Abstract
Background
In patients with end‐stage kidney disease, sudden cardiac death is more frequent after a long interdialytic interval, within 6 hours after the end of a hemodialysis session. We hypothesized that the occurrence of paroxysmal arrhythmias is associated with changes in heart rate and heart rate variability in different phases of hemodialysis.
Methods and Results
We conducted a prospective ancillary study of the Predictors of Arrhythmic and Cardiovascular Risk in End Stage Renal Disease cohort. Continuous ECG monitoring was performed using an ECG patch, and short‐term heart rate variability was measured for 3 minutes every hour (by root mean square of the successive normal‐to‐normal intervals, spectral analysis, Poincaré plot, and entropy), up to 300 hours. Out of enrolled participants (n=28; age 54±13 years; 57% men; 96% black; 33% with a history of cardiovascular disease; left ventricular ejection fraction 70±9%), arrhythmias were detected in 13 (46%). Nonsustained ventricular tachycardia occurred more frequently during/posthemodialysis than pre‐/between hemodialysis (63% versus 37%, P=0.015). In adjusted for cardiovascular disease time‐series analysis, nonsustained ventricular tachycardia was preceded by a sudden heart rate increase (by 11.2 [95% CI 10.1–12.3] beats per minute; P<0.0001). During every‐other‐day dialysis, root mean square of the successive normal‐to‐normal intervals had a significant circadian pattern (Mesor 10.6 [ 95% CI 0.9–11.2] ms; amplitude 1.5 [95% CI 1.0–3.1] ms; peak at 02:01 [95% CI 20:22–03:16] am; P<0.0001), which was replaced by a steady worsening on the second day without dialysis (root mean square of the successive normal‐to‐normal intervals −1.41 [95% CI −1.67 to −1.15] ms/24 h; P<0.0001).
Conclusions
Sudden increase in heart rate during/posthemodialysis is associated with nonsustained ventricular tachycardia. Every‐other‐day hemodialysis preserves circadian rhythm, but a second day without dialysis is characterized by parasympathetic withdrawal.
Keywords: electrocardiography, heart rate/heart rate variability, hemodialysis, trigger clusters, ventricular arrhythmia
Subject Categories: Electrophysiology, Arrhythmias, Autonomic Nervous System, Mechanisms, Electrocardiology (ECG)
Clinical Perspective
What Is New?
Nonsustained ventricular tachycardia is more frequent during hemodialysis or within 6 hours posthemodialysis.
In incident hemodialysis patients, heart rate sharply increases before nonsustained ventricular tachycardia events, suggesting a triggered ventricular tachycardia mechanism.
Every‐other‐day hemodialysis preserves physiological circadian rhythm in heart rate and heart rate variability, whereas the extension of the interdialytic period for the second day abolishes circadian rhythm and shows a steady deterioration in autonomic disbalance.
What Are the Clinical Implications?
The harmful effect of the 2‐day interval without hemodialysis suggests that more frequent dialysis should be considered the preferred prescribed treatment schedule.
Improvement of patient adherence—avoiding missing hemodialysis sessions—can improve patient outcomes.
Diagnosis of autonomic imbalance in end‐stage kidney disease patients presenting with bradycardia is difficult. In end‐stage kidney disease, an autonomic disbalance is manifested by parasympathetic withdrawal and impaired baroreflex sensitivity, and use of β‐blockers attenuates association of heart rate with nonsustained ventricular tachycardia, suggesting potential treatment benefit of β‐blocker use, which should be studied further.
Introduction
Sudden cardiac death (SCD) is the most common cause of death in end‐stage kidney disease (ESKD) patients receiving dialysis.1, 2 Both tachy‐ and bradyarrhythmias may play a causative role in SCD in ESKD.3, 4, 5 Importantly, the phases of hemodialysis relate to the rate of SCD.6, 7, 8, 9 Mortality increases after a long interdialytic interval, within 6 hours after the end of a hemodialysis session.6, 7, 8 The dialysis procedure itself triggers multiple mechanisms that can increase the propensity to cardiac arrhythmias.10 Oscillations in electrolyte levels and fluid volume, a pro‐inflammatory state, repetitive myocardial injury from uremia and other toxins,10 and myocardial stunning11 are hemodialysis‐specific risk factors for SCD. A SCD event exemplifies a perfect storm, requiring both a susceptible substrate and a trigger.12 Together the exposure to SCD risk factors related to fluctuating hemodialysis sessions and traditional SCD triggers can explain the extremely high rate of SCD in dialysis patients.
Autonomic imbalance of the heart is traditionally associated with SCD. Under normal conditions, the autonomic system controls heart rate and rhythm via a balance between the parasympathetic and sympathetic systems. Heart rate variability (HRV) is a measure of fluctuations in the autonomic system and baroreflex sensitivity.13, 14 Parasympathetic withdrawal and increased sympathetic input decreases HRV and triggers SCD.12 Previous studies using 24‐hour Holter electrocardiogram recordings reported the association of depressed HRV15 with increased mortality in ESKD patients.16, 17, 18 However, 24‐ or even 48‐hour Holter ECG recordings are too short to study the association of fluctuating autonomic tone with the standard 7‐day hemodialysis schedule. Whether autonomic imbalance associates with both the dialytic cycle and cardiac arrhythmias in dialysis patients remains unknown. Longitudinal changes in autonomic tone before, during, and after hemodialysis procedures, along with their association with brady‐ and tachyarrhythmias, have not been previously studied.
To address this knowledge gap, we conducted an ancillary prospective study in a subset of incident hemodialysis patients, enrolled in the PACE (Predictors of Arrhythmic and Cardiovascular Risk in End Stage Renal Disease) study.2, 19 We hypothesized that in incident hemodialysis patients, clinical characteristics and paroxysmal arrhythmias are associated with periodic changes in heart rate and HRV in different phases of hemodialysis.
Methods
Developed for the data analysis, software code is available at GitHub and can be accessed at https://github.com/Tereshchenkolab/HRV. In order to minimize the possibility of unintentionally sharing information that can be used to re‐identify private information, a subset of the deidentified data generated for this study is available at GitHub.20
Study Population
We conducted a prospective ancillary study within PACE.19 Both the parental PACE study and this ancillary study were approved by the Johns Hopkins Institutional Review Board, and all participants provided written informed consent.
The PACE study design has been previously described.2, 19 Briefly, the study enrolled adult ESKD patients who had started in‐center hemodialysis within 6 months of enrollment. Patients on home hemodialysis, peritoneal dialysis, in hospice or skilled nursing facility, and patients with an implanted pacemaker or cardioverter‐defibrillator were not eligible. At enrollment, participants underwent comprehensive cardiovascular evaluation, which included several types of ECG, echocardiogram, cardiac computed tomography, and coronary angiography.
This ancillary study included randomly selected PACE participants who underwent baseline cardiovascular evaluation and agreed to undergo continuous ECG monitoring for at least 7 days.
Clinical Covariates
Prevalent coronary artery disease (CAD), cerebrovascular disease (CVD), congestive heart failure (CHF), hypercholesterolemia, hypertension, and diabetes mellitus were determined by participant's self‐report and physician's diagnosis recorded in the medical record. Participants were asked to bring in their medications, and medication use was recorded during the baseline visit. To assess subjective postdialysis recovery time, participants answered the question: “How long does it take you to recover from a dialysis session?” during a telephone interview, conducted within 30 days of ECG monitoring.
Echocardiography was performed at the PACE echocardiographic core laboratory,19 where left ventricular ejection fraction, left ventricular dimensions, and left ventricular mass index were measured as previously described.19 Left ventricular hypertrophy was defined as an echocardiographic left ventricular mass index ≥116 g/m2 in males or ≥104 g/m2 in females.
Changes in weight and sitting systolic and diastolic blood pressure before and after each dialysis session were recorded for this study's participants.
Dialysis bath (dialysate concentrations of calcium and potassium) data are reported as a 3‐month average. All participants received a dialysate Mg concentration of 1.0 mEq/L, and a dialysate bicarbonate concentration of 40 mmol/L. Three‐month averaged intradialytic weight change was calculated. Serum calcium and potassium were assessed as a 3‐month average of predialysis measures before the study clinic visit.
Continuous ECG Monitoring Using ECG Patch
Continuous ECG monitoring was performed using an ECG patch (Zio Patch, iRhythm Technologies, Inc., San Francisco, CA). During the study visit, a study coordinator applied the device over the left pectoral region,21 and instructed the participant to activate a trigger button in the event of cardiac symptoms (presumed arrhythmia). Participants were instructed to wear the adhesive ECG patch for as long as possible, with the goal of obtaining at least 7 days of continuous ECG recording. After completion of the ECG recording, participants mailed the ECG patch to iRhythm Technologies, Inc, which provided their standard US Food and Drug Administration–approved report to the study investigators—via a secure website. The Zio Patch report was reviewed within 24 hours by the study investigators, and clinically important findings were communicated with participants and their healthcare providers. In addition, continuously recorded raw digital ECG signal was provided by iRhythm Technologies, Inc for further analyses.
Diagnosis of cardiac arrhythmias
Per the standard iRhythm Technologies protocol, the following arrhythmias were diagnosed: atrial fibrillation (AF) or flutter (>4 beats), supraventricular tachycardia (>4 beats), pause >3 s, atrioventricular block of the second or the third degree, ventricular tachycardia (VT, >4 beats), or polymorphic VT/ventricular fibrillation. All arrhythmic events captured by the Zio Patch report were reviewed and validated by at least 2 study investigators (LGT, RSP, SH).
