Abstract
Primary renal lymphoma (PRL) is a rare lymphoid malignancy with only a few cases reported in the literature. We performed a population-based study of PRL to determine its incidence, clinical characteristics and factors associated with survival using the Surveillance, Epidemiology, and End Results (SEER) database. We identified 723 patients with PRL. The most common histological subtype of PRL was diffuse large B-cell lymphoma (56.3%). The incidence and mortality rate of PRL was 0.053/100,000 person-years and 0.036/100,000 person-years, respectively. The incidence rate of PRL was increasing significantly with an annual percentage change (APC) of 3.45% (p < 0.001). The 1-year and 5-year relative survival (RS) rates of patients with PRL were 78% and 64%. The RS of patients diagnosed between 2000 to 2013 was better than that of patients diagnosed between 1980–1999. A multivariate Cox hazards regression analysis revealed that older age, male gender, diagnosis before 2000, advanced stage, not receiving surgical treatment, and DLBCL or T/NK cell lymphoma type were independent predictors of unfavorable survival.
Subject terms: Disease prevention, Cancer, Urological cancer
Introduction
Primary renal lymphoma (PRL) is a rare disease that comprises less than 1% of extranodal lymphomas1. It is defined as lymphoma involving the kidney without prior lymphatic disease beyond the kidney2. The etiology of PRL is not clear. As a normal kidney does not contain lymphoid tissue3, it has been postulated that PRL originates from the renal capsule, which is rich in lymphatic tissue and penetrates the renal parenchyma4. Another hypothesis is that lymphoid cells are attracted by the chronic inflammatory conditions of the kidney and develop lymphoma eventually5,6. Clinical presentation of PRL is nonspecific and includes such symptoms as gross hematuria, flank pain, loss of weight, fever, or acute/chronic kidney failure7. Renal biopsy is the gold standard for diagnosis8.
As a result of the rarity of this malignancy, there are very limited data on its incidence, optimal management and clinical outcome, mainly from case reports or case series9. Up to our knowledge, only 70 cases were reported as stated in a literature review conducted in 20162. Using data from the Surveillance, Epidemiology, and End Results (SEER) database, our study aimed to examine the incidence, clinical characteristics and survival of patients with PRL as well as to determine prognostic factors in a population-based cohort in the United States.
Materials and Methods
Data source
The SEER database is a program of the National Cancer Institute, representing nearly 30% of the US population from 18 registries currently. This population-based program collects information on patient demographics, morphology and site of primary tumor, follow-up and outcomes. Further information about SEER can be found on its web site10.
Study population and variables
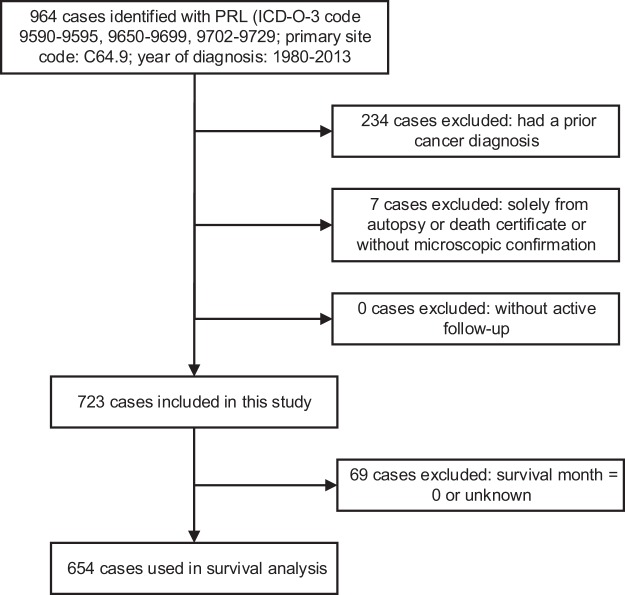
We obtained clinical data for patients with PRL from the SEER 18 (1973–2013, Nov 2015 Sub) database released in April 2016 using SEER*Stat software (Version 8.3.5; NCI; Bethesda, MD)11. We used International Classification of Diseases for Oncology, 3rd edition (ICD-O-3) histologic codes 9590–9595, 9650–9699 and 9702–9729 for lymphoma and primary site code C64.9 for the kidney to identify all patients with PRL diagnosed between 1980 and 2013 (Fig. 1). We excluded patients who had a prior cancer diagnosis, as a prior cancer diagnosis or its treatment might have unknown underlying impacts on outcome of subsequent primary cancer12. We also excluded cases without microscopic confirmation or active follow-up (Fig. 1). In addition, we obtained survival data on patients with primary nodal diffuse large B-cell lymphoma (DLBCL) from the same period for comparing survival with primary renal DLBCL13. To estimate long-term incidence, we obtained a cohort of patients with PRL from the SEER-9 database from 1980 to 201311.
Figure 1.
Flow diagram of patient selection within the SEER database between 1980 and 2013.
The following patient-specific information was extracted: age at diagnosis, gender, race, year of diagnosis, marital status, laterality, morphological subtype, cancer stage, therapy type, the length of survival and the cause of death as recorded in the database. Cancer stages reported were according to the Ann Arbor staging system of American Joint Committee on Cancer (AJCC) (7th edition)14.
