ABSTRACT
Comparative physiologists are often interested in adaptive physiological phenomena found in unconventional model organisms; however, research on these species is frequently constrained by the limited availability of investigative tools. Here, we propose that induced pluripotent stem cells (iPSCs) from unconventional model organisms may retain certain species-specific features that can consequently be investigated in depth in vitro; we use hibernating mammals as an example. Many species (including ground squirrels, bats and bears) can enter a prolonged state of physiological dormancy known as hibernation to survive unfavorable seasonal conditions. Our understanding of the mechanisms underpinning the rapid transition and adaptation to a hypothermic, metabolically suppressed winter torpor state remains limited partially because of the lack of an easily accessible model. To address the fascinating unanswered questions underlying hibernation biology, we have developed a powerful model system: iPSCs from a hibernating species, the thirteen-lined ground squirrel (Ictidomys tridecemlineatus). These stem cells can potentially be differentiated into any cell type, and can be used for the analysis of cell-autonomous mechanisms that facilitate adaptation to hibernation and for comparisons with non-hibernators. Furthermore, we can manipulate candidate molecular and cellular pathways underlying relevant physiological phenomena by pharmacological or RNAi-based methods, and CRISPR/Cas9 gene editing. Moreover, iPSC strategies can be applied to other species (e.g. seals, naked mole rats, humming birds) for in vitro studies on adaptation to extreme physiological conditions. In this Commentary, we discuss factors to consider when attempting to generate iPSCs from unconventional model organisms, based on our experience with the thirteen-lined ground squirrel.
KEY WORDS: Stem cells, Hibernation, Torpor, Physiological dormancy, Reprogramming, Adaptation, Tau hyperphosphorylation
Summary: Stem cell techniques could benefit comparative studies of unconventional organisms. As an example, here we describe some key considerations in deriving and utilizing induced pluripotent stem cells from a hibernator.
Introduction
Historically, scientists have primarily studied a handful of domesticated species as ‘model organisms’ (Davis, 2004). Powerful research tools have been tailored to these models, resulting in numerous advances in genetics, cell biology and physiology. Nonetheless, for every physiological question there is an organism that makes the ideal test species (Krogh, 1929). When existing model organisms meet their limits in answering particular questions, the catalog of model organisms shall expand (Goldstein and King, 2016; Russell et al., 2017). For example, mice and humans cannot survive certain extreme physiological conditions to which other mammals are naturally adapted. Deep-diving marine mammals such as seals have attracted research interest based on their specialized inflammatory responses and tolerance of hypoxia and ischemia–reperfusion stresses associated with their diving behavior (Bagchi et al., 2018; Meir et al., 2009; Vázquez-Medina et al., 2012). The naked mole rat is known for its extreme tolerance of hypoxia (Pamenter et al., 2018; Park et al., 2017), as well as its longevity and robust cancer resistance (Buffenstein, 2005; Liang et al., 2010; Tian et al., 2013). Lastly, and most germane to this Commentary, hibernating mammals, such as some ground squirrels and bears, can actively lower their metabolic rates and body temperature (Carey et al., 2003; Geiser, 2004; Staples, 2016; Toien et al., 2011; Treat et al., 2018) and tolerate hypoxia and ischemia (Dave et al., 2006; Drew et al., 2016).
Glossary.
Direct reprogramming
Techniques that directly convert a certain type of cell into another defined cell type without the need for acquiring iPSCs.
Embryoid body
A free-floating three-dimensional aggregate of stem cells.
Induced pluripotent stem cells (iPSCs)
In 2006, Kazutoshi Takahashi and Shinya Yamanaka reported a novel method in which four transcription factors were introduced into cultured fibroblast cells, reprogramming these somatic cells into iPSCs that can proliferate indefinitely and differentiate into many other cell types in a culture dish (see Takahashi and Yamanaka, 2006).
Organoid
A miniature of an organ derived from an aggregate of stem cells. Organoids have histological structure and cell-type composition similar to those of the actual organ, and even have primitive functions.
Reprogramming
The process of reverting a defined type of cells into iPSCs.
Stem cells
Cells in an organism that can divide into more cells of the same type or differentiate into other types of cells.
Teratoma
A tumor composed of tissues or cells derived from the three different germ layers: ectoderm, mesoderm and endoderm. In the stem cell biology field, using mouse models as the host for exogenous teratoma formation has become a standard assay to verify the in vivo pluripotency of the tested stem cell line.
Transdifferentiation
Conversion of a certain somatic cell type into another cell type without producing an intermediate pluripotent state.
It is well known to the readers of Journal of Experimental Biology that unconventional model organisms offer exciting possibilities for comparative physiology and systems biology, especially with the explosion of next-generation sequencing and proteomics-based methods. However, housing and breeding live animals is often onerous and expensive. Conducting studies with statistically reasonable sample sizes can be labor intensive and cost prohibitive. Furthermore, even though researchers have made exciting progress on gene-editing techniques in recent years, it still can be difficult to conduct genetic analyses on many unconventional model organisms, because they may have long breeding cycles, limited numbers of offspring and/or unsequenced/unannotated genomes. The inability to pinpoint and mutate a genetic locus holds most studies at the level of the descriptive, and thus mechanistic insight is difficult to attain. Stem cells (see Glossary) derived from these organisms may maintain critical biological features of the animal while simplifying sample collection and storage, facilitating experimental manipulation and reducing costs. For example, stem cells derived from the naked mole rat reflect the cancer-resistant phenotype of the whole animal: they are incompetent to form teratomas (see Glossary) in mice because they maintain their species-specific activation of a tumor suppressor called alternative reading frame (ARF) (Miyawaki et al., 2016). Importantly, stem cell-based models greatly facilitate pharmacological manipulation, RNA interference (RNAi)-mediated knockdown and CRISPR/Cas9 editing. These tools give researchers the opportunity to conduct mechanistic inquiries and genetic screens that have previously only been possible in conventional model systems.