Phases of Hemodialysis
To determine the association of the intermittent hemodialysis sessions with heart rate and HRV time‐series, we used hemodialysis phases accepted in the nephrology field relative to their proximity to a hemodialysis treatment (Figure 1). Every study participant underwent hemodialysis (4–5 hours, phase 1), postdialysis (6 hours immediately after hemodialysis, phase 2), between‐hemodialysis (variable length, phases 3, 3–5, and 3–7), and predialysis (6 hours preceding dialysis, phases 4, 6, and 8) phases. Over the course of the 7‐day cycle, dialysis treatment–adherent study participants were dialyzed every other day for 5 days (either Monday/Wednesday/Friday or Tuesday/Thursday/Saturday; a total of 3 treatments weekly) and then experienced a 2‐day‐long interdialytic interval. For example, for an adherent Monday–Friday schedule, the regular interdialytic period was ≈32 hours, and the length of the prolonged interdialytic period over the weekend was much longer at about 56 hours. Nonadherent participants (n=4) did not follow this standard dialytic pattern. Their treatment schedule was interrupted by missed hemodialysis treatments, leading to a variable prolonged interdialytic period of at least 72 hours or longer.
Figure 1.
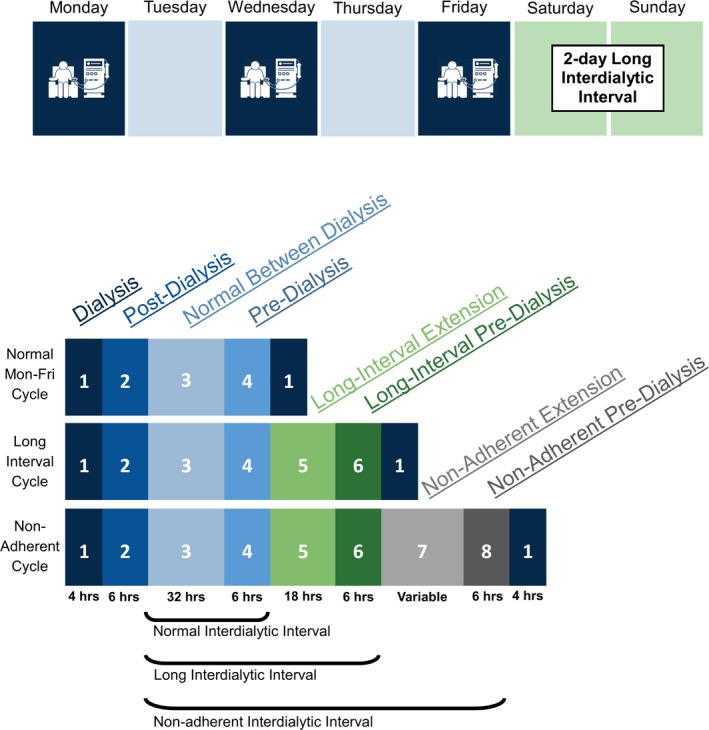
A schematic presentation of the dialytic cycles used for analysis. Blue color indicates phases within 48 hours of the last treatment, green color highlights the phases included to analyze the extra 24 hours between treatments in the 2‐day‐long interdialytic interval, and gray color symbolizes the phases that were included to analyze >72‐hour interdialytic intervals for those who were nonadherent.
HRV Measurements
A raw single‐lead digital ECG signal (sampling rate 200 Hz; amplitude resolution 4.88 μV) was analyzed in the Tereshchenko laboratory at the Oregon Health & Science University. Figure 2 shows examples of the ECG signal and detected cardiac arrhythmia. A custom MATLAB (The MathWorks, Inc, Natick, MA) software application was developed (NMR, EAPA, MMK; provided at https://github.com/Tereshchenkolab/HRV) to automatically detect QRS complexes and select a single 3‐minute normal sinus rhythm epoch for each hour of recording. The algorithm automatically eliminated epochs with premature R2 beats if the R1R2 interval was shorter than the preceding R0R1 interval by 15% or greater. The algorithm similarly eliminated epochs with a sudden pause, if subsequently the R1R2 interval was longer than the preceding R0R1 interval by 15% or greater, to remove epochs with blocked premature atrial or His extrasystoles, or intermittent sinoatrial or atrioventricular block. Traditionally, in Holter ECG analysis, the premature atrial beat was defined by a coupling interval of <80% of the mean RR interval.22 We applied a more stringent threshold after manually reviewing our ECG data with thresholds ranging from 2% to 20%. A sliding 3‐minute window approach was used to scan the entirety of the data: when a premature beat (or sudden pause) was detected, the premature beat and subsequent compensatory pause were skipped, and a new 3‐minute window search started thereafter again. If the algorithm did not find a continuous 3‐minute epoch of sinus rhythm in an entire hour, the software would change the R‐peak detection algorithm23 and repeated all steps described above. The first R‐peak detection algorithm used was a Pan‐Tompkins,24 followed by principal component analysis,25 and then parabolic fitting.26 Because the magnitude of R and S peaks varied within and between patients, the dominant peak of the QRS complex varied during this ECG monitoring. We paid special attention to ensure consistent signs of the dominant QRS peak for the entire 3‐minute epoch. The greatest average Manhattan distance from baseline to the highest positive (R) peak and highest negative (S) peak was calculated to identify the best dominant peak for each 3‐minute epoch. The accuracy of consistent dominant (R or S) peaks detection, and accuracy of the selection of consecutive normal sinus beat were validated on a data subset by the investigator (NMR), with the aid of a graphical display.
Figure 2.
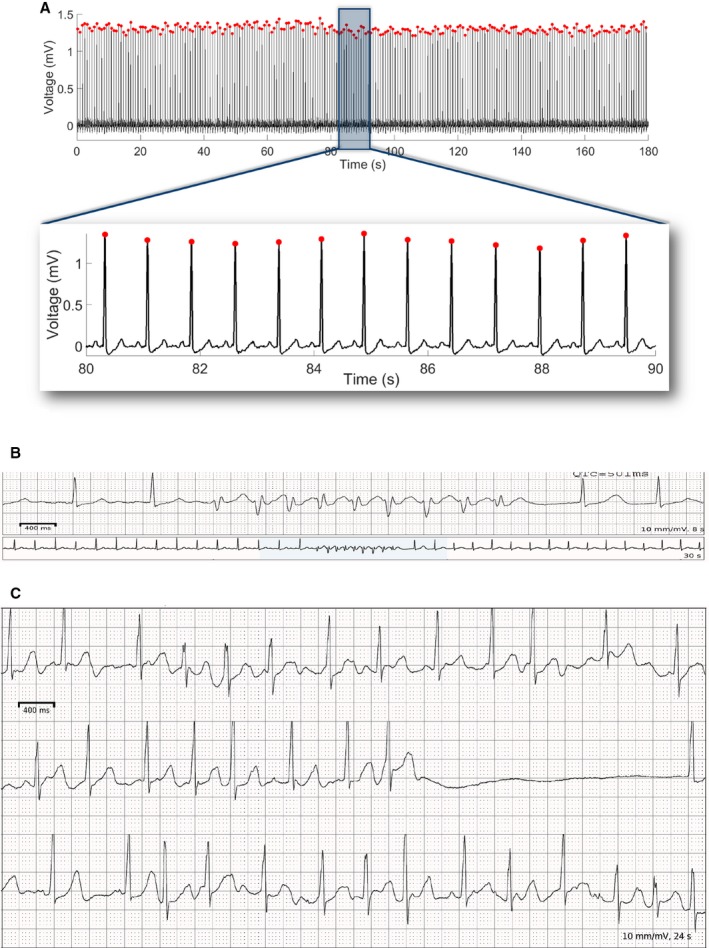
A representative example of (A) a single‐lead ECG with detected R‐peaks and measured RR′ intervals. A 3‐minute epoch is shown, with mean heart rate 78 bpm, rMSSD of 11.5 ms, LF power of 2.4 s2, HF power of 6.3 s2, LF/HF ratio of 0.38, SD 1 of 8.2 ms, SD 2 of 19.7 ms, SD 12 ratio of 0.41, sample entropy of 2.0, and Renyi entropy of 1.4. A 10‐s portion is displayed for closer examination of ECG morphology. B, Polymorphic ventricular tachycardia, and (C) atrial fibrillation and a pause in a study participant. bpm indicates beats per minute; HF, high frequency; LF, low frequency; rMSSD, root mean square of the successive normal sinus to normal sinus intervals differences.
HRV was measured according to the Standards.27, 28 Developed (by MMK) MATLAB (the MathWorks, Inc, Natick, MA) software code is provided at https://github.com/Tereshchenkolab/HRV.
Time‐domain HRV measures
Heart rate and the root mean square of the successive normal sinus to normal sinus (NN) intervals differences (rMSSD) were calculated for each 3‐minute epoch selected per hour.
Frequency‐domain HRV measures
The low‐frequency (LF; 0.04–0.15 Hz) power, high‐frequency (HF; 0.15–0.4 Hz) power, and LF/HF ratio of powers were calculated for each 3‐minute epoch.
Nonlinear HRV measures
Quantitative analysis of the Poincaré plot was performed.28 The Poincaré plot was derived from every 3‐minute NN data epoch by plotting the values NNn+1 against the values of NNn. SD1 was calculated as the SD of the cloud of points in the direction perpendicular to the line‐of‐identity. SD2 was calculated as the SD of the cloud of points in the direction of the line‐of‐identity. SD1/SD2 ratio was called SD12.
Entropy
To quantify the entropy rate on a short‐length NN series, we elected to measure sample entropy28 and Renyi entropy for each 3‐minute epoch. Renyi entropy was calculated as described by Cornforth et al.29 We used an α value equal to 4 because of previous data suggesting that positive α (1–5) provides the best discrimination of cardiac autonomic neuropathy, and based on the sampling rate of our data.30
Statistical Analysis
Normality of the distribution of continuous variables was evaluated using standardized normal probability plots. For comparison of clinical characteristics in participants with versus without detected arrhythmia, normally distributed continuous variables were presented as means±SD and were compared using a t test. Fisher exact test was used to compare categorical variables.