Statistical analysis
We calculated the long-term incidence rates, mortality rates and corresponding annual percentage changes (APCs) between 1980 and 2013. The incidence rates were age-adjusted to the 2000 US standard population and expressed per 100,000 person-years. A log-linear model was used to calculate APCs of incidence and mortality rates. If there were no cases or deaths recorded in a certain year, the incidence or mortality rate in that year would be zero, which was replaced with a small positive constant (0.0001) for logarithmic transformation15. The significance level was calculated using Student’s t test16. We analyzed the overall survival (OS), disease-specific survival (DSS) and relative survival (RS) using the Kaplan-Meier method. OS rate is defined as the percentage of survivors (all causes of death) after a duration of follow-up time. DSS rate is defined as the percentage of patients who have not died from PRL (rather than from other causes) in a defined period of time17. RS is defined as the ratio of the proportion of observed survivors in a cohort of cancer patients to the proportion of expected survivors in a comparable set of cancer free individuals. The Ederer method was used to calculate RS18. The cases whose survival month was 0 or unknown were excluded in the survival analysis (Fig. 1). Univariate and multivariate analysis was done by a Cox proportional hazards model to assess the prognostic effect of the various clinical variables.
All analyses were performed using SEER*Stat software (Version 8.3.5; NCI; Bethesda, MD), Joinpoint Regression Program (version 4.6.0.0; Information Management Services; Calverton, MD), SPSS Statistics software (Version 22; IBM Corporation; Armonk, NY) and STATA MP (version 15.0; StataCorp LP; College Station, TX). Tests were two-tailed, with a p-value of less than 0.05 considered statistically significant.
Ethical approval
This article does not contain any studies with human participants or animals.
Results
Demographic and clinical characteristics
Overall, 723 patients with PRL were identified in the SEER database and were included in the current analysis (Fig. 1). The demographic and clinical characteristics of these patients are shown in Table 1. The mean age at diagnosis was 63.7 (±17.7) years. The majority of the patients were male (62.9%), while the proportion of males in the US general population was 49.1% (Table 1 and Supplementary Table 3). Most patients had a unilateral involvement of the left side (48.3%) or right side (39.1%), while 57 patients (7.9%) had bilateral presentation (Table 1). Patients younger than 18 had a significantly higher proportion (39.1%) of bilateral involvement than adults (6.9%) (P < 0.001) (Table S1). A total of 243 patients (33.6%) underwent surgery, and 61 patients (8.4%) received radiotherapy. The preponderance of PRL was non-Hodgkin lymphoma (NHL) (93.2%). The most common NHL subtype identified was DLBCL (56.3%), followed by follicular lymphoma (9.0%) and marginal zone lymphoma (MZL) (7.8%). There were only 5 patients (0.7%) with Hodgkin lymphoma (HL) (Table 1). In total, 423 patients (58.5%) died during the study period. 59.8% of the deaths resulted from PRL. Other major causes of death included cardiovascular diseases (10.9%), other malignant cancers (10.6%), infectious diseases (7.6%) and renal diseases (1.7%) (Supplementary Table 1).
Table 1.
Demographic and clinical characteristics of patients with primary renal lymphoma (PRL).
| Characteristics | No. of Patient (n = 723) |
|---|---|
| Age | |
| mean age (±SD) | 63.7 (±17.7) |
| range | 2–95 |
| ≤60 | 255 (35.3%) |
| 61–80 | 369 (51.0%) |
| >80 | 99 (13.7%) |
| Sex | |
| Female | 268 (37.1%) |
| Male | 455 (62.9%) |
| Race | |
| White | 616 (85.2%) |
| Black | 59 (8.2%) |
| Asian or Pacific Islander | 42 (5.8%) |
| American Indian | 3 (0.4%) |
| Unknown | 3 (0.4%) |
| Year | |
| 1980–1999 | 171 (23.7%) |
| 2000–2013 | 552 (76.4%) |
| Laterality | |
| Bilateral | 57 (7.9%) |
| Left | 349 (48.3%) |
| Right | 283 (39.1%) |
| Unknown | 34 (4.7%) |
| Stage | |
| Stage I | 188 (26.0%) |
| Stage II | 178 (24.6%) |
| Stage III | 45 (6.2%) |
| Stage IV | 263 (36.4%) |
| Unknown | 49 (6.8%) |
| Surgery | |
| Surgery | 243 (33.6%) |
| Non-surgery | 462 (63.9%) |
| Unknown | 18 (2.5%) |
| Histology | |
| Hodgkin lymphoma | 5 (0.7%) |
| Non-Hodgkin lymphoma | 674 (93.2%) |
| DLBCL | 407 (56.3%) |
| Follicular lymphoma | 65 (9.0%) |
| Marginal zone lymphoma | 56 (7.8%) |
| Burkitt’s lymphoma | 25 (3.5%) |
| Small lymphatic lymphoma | 20 (2.8%) |
| Continued | |
| Characteristics | No. of Patient (n = 723) |
| T/NK cell lymphoma | 10 (1.4%) |
| Non-Hodgkin lymphoma, NOS | 91 (12.6%) |
| Lymphoma, NOS | 44 (6.1%) |
Note: SD: standard deviation; DLBCL: diffuse large B-cell lymphoma; NOS: not otherwise specified.