The development of induced pluripotent stem cells (iPSCs; see Glossary) (Takahashi and Yamanaka, 2006) has revolutionized the in vitro study of human cellular physiology and human diseases by allowing reprogramming (see Glossary) of terminally differentiated cells (such as skin fibroblasts) into stem cells that can then be differentiated into various cell types and tissue organoids (see Glossary; Avior et al., 2016; Grskovic et al., 2011). Furthermore, there is on-going development and optimization of techniques for making iPSCs (Li and Izpisua Belmonte, 2016), which may facilitate the creation of iPSCs from non-mammalian vertebrates and invertebrates (Lu et al., 2012; Rossello et al., 2013). Here, we describe our experience generating iPSCs from a mammalian hibernator – the thirteen-lined ground squirrel (Ictidomys tridecemlineatus) – and how we use this cell culture platform to study biological features such as hibernation and cold adaptation (Ou et al., 2018). Hopefully, our experience – and others' success in generating iPSCs from naked mole rats (Miyawaki et al., 2016; Tan et al., 2017) – can motivate comparative biologists to expand their arsenal of research tools with stem cell-related techniques, allowing them to address complex biological questions on a simplified in vitro platform.
Challenges in developing cell culture-based systems to study unconventional models in a dish
Cultured somatic cells can be reprogrammed into iPSCs by the induced expression of four transcription factors – for human cells these are OCT4 (POU5F1), SOX2, KLF4 and cMYC (OSKM; see Fig. 1). The advantage of working with pluripotent stem cells is that they have high proliferative capacity and can potentially be differentiated into any desired cell type. Deriving iPSCs or embryonic stem cells (ESCs) from relevant unconventional model organisms is an efficient and effective way to obtain gene-editable cell lines that benefit comparative physiology studies. However, most stem cell protocols have been designed for cells derived from humans and mice; although these protocols can serve as excellent points of reference, trial-and-error optimization is required in order to establish suitable protocols for unconventional models. With this in mind, here, we discuss our experience in generating and observing thirteen-lined ground squirrel iPSCs, in the hope that this might be instructive to comparative physiologists interested in making iPSCs from other organisms (summarized in Fig. 2).
Fig. 1.
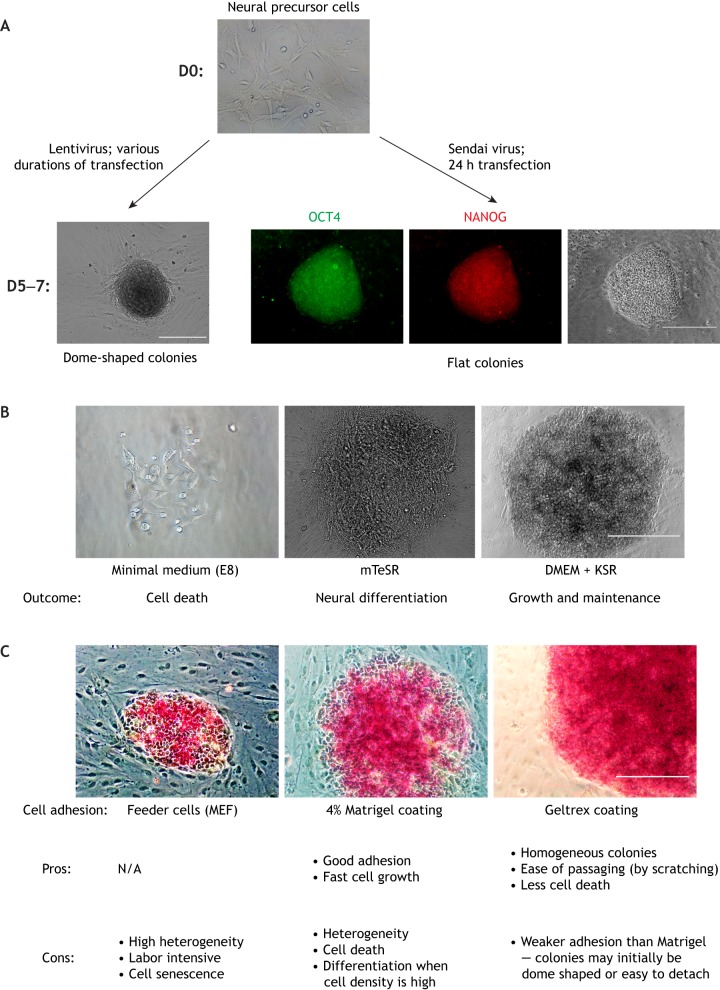
Generation and expansion of thirteen-lined ground squirrel induced pluripotent stem cells (iPSCs). (A) Lentivirus transfection of thirteen-lined ground squirrel primary neural precursor cells (from postnatal day 2 animals) with the OSKM (OCT4, SOX2, KLF4 and cMYC) transcription factor genes led to the formation of dome-shaped colonies that could not be expanded (i.e. displayed slow/no growth and therefore were not passaged). Sendai virus transfection of the primary cells with OSKM genes for 24 h led to the formation of flat colonies that stained positive for the pluripotency markers OCT4 and NANOG, and that could be passaged by using dispase or scratching with pipette tips. D0, day 0 of culture; D2, day 2 of culture, etc. (B,C) Culture medium (B) and cell adhesion (C) strongly affect the expansion and maintenance of thirteen-lined ground squirrel iPSC colonies. E8 and mTeSR, standard defined media optimized for human iPSC and embryonic stem cell cultures; DMEM, Dulbecco's modified Eagle medium; KSR, knockout serum replacement; MEF, mouse embryonic fibroblasts. Scale bars: 200 µm (A); 400 µm (B,C).
Fig. 2.