The main data set was structured as a panel of time‐series, as HRV was measured at the beginning of each hour (assuming equal intervals between 3‐minute epochs). The assumption of equal intervals between 3‐minute epochs was confirmed for 81% of epochs starting in the first second (44%), within the first 10 minutes (76%), or within the first 15 minutes (81%) of each hour. The assumption of equal intervals between 3‐minute epochs was violated for 10% of epochs starting in the second half of an hour. To test the robustness of our findings, we conducted a sensitivity analysis after exclusion of epochs that violated the equal intervals assumption required for time‐series analysis.
Within‐subject and between‐subjects SDs were reported for each phase of dialysis. Paired comparison of HRV in different phases of the dialytic cycle was performed using analysis of variance within subjects (for repeated measures). As previous studies reported an increased risk of SCD in the postdialysis phase after the long interdialytic weekend,31 we performed paired comparisons of HRV in the postdialysis phase that followed: (1) normal hemodialysis versus (2) 2‐day‐long interdialytic interval versus (3) longer than 72‐hour interdialytic interval in nonadherent participants.
To determine whether demographic and clinical characteristics, paroxysmal cardiac arrhythmias, and dialytic and circadian (24‐hour) cycles are associated with time‐series of heart rate and HRV metrics, we constructed autoregressive conditional heteroscedasticity (ARCH) and generalized autoregressive conditionally heteroscedastic (GARCH) panel models.32 In ARCH time‐series analysis, both the mean and variance of the HRV metric were modeled as time dependent, which allowed modeling volatility that can arise in response to the dialysis procedure or other unmeasured factors. Time series of heart rate and HRV metrics (1‐by‐1) served as an outcome. To identify our ARCH/GARCH model, we first explored the autocorrelation function (ACF) and partial autocorrelation (PACF) function of the HRV time‐series, and ACF/PACF of squared HRV variables’ values. Because the ACF and PACF of heart rate and HRV time‐series represented white noise, but ACF/PACF of squared series tapered (autoregressive of order 1), we constructed ARCH(1/1)/GARCH(1) models.
To determine whether demographic and clinical characteristics (including use of β‐blockers) are associated with heart rate and HRV time‐series, age, sex, race, prevalent CAD, CHF, CVD, history of AF, diabetes mellitus, Charlson comorbidity index, and postdialysis recovery time were included in each ARCH model.
To determine whether paroxysmal cardiac arrhythmias are associated with time‐series of heart rate and HRV metrics, separate ARCH models were constructed for each type of paroxysmal arrhythmia (VT, AF, supraventricular tachycardia, and pause). ARCH models were adjusted for age, sex, race, prevalent CAD, CHF, CVD, AF, Charlson comorbidity index, and postdialysis recovery time.
To describe the circadian (24‐hour) rhythm—while accounting for multiple 24‐hour cycles analyzed for the same patient (longitudinal/panel data structure)—we constructed periodic regression models with fixed (within) estimators. We used periodic regression to analyze the behavior of heart rate and HRV, which vary in a circular‐scale 24‐hour cycle. We converted the circular variable “hour in a 24‐hour cycle” from cyclic (hours of the day) to angular (radians) format, and then to trigonometric format (paired units sine and cosine). We studied whether heart rate and HRV time‐series responded in a periodic way to the 24‐hour cycle. We estimated the periodic mean (Mesor) for heart rate and HRV metrics, and calculated an amplitude (A) of variation about the Mesor in the modeled cycle:
We calculated phase angle (acrophase φ) as a time within the 24‐hour cycle when heart rate/HRV is maximized (peak location): φ=arctan(sinβ/cosβ). The minimum is located 12 hours (0.5 cycles) away from the maximum. Because dialysis and the immediate postdialytic phase are well known to significantly affect heart rate and HRV,31, 33 dialysis (phase 1) and postdialytic phase 2 were excluded from periodic regression analyses. We stratified circadian rhythm analyses by the type of interdialytic phase: in a regular dialytic schedule (phases 3–4), second‐day interdialytic extension (phases 5–6), and interdialytic extension above 72 hours (phases 7–8 in nonadherent participants missing dialysis), as shown in Figure 1.
To determine whether the dialytic cycle is associated with the heart rate and HRV time‐series after removal of the effect of circadian rhythm, we constructed periodic panel ARCH/GARCH models to determine a change in HRV per hour of remoteness from the first dialysis hour. ARCH/GARCH models were adjusted for age, sex, race, prevalent CAD, CHF, CVD, AF, diabetes mellitus, Charlson comorbidity index, and postdialysis recovery time. In addition, circadian 24‐hour cycles (paired units sine and cosine) were included in each ARCH model and served to adjust for circadian periodicity.
Sensitivity analyses
To test the robustness of our findings, we excluded 10% of epochs that violated the equal intervals assumption that is required for time‐series ARCH models. Furthermore, we adjusted ARCH models for weight and sitting systolic and diastolic blood pressure changes before and after every hemodialysis session during ECG monitoring.
Statistical analysis was performed using STATA MP 15.1 (StataCorp LP, College Station, TX).
Results
Study Population
The study population (Table 1) included 28 PACE participants (mean age 54±13 years; 57% men; 96% black). Approximately one third of the population had a history of structural heart disease with normal left ventricular ejection fraction. The average dialysis recovery time was 16 minutes. All study participants had hypertension and used antihypertensive medications; 83% were on β‐blockers. Most of the study participants received identical dialysate: >70% participants received dialysate concentration of potassium equal to 2 mmol/L and calcium equal to 2.5 mmol/L.
Table 1.
Clinical Characteristics of Study Participants With and Without Any Clinically Significant Cardiac Arrhythmias Diagnosed During ECG Monitoring
| Characteristic | Total (n=28) |
|---|---|
| Age (SD), y | 53.9 (12.5) |
| Male, n (%) | 16 (57.1) |
| Black, n (%) | 27 (96.4) |
| Body mass index (SD), kg/m2 | 29.4 (7.4) |
| Cause of ESKD: | |
| Glomerulonephritis, n (%) | 3 (10.7) |
| Hypertension, n (%) | 5 (17.9) |
| Diabetes mellitus, n (%) | 12 (42.9) |
| HIV/other/genetic/obstruction, n (%) | 7 (25.0) |
| Unknown, n (%) | 1 (3.6) |
| Hypertension, n (%) | 28 (100) |
| Diabetes mellitus, n (%) | 16 (57.1) |
| Coronary artery disease, n (%) | 8 (28.6) |
| Heart failure, n (%) | 9 (32.1) |
| Cerebrovascular disease, n (%) | 8 (28.6) |
| History of atrial fibrillation, n (%) | 10 (35.7) |
| Hypercholesterolemia, n (%) | 19 (67.9) |
| Smoker current or former, n (%) | 15 (53.6) |
| Drinker current or former, n (%) | 23 (82.1) |
| Use of β‐blockers, n (%) | 19/23 (82.6) |
| Charlson comorbidity index (SD) | 5.5 (2.2) |
| Dialysis recovery time (SD), min | 15.9 (3.3) |
| LV ejection fraction (SD), % | 70.3 (9.3) |
| LV internal dimension diastole (SD), cm | 5.3 (0.7) |
| LV internal dimension systole (SD), cm | 3.1 (0.7) |
| LV mass index (SD), g/m2 | 146.0 (53.2) |
| LV hypertrophy, n (%) | 17 (70.8) |
| 3‐mo averaged calcium (SD), mg/dL | 8.52 (0.65) |
| 3‐mo averaged potassium (SD), mEq/L | 4.34 (0.39) |
| 3‐mo averaged intradialytic weight change (SD), kg | 2.09 (0.97) |
| 3‐mo averaged dialysis bath calcium concentration (SD), mmol/L | 2.4 (0.2) |
| 3‐mo averaged dialysis bath potassium concentration (SD), mmol/L | 2.2 (0.4) |
Three‐month‐averaged values are from the start of dialysis. ESKD indicates end‐stage kidney disease; HIV, human immunodeficiency virus; LV, left ventricular.
Cardiac Arrhythmia Events
Almost half of the participants (n=13, 46%) had arrhythmias detected during monitoring (Table 2; Figure 2). Except for 1 patient with 46% paroxysmal AF burden, all detected events were nonsustained (NS), lasting <30 seconds, and asymptomatic. NSVT occurred more frequently during hemodialysis or within 6 hours posthemodialysis, as compared with pre‐ or between‐hemodialysis (63% versus 37%, P=0.015). Supraventricular tachycardia occurred more frequently pre‐ or between‐hemodialysis as compared with during hemodialysis or posthemodialysis (84% versus 16%, P=0.015). All patients with NSVT were free from CAD at baseline.
Table 2.
Cardiac Arrhythmia Events Timeline
| Events 6‐h Predialysis, n (%) | Events During Dialysis, n (%) | Events 6‐h Postdialysis, n (%) | Events Between Dialysis, n (%) | Number of Patients (% of population) | |
|---|---|---|---|---|---|
| Total number of events, n=47 | 12 (26) | 8 (17) | 9 (19) | 18 (38) | 13 (46) |
| VT, n=8 | 1 (13) | 2 (25) | 3 (38) | 2 (25) | 4 (15) |
| SVT, n=17 | 5 (29) | 1 (6) | 2 (12) | 9 (53) | 9 (32) |
| AF, n=21 | 6 (29) | 4 (19) | 4 (19) | 7 (33) | 2 (7) |
| Pauses >3 s, n=1 | 0 | 1 (100) | 0 | 0 | 1 (3.5) |
AF indicates atrial fibrillation; SVT, supraventricular tachycardia; VT, ventricular tachycardia.