Incidence and mortality
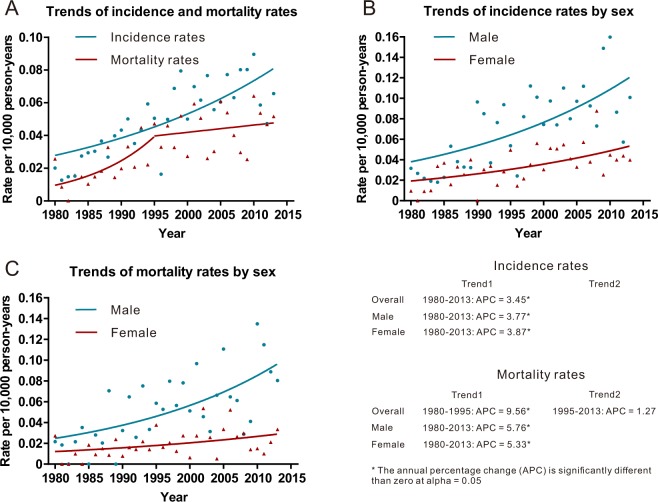
The age-adjusted incidence and mortality rate during the study period were 0.053 per 100,000 person-years (95% confidence interval [CI], 0.048–0.058) and 0.036 per 100,000 person-years (95% CI, 0.032–0.040), respectively. Overall, the incidence of PRL increased from 0.020 per 100 000 person-years (95% CI, 0.005–0.052) in 1980 to 0.066 per 100 000 person-years (95% CI, 0.041–0.100) in 2013, generating an APC of 3.45% (95% CI, 2.32–4.59; p < 0.001) (Fig. 2 and Supplementary Table 4). The mortality due to PRL increased markedly from 1980 to 1995 (APC, 9.59%; 95% CI, 1.35–18.44; p = 0.023), and remained stable in the years between 1995 and 2013 (APC, 1.27%; 95% CI, −2.24–4.91; p = 0.470) (Fig. 2 and Supplementary Table 5).
Figure 2.
Incidence and mortality rates of primary renal lymphoma (PRL) and annual percentage change (APC) trends of PRL. (A) Incidence rates and relative APC trends. (B) Mortality rates and APC trends. Notes: incidence and mortality rates were age-adjusted to the 2000 US standard population and expressed per 100,000 person-years.
The incidence rate of PRL among male population (0.078 per 100,000 person-years; 95% CI, 0.069–0.088) was higher than that of females (0.035 per 100,000 person-years; 95% CI, 0.030–0.041), both of which were increasing significantly in the study period (male: APC = 3.77% [95% CI, 2.26–5.30; p < 0.001]; female: APC = 3.87% [95% CI, 0.88–6.94; p = 0.009]). Compared to female patients, male patients also had higher mortality rate of PRL (male: 0.059 per 100,000 person-years [95% CI, 0.051–0.068]; female: 0.020 per 100,000 person-years [95% CI, 0.017–0.025]).
Survival
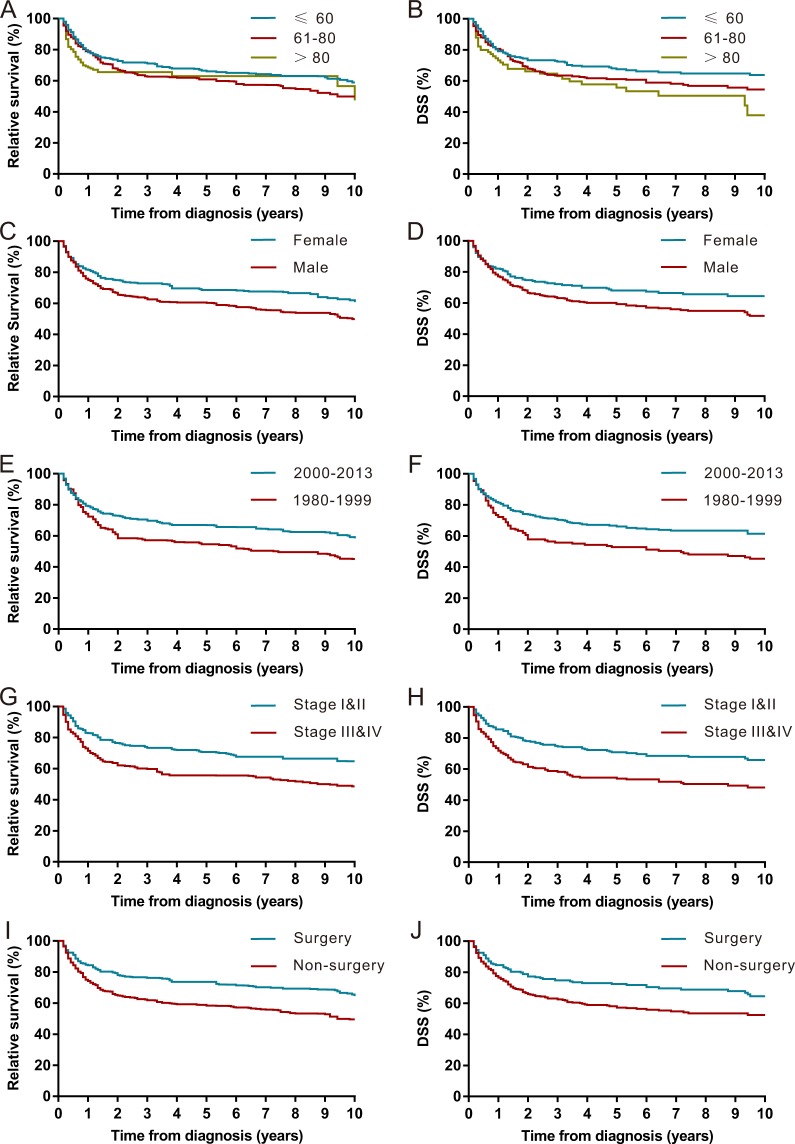
The Kaplan-Meier survival analysis revealed that the RS rates of PRL at 1 year, 5 years and 10 years were 78%, 64% and 55%, respectively (Fig. 3 and Table 2). The RS for patients diagnosed between 2000 to 2013 was better than that of patients diagnosed between 1980–1999 (Fig. 4). A Kaplan-Meier survival analysis also demonstrated a RS and DSS advantage for patients who were younger, were female, had disease of early stage (stage I and II), received surgery and were diagnosed with MZL (Table 2, Fig. 4 and Supplementary Fig. 1).
Figure 3.
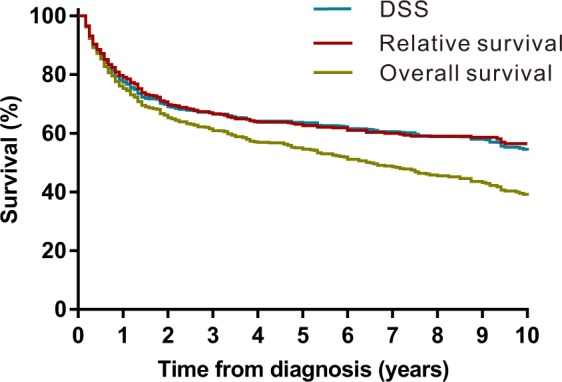