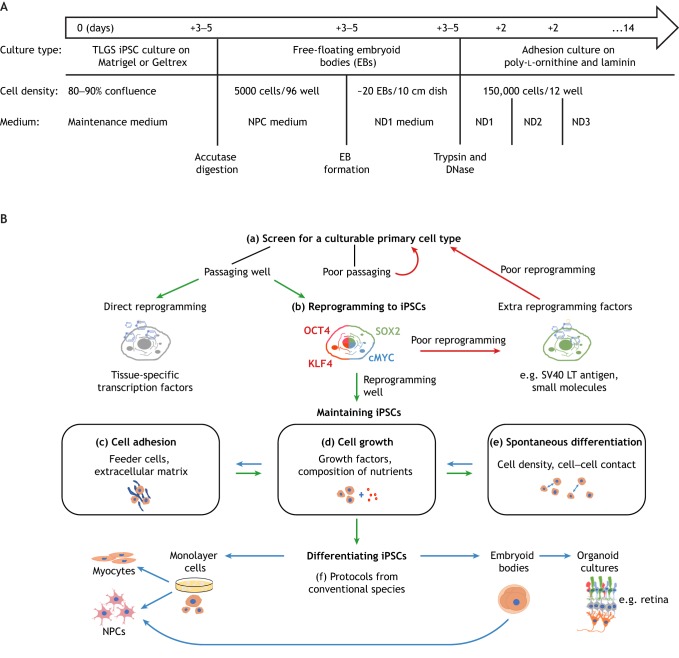
Work flowcharts for developing iPSC-derived cell culture platforms from thirteen-lined ground squirrels and other unconventional model organisms. It is worth noting that iPSCs from non-mammalian vertebrate species and invertebrate species have been successfully developed. (A) A work flowchart for effective differentiation of thirteen-lined ground squirrel iPSCs into neurons. Maintenance medium: DMEM/F12, 1× MEM non-essential amino acids, 40 ng ml−1 FGF-2, 15% (v/v) KSR (knockout serum replacement), 1× antibiotic-antimycotic. NPC (neural precursor cell) medium: DMEM, 5% KSR, B27 without vitamin A, 2 mmol l−1 l-glutamine, 1× antibiotic-antimycotic, 10 ng ml−1 NOGGIN, 20 ng ml−1 EGF and 10 ng ml−1 FGF-2. ND1 medium: DMEM, 5% KSR, B27 without vitamin A, 2 mmol l−1 l-glutamine, 1× antibiotic-antimycotic, 10 ng ml−1 BDNF, 2.5 ng ml−1 GDNF and 10 ng ml−1 activin A; ND2 medium: ND1 without activin A; ND3 medium: ND2 without KSR. For further information on cell culture-related reagents, see Ou et al. (2018). (B) Based on our experience working with thirteen-lined ground squirrel iPSCs and others' experience with iPSCs of naked mole rats and conventional species, this flowchart can act as a guide to developing iPSCs or other culturable cell types from unconventional model organisms. Note that a–f describe key factors that could greatly impact the outcome of the whole procedure – these steps require optimization for each cell type and each species; c–e are interactive factors that may require lots of trial-and-error optimization.
Choice of primary cell types
When deriving thirteen-lined ground squirrel iPSCs, we attempted to culture primary skin fibroblasts, skeletal muscle fibroblasts, astrocytes and bone marrow cells from adult thirteen-lined ground squirrels. In our hands, these cell types either lacked the proliferative capacity to generate enough cells for reprogramming or failed to form iPSC-like colonies following Sendai virus-mediated transfection of the OSKM transcription factor genes. We eventually succeeded by using neural precursor cells obtained from thirteen-lined ground squirrel postnatal day 0–2 pups (Fig. 1A), albeit with a low reprogramming efficiency (4–20 colonies per 105 transfected cells). Other work has shown that skin fibroblasts from adult naked mole rats also have very low reprogramming efficiencies (Miyawaki et al., 2016; Tan et al., 2017), whereas embryonic fibroblasts from this species appear to have a higher reprogramming rate. We conclude that using immature cells with high proliferative capacity may increase the chances of making iPSCs from the species of interest.
Reprogramming strategies
The low reprogramming efficiencies we observed with cells from thirteen-lined ground squirrels could be because commercially available virus-based reprogramming kits contain human or mouse OSKM; hence, they may not efficiently reprogram cells from other species. Predicted thirteen-lined ground squirrel OSKM proteins share high sequence homology with the human proteins (thirteen-lined ground squirrel predicted protein sequences and protein BLAST service are available at the NCBI website). In the case of naked mole rats, the use of species-specific OSKM did not improve reprogramming efficiency; instead, a combination of OSKM and LT (SV40 large T antigen, a viral oncoprotein that inactivates the p53 and pRb pathways) was found to greatly enhance reprogramming efficiency (Tan et al., 2017).
Another caveat in virus-based reprogramming is that there is variable susceptibility to viral transfection among species (Miyawaki et al., 2016). With the aim of replacing viral delivery of the OSKM factors, researchers have been developing techniques such as stem cell-reprogramming small molecules (Borgohain et al., 2019; Lyssiotis et al., 2009; Yu et al., 2014), which may provide an effective solution to circumvent this issue. For organisms where primary cell types are easy to obtain and expand in the laboratory, instead of making iPSCs, other cell types – such as neurons (Ambasudhan et al., 2011), cardiac progenitors (Lalit et al., 2016), hepatocytes (Huang et al., 2014) and pancreatic cells (Li et al., 2014) – may be created by direct reprogramming (see Glossary; Fig. 2); these techniques can induce expression of tissue/cell type-specific transcription factors or promote somatic cellular transdifferentiation (see Glossary). Because we found that many types of primary cells from adult and neonatal thirteen-lined ground squirrels cannot sustain large-scale expansion and multiple passages, direct reprogramming of thirteen-lined ground squirrel primary somatic cells was not an option.