Dynamic Time Series of Heart Rate and HRV
The average duration of analyzed continuous ECG recording was 6.5±2.3 days. All 28 participants had at least 1 ECG recording over a 2‐day‐long interdialytic interval, and 4 participants missed 1 to 2 dialysis sessions during ECG monitoring.
Average heart rate was fastest within the first 6 hours postdialysis (Table 3) and gradually slowed down afterward. Short‐term HRV (rMSSD, HF power, Poincaré SD1) decreased posthemodialysis, then recovered by the time of the next regular hemodialysis session. However, if hemodialysis was not performed within the next 72 hours, short‐term HRV gradually diminished further. In an unadjusted paired comparison, there were no statistically significant differences in HRV posthemodialysis after different durations of the preceding interdialytic interval (Table S1).
Table 3.
Heart Rate and HRV in Different Phases of the Dialytic Cycle
| HRV Measure | Phase 1 N=323; n=26 T=12.4 | Phase 2 N=480; n=26 T=18.5 | Phase 3 N=2554; n=28 T=91.2 | Phase 4 N=438; n=27 T=16.2 | Phase 5 N=428; n=28 T=15.3 | Phase 6 N=135; n=26 T=5.2 | Phase 7 N=221; n=4 T=55.3 | Phase 8 N=15; n=3 T=5 | Within ANOVA P |
|---|---|---|---|---|---|---|---|---|---|
| Heart rate (SD), bpm | 74.1 (11.3) | 81.3 (13.4) | 77.2 (12.2) | 75.9 (11.5) | 77.6 (14.1) | 73.9 (12.2) | 70.8 (8.0) | 71.0 (5.4) | <0.0001 |
| SD between | 10.4 | 11.3 | 10.1 | 10.3 | 12.9 | 13.2 | 9.8 | 5.3 | |
| SD within | 6.1 | 7.6 | 7.7 | 6.0 | 6.0 | 4.4 | 4.3 | 3.1 | |
| rMSSD (SD), ms | 12.6 (7.8) | 9.7 (5.2) | 11.0 (6.4) | 11.1 (6.7) | 11.1 (5.6) | 11.8 (5.6) | 11.3 (4.1) | 9.2 (2.3) | <0.0001 |
| SD between | 6.1 | 3.7 | 4.1 | 5.2 | 3.7 | 4.3 | 2.7 | 2.1 | |
| SD within | 4.6 | 3.7 | 5.1 | 5.1 | 4.4 | 4.2 | 3.6 | 1.7 | |
| HF power (SD), s2 | 4.6 (2.0) | 4.1 (1.8) | 4.3 (1.9) | 4.4 (2.0) | 4.5 (2.0) | 4.6 (2.2) | 5.8 (2.2) | 5.1 (2.9) | <0.0001 |
| SD between | 1.5 | 1.3 | 1.4 | 1.5 | 1.5 | 1.7 | 1.9 | 2.9 | |
| SD within | 1.3 | 1.4 | 1.4 | 1.4 | 1.3 | 1.4 | 1.4 | 0.9 | |
| LF power (SD), s2 | 3.1 (1.0) | 3.0 (1.0) | 3.1 (1.0) | 2.9 (0.9) | 3.0 (1.0) | 3.0 (1.0) | 3.1 (1.0) | 3.0 (0.8) | 0.068 |
| SD between | 0.6 | 0.6 | 0.6 | 0.5 | 0.6 | 0.7 | 0.8 | 0.3 | |
| SD within | 0.8 | 0.8 | 0.8 | 0.8 | 0.8 | 0.8 | 0.8 | 0.7 | |
| LF/HF ratio (SD) | 0.82 (0.46) | 0.89 (0.48) | 0.87 (0.46) | 0.84 (0.45) | 0.84 (0.47) | 0.80 (0.43) | 0.66 (0.39) | 0.88 (0.58) | 0.006 |
| SD between | 0.36 | 0.36 | 0.35 | 0.37 | 0.36 | 0.33 | 0.37 | 0.61 | |
| SD within | 0.27 | 0.31 | 0.32 | 0.32 | 0.29 | 0.26 | 0.24 | 0.16 | |
| Poincaré SD1 (SD), ms | 9.0 (5.6) | 6.9 (3.7) | 7.8 (4.6) | 7.9 (4.8) | 7.8 (4.0) | 8.4 (3.9) | 8.0 (2.9) | 6.5 (1.6) | <0.0001 |
| SD between | 4.3 | 2.6 | 2.9 | 3.7 | 2.6 | 3.0 | 1.9 | 1.5 | |
| SD within | 3.3 | 2.6 | 3.6 | 3.6 | 3.1 | 2.9 | 2.6 | 1.2 | |
| Poincaré SD2 (SD), ms | 25.8 (18.7) | 21.6 (15.9) | 23.8 (18.4) | 23.7 (17.2) | 22.4 (16.5) | 24.4 (16.5) | 20.3 (15.3) | 23.5 (17.0) | 0.002 |
| SD between | 15.2 | 11.1 | 14.9 | 19.6 | 12.1 | 12.5 | 13.7 | 16.6 | |
| SD within | 11.1 | 11.6 | 13.5 | 11.4 | 12.2 | 10.7 | 10.1 | 7.4 | |
| SD12 (SD), % | 0.44 (0.25) | 0.42 (0.25) | 0.43 (0.24) | 0.43 (0.27) | 0.46 (0.28) | 0.45 (0.27) | 0.56 (0.33) | 0.47 (0.32) | 0.461 |
| SD between | 0.20 | 0.19 | 0.19 | 0.20 | 0.20 | 0.20 | 0.34 | 0.33 | |
| SD within | 0.16 | 0.17 | 0.18 | 0.20 | 0.19 | 0.18 | 0.20 | 0.12 | |
| Sample Entropy (SD) | 1.49 (0.40) | 1.39 (0.46) | 1.39 (0.44) | 1.43 (0.44) | 1.42 (0.44) | 1.43 (0.47) | 1.52 (0.43) | 1.25 (0.62) | 0.0004 |
| SD between | 0.25 | 0.26 | 0.21 | 0.24 | 0.23 | 0.29 | 0.23 | 0.73 | |
| SD within | 0.35 | 0.40 | 0.39 | 0.38 | 0.37 | 0.38 | 0.38 | 0.30 | |
| Renyi entropy (SD) | 1.31 (0.31) | 1.33 (0.28) | 1.31 (0.30) | 1.33 (0.29) | 1.31 (0.31) | 1.29 (0.31) | 1.23 (0.33) | 1.30 (0.36) | 0.758 |
| SD between | 0.17 | 0.13 | 0.09 | 0.17 | 0.12 | 0.15 | 0.13 | 0.31 | |
| SD within | 0.27 | 0.26 | 0.29 | 0.27 | 0.29 | 0.28 | 0.31 | 0.24 |
bpm indicates beats per minute; HRV, heart rate variability; N, number of epochs; n, number of participants; rMSSD, root mean square of the successive normal sinus to normal sinus intervals differences; SD1, Poincaré plot SD perpendicular the line of identity; SD2, Poincaré plot SD along the line of identity; T, average number of hours.
Association of Clinical Characteristics With Heart Rate and HRV Time Series
During the regular hemodialysis schedule (phases 1–4), prevalent cardiovascular disease and its risk factors were associated with faster heart rate and further depressed HRV (Figure 3 and Table S2). History of AF and use of β‐blockers were associated with slower heart rate.
Figure 3.
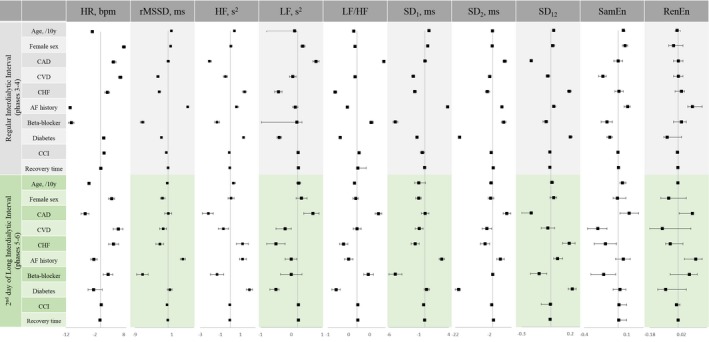
Association of demographic and clinical characteristics with heart rate and short‐term HRV in ARCH models, stratified by the type of dialytic cycle. Beta‐coefficient with 95% CI is shown. AF indicates atrial fibrillation; bpm, beats per minute; CAD, coronary artery disease; CCI, Charlson comorbidity index; CHF, congestive heart failure; CVD, cerebrovascular disease; HF, high‐frequency power; HR, heart rate; HRV, heart rate variability; LF, low‐frequency power; RenEn, Renyi entropy; rMDDS, root mean square of the successive normal sinus to normal sinus intervals differences; SamEn, sample entropy; SD 1, the SD of the Poincare plot cloud of points in the direction perpendicular to the line‐of‐identity; SD 12, SD 1/SD 2 ratio; SD 2, the SD of the cloud of points in the direction of the line‐of‐identity.
In contrast, during the second day without hemodialysis (phases 5–6), traditional cardiovascular risk factors were associated with slower heart rates. Female sex and prevalent CAD were associated with increased SD2 and LF power, suggesting greater sympathetic predominance. Interestingly, diabetes mellitus was associated with a smaller SD2 and LF power.
In our study, 4 participants (100% black; 2 males and 2 females) missed hemodialysis for >72 hours. They were CAD‐ and CHF‐free, with a high Charlson comorbidity index (6.8±1.2). During missed hemodialysis (phases 7–8), diabetes mellitus and longer perceived dialysis recovery time were associated with greater degree of HRV depression (Table S2 through S4).