Kaplan–Meier estimate of overall survival (OS), relative survival (RS) and disease-specific survival (DSS) of patients with primary renal lymphoma (PRL).
Table 2.
Relative Survival (RS) rates and disease-specific survival (DSS) rates of patients with primary renal lymphoma (PRL) for different clinical characteristics.
| Variables | RS rates | DSS rates | ||||
|---|---|---|---|---|---|---|
| 1-year (95% CI) | 5-year (95% CI) | 10-year (95% CI) | 1-year (95% CI) | 5-year (95% CI) | 10-year (95% CI) | |
| Overall | 78% (74–81) | 64% (59–68) | 55% (34–43) | 79% (76–82) | 63% (58–67) | 57% (52–61) |
| Age | ||||||
| ≤60 | 78% (72–83) | 66% (59–72) | 67% (60–73) | 79% (73–84) | 68% (61–73) | 64% (57–70) |
| 61–80 | 79% (74–83) | 61% (54–67) | 61% (54–67) | 81% (76–85) | 61% (55–67) | 55% (48–61) |
| >80 | 69% (56–78) | 63% (45–77) | 63% (45–77) | 73% (62–81) | 56% (43–66) | 38% (21–55) |
| Sex | ||||||
| Male | 75% (70–79) | 60% (54–66) | 50% (41–58) | 77% (73–81) | 59% (54–64) | 52% (45–58) |
| Female | 82% (76–86) | 69% (61–75) | 61% (50–70) | 82% (77–87) | 68% (61–74) | 65% (57–71) |
| Race | ||||||
| White | 78% (74–82) | 65% (59–70) | 65% (59–70) | 80% (76–83) | 63% (59–67) | 57% (52–62) |
| Other | 73% (61–81) | 59% (45–70) | 59% (45–70) | 74% (63–82) | 59% (47–69) | 54% (40–67) |
| Year of diagnosis | ||||||
| 1980–1999 | 72% (64–79) | 55% (45–63) | 45% (35–55) | 72% (64–79) | 53% (44–61) | 45% (37–53) |
| 2000–2013 | 79% (75–83) | 67% (61–72) | 58% (49–66) | 81% (78–85) | 66% (61–70) | 61% (56–67) |
| Laterality | ||||||
| Right | 79% (73–83) | 61% (53–68) | 49% (38–58) | 82% (77–86) | 62% (55–68) | 54% (46–62) |
| Left | 77% (71–81) | 66% (58–72) | 59% (48–68) | 77% (72–82) | 63% (57–68) | 57% (50–63) |
| Bilateral | 72% (58–83) | 59% (44–72) | 56% (38–71) | 71% (57–82) | 61% (46–73) | 58% (42–70) |
| Stage | ||||||
| Stage I & II | 83% (78–87) | 71% (64–77) | 65% (55–73) | 86% (81–89) | 71% (65–76) | 66% (59–72) |
| Stage III & IV | 72% (65–77) | 56% (49–62) | 49% (39–58) | 72% (66–77) | 54% (47–60) | 48% (41–55) |
| Surgery | ||||||
| Surgery | 84% (78–89) | 74% (66–80) | 65% (53–74) | 85% (79–89) | 72% (66–78) | 65% (57–71) |
| Non-surgery | 74% (69–78) | 58% (52–64) | 50% (41–58) | 77% (72–80) | 57% (52–62) | 53% (47–58) |
| Histology | ||||||
| Marginal zone lymphoma | 94% (79–98) | 87% (59–97) | 79% (47–93) | 98% (87–100) | 82% (63–92) | 82% (63–92) |