Cell culture conditions
During the optimization of culture conditions for the maintenance and expansion of the thirteen-lined ground squirrel iPSCs, we observed that the most important variables affecting the growth and maintenance of these iPSC colonies were the culture medium and coating material for the culture vessels (Fig. 1B,C). For example, no thirteen-lined ground squirrel iPSC colonies form following OSKM transfection when the culture medium contains leukemia inhibitory factor (LIF), a key factor in making and maintaining mouse and rat iPSCs and ESCs. Likewise, almost no naked mole rat iPSC colonies form in LIF-containing medium unless the cells are also transfected with the LT antigen (Tan et al., 2017). Thirteen-lined ground squirrel iPSCs and iPSC-derived cells do not grow well in minimal medium unless it is supplemented with knock-out serum or palmitate, which serve as sources for lipid metabolism, suggesting that cultured thirteen-lined ground squirrel cells thrive on a protein/lipid-rich diet as do live animals (Merriman et al., 2012). Therefore, in order to better maintain the iPSC or a defined cell type of any unconventional model, it may be helpful to consider the diet of the whole animal and/or the microenvironment suitable for the cell type of that animal, including factors such as osmolarity, pH, oxygen and nutrient composition.
In our hands, different cellular adhesion cues had a large impact on the growth of thirteen-lined ground squirrel iPSCs and their spontaneous differentiation. When the rate of spontaneous differentiation is high, iPSCs rapidly produce other cell types, resulting in a highly heterogeneous culture. Therefore, these conditions need to be optimized in order to successfully maintain and expand iPSCs from unconventional models.
Optimizing differentiation protocols
Differentiating iPSCs from unconventional model animals into the cell type of interest often requires modification and optimization of currently existing protocols. We observed that undifferentiated thirteen-lined ground squirrel iPSC colonies can very quickly overgrow into dense mats of cells, which prevents them from differentiating efficiently – particularly into neurons. To circumvent this problem, we have developed a protocol derived from the Columbia University Stem Cell Core mouse iPSC neuronal differentiation procedure; this protocol allows us to differentiate thirteen-lined ground squirrel iPSCs into neurons via intermediate embryoid bodies (see Glossary; Fig. 2A). Theoretically, this method should be useful for the differentiation of thirteen-lined ground squirrel iPSCs into any cell type, but we have not yet attempted to produce non-neural cells.
Utilizing iPSCs in comparative physiology research
Naked mole rat iPSCs are incompetent in tumor formation (Miyawaki et al., 2016) and proliferate slowly (Tan et al., 2017), apparently emulating the in vivo features of this species. This ability to use iPSCs to investigate features that are observed at the level of the whole animal may be relevant to any organism of interest to comparative physiology. In our case, we were interested to determine whether thirteen-lined ground squirrel iPSCs and iPSC-derived somatic cell types may contain some key features of this hibernator that could be evaluated in vitro and compared with the in vivo thirteen-lined ground squirrel physiology, or with that of the same cell type from other organisms. Below, we use our work on thirteen-lined ground squirrel iPSCs to provide examples of how iPSCs can be used to shed light on the mechanisms underlying whole-organism function.
Autonomous cold and metabolic adaptations
Some mammals are adapted to drastic and repetitive physiological changes, as demonstrated by the diving behavior of seals and the torpor–interbout arousal cycles in small hibernators. In small hibernators, cycles of torpor and arousal are characterized by the rise and fall of the hibernators' body temperature between near-freezing and 37°C (Andrews, 2007; Carey et al., 2003). The cells and tissues of these species must have evolved intrinsic mechanisms to survive these physiological challenges, and these mechanisms may be easier to uncover in a cell culture system than in the whole animal. Unlike human cells, cold-exposed thirteen-lined ground squirrel iPSC-derived neurons lack canonical stress responses in the mitochondria and lysosomes, and maintain a relatively stable transcriptome. These cells also protect microtubule integrity that otherwise would be destroyed at low temperatures in the neurons of non-hibernating mammals (Ou et al., 2018). Although the underlying molecular mechanisms of these adaptations are yet to be elucidated, such remarkable neuronal protection in thirteen-lined ground squirrels may be linked with the cellular lipid metabolic activities, which produce large amounts of free fatty acids that could uncouple mitochondria (Dedukhova et al., 1991; Skulachev, 1991) and prevent cold-triggered cytotoxic mitochondrial hyperpolarization. To this end, the thirteen-lined ground squirrel iPSC system permits various measurements of cellular responses to changes in culture conditions. Moreover, by utilizing gene-editing methods, one can generate various mutant thirteen-lined ground squirrel cell lines to target candidate cellular and genetic pathways that underpin mammalian adaptation to low temperature and metabolic stress.
Neuronal tau phosphorylation in physiology and pathology
Thirteen-lined ground squirrel iPSCs may also be of use in investigating mechanisms of neuroprotection, which is an important aspect of a hibernator's physiology, with implications for neurodegenerative disease. For example, hyperphosphorylation of the microtubule-associated protein tau is a hallmark of neurodegenerative disease, and phosphorylation of tau is often referred to as a causative event in disease progression (Gong and Iqbal, 2008). However, European ground squirrels (Spermophilus citellus) accumulate hyperphosphorylated tau proteins within the brain during hibernation (Arendt et al., 2003; Stieler et al., 2009), particularly in the entorhinal cortex, hippocampus and isocortical areas (Arendt et al., 2003). These phosphorylation events occur at several residues associated with human pathology. Importantly, hyperphosphorylation of ground squirrel tau is reversed upon exit from hibernation by an unknown mechanism that operates under physiological conditions. These findings have been extended to other obligate hibernators such as black bears (Ursus americanus) and arctic ground squirrels (Urocitellus parryii), as well as to facultative hibernators such as Syrian hamsters (Mesocricetus auratus) (Härtig et al., 2007; Stieler et al., 2011; Su et al., 2008). These findings are at odds with the hypothesis that tau hyperphosphorylation is obligatorily pathological. Accumulating evidence suggests that, instead of promoting dysfunction, hyperphosphorylation can serve to prevent or reverse protein aggregation (Carpenter et al., 2018; Monahan et al., 2017). Therefore, tau hyperphosphorylation represents a physiological process that could be key to understanding how aggregated tau is cleared from neurons.