Association of Heart Rate and HRV With Cardiac Arrhythmias
In adjusted analysis, significantly increased heart rate in the beginning of an hour (as compared with the preceding hour, by 11.2 [95% CI 10.1–12.3] beats per minute [P<0.0001]) was associated with paroxysmal VT events occurring at any time within the same hour. That association remained significant after further adjustment for the use of β‐blockers (+8.5 [95% CI 7.1–9.8] beats per minute; P<0.0001). There was no association of VT events with HRV. There were no associations of other types of observed arrhythmias with heart rate or HRV.
Circadian Rhythm in Heart Rate and HRV During Different Types of Dialytic Cycles
During the interdialytic period in the regular dialytic cycle (phases 3–4), heart rate and all HRV parameters demonstrated a significant circadian pattern—as expected (Figure 4 and Table S5). Short‐term HRV (rMSSD and SD1) peaked at night, whereas heart rate and SD12 peaked during the day.
Figure 4.
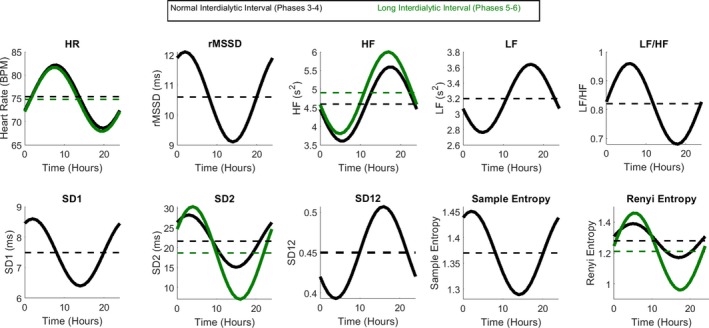
Circadian rhythm in heart rate and HRV during a 1‐day‐ (black) and 2‐day‐long (green) interdialytic interval. Mesor is shown as a dashed line. BPM indicates beats per minute; HF, high‐frequency power; HR, heart rate; HRV, heart rate variability; LF, low‐frequency power; rMDDS, root mean square of the successive normal sinus to normal sinus intervals differences; SD 1, the SD of the Poincare plot cloud of points in the direction perpendicular to the line‐of‐identity; SD 12, SD 1/SD 2 ratio; SD 2, the SD of the cloud of points in the direction of the line‐of‐identity.
In contrast, the second day of the long interdialytic interval (phases 5–6) abolished circadian rhythms, with few exceptions. Periodic changes in heart rate remained largely unchanged across all interdialytic periods, but its acrophase shifted to the afternoon in the ultralong (missed dialysis >72 hours) interdialytic interval (phases 7–8). In phases 5 to 6, the amplitude of circadian rhythm in Renyi entropy and SD2 increased, whereas circadian rhythm in other HRV metrics dissipated. During phases 7 to 8, we observed a distorted periodic pattern in short‐term HRV (rMSSD, SD1) with acrophase in the afternoon, whereas circadian rhythm in other HRV metrics remained eliminated.
Association of the Phases of Hemodialysis With Heart Rate and HRV Time‐Series
In fully adjusted ARCH models (Figure 5 and Table S6), phase 1 dialysis and postdialytic phase 2 were characterized by gradual improvement of parasympathetic tone (increasing rMSSD and SD1). During the regular interdialytic interval in every‐other‐day hemodialysis cycle (phases 3–4), we observed very few significant trends: a slight decrease in rMSSD and SD1, and the increase in SD2 and sample entropy. In contrast, during the second day of a 2‐day‐long interdialytic interval (phases 5–6), there were significant and meaningful changes in all HRV parameters: heart rate gradually increased, short‐term HRV (rMSSD, HF power, SD1) and SD12 decreased, whereas intermediate HRV (SD2, LF/HF ratio, Renyi entropy) increased, suggesting depressed parasympathetic and increased sympathetic influences. Missing dialysis for >72 hours was associated with a steady increase in SD12 ratio and HF power (Table S6).
Figure 5.
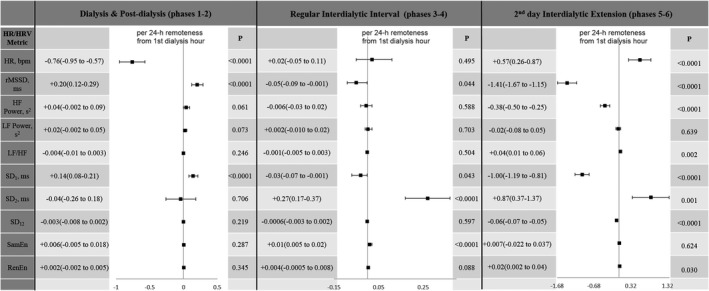
Association of the phase of dialysis with heart rate and HRV after adjustment for circadian rhythm and clinical characteristics. bpm indicates beats per minute; HF, high‐frequency power; HR, heart rate; HRV, heart rate variability; LF, low‐frequency power; RenEn, Renyi entropy; rMDDS, root mean square of the successive normal sinus to normal sinus intervals differences; SamEn, sample entropy; SD 1, the SD of the Poincaré plot cloud of points in the direction perpendicular to the line‐of‐identity; SD 12, SD 1/SD 2 ratio; SD 2, the SD of the cloud of points in the direction of the line‐of‐identity.
Sensitivity analysis, after exclusion of 3‐minute epochs that started in the second half of an hour and thus violated the equal intervals assumption, provided consistently similar results (Table S7). Further adjustment for changes in weight and sitting systolic and diastolic blood pressure before and after dialysis yielded similar results (Table S8).
Discussion
This continuous ECG‐monitoring study of incident in‐center hemodialysis patients revealed several important findings. First, we observed an association of the phases of dialysis with cardiac arrhythmias; specifically, NSVT events were more frequent during hemodialysis or within 6 hours posthemodialysis. In the time‐series analysis adjusted for cardiovascular disease and risk factors, we demonstrated a sharp increase in heart rate preceding NSVT events, suggesting a triggered VT mechanism,34 likely because of sympathetic activation. Second, we found that the hemodialysis schedule dramatically influenced the autonomic tone. An every‐other‐day dialysis schedule preserved physiological circadian rhythm in heart rate and HRV, whereas the extension of the interdialytic period for the second day abolished circadian rhythm and displayed a steady deterioration. The 2‐days‐off dialysis led to progressively decreasing parasympathetic tone.
Dialysis Phase and Paroxysmal Cardiac Arrhythmias During ECG Monitoring
SCD is more frequent after the long interdialytic interval, during hemodialysis, or within 6 to 12 hours after the end of a hemodialysis session.6, 7, 8 Our observation was consistent with implantable loop recorder studies35 that confirmed the same pattern: NSVT events were more frequent during and postdialysis, and the reasons could be multifactorial.10 We must emphasize that NSVT events are not equivalent to SCD. The predictive value of NSVT for SCD in ESKD remains unknown. NSVT is associated with SCD in many,36, 37, 38, 39 but not all40 populations.
Importantly, after rigorous adjustment for cardiovascular disease and associated risk factors, including use of β‐blockers, we observed that suddenly increased heart rate precedes subsequent NSVT events. Such clinical manifestation is typical for triggered VT, which can manifest as polymorphic VT (Figure 2). The triggered VT mechanism can potentially41, 42—at least partially—explain recent results of the ICD2 trial, which showed that prophylactic ICD therapy did not reduce the rate of SCD or all‐cause mortality in ESKD patients.43
Use of β‐blockers44 can be a potential preventive intervention in such patients.3 Of note, we observed an attenuated association of heart rate with VT events even after adjustment for the use of β‐blockers, in spite of 83% of participants taking β‐blockers, which highlights the possible importance of appropriate β‐blocker dose titration.
Dynamic Changes in Autonomic Tone in the Dialytic Cycle
Despite multiple studies reporting unfavorable effects of the long 2‐day interdialytic interval6, 7, 8, in‐center hemodialysis is typically prescribed 3 times per week. Our study is the first longitudinal study of heart rate and HRV time‐series across multiple hemodialysis sessions, allowing direct paired comparison of the effect of the 2‐day interdialytic interval. Our findings add to the growing evidence of the harmful consequences of a prolonged interdialytic interval. While every‐other‐day hemodialysis preserved relatively normal cardiovascular autonomic tone, a second day without hemodialysis was characterized by parasympathetic withdrawal and a steady increase in sympathetic predominance, which may explain the previously observed increased rate of SCD after the long interdialytic interval.6, 7, 8 Mounting evidence of the harmful effect of the 2‐day interval without hemodialysis suggests that more frequent45, 46 dialysis should be considered the preferred prescribed treatment schedule. Whether more frequent hemodialysis attenuates arrhythmic propensity remains to be determined.
HRV in ESKD Patients on Hemodialysis, and Effect of Missing Dialysis
In addition to the work in previous implantable loop recorder studies—which similarly detected arrhythmias—we also analyzed time‐series of heart rate and HRV, which allowed us to shed light on the underlying mechanisms. HRV reflects a dynamic bidirectional interaction between the heart and the respiratory system, regulated by the autonomic nervous system14 and estimating sympathovagal balance.47, 48 Our study highlights the unique features of the HRV‐manifestation of autonomic imbalance in ESKD patients presenting with bradycardia because of direct negative chronotropic and dromotropic effects of hyperkalemia, hypocalcemia, and possible uremic toxins. Autonomic imbalance can diminish the cholinergic anti‐inflammatory pathway, leading to an exaggerated cytokine response and inflammation49. Similar to other studies,50 we observed that heart rate increases during and immediately after hemodialysis.51 In our study participants, autonomic imbalance was manifested largely by parasympathetic withdrawal. Unlike in CHF studies,52, 53 we observed LF/HF ratio <1, likely because of impaired baroreflex sensitivity, or a blunted sympathetic response in our study participants. Moak et al13 showed that LF power reflects baroreflex‐mediated changes in cardiovagal and sympathetic noradrenergic outflows. In the case of baroreflex failure, LF power is reduced, regardless of the status of cardiac sympathetic innervation. Normally, acute removal of fluid during a hemodialysis session activates the baroreflex. Several groups of investigators54, 55, 56, 57 observed worse clinical outcomes in ESKD patients with a blunted sympathetic response and a low (<1) LF/HF power ratio, which was characteristic of our patient population. Blunted sympathetic response on volume removal can be genetically determined by the polymorphism in the gene for angiotensin‐converting enzyme.50 Inadequate baroreflex sensitivity can cause intradialytic hypotension,58 a well‐known risk marker of adverse clinical outcomes in ESKD.