| Follicular lymphoma | 88% (74–95) | 71% (53–83) | 52% (27–72) | 90% (78–95) | 70% (56–81) | 57% (39–72) |
| Small lymphatic lymphoma | 85% (58–95) | 66% (34–85) | 48% (12–78) | 84% (59–95) | 72% (44–87) | 60% (28–81) |
| Burkitt’s lymphoma | 78% (54–90) | 69% (43–85) | 55% (48–63) | 77% (54–90) | 67% (43–83) | 54% (48–60) |
| DLBCL | 71% (65–75) | 59% (53–64) | 69% (43–85) | 73% (68–77) | 58% (53–64) | 67% (43–83) |
| T/NK cell lymphoma | 51% (23–73) | 35% (12–60) | 35% (12–60) | 57% (28–78) | 39% (14–64) | 39% (14–64) |
Note: RS: relative survival; DSS: disease-specific survival; CI: confidence interval; DLBCL: diffuse large B-cell lymphoma.
Figure 4.
Kaplan–Meier estimate of relative survival (RS) and disease-specific survival (DSS) of patients with primary renal lymphoma (PRL) by different clinical factors. (A) RS by age. (B) DSS by age. (C) RS by gender. (D) DSS by gender. (E) RS by year of diagnosis. (F) DSS by year of diagnosis. (G) RS by Ann Arbor Stage. (H) DSS by Ann Arbor stage. (I) RS by surgery. (J) DSS by surgery.
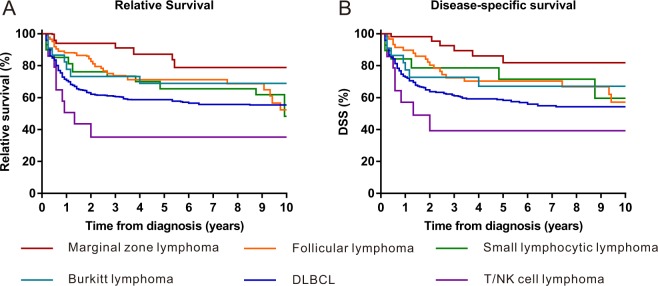
As the most prevalent subtype, the estimated 1-year, 5-year and 10-year RS rates for DLBCL were 71%, 59% and 55%, respectively (Table 2, Fig. 5). Compared with nodal DLBCL, we did not find a survival advantage for renal DLBCL (Supplementary Fig. 2).
Figure 5.
Kaplan–Meier estimate of relative survival (RS) and disease-specific survival (DSS) of patients with primary renal lymphoma (PRL) by histological subtype. (A) RS. (B) DSS.
In the univariable analysis, older age (>80 years old vs <60 years old: HR = 2.98, p < 0.001), male gender (HR = 1.33, p = 0.012), diagnosis before 2000 (HR = 1.33, p = 0.014), advanced stage (HR = 1.40, p < 0.001), not receiving treatment with surgery (HR = 1.41, p = 0.003) and a histologic subtype of DLBCL (HR = 2.24, p = 0.005) were associated with unfavorable survival for patients with PRL (Table 3). The results of multivariable analysis further demonstrated the correlations between worse survival for patients with PRL and older age (>80 years old vs <60 years old: HR = 3.34, p < 0.001), male gender (HR = 1.38, p = 0.005), diagnosis before 2000 (HR = 1.40, p = 0.007), advanced stage (HR = 1.39, p = 0.004), not receiving treatment with surgery (HR = 1.50, p = 0.001) and the histologic subtype of DLBCL (HR = 2.48, p = 0.002) or T/NK cell lymphoma (HR = 4.37, p = 0.021) (Table 3).
Table 3.