To this end, neurons derived from iPSCs of thirteen-lined ground squirrels or other hibernators offer unparalleled advantages as a model system. Compared with live-animal systems, iPSCs can be rapidly grown and differentiated, allowing more experiments to be run. It is simpler and more efficient to apply cold stimuli and rewarming protocols to cell cultures than to live animals. Cellular responses in thirteen-lined ground squirrel neurons could be compared with those of control monolayer neuronal cultures or brain organoids derived from hibernators, humans and/or mice. By investigating the ability of ground squirrel neurons to reverse tau hyperphosphorylation upon exit from hibernation or removal of cold stimuli, we have an opportunity to elucidate the mechanisms underlying neuroprotection and neuropathology.
Organoid culture for developmental biology and physiology
Thirteen-lined ground squirrel iPSCs also provide opportunities to further our understanding of developmental biology. Ever since retina-like organoids were first successfully developed from mouse ESC aggregates (Eiraku et al., 2011), stem cell biologists have pushed the frontier of in vitro modeling of tissue/organ development from two-dimensional monolayer cell cultures to three-dimensional organoid cultures (Dutta et al., 2017). Using retina research as an example, a key question is the development of the cone-enriched fovea region in primates. Like humans, the thirteen-lined ground squirrel is a diurnal animal, and it has a cone photoreceptor-dominant retina with high visual acuity (Merriman et al., 2016). Using the thirteen-lined ground squirrel as a model for studying cone photoreceptor development is attractive but technically challenging, because no transgenic thirteen-lined ground squirrels have been generated in the laboratory, and the animals breed only once a year, in spring (Merriman et al., 2012; Vaughan et al., 2006). Thirteen-lined ground squirrel iPSC-derived retinal organoids will allow real-time microscopic observation of cone photoreceptor development, as well as facilitating pharmacological and genetic manipulation of relevant pathways and molecules. Retinal organoids derived from thirteen-lined ground squirrel iPSCs could be directly compared with those derived from human or mouse iPSCs and ESCs to reveal the mechanism of cone dominance in the retina. Likewise, in the future, iPSC- or ESC-derived organoids representing heart, lung, liver or other organs from unconventional model organisms may be developed in vitro. This would permit researchers to study their unique features in areas such as development, metabolism and aging, allowing comparisons with conventional model organisms.
Conclusions
Our experience working with thirteen-lined ground squirrel iPSCs (Ou et al., 2018) and that of others working with naked mole rat iPSCs (Miyawaki et al., 2016; Tan et al., 2017) clearly demonstrates that iPSCs can retain at least some species-specific cellular features that can be more easily investigated in vitro than in vivo. In work propelled by the fast-developing stem cell field, iPSCs have been successfully generated from non-mammalian vertebrate and invertebrate species (Rossello et al., 2013), offering a great opportunity for comparative biologists to cultivate cells and even tissues from rare organisms or from those which cannot be bred in the laboratory. For example, hummingbirds have attracted comparative biologists' interests because of their exceptional visual (Gaede et al., 2017) and metabolic (Suarez and Welch, 2017; Welch and Suarez, 2007) adaptations that facilitate their rapid flight. If hummingbird iPSCs were available, brain and retina organoids or other suitable cell types could be derived, and nutrients in the culture media could be manipulated and monitored, paving the way to the in vitro discovery of the neuronal and metabolic adaptive mechanisms that underlie the extraordinary performance of the whole animal. Furthermore, the use of stem cell techniques has the potential to expand the reach of comparative physiological research, as cellular physiological responses to a unified, easily controllable stimulus could be directly compared across multiple organisms. Undoubtedly, mechanisms discovered from stem cell-based studies in diverse species would provide unprecedented insights for in vivo comparative biological research.
It should be expected that working with iPSCs or ESCs of unconventional research species would necessitate extensive protocol optimization (Fig. 2B). Such optimization mirrors a learning process that scientists working with conventional model organisms underwent over decades with a high level of resource investment. In our case, we started to learn stem cell techniques by practicing with adult thirteen-lined ground squirrel materials; after attempting to generate iPSCs for about 8 months, we sought to use neonatal thirteen-lined ground squirrel cells. Our previous efforts had produced a reasonable setup for performing the relevant experiments, so we found that only four thirteen-lined ground squirrel pups were needed to successfully produce thirteen-lined ground squirrel iPSCs within 2 months. We are now beginning to take the necessary steps to develop thirteen-lined ground squirrel iPSCs as an in vitro model system to study hibernation biology.
It is worth noting that the thirteen-lined ground squirrel pups we used to make iPSCs had never experienced hibernation, but neurons derived from these iPSCs demonstrated superior cold tolerance compared with neurons from rats and humans (Ou et al., 2018). We have not verified whether such cold tolerance results from the contribution of genetic or epigenetic factors or both. If technically possible, it would be interesting to test whether future iPSC lines derived from adult thirteen-lined ground squirrels at certain physiological stages (e.g. entry to hibernation, deep torpor, interbout arousal) would more precisely emulate features of in vivo hibernation, such as those relating to the regulation of cellular metabolism (Staples, 2014) and tolerance to hypoxia and reperfusion (Larson et al., 2014).
Cell fate-reprogramming techniques provide comparative physiologists with an unprecedented opportunity not only to advance knowledge on the cell biology and genetics of unconventional model species but also to introduce rapidly developing techniques such as gene editing and sequencing-based approaches to fuel research in these new models. As the development of cellular and molecular tools makes research on unusual animals easier and less costly, their scientific value and potential can only improve. We hope that the evolving state of life science and technology will facilitate the development of many more model organisms, such that previously unattainable biological and medical advances may be better addressed with suitable novel model systems.