In participants who missed 1 to 2 hemodialysis sessions during ECG monitoring, as expected, heart rate further slowed, HRV progressively diminished, and circadian rhythm in HRV remained vanished or distorted—all because of deepened autonomic imbalance. Of note, patients who missed hemodialysis were characterized by prominent bradycardia. Several recent studies reported bradyarrhythmias during monitoring,5, 9, 35, 59 but did not comment (or possibly did not have available data) on patients’ adherence to treatment. Improvement of patient adherence—avoiding missing hemodialysis sessions—can improve patient outcomes.
Circadian Rhythm in Heart Rate and HRV
Our finding of disrupted circadian rhythm aligns with previously reported autonomic dysregulation during the normal wake/sleep cycle in ESKD.60 Two mechanisms61 are responsible for circadian rhythm in heart rate: (1) central circadian clock in the suprachiasmatic nucleus in the hypothalamus, acting via the autonomic nervous system, and (2) a local circadian clock in the heart itself. In our study participants, the circadian rhythm in heart rate remained relatively preserved during the prolonged interdialytic interval, whereas the circadian rhythm in HRV was largely abolished. Changes in the acrophase of the circadian rhythm in heart rate during the ultralong interdialytic (missed hemodialysis) interval—peaking in the afternoon instead of morning hours—may reflect a switch in the circadian clock from central to local regulatory mechanisms. A better understanding of circadian rhythms in heart rate and HRV may help to improve medical management and clinical outcomes in ESKD patients on hemodialysis. Chan et al45 studied the effect of daily dialysis versus every‐other‐day dialysis and found that daily dialysis improved vagal modulation of the heart and increased short‐term HRV, which is consistent with our study findings. Further studies of the interplay between circadian rhythm and dialytic cycle are needed to develop an optimal treatment schedule for ESKD patients.
Limitations
The strengths of this study arise from the continuous ECG monitoring, providing an opportunity for our study—with multiple 24‐hour and dialytic cycles for the same participants—to significantly improve robustness and reduce error in estimations. Other strengths include the use of advanced statistical modeling of time‐series (ARCH/GARCH) and appropriate use of explicit periodic regression.
However, limitations of the study should be considered. Use of a single‐lead ECG poses objective challenges for discrimination of supraventricular arrhythmia from normal sinus rhythm. Everyday physical activity manifests through noise and artifacts. To ensure analysis of normal sinus rhythm, we implemented rigorous quality control procedures, which included semi‐automated analysis of the data, and manual review of ECGs. To improve the quality of included ECG data, we elected to study 3‐minute epochs of continuously normal (uninterrupted) clean sinus rhythm.
Impaired baroreflex sensitivity can be measured by heart rate turbulence, which was not measured in this study. Further study of heart rate turbulence in ESKD patients on dialysis is needed. Results of frequency‐domain HRV should be considered with caution. We measured HRV on each 3‐minute segment, which at least partially explains the differences between HF and LF power reported in our study as compared with the 24‐hour power spectrum analyses.52, 53 To overcome limitations of isolated HRV metrics, we evaluated several HRV measures presumably reflecting parasympathetic tone (rMSSD, SD1, HF power), and sympathovagal balance (SD2, SD12, LF/HF ratio). Consistent findings across the full set of HRV metrics increase the validity of our results.
The size of this study is relatively small, and participants were predominantly black, which limits generalizability. Nevertheless, these were participants with known structural heart disease and in‐depth data for a prolonged period of time to compare using within‐person comparison. On average, the study participant had 156±55 hours of data, providing satisfactory power for the analyses of heart rate and short‐term HRV (rMSSD, SD1) using the periodic regression and time‐series analysis, in all stratified time intervals. However, the statistical powers of the stratified ARCH analyses of LF/HF power, SD12, and entropy measures were not sufficient in phases 3 to 4 and 5 to 6. Thus, some of the nonsignificant findings might be because of low statistical power.
The assumption of equal intervals between 3‐minute epochs was violated for ≈10% of epochs. To address this limitation, sensitivity analyses were performed and epochs that started in the second half of an hour were excluded from ARCH models, which did not change the association of dialytic cycle with HRV time‐series. Only 4 study participants missed hemodialysis, and therefore, results of this subgroup analysis should be considered with caution. The reason for the missed hemodialysis sessions was unknown.
Clinical Implications
In summary, this prospective continuous ECG‐monitoring study showed that cardiac arrhythmias are common in hemodialysis patients. In time‐series analysis, paroxysms of VT are preceded by faster (than in a previous hour) heart rate, suggesting a triggered mechanism of VT. Every‐other‐day hemodialysis preserves physiological circadian rhythm in heart rate and HRV, while the prolonged interdialytic interval abolishes the circadian rhythm and displays a steadily worsening autonomic imbalance. The armamentarium of devices capable of continuously monitoring ECG is fast‐growing. Further studies are needed to develop an individualized prediction of cardiac arrhythmias based on analyses of heart rate and HRV time‐series.
Sources of Funding
The PACE Study was supported by the NIH grant R01DK072367 (to Parekh). This study was supported in part by the NIH grant HL118277 (to Tereshchenko).
Disclosures
None.
Supporting information
Table S1. Comparison of Heart Rate and HRV Post‐Dialysis in a Regular Dialytic (every other day) Cycle, After 2‐Day‐Long Interdialytic Interval, and After Missed Dialysis for More Than 72 h
Table S2. Association of Demographic and Clinical Characteristics With Heart Rate and Short‐Term HRV in ARCH Models, Stratified by the Type of Dialytic Cycle
Table S3. Association of Demographic and Clinical Characteristics With Intermediate HRV in ARCH Models, Stratified by the Type of Dialytic Cycle
Table S4. Association of Demographic and Clinical Characteristics With Entropy in ARCH Models, Stratified by the Type of Dialytic Cycle
Table S5. Comparison of Circadian Rhythm in Heart Rate and HRV During Different Interdialytic Phases
Table S6. Association of Phase of Dialysis With Heart Rate and HRV After Adjustment for Circadian Rhythm, Clinical Characteristics
Table S7. Sensitivity Analysis After Exclusion of Epochs Starting in the Second Half of an Hour: Association of the Dialytic Cycle With HRV
Table S8. Sensitivity Analysis Adjusted for Weight and Blood Pressure Change: Association of the Dialytic Cycle With HRV (N=7). All 7 Participants Were on Beta‐Blockers
(J Am Heart Assoc. 2019;8:e013748 DOI: 10.1161/JAHA.119.013748.)
References
- 1. United States Renal Data System. 2018. USRDS annual data report: Epidemiology of kidney disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2018.