Univariate and multivariate Cox analysis of survival in patients with primary renal lymphoma (PRL) by different clinical variables.
| Variables | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR | p | HR | p | |
| Age | ||||
| ≤60 | ||||
| 61–80 | 1.86 (1.46–2.38) | <0.001 | 1.90 (1.47–2.44) | <0.001 |
| >80 | 2.98 (2.13–4.18) | <0.001 | 3.34 (2.34–4.77) | <0.001 |
| Sex | ||||
| Female | ||||
| Male | 1.33 (1.06–1.65) | 0.012 | 1.38 (1.10–1.73) | 0.005 |
| Race | ||||
| White | ||||
| Other | 0.92 (0.66–1.27) | 0.598 | 1.19 (0.85–1.67) | 0.314 |
| Unknown | 1.05 (0.14–7.51) | 0.958 | 0.84 (0.12–6.13) | 0.864 |
| Year | ||||
| 1980–1999 | 1.33 (1.06–1.68) | 0.014 | 1.40 (1.10–1.80) | 0.007 |
| 2000–2013 | ||||
| Laterality | ||||
| Right | ||||
| Left | 0.89 (0.71–1.10) | 0.282 | 0.89 (0.71–1.11) | 0.311 |
| Bilateral | 0.79 (0.53–1.18) | 0.250 | 0.78 (0.51–1.19) | 0.247 |
| Unknown | 0.66 (0.35–1.26) | 0.206 | 0.58 (0.29–1.13) | 0.111 |
| Stage | ||||
| Stage I & II | ||||
| Stage III & IV | 1.40 (1.12–1.74) | <0.001 | 1.39 (1.11–1.75) | 0.004 |
| Unknown | 1.95 (1.35–2.81) | <0.001 | 1.67 (1.14–2.47) | 0.009 |
| Surgery | ||||
| Surgery | ||||
| Non-surgery | 1.41 (1.13–1.76) | 0.003 | 1.50 (1.18–1.91) | 0.001 |
| Unknown | 1.49 (0.83–2.65) | 0.181 | 1.46 (0.79–2.70) | 0.224 |
| Histology | ||||
| Marginal zone lymphoma | ||||
| Follicular lymphoma | 2.01 (1.06–3.79) | 0.032 | 1.97 (1.04–3.73) | 0.039 |
| Small lymphatic lymphoma | 2.03 (0.93–4.47) | 0.076 | 2.21 (1.00–4.89) | 0.051 |
| Burkitt’s lymphoma | 1.18 (0.49–2.86) | 0.708 | 1.51 (0.60–3.77) | 0.381 |
| DLBCL | 2.24 (1.28–3.94) | 0.005 | 2.48 (1.40–4.38) | 0.002 |
| T/NK cell lymphoma | 3.03 (0.99–9.29) | 0.053 | 4.37 (1.40–13.57) | 0.011 |
| Other | 2.18 (1.21–3.93) | 0.010 | 2.04 (1.11–3.73) | 0.021 |
Note: HR: Hazard ratios; CI: confidence interval; DLBCL: diffuse large B-cell lymphoma; *Included lymphoma, NOS (not otherwise specified); non-Hodgkin lymphoma, NOS and Hodgkin lymphoma.
Discussion
In this study, we performed an analysis of a population-based cohort of patients with a diagnosis of PRL as a first primary cancer in the US National Cancer Institute database. To our knowledge, this is the largest evaluation of PRL to date.
The very existence of PRL has been controversial. In recent years, increasing case reports of PRL have confirmed the presence of the disease2,19–23. We also observed an increased incidence rate of PRL from 1980 to 2013 in both male and female patients. Further investigation is required to discern whether our reported increase in PRL incidence is true or related to an increase in the recognition of this disease as an entity over time2,24.
The median OS of patients with PRL that we calculated was 77 months, which is better than the OS found by a previous review of 49 patients2. We observed a discrepancy in survival by gender in our analysis. Females had better prognoses than males. In the Cox proportional hazards model, age greater than 60 years was associated with a poor prognosis. This association is well established in NHL as 60 years is the cutoff value as a component of the International Prognostic Index (IPI)25. In our model, Ann Arbor stage was also a determinant.
An improvement in prognosis after the year 2000 was also noted. We observed increased 5-year RS and DSS rates since 2000. We also found that both the incidence and mortality rates elevated with time. However, the trends in incidence increased faster than that of mortality since 1995. Thus, the better survival of PRL observed in our study since 2000 may reflect improved diagnostics in the modern era. Besides, the introduction of rituximab may also account for this improvement in prognosis, as the outcomes of patients with CD20-positive B lymphoma were significantly improved by the targeted therapy of rituximab9,26–29.
PRL is usually unilateral, whereas it can also be bilateral2. Here, we identified 7.9% of the cases with bilateral involvement with a slight predominance of left involvement. Previous studies suggested that PRL generally appeared to be bilateral in patients who were younger; these studies also showed a shorter survival time with bilateral PRL than with unilateral PRL2. In our study, patients younger than 18 also had a higher percentage of bilateral involvement. However, we did not observe a survival difference between unilateral and bilateral PRL.
Our data suggested that NHL constituted the majority of PRL, and the most prevalent subtype was DLBCL. HL accounted for only 0.7% of all lymphomas in our study. As an indolent type, MZL was associated with a good prognosis with a 1-year RS rate of 94% and a 5-year RS rate of 87%, while T-cell or NK-cell lymphoma revealed particularly poor outcomes, with a 1-year RS of 51%. We did not find a significant difference in survival in patients with renal DLBCL compared with nodal DLBCL.