Acknowledgements
We thank Dr Barbara S. Mallon at the Stem Cell Unit of the National Institute of Neurological Disorders and Stroke, and Dr Dana K. Merriman at the Department of Biology, University of Wisconsin-Oshkosh for their support and advice on the thirteen-lined ground squirrel iPSC project. We also thank Dr Barbara Corneo at the Columbia University Medical Center Stem Cell Initiative for her training and support, particularly with regards to development of the embryoid body neuronal differentiation protocol. L.E.B. would also like to thank Dr Michael Shelanski for guidance.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Funding
This work was supported by the National Institutes of Health (1ZIAEY000488-10 to W.L.; R35GM124633 to L.E.B.) and the Schaefer Scholars Award (to L.E.B.). Deposited in PMC for release after 12 months.
References
- Ambasudhan R., Talantova M., Coleman R., Yuan X., Zhu S., Lipton S. A. and Ding S. (2011). Direct reprogramming of adult human fibroblasts to functional neurons under defined conditions. Cell Stem Cell 9, 113-118. 10.1016/j.stem.2011.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews M. T. (2007). Advances in molecular biology of hibernation in mammals. BioEssays 29, 431-440. 10.1002/bies.20560 [DOI] [PubMed] [Google Scholar]
- Arendt T., Stieler J., Strijkstra A. M., Hut R. A., Rüdiger J., Van der Zee E. A., Harkany T., Holzer M. and Härtig W. (2003). Reversible paired helical filament-like phosphorylation of tau is an adaptive process associated with neuronal plasticity in hibernating animals. J. Neurosci. 23, 6972-6981. 10.1523/JNEUROSCI.23-18-06972.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avior Y., Sagi I. and Benvenisty N. (2016). Pluripotent stem cells in disease modelling and drug discovery. Nat. Rev. Mol. Cell Biol. 17, 170-182. 10.1038/nrm.2015.27 [DOI] [PubMed] [Google Scholar]
- Bagchi A., Batten A. J., Levin M., Allen K. N., Fitzgerald M. L., Huckstadt L. A., Costa D. P., Buys E. S. and Hindle A. G. (2018). Intrinsic anti-inflammatory properties in the serum of two species of deep-diving seal. J. Exp. Biol. 221, jeb178491 10.1242/jeb.178491 [DOI] [PubMed] [Google Scholar]
- Borgohain M. P., Haridhasapavalan K. K., Dey C., Adhikari P. and Thummer R. P. (2019). An insight into DNA-free reprogramming approaches to generate integration-free induced pluripotent stem cells for prospective biomedical applications. Stem Cell Rev. 15, 286-313. 10.1007/s12015-018-9861-6 [DOI] [PubMed] [Google Scholar]
- Buffenstein R. (2005). The naked mole-rat: a new long-living model for human aging research. J. Gerontol. A Biol. Sci. Med. Sci. 60, 1369-1377. 10.1093/gerona/60.11.1369 [DOI] [PubMed] [Google Scholar]
- Carey H. V., Andrews M. T. and Martin S. L. (2003). Mammalian hibernation: cellular and molecular responses to depressed metabolism and low temperature. Physiol. Rev. 83, 1153-1181. 10.1152/physrev.00008.2003 [DOI] [PubMed] [Google Scholar]
- Carpenter K., Bell R. B., Yunus J., Amon A. and Berchowitz L. E. (2018). Phosphorylation-mediated clearance of amyloid-like assemblies in meiosis. Dev. Cell 45, 392-405.e396. 10.1016/j.devcel.2018.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dave K. R., Prado R., Raval A. P., Drew K. L. and Perez-Pinzon M. A. (2006). The arctic ground squirrel brain is resistant to injury from cardiac arrest during euthermia. Stroke 37, 1261-1265. 10.1161/01.STR.0000217409.60731.38 [DOI] [PubMed] [Google Scholar]
- Davis R. H. (2004). The age of model organisms. Nat. Rev. Genet. 5, 69-76. 10.1038/nrg1250 [DOI] [PubMed] [Google Scholar]
- Dedukhova V. I., Mokhova E. N., Skulachev V. P., Starkov A. A., Arrigoni-Martelli E. and Bobyleva V. A. (1991). Uncoupling effect of fatty acids on heart muscle mitochondria and submitochondrial particles. FEBS Lett. 295, 51-54. 10.1016/0014-5793(91)81382-I [DOI] [PubMed] [Google Scholar]
- Drew K. L., Wells M., McGee R., Ross A. P. and Kelleher-Andersson J. (2016). Arctic ground squirrel neuronal progenitor cells resist oxygen and glucose deprivation-induced death. World J. Biol. Chem. 7, 168-177. 10.4331/wjbc.v7.i1.168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta D., Heo I. and Clevers H. (2017). Disease modeling in stem cell-derived 3D organoid systems. Trends Mol. Med. 23, 393-410. 10.1016/j.molmed.2017.02.007 [DOI] [PubMed] [Google Scholar]
- Eiraku M., Takata N., Ishibashi H., Kawada M., Sakakura E., Okuda S., Sekiguchi K., Adachi T. and Sasai Y. (2011). Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature 472, 51-56. 10.1038/nature09941 [DOI] [PubMed] [Google Scholar]
- Gaede A. H., Goller B., Lam J. P. M., Wylie D. R. and Altshuler D. L. (2017). Neurons responsive to global visual motion have unique tuning properties in hummingbirds. Curr. Biol. 27, 279-285. 10.1016/j.cub.2016.11.041 [DOI] [PubMed] [Google Scholar]
- Geiser F. (2004). Metabolic rate and body temperature reduction during hibernation and daily torpor. Annu. Rev. Physiol. 66, 239-274. 10.1146/annurev.physiol.66.032102.115105 [DOI] [PubMed] [Google Scholar]