- 2. Tereshchenko LG, Kim ED, Oehler A, Meoni LA, Ghafoori E, Rami T, Maly M, Kabir M, Hawkins L, Tomaselli GF, Lima JA, Jaar BG, Sozio SM, Estrella M, Kao WH, Parekh RS. Electrophysiologic substrate and risk of mortality in incident hemodialysis. J Am Soc Nephrol. 2016;27:3413–3420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sacher F, Jesel L, Borni‐Duval C, De Precigout V, Lavainne F, Bourdenx JP, Haddj‐Elmrabet A, Seigneuric B, Keller A, Ott J, Savel H, Delmas Y, Bazin‐Kara D, Klotz N, Ploux S, Buffler S, Ritter P, Rondeau V, Bordachar P, Martin C, Deplagne A, Reuter S, Haissaguerre M, Gourraud JB, Vigneau C, Mabo P, Maury P, Hannedouche T, Benard A, Combe C. Cardiac rhythm disturbances in hemodialysis patients: early detection using an implantable loop recorder and correlation with biological and dialysis parameters. JACC Clin Electrophysiol. 2018;4:397–408. [DOI] [PubMed] [Google Scholar]
- 4. Makar MS, Pun PH. Sudden cardiac death among hemodialysis patients. Am J Kidney Dis. 2017;69:684–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Turakhia MP, Blankestijn PJ, Carrero JJ, Clase CM, Deo R, Herzog CA, Kasner SE, Passman RS, Pecoits‐Filho R, Reinecke H, Shroff GR, Zareba W, Cheung M, Wheeler DC, Winkelmayer WC, Wanner C, Conference Participation. Chronic kidney disease and arrhythmias: conclusions from a Kidney Disease: improving Global Outcomes (KDIGO) Controversies Conference. Eur Heart J. 2018;39:2314–2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bleyer AJ, Hartman J, Brannon PC, Reeves‐Daniel A, Satko SG, Russell G. Characteristics of sudden death in hemodialysis patients. Kidney Int. 2006;69:2268–2273. [DOI] [PubMed] [Google Scholar]
- 7. Foley RN, Gilbertson DT, Murray T, Collins AJ. Long interdialytic interval and mortality among patients receiving hemodialysis. N Engl J Med. 2011;365:1099–1107. [DOI] [PubMed] [Google Scholar]
- 8. Perl J, Chan CT. Timing of sudden death relative to the hemodialysis procedure. Nat Clin Pract Nephrol. 2006;2:668–669. [DOI] [PubMed] [Google Scholar]
- 9. Wong MC, Kalman JM, Pedagogos E, Toussaint N, Vohra JK, Sparks PB, Sanders P, Kistler PM, Halloran K, Lee G, Joseph SA, Morton JB. Temporal distribution of arrhythmic events in chronic kidney disease: highest incidence in the long interdialytic period. Heart Rhythm. 2015;12:2047–2055. [DOI] [PubMed] [Google Scholar]
- 10. Tereshchenko LG, Posnack NG. Does plastic chemical exposure contribute to sudden death of patients on dialysis? Heart Rhythm. 2019;16:312–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Burton JO, Jefferies HJ, Selby NM, McIntyre CW. Hemodialysis‐induced cardiac injury: determinants and associated outcomes. Clin J Am Soc Nephrol. 2009;4:914–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zipes DP, Wellens HJ. Sudden cardiac death. Circulation. 1998;98:2334–2351. [DOI] [PubMed] [Google Scholar]
- 13. Moak JP, Goldstein DS, Eldadah BA, Saleem A, Holmes C, Pechnik S, Sharabi Y. Supine low‐frequency power of heart rate variability reflects baroreflex function, not cardiac sympathetic innervation. Heart Rhythm. 2007;4:1523–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Saul JP, Berger RD, Albrecht P, Stein SP, Chen MH, Cohen RJ. Transfer function analysis of the circulation: unique insights into cardiovascular regulation. Am J Physiol. 1991;261:H1231–H1245. [DOI] [PubMed] [Google Scholar]
- 15. Secemsky EA, Verrier RL, Cooke G, Ghossein C, Subacius H, Manuchehry A, Herzog CA, Passman R. High prevalence of cardiac autonomic dysfunction and T‐wave alternans in dialysis patients. Heart Rhythm. 2011;8:592–598. [DOI] [PubMed] [Google Scholar]
- 16. Fukuta H, Hayano J, Ishihara S, Sakata S, Mukai S, Ohte N, Ojika K, Yagi K, Matsumoto H, Sohmiya S, Kimura G. Prognostic value of heart rate variability in patients with end‐stage renal disease on chronic haemodialysis. Nephrol Dial Transplant. 2003;18:318–325. [DOI] [PubMed] [Google Scholar]
- 17. Oikawa K, Ishihara R, Maeda T, Yamaguchi K, Koike A, Kawaguchi H, Tabata Y, Murotani N, Itoh H. Prognostic value of heart rate variability in patients with renal failure on hemodialysis. Int J Cardiol. 2009;131:370–377. [DOI] [PubMed] [Google Scholar]
- 18. Suzuki M, Hiroshi T, Aoyama T, Tanaka M, Ishii H, Kisohara M, Iizuka N, Murohara T, Hayano J. Nonlinear measures of heart rate variability and mortality risk in hemodialysis patients. Clin J Am Soc Nephrol. 2012;7:1454–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Parekh RS, Meoni LA, Jaar BG, Sozio SM, Shafi T, Tomaselli GF, Lima JA, Tereshchenko LG, Estrella MM, Kao WH. Rationale and design for the Predictors of Arrhythmic and Cardiovascular Risk in End Stage Renal Disease (PACE) study. BMC Nephrol. 2015;16:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tereshchenko Larisa G. PACE deidentified ECGpatch HRV dataset 28pts. Available at: https://github.com/Tereshchenkolab/HRV: GitHub; August 13, 2019. Accessed August 13, 2019.
- 21. Steinberg JS, Varma N, Cygankiewicz I, Aziz P, Balsam P, Baranchuk A, Cantillon DJ, Dilaveris P, Dubner SJ, El‐Sherif N, Krol J, Kurpesa M, La Rovere MT, Lobodzinski SS, Locati ET, Mittal S, Olshansky B, Piotrowicz E, Saxon L, Stone PH, Tereshchenko L, Turitto G, Wimmer NJ, Verrier RL, Zareba W, Piotrowicz R. 2017 ISHNE‐HRS expert consensus statement on ambulatory ECG and external cardiac monitoring/telemetry. Heart Rhythm. 2017;14:e55–e96. [DOI] [PubMed] [Google Scholar]
- 22. Conen D, Adam M, Roche F, Barthelemy J‐C, Dietrich DF, Imboden M, Künzli N, Eckardstein Av, Regenass S, Hornemann T, Rochat T, Gaspoz J‐M, Probst‐Hensch N, Carballo D. Premature atrial contractions in the general population. Circulation. 2012;126:2302–2308. [DOI] [PubMed] [Google Scholar]
- 23. Pahlm O, Sörnmo L. Software QRS detection in ambulatory monitoring—a review. Med Biol Eng Compu. 1984;22:289–297. [DOI] [PubMed] [Google Scholar]
- 24. Pan J, Tompkins W. A real‐time QRS detection algorithm. IEEE Transac Bio‐Med Eng. 1985;32:230–236. [DOI] [PubMed] [Google Scholar]
- 25. Castells F, Laguna P, Sörnmo L, Bollmann A, Roig JM. Principal component analysis in ECG signal processing. EURASIP J Adv Signal Process. 2007;2007:074580. [Google Scholar]
- 26. Manriquez AI, Zhang Q. An algorithm for QRS onset and offset detection in single lead electrocardiogram records. Conf Proc IEEE Eng Med Biol Soc. 2007;2007:541–544. [DOI] [PubMed] [Google Scholar]
- 27. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- 28. Sassi R, Cerutti S, Lombardi F, Malik M, Huikuri HV, Peng CK, Schmidt G, Yamamoto Y. Advances in heart rate variability signal analysis: joint position statement by the e‐Cardiology ESC Working Group and the European Heart Rhythm Association co‐endorsed by the Asia Pacific Heart Rhythm Society. Europace. 2015;17:1341–1353. [DOI] [PubMed] [Google Scholar]
- 29. Cornforth DJ, Tarvainen MP, Jelinek HF. How to calculate Renyi entropy from heart rate variability, and why it matters for detecting cardiac autonomic neuropathy. Front Bioeng Biotechnol. 2014;2:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Cornforth DJ, Tarvainen MP, Jelinek HF. Using Renyi entropy to detect early cardiac autonomic neuropathy. Conf Proc IEEE Eng Med Biol Soc. 2013;2013:5562–5565. [DOI] [PubMed] [Google Scholar]
- 31. Rhee CM, Chou JA, Kalantar‐Zadeh K. Dialysis prescription and sudden death. Semin Nephrol. 2018;38:570–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Higgins ML, Bera A. A class of nonlinear ARCH models. Int Econ Rev. 1992;33:137–158. [Google Scholar]
- 33. Weise F, London GM, Pannier BM, Guerin AP, Elghozi JL. Effect of hemodialysis on cardiovascular rhythms in end‐stage renal failure. Kidney Int. 1995;47:1443–1452. [DOI] [PubMed] [Google Scholar]
- 34. Josephson ME. Clinical cardiac electrophysiology : techniques and interpretations, 4th ed Philadelphia, PA: Wolters Kluwer/Lippincott Williams & Wilkins, Health; 2008. [Google Scholar]
- 35. Roy‐Chaudhury P, Tumlin JA, Koplan BA, Costea AI, Kher V, Williamson D, Pokhariyal S, Charytan DM, Williamson D, Roy‐Chaudhury P, Tumlin J, Kher V, Reddy V, Prakash KC, Charytan D, Tiwari SC, Pokhariyal S, Podoll A, Jasuja S, Walters GL, Wangsnes K, Costea A, Tombul S, Singh B, Mishra B, Yalagudri S, Shelke A, Narasimhan C, Karthigesan AM, Oomman A, Kumar KPP, Koplan B, Kaul U, Ghose T, Gupta R, Sethi A, Kumar N, Hariharan R, Sardana R, Wahab A, Khanna NN, Smith M, Kamath S, Galphin C, Sodhi P, Chakravarthy R, Budithi SR, McCausland F, Gulati S, Dijoo M, Singh U, Jain S, Saxena V, Sagar G, Charytan D, Fissell R, Foley R, Herzog CA, McCullough P, Rogers JD, Tumlin JA, Zimetbaum P, Assar M, Kremers M, Winkelmayer WC. Primary outcomes of the Monitoring in Dialysis Study indicate that clinically significant arrhythmias are common in hemodialysis patients and related to dialytic cycle. Kidney Int. 2018;93:941–951. [DOI] [PubMed] [Google Scholar]
- 36. Wang W, Lian Z, Rowin EJ, Maron BJ, Maron MS, Link MS. Prognostic implications of nonsustained ventricular tachycardia in high‐risk patients with hypertrophic cardiomyopathy. Circ Arrhythm Electrophysiol. 2017;10:e004604. [DOI] [PubMed] [Google Scholar]