The role of surgery in PRL is controversial, and evidence is based only on case series or literature reviews9,30,31. Belbaraka R et al. recommended chemotherapy regimens of R-CHOP to manage PRL rather than surgery30. However, Chen X. et al. reported a better outcome for patients who received surgical treatment2. In the current study with a large population, we observed that surgical treatment was associated with a significant improvement in survival. The use of radiation in PRL is also poorly studied. Here, we did not find a survival advantage for patients who received radiotherapy.
Our study has several limitations. First, the diagnosis of PRL has been controversial2. To reduce the risk of including secondary renal involvement of terminal systemic or adjacent lymphoma, our study focused on a diagnosis of lymphoma and a primary location in the kidney with no history of prior cancer diagnosis, as defined by the ICD-O-3. Second, there is a lack of treatment information on chemotherapy from the SEER database. It is not possible to know the actual proportions of patients who received chemotherapy, and such data are important because chemotherapy plays an important role in the management of lymphoma. Third, the SEER database does not provide other objective prognostic factors (such as the IPI, serum lactate dehydrogenase level) or other laboratory parameters in order to perform further analysis.
Nevertheless, based on a large population, the SEER database remains a valuable source for studying such rare kinds of cancers despite these limitations. This population-based approach can normalize the outcome analysis from potential selection biases, standardize both category and outcome definitions, and provide reasonable statistical utility to identify prognostic factors.
Conclusion
In this study, we adopted a population-based dataset to examine the incidence, clinical characteristics and factors associated with survival of an extremely rare disease. The incidence rate of PRL is increasing over time. Older age, male gender, diagnosis before 2000, advanced stage, not receiving treatment with surgery, and DLBCL type or T/NK cell lymphoma type were associated with worse survival of patients with PRL in the multivariate analyses.
Supplementary information
Acknowledgements
This work had no financial support.
Author contributions
J.C., Y.Z. and J.P. analyzed the data and wrote the paper. S.L. and S.Y. analyzed the data. P.Y., X.W., H.T., H.L. and W.W. helped in data collection, data analysis and manuscript writing. B.W.: designed the study and wrote the paper. All authors reviewed the manuscript.
Data availability
The data is available on the Surveillance, Epidemiology, and End Results (SEER, http://seer.cancer.gov) database.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Jiayuan Chen and Jiangtong Peng.
Supplementary information
is available for this paper at 10.1038/s41598-019-51635-6.
References
- 1.Freeman C, Berg JW, Cutler SJ. Occurrence and prognosis of extranodal lymphomas. Cancer. 1972;29:252–260. doi: 10.1002/1097-0142(197201)29:1<252::AID-CNCR2820290138>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 2.Chen X, et al. Primary renal lymphoma: A case report and literature review. Oncology letters. 2016;12:4001–4008. doi: 10.3892/ol.2016.5173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farrow G, Harrison E, Utz D. Sarcomas and sarcomatoid and mixed malignant tumours of the kidney in adults - Part II. Cancer. 1968;22:551–555. doi: 10.1002/1097-0142(196809)22:3<551::AID-CNCR2820220309>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 4.Betta P, Bottero G, Cosimi M, Musante F. Primary renal lymphoma. Eur Urol. 1986;12:352–354. doi: 10.1159/000472654. [DOI] [PubMed] [Google Scholar]
- 5.Duanay P. Linfosarcoma y linfosarcomatosis des los rinones: part II. Rev Med Trop Parasitol Bacteriol Clin Lab. 1940;6:213. [Google Scholar]
- 6.Gellrich J, et al. Primary renal non-Hodgkin’s lymphoma-a difficult differential diagnosis. Onkologie. 2002;25:273–277. doi: 10.1159/000064322. [DOI] [PubMed] [Google Scholar]
- 7.Okuno S, Hoyer J, Ristow K, Witzig T. Primary renal non-Hodgkin’s lymphoma. An unusual extranodal site. Cancer. 1995;75:2258–2261. doi: 10.1002/1097-0142(19950501)75:9<2258::AID-CNCR2820750911>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 8.Al-Salam S, Shaaban A, Alketbi M, Haq NU, Abouchacra S. Acute kidney injury secondary to renal large B-cell lymphoma: Role of early renal biopsy. International urology and nephrology. 2011;43:237–240. doi: 10.1007/s11255-010-9728-5. [DOI] [PubMed] [Google Scholar]
- 9.Ahmad AH, MacLennan GT, Listinsky C. Primary renal lymphoma: a rare neoplasm that may present as a primary renal mass. Journal Urol. 2005;172:239. doi: 10.1097/01.ju.0000148570.46368.c0. [DOI] [PubMed] [Google Scholar]
- 10.Zembower TR. Epidemiology of infections in cancer patients. Cancer treatment and research. 2014;161:43–89. doi: 10.1007/978-3-319-04220-6_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Surveillance, Epidemiology, and End Results (SEER) Program. Research Data (1973-2013), National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, based on November 2015 SEER data submission, posted to the SEER web site. Available at, http://www.seer.cancer.gov, (accessed April 2018).
