/57.1.P47. [DOI] [PubMed] [Google Scholar]
- 23.Driscoll I, et al. Relationships between caffeine intake and risk for probable dementia or global cognitive impairment: the Women’s Health Initiative Memory Study. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2016;71:1596–1602. doi: 10.1093/gerona/glw078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilson RS, et al. Early and late life cognitive activity and cognitive systems in old age. J. Int. Neuropsychol. Soc. 2005;11:400–407. doi: 10.1017/S1355617705050459. [DOI] [PubMed] [Google Scholar]
- 25.Wilson RS, Scherr PA, Schneider JA, Tang Y, Bennett DA. Relation of cognitive activity to risk of developing Alzheimer disease. Neurology. 2007;69:1911–1920. doi: 10.1212/01.wnl.0000271087.67782.cb. [DOI] [PubMed] [Google Scholar]
- 26.Ko K, et al. Early-life cognitive activity is related to reduced neurodegeneration in Alzheimer signature regions in late life. Front. Aging Neurosci. 2018;10:70. doi: 10.3389/fnagi.2018.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DeCarli C, et al. Memory impairment, but not cerebrovascular disease, predicts progression of MCI to dementia. Neurology. 2004;63:220–227. doi: 10.1212/01.WNL.0000130531.90205.EF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim JY, et al. Standardization of the korean version of the geriatric depression scale: reliability, validity, and factor structure. Psychiatry Investig. 2008;5:232–238. doi: 10.4306/pi.2008.5.4.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wenham PR, Price WH, Blandell G. Apolipoprotein E genotyping by one-stage PCR. Lancet. 1991;337:1158–1159. doi: 10.1016/0140-6736(91)92823-K. [DOI] [PubMed] [Google Scholar]
- 30.Park JC, et al. Plasma tau/amyloid-beta1–42 ratio predicts brain tau deposition and neurodegeneration in Alzheimer's disease. Brain. 2019;142:771–786. doi: 10.1093/brain/awy347. [DOI] [PubMed] [Google Scholar]
- 31.Reiman EM, et al. Fibrillar amyloid-beta burden in cognitively normal people at 3 levels of genetic risk for Alzheimer's disease. Proc. Natl Acad. Sci. USA. 2009;106:6820–6825. doi: 10.1073/pnas.0900345106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jack CR, et al. Age-specific population frequencies of cerebral beta-amyloidosis and neurodegeneration among people with normal cognitive function aged 50–89 years: a cross-sectional study. Lancet Neurol. 2014;13:997–1005. doi: 10.1016/S1474-4422(14)70194-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klunk WE. Amyloid imaging as a biomarker for cerebral beta-amyloidosis and risk prediction for Alzheimer dementia. Neurobiol. Aging. 2011;32(Suppl 1):S20–S36. doi: 10.1016/j.neurobiolaging.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gottesman RF, et al. Association between midlife vascular risk factors and estimated brain amyloid deposition. J. Am. Med. Assoc. 2017;317:1443–1450. doi: 10.1001/jama.2017.3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsai JZ, et al. Automated segmentation and quantification of white matter hyperintensities in acute ischemic stroke patients with cerebral infarction. PloS ONE. 2014;9:e104011. doi: 10.1371/journal.pone.0104011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moon SW, et al. The ankle-brachial index is associated with cerebral beta-amyloid deposition in cognitively normal older adults. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2019;74:1141–1148. doi: 10.1093/gerona/gly157. [DOI] [PubMed] [Google Scholar]
- 37.Li S, et al. Caffeine, through adenosine a3 receptor-mediated actions, suppresses amyloid-beta protein precursor internalization and amyloid-beta generation. J. Alzheimer Dis. 2015;47:73–83. doi: 10.3233/JAD-142223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cao C, et al. Caffeine synergizes with another coffee component to increase plasma GCSF: linkage to cognitive benefits in Alzheimer's mice. J. Alzheimer Dis. 2011;25:323–335. doi: 10.3233/JAD-2011-110110. [DOI] [PubMed] [Google Scholar]
- 39.Gelber RP, Petrovitch H, Masaki KH, Ross GW, White LR. Coffee intake in midlife and risk of dementia and its neuropathologic correlates. J. Alzheimer Dis. 2011;23:607–615. doi: 10.3233/JAD-2010-101428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grobbee DE, et al. Coffee, caffeine, and cardiovascular disease in men. N. Engl. J. Med. 1990;323:1026–1032. doi: 10.1056/NEJM199010113231504. [DOI] [PubMed] [Google Scholar]
- 41.Mostofsky E, Schlaug G, Mukamal KJ, Rosamond WD, Mittleman MA. Coffee and acute ischemic stroke onset: the Stroke Onset Study. Neurology. 2010;75:1583–1588. doi: 10.1212/WNL.0b013e3181fb443d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ritchie K, et al. Caffeine, cognitive functioning, and white matter lesions in the elderly: establishing causality from epidemiological evidence. J. Alzheimer Dis. 2010;20(Suppl 1):S161–S166. doi: 10.3233/JAD-2010-1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jack CR, et al. Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. Lancet Neurol. 2010;9:119–128. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jack CR, et al. Tracking pathophysiological processes in Alzheimer's disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12:207–216. doi: 10.1016/S1474-4422(12)70291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Watson EJ, Kohler M, Banks S, Coates AM. Validation and reproducibility of an Australian caffeine food frequency questionnaire. Int J. Food Sci. Nutr. 2017;68:617–626. doi: 10.1080/09637486.2016.1268102. [DOI] [PubMed] [Google Scholar]
- 46.Nuhu AA. Bioactive micronutrients in coffee: recent analytical approaches for characterization and quantification. ISRN Nutr. 2014;2014:384230. doi: 10.1155/2014/384230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fukuyama Kazuya, Kakio Shota, Nakazawa Yosuke, Kobata Kenji, Funakoshi-Tago Megumi, Suzuki Toshiharu, Tamura Hiroomi. Roasted Coffee Reduces β-Amyloid Production by Increasing Proteasomal β-Secretase Degradation in Human Neuroblastoma SH-SY5Y Cells. Molecular Nutrition & Food Research. 2018;62(21):1800238. doi: 10.1002/mnfr.201800238. [DOI] [PubMed] [Google Scholar]
- 48.Dhouafli Zohra, Cuanalo-Contreras Karina, Hayouni El Akrem, Mays Charles E., Soto Claudio, Moreno-Gonzalez Ines. Inhibition of protein misfolding and aggregation by natural phenolic compounds. Cellular and Molecular Life Sciences. 2018;75(19):3521–3538. doi: 10.1007/s00018-018-2872-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Turunc Bayrakdar E, Uyanikgil Y, Kanit L, Koylu E, Yalcin A. Nicotinamide treatment reduces the levels of oxidative stress, apoptosis, and PARP-1 activity in Abeta(1–42)-induced rat model of Alzheimer's disease. Free Radic. Res. 2014;48:146–158. doi: 10.3109/10715762.2013.857018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table1, Supplementary Table 2, Supplementary Table 3
Data Availability Statement
The datasets generated and analyzed during the present study are not publicly available, owing to ethics considerations and privacy restrictions. Data may be obtained from the corresponding author after approval by the Institutional Review Board of the Seoul National University Hospital, South Korea has been sought.