- Goldstein B. and King N. (2016). The future of cell biology: emerging model organisms. Trends Cell Biol. 26, 818-824. 10.1016/j.tcb.2016.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong C.-X. and Iqbal K. (2008). Hyperphosphorylation of microtubule-associated protein tau: a promising therapeutic target for Alzheimer disease. Curr. Med. Chem. 15, 2321-2328. 10.2174/092986708785909111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grskovic M., Javaherian A., Strulovici B. and Daley G. Q. (2011). Induced pluripotent stem cells--opportunities for disease modelling and drug discovery. Nat. Rev. Drug Discov. 10, 915-929. 10.1038/nrd3577 [DOI] [PubMed] [Google Scholar]
- Härtig W., Stieler J., Boerema A. S., Wolf J., Schmidt U., Weissfuss J., Bullmann T., Strijkstra A. M. and Arendt T. (2007). Hibernation model of tau phosphorylation in hamsters: selective vulnerability of cholinergic basal forebrain neurons - implications for Alzheimer's disease. Eur. J. Neurosci. 25, 69-80. 10.1111/j.1460-9568.2006.05250.x [DOI] [PubMed] [Google Scholar]
- Huang P., Zhang L., Gao Y., He Z., Yao D., Wu Z., Cen J., Chen X., Liu C., Hu Y. et al. (2014). Direct reprogramming of human fibroblasts to functional and expandable hepatocytes. Cell Stem Cell 14, 370-384. 10.1016/j.stem.2014.01.003 [DOI] [PubMed] [Google Scholar]
- Krogh A. (1929). The progress of physiology. Science 70, 200-204. 10.1126/science.70.1809.200 [DOI] [PubMed] [Google Scholar]
- Lalit P. A., Salick M. R., Nelson D. O., Squirrell J. M., Shafer C. M., Patel N. G., Saeed I., Schmuck E. G., Markandeya Y. S., Wong R. et al. (2016). Lineage reprogramming of fibroblasts into proliferative induced cardiac progenitor cells by defined factors. Cell Stem Cell 18, 354-367. 10.1016/j.stem.2015.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson J., Drew K. L., Folkow L. P., Milton S. L. and Park T. J. (2014). No oxygen? No problem! Intrinsic brain tolerance to hypoxia in vertebrates. J. Exp. Biol. 217, 1024-1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M. and Izpisua Belmonte J. C. (2016). Looking to the future following 10 years of induced pluripotent stem cell technologies. Nat. Protoc. 11, 1579-1585. 10.1038/nprot.2016.108 [DOI] [PubMed] [Google Scholar]
- Li K., Zhu S., Russ H. A., Xu S., Xu T., Zhang Y., Ma T., Hebrok M. and Ding S. (2014). Small molecules facilitate the reprogramming of mouse fibroblasts into pancreatic lineages. Cell Stem Cell 14, 228-236. 10.1016/j.stem.2014.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang S., Mele J., Wu Y., Buffenstein R. and Hornsby P. J. (2010). Resistance to experimental tumorigenesis in cells of a long-lived mammal, the naked mole-rat (Heterocephalus glaber). Aging Cell 9, 626-635. 10.1111/j.1474-9726.2010.00588.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y., West F. D., Jordan B. J., Mumaw J. L., Jordan E. T., Gallegos-Cardenas A., Beckstead R. B. and Stice S. L. (2012). Avian-induced pluripotent stem cells derived using human reprogramming factors. Stem Cells Dev. 21, 394-403. 10.1089/scd.2011.0499 [DOI] [PubMed] [Google Scholar]
- Lyssiotis C. A., Foreman R. K., Staerk J., Garcia M., Mathur D., Markoulaki S., Hanna J., Lairson L. L., Charette B. D., Bouchez L. C. et al. (2009). Reprogramming of murine fibroblasts to induced pluripotent stem cells with chemical complementation of Klf4. Proc. Natl. Acad. Sci. USA 106, 8912-8917. 10.1073/pnas.0903860106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meir J. U., Champagne C. D., Costa D. P., Williams C. L. and Ponganis P. J. (2009). Extreme hypoxemic tolerance and blood oxygen depletion in diving elephant seals. Am. J. Physiol. Regul. Integr. Comp. Physiol. 297, R927-R939. 10.1152/ajpregu.00247.2009 [DOI] [PubMed] [Google Scholar]
- Merriman D. K., Lahvis G., Jooss M., Gesicki J. A. and Schill K. (2012). Current practices in a captive breeding colony of 13-lined ground squirrels (Ictidomys tridecemlineatus). Lab. Anim. (NY) 41, 315-325. 10.1038/laban.150 [DOI] [PubMed] [Google Scholar]
- Merriman D. K., Sajdak B. S., Li W. and Jones B. W. (2016). Seasonal and post-trauma remodeling in cone-dominant ground squirrel retina. Exp. Eye Res. 150, 90-105. 10.1016/j.exer.2016.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyawaki S., Kawamura Y., Oiwa Y., Shimizu A., Hachiya T., Bono H., Koya I., Okada Y., Kimura T., Tsuchiya Y. et al. (2016). Tumour resistance in induced pluripotent stem cells derived from naked mole-rats. Nat. Commun. 7, 11471 10.1038/ncomms11471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monahan Z., Ryan V. H., Janke A. M., Burke K. A., Rhoads S. N., Zerze G. H., O'Meally R., Dignon G. L., Conicella A. E., Zheng W. et al. (2017). Phosphorylation of the FUS low-complexity domain disrupts phase separation, aggregation, and toxicity. EMBO J. 36, 2951-2967. 10.15252/embj.201696394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou J., Ball J. M., Luan Y., Zhao T., Miyagishima K. J., Xu Y., Zhou H., Chen J., Merriman D. K., Xie Z. et al. (2018). iPSCs from a hibernator provide a platform for studying cold adaptation and its potential medical applications. Cell 173, 851-863.e816. 10.1016/j.cell.2018.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pamenter M. E., Lau G. Y., Richards J. G. and Milsom W. K. (2018). Naked mole rat brain mitochondria electron transport system flux and H(+) leak are reduced during acute hypoxia. J. Exp. Biol. 221 10.1242/jeb.171397 [DOI] [PubMed] [Google Scholar]