- 12.Zhou, H. et al. Impact of prior cancer history on the overall survival of patients newly diagnosed with cancer: A pan-cancer analysis of the SEER database. Int J Cancer, 10.1002/ijc.31543 (2018). [DOI] [PubMed]
- 13.Gundrum JD, Mathiason MA, Moore DB, Go RS. Primary testicular diffuse large B-cell lymphoma: a population-based study on the incidence, natural history, and survival comparison with primary nodal counterpart before and after the introduction of rituximab. J Clin Oncol. 2009;27:5227–5232. doi: 10.1200/JCO.2009.22.5896. [DOI] [PubMed] [Google Scholar]
- 14.Edge, S. B. et al. Lymphoid Neoplasms. In: AJCC Cancer Staging Manual, 7th Edition. Springer, 605-628 (2010).
- 15.Johnson S, Rausser GC. Effects of misspecifications of log-linear functions when sample values are zero or negative. American Journal of Agricultural Economics. 1971;53:120–124. doi: 10.2307/3180308. [DOI] [Google Scholar]
- 16.Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19:335–351. doi: 10.1002/(SICI)1097-0258(20000215)19:3<335::AID-SIM336>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 17.Gschwend JE, Dahm P, Fair WR. Disease specific survival as endpoint of outcome for bladder cancer patients following radical cystectomy. Eur Urol. 2002;41:440–448. doi: 10.1016/S0302-2838(02)00060-X. [DOI] [PubMed] [Google Scholar]
- 18.Ederer F, Axtell LM, Cutler SJ. The relative survival rate: a statistical methodology. Natl Cancer Inst Monogr. 1961;6:101–121. [PubMed] [Google Scholar]
- 19.Butani L, Ducore J. Primary Renal Lymphoma Presenting as End-Stage Renal Disease. Case reports in medicine. 2017;2017:9210648. doi: 10.1155/2017/9210648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hagihara M, Hua J, Iwaki Y, Inoue M, Sato T. Primary renal lymphoma: a case report and literature review. Internal medicine. 2015;54:2655–2659. doi: 10.2169/internalmedicine.54.3368. [DOI] [PubMed] [Google Scholar]
- 21.Geetha N, Shahid A, Rajan V, Jacob PM. Primary renal lymphoma-a case report. Ecancermedicalscience. 2014;8:466. doi: 10.3332/ecancer.2014.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kocher NJ, Leung S, Sarwani NE, Warrick JI, Raman JD. Synchronous Primary Renal Lymphoma and Renal Cell Carcinoma: Collision Tumors in the Same Kidney. Journal of endourology case reports. 2017;3:87–89. doi: 10.1089/cren.2017.0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shetty S, Singh AC, Babu V. Primary Renal Lymphoma - A Case Report and Review of Literature. Journal of clinical and diagnostic research: JCDR. 2016;10:XD05–XD07. doi: 10.7860/JCDR/2016/20901.8577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saad AM, et al. Characteristics, survival and incidence rates and trends of primary cardiac malignancies in the United States. Cardiovascular pathology: the official journal of the Society for Cardiovascular Pathology. 2018;33:27–31. doi: 10.1016/j.carpath.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 25.Project., I. N.-H. s. L. P. F. A predictive model for aggressive non-Hodgkin’s lymphoma. N Engl J Med. 1993;329:987–994. doi: 10.1056/NEJM199309303291402. [DOI] [PubMed] [Google Scholar]
- 26.Czuczman M, et al. Treatment of patients with low-grade B-cell lymphoma with the combination of chimeric anti-CD20 monoclonal antibody and CHOP chemotherapy. J Clin Oncol. 1999;17:268–276. doi: 10.1200/JCO.1999.17.1.268. [DOI] [PubMed] [Google Scholar]
- 27.Sehn LH, et al. Introduction of combined CHOP plus rituximab therapy dramatically improved outcome of diffuse large B-cell lymphoma in British Columbia. J Clin Oncol. 2005;23:5027–5033. doi: 10.1200/JCO.2005.09.137. [DOI] [PubMed] [Google Scholar]
- 28.Habermann T, et al. Rituximab-CHOP versus CHOP alone or with maintenance rituximab in older patients with diffuse large B-cell lymphoma. J Clin Oncol. 2006;24:3121–3127. doi: 10.1200/JCO.2005.05.1003. [DOI] [PubMed] [Google Scholar]
- 29.Persky DO, et al. Phase II study of rituximab plus three cycles of CHOP and involved-field radiotherapy for patients with limited-stage aggressive B-cell lymphoma: Southwest Oncology Group study 0014. J Clin Oncol. 2008;26:2258–2263. doi: 10.1200/JCO.2007.13.6929. [DOI] [PubMed] [Google Scholar]
- 30.Belbaraka R, Elyoubi MB, Boutayeb S, Errihani H. Primary renal non-Hodgkin lymphoma: An unusual diagnosis for a renal mass. Indian J Cancer. 2011;48:255–256. doi: 10.4103/0019-509X.82880. [DOI] [PubMed] [Google Scholar]
- 31.O’Riordan E, Reeve R, Houghton JB, O’Donoghue DJ, Waldek S. Primary bilateral T-cell renal lymphoma presenting with sudden loss of renal function. Nephrol Dial Transplant. 2001;16:1487–1489. doi: 10.1093/ndt/16.7.1487. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data is available on the Surveillance, Epidemiology, and End Results (SEER, http://seer.cancer.gov) database.