- 37. Priori SG, Napolitano C, Memmi M, Colombi B, Drago F, Gasparini M, DeSimone L, Coltorti F, Bloise R, Keegan R, Filho FESC, Vignati G, Benatar A, DeLogu A. Clinical and molecular characterization of patients with catecholaminergic polymorphic ventricular tachycardia. Circulation. 2002;106:69–74. [DOI] [PubMed] [Google Scholar]
- 38. Cadrin‐Tourigny J, Bosman LP, Nozza A, Wang W, Tadros R, Bhonsale A, Bourfiss M, Fortier A, Lie OH, Saguner AM, Svensson A, Andorin A, Tichnell C, Murray B, Zeppenfeld K, van den Berg MP, Asselbergs FW, Wilde AAM, Krahn AD, Talajic M, Rivard L, Chelko S, Zimmerman SL, Kamel IR, Crosson JE, Judge DP, Yap SC, van der Heijden JF, Tandri H, Jongbloed JDH, Guertin MC, van Tintelen JP, Platonov PG, Duru F, Haugaa KH, Khairy P, Hauer RNW, Calkins H, Te Riele A, James CA. A new prediction model for ventricular arrhythmias in arrhythmogenic right ventricular cardiomyopathy. Eur Heart J. 2019;40:1850–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Scirica BM, Braunwald E, Belardinelli L, Hedgepeth CM, Spinar J, Wang W, Qin J, Karwatowska‐Prokopczuk E, Verheugt FW, Morrow DA. Relationship between nonsustained ventricular tachycardia after non‐ST‐elevation acute coronary syndrome and sudden cardiac death: observations from the metabolic efficiency with ranolazine for less ischemia in non‐ST‐elevation acute coronary syndrome‐thrombolysis in myocardial infarction 36 (MERLIN‐TIMI 36) randomized controlled trial. Circulation. 2010;122:455–462. [DOI] [PubMed] [Google Scholar]
- 40. Teuwen CP, Ramdjan TT, Gotte M, Brundel BJ, Evertz R, Vriend JW, Molhoek SG, Reinhart Dorman HG, van Opstal JM, Konings TC, van der Voort P, Delacretaz E, Wolfhagen NJ, van Gastel V, de Klerk P, Theuns DA, Witsenburg M, Roos‐Hesselink JW, Triedman JK, Bogers AJ, de Groot NM. Non‐sustained ventricular tachycardia in patients with congenital heart disease: an important sign? Int J Cardiol. 2016;206:158–163. [DOI] [PubMed] [Google Scholar]
- 41. Miyake CY, Webster G, Czosek RJ, Kantoch MJ, Dubin AM, Avasarala K, Atallah J. Efficacy of implantable cardioverter defibrillators in young patients with catecholaminergic polymorphic ventricular tachycardia: success depends on substrate. Circ Arrhythm Electrophysiol. 2013;6:579–587. [DOI] [PubMed] [Google Scholar]
- 42. Roston TM, Vinocur JM, Maginot KR, Mohammed S, Salerno JC, Etheridge SP, Cohen M, Hamilton RM, Pflaumer A, Kanter RJ, Potts JE, LaPage MJ, Collins KK, Gebauer RA, Temple JD, Batra AS, Erickson C, Miszczak‐Knecht M, Kubuš P, Bar‐Cohen Y, Kantoch M, Thomas VC, Hessling G, Anderson C, Young M‐L, Cabrera Ortega M, Lau YR, Johnsrude CL, Fournier A, Kannankeril PJ, Sanatani S. Catecholaminergic polymorphic ventricular tachycardia in children: analysis of therapeutic strategies and outcomes from an international multicenter registry. Circ Arrhyth Electrophysiol. 2015;8:633–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jukema JW, Timal RJ, Rotmans JI, Hensen LCR, Buiten MS, de Bie MK, Putter H, Zwinderman AH, van Erven L, Krol‐van Straaten MJ, Hommes N, Gabreels B, van Dorp W, van Dam B, Herzog CA, Schalij MJ, Rabelink TJ. Prophylactic use of implantable cardioverter‐defibrillators in the prevention of sudden cardiac death in dialysis patients. Circulation. 2019;139:2628–2638. [DOI] [PubMed] [Google Scholar]
- 44. Tory K, Horvath E, Suveges Z, Fekete A, Sallay P, Berta K, Szabo T, Szabo AJ, Tulassay T, Reusz GS. Effect of propranolol on heart rate variability in patients with end‐stage renal disease: a double‐blind, placebo‐controlled, randomized crossover pilot trial. Clin Nephrol. 2004;61:316–323. [DOI] [PubMed] [Google Scholar]
- 45. Chan CT, Chertow GM, Daugirdas JT, Greene TH, Kotanko P, Larive B, Pierratos A, Stokes JB. Effects of daily hemodialysis on heart rate variability: results from the Frequent Hemodialysis Network (FHN) Daily Trial. Nephrol Dial Transplant. 2014;29:168–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gul A, Miskulin DC, Harford A, Zager P. In‐center hemodialysis: time for a paradigm shift. J Am Soc Nephrol. 2018;29:2452–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sedaghat G, Gardner RT, Kabir MM, Ghafoori E, Habecker BA, Tereshchenko LG. Correlation between the high‐frequency content of the QRS on murine surface electrocardiogram and the sympathetic nerves density in left ventricle after myocardial infarction: experimental study. J Electrocardiol. 2017;50:323–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Anrep G, Pascual W, Rössler R. Respiratory variations of the heart rate‐II—the central mechanism of the respiratory arrhythmia and the inter‐relations between the central and the reflex mechanisms. Proc R Soc London B Biol Sci. 1936;119:218–230. [Google Scholar]
- 49. Seibert E, Zohles K, Ulrich C, Kluttig A, Nuding S, Kors JA, Swenne CA, Werdan K, Fiedler R, Girndt M. Association between autonomic nervous dysfunction and cellular inflammation in end‐stage renal disease. BMC Cardiovasc Disord. 2016;16:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ribas Ribeiro L, Flores de Oliveira J, Bueno Orcy R, Castilho Barros C, Dame Hense J, Santos F, Irigoyen MC, Gonzalez MC, Oses JP, Bohlke M. Exploring the complexity: the interplay between the angiotensin‐converting enzyme insertion/deletion polymorphism and the sympathetic response to hemodialysis. Am J Physiol Heart Circ Physiol. 2018;315:H1002–H1011. [DOI] [PubMed] [Google Scholar]
- 51. Waks JW, Tereshchenko LG, Parekh RS. Electrocardiographic predictors of mortality and sudden cardiac death in patients with end stage renal disease on hemodialysis. J Electrocardiol. 2016;49:848–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Stein PK, Tereshchenko L, Domitrovich PP, Kleiger RE, Perez A, Deedwania P. Diastolic dysfunction and autonomic abnormalities in patients with systolic heart failure. Eur J Heart Fail. 2007;9:364–369. [DOI] [PubMed] [Google Scholar]
- 53. Kleiger RE, Stein PK, Bigger JT Jr. Heart rate variability: measurement and clinical utility. Ann Noninvasive Electrocardiol. 2005;10:88–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Chou YH, Huang WL, Chang CH, Yang CCH, Kuo TBJ, Lin SL, Chiang WC, Chu TS. Heart rate variability as a predictor of rapid renal function deterioration in chronic kidney disease patients. Nephrology. 2019;24:806–813. [DOI] [PubMed] [Google Scholar]
- 55. Pei J, Tang W, Li LX, Su CY, Wang T. Heart rate variability predicts mortality in peritoneal dialysis patients. Ren Fail. 2015;37:1132–1137. [DOI] [PubMed] [Google Scholar]
- 56. Huang J‐C, Chen C‐F, Chang C‐C, Chen S‐C, Hsieh M‐C, Hsieh Y‐P, Chen H‐C. Effects of stroke on changes in heart rate variability during hemodialysis. BMC Nephrol. 2017;18:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Poulikakos D, Hnatkova K, Banerjee D, Malik M. Association of QRS‐T angle and heart rate variability with major cardiac events and mortality in hemodialysis patients. Ann Noninvasive Electrocardiol. 2018;23:e12570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Park S, Kim WJ, Cho NJ, Choi CY, Heo NH, Gil HW, Lee EY. Predicting intradialytic hypotension using heart rate variability. Sci Rep. 2019;9:2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wong MCG, Kalman JM, Pedagogos E, Toussaint N, Vohra JK, Sparks PB, Sanders P, Kistler PM, Halloran K, Lee G, Joseph SA, Morton JB. Bradycardia and asystole is the predominant mechanism of sudden cardiac death in patients with chronic kidney disease. J Am Coll Cardiol. 2015;65:1263–1265. [DOI] [PubMed] [Google Scholar]
- 60. Roumelioti ME, Ranpuria R, Hall M, Hotchkiss JR, Chan CT, Unruh ML, Argyropoulos C. Abnormal nocturnal heart rate variability response among chronic kidney disease and dialysis patients during wakefulness and sleep. Nephrol Dial Transplant. 2010;25:3733–3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Black N, D'Souza A, Wang Y, Piggins H, Dobrzynski H, Morris G, Boyett MR. Circadian rhythm of cardiac electrophysiology, arrhythmogenesis, and the underlying mechanisms. Heart Rhythm. 2019;16:298–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Comparison of Heart Rate and HRV Post‐Dialysis in a Regular Dialytic (every other day) Cycle, After 2‐Day‐Long Interdialytic Interval, and After Missed Dialysis for More Than 72 h
Table S2. Association of Demographic and Clinical Characteristics With Heart Rate and Short‐Term HRV in ARCH Models, Stratified by the Type of Dialytic Cycle
Table S3. Association of Demographic and Clinical Characteristics With Intermediate HRV in ARCH Models, Stratified by the Type of Dialytic Cycle
Table S4. Association of Demographic and Clinical Characteristics With Entropy in ARCH Models, Stratified by the Type of Dialytic Cycle
Table S5. Comparison of Circadian Rhythm in Heart Rate and HRV During Different Interdialytic Phases
Table S6. Association of Phase of Dialysis With Heart Rate and HRV After Adjustment for Circadian Rhythm, Clinical Characteristics
Table S7. Sensitivity Analysis After Exclusion of Epochs Starting in the Second Half of an Hour: Association of the Dialytic Cycle With HRV
Table S8. Sensitivity Analysis Adjusted for Weight and Blood Pressure Change: Association of the Dialytic Cycle With HRV (N=7). All 7 Participants Were on Beta‐Blockers