- Park T. J., Reznick J., Peterson B. L., Blass G., Omerbasic D., Bennett N. C., Kuich P., Zasada C., Browe B. M., Hamann W. et al. (2017). Fructose-driven glycolysis supports anoxia resistance in the naked mole-rat. Science 356, 307-311. 10.1126/science.aab3896 [DOI] [PubMed] [Google Scholar]
- Rossello R. A., Chen C. C., Dai R., Howard J. T., Hochgeschwender U. and Jarvis E. D. (2013). Mammalian genes induce partially reprogrammed pluripotent stem cells in non-mammalian vertebrate and invertebrate species. Elife 2, e00036 10.7554/eLife.00036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J. J., Theriot J. A., Sood P., Marshall W. F., Landweber L. F., Fritz-Laylin L., Polka J. K., Oliferenko S., Gerbich T., Gladfelter A. et al. (2017). Non-model model organisms. BMC Biol. 15, 55 10.1186/s12915-017-0391-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skulachev V. P. (1991). Fatty acid circuit as a physiological mechanism of uncoupling of oxidative phosphorylation. FEBS Lett. 294, 158-162. 10.1016/0014-5793(91)80658-P [DOI] [PubMed] [Google Scholar]
- Staples J. F. (2014). Metabolic suppression in mammalian hibernation: the role of mitochondria. J. Exp. Biol. 217, 2032-2036. 10.1242/jeb.092973 [DOI] [PubMed] [Google Scholar]
- Staples J. F. (2016). Metabolic flexibility: hibernation, torpor, and estivation. Comp. Physiol. 6, 737-771. 10.1002/cphy.c140064 [DOI] [PubMed] [Google Scholar]
- Stieler J. T., Bullmann T., Kohl F., Barnes B. M. and Arendt T. (2009). PHF-like tau phosphorylation in mammalian hibernation is not associated with p25-formation. J. Neural. Transm. (Vienna) 116, 345-350. 10.1007/s00702-008-0181-x [DOI] [PubMed] [Google Scholar]
- Stieler J. T., Bullmann T., Kohl F., Tøien O., Bruckner M. K., Härtig W., Barnes B. M. and Arendt T. (2011). The physiological link between metabolic rate depression and tau phosphorylation in mammalian hibernation. PLoS ONE 6, e14530 10.1371/journal.pone.0014530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su B., Wang X., Drew K. L., Perry G., Smith M. A. and Zhu X. (2008). Physiological regulation of tau phosphorylation during hibernation. J. Neurochem. 105, 2098-2108. 10.1111/j.1471-4159.2008.05294.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez R. K. and Welch K. C. (2017). Sugar metabolism in hummingbirds and nectar bats. Nutrients 9, E743 10.3390/nu9070743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K. and Yamanaka S. (2006). Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663-676. 10.1016/j.cell.2006.07.024 [DOI] [PubMed] [Google Scholar]
- Tan L., Ke Z., Tombline G., Macoretta N., Hayes K., Tian X., Lv R., Ablaeva J., Gilbert M., Bhanu N. V. et al. (2017). Naked mole rat cells have a stable epigenome that resists iPSC reprogramming. Stem Cell Reports 9, 1721-1734. 10.1016/j.stemcr.2017.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian X., Azpurua J., Hine C., Vaidya A., Myakishev-Rempel M., Ablaeva J., Mao Z., Nevo E., Gorbunova V. and Seluanov A. (2013). High-molecular-mass hyaluronan mediates the cancer resistance of the naked mole rat. Nature 499, 346-349. 10.1038/nature12234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toien O., Blake J., Edgar D. M., Grahn D. A., Heller H. C. and Barnes B. M. (2011). Hibernation in black bears: independence of metabolic suppression from body temperature. Science 331, 906-909. 10.1126/science.1199435 [DOI] [PubMed] [Google Scholar]
- Treat M. D., Scholer L., Barrett B., Khachatryan A., McKenna A. J., Reyes T., Rezazadeh A., Ronkon C. F., Samora D., Santamaria J. F. et al. (2018). Extreme physiological plasticity in a hibernating basoendothermic mammal, Tenrec ecaudatus. J. Exp. Biol. 221, jeb185900 10.1242/jeb.185900 [DOI] [PubMed] [Google Scholar]
- Vaughan D. K., Gruber A. R., Michalski M. L., Seidling J. and Schlink S. (2006). Capture, care, and captive breeding of 13-lined ground squirrels, Spermophilus tridecemlineatus. Lab. Anim. (NY) 35, 33-40. 10.1038/laban0406-33 [DOI] [PubMed] [Google Scholar]
- Vázquez-Medina J. P., Zenteno-Savín T., Elsner R. and Ortiz R. M. (2012). Coping with physiological oxidative stress: a review of antioxidant strategies in seals. J. Comp. Physiol. B 182, 741-750. 10.1007/s00360-012-0652-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch K. C. Jr and Suarez R. K. (2007). Oxidation rate and turnover of ingested sugar in hovering Anna's (Calypte anna) and rufous (Selasphorus rufus) hummingbirds. J. Exp. Biol. 210, 2154-2162. 10.1242/jeb.005363 [DOI] [PubMed] [Google Scholar]
- Yu C., Liu K., Tang S. and Ding S. (2014). Chemical approaches to cell reprogramming. Curr. Opin. Genet. Dev. 28, 50-56. 10.1016/j.gde.2014.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]