Abstract
Background
Whether marine omega‐3 supplementation is associated with reduction in risk of cardiovascular disease (CVD) remains controversial.
Methods and Results
This meta‐analysis included study‐level data from 13 trials. The outcomes of interest included myocardial infarction, coronary heart disease (CHD) death, total CHD, total stroke, CVD death, total CVD, and major vascular events. The unadjusted rate ratios were calculated using a fixed‐effect meta‐analysis. A meta‐regression was conducted to estimate the dose–response relationship between marine omega‐3 dosage and risk of each prespecified outcome. During a mean treatment duration of 5.0 years, 3838 myocardial infarctions, 3008 CHD deaths, 8435 total CHD events, 2683 strokes, 5017 CVD deaths, 15 759 total CVD events, and 16 478 major vascular events were documented. In the analysis excluding REDUCE‐IT (Reduction of Cardiovascular Events with Icosapent Ethyl‐Intervention Trial), marine omega‐3 supplementation was associated with significantly lower risk of myocardial infarction (rate ratio [RR] [95% CI]: 0.92 [0.86, 0.99]; P=0.020), CHD death (RR [95% CI]: 0.92 [0.86, 0.98]; P=0.014), total CHD (RR [95% CI]: 0.95 [0.91, 0.99]; P=0.008), CVD death (RR [95% CI]: 0.93 [0.88, 0.99]; P=0.013), and total CVD (RR [95% CI]: 0.97 [0.94, 0.99]; P=0.015). Inverse associations for all outcomes were strengthened after including REDUCE‐IT while introducing statistically significant heterogeneity. Statistically significant linear dose–response relationships were found for total CVD and major vascular events in the analyses with and without including REDUCE‐IT.
Conclusions
Marine omega‐3 supplementation lowers risk for myocardial infarction, CHD death, total CHD, CVD death, and total CVD, even after exclusion of REDUCE‐IT. Risk reductions appeared to be linearly related to marine omega‐3 dose.
Keywords: cardiovascular diseases, fish oil, marine omega‐3 supplementation, meta‐analysis, randomized controlled trials
Subject Categories: Cardiovascular Disease, Diet and Nutrition, Epidemiology
Clinical Perspective
What Is New?
We updated previous meta‐analyses by adding 3 recent large randomized controlled clinical trials, increasing sample size by 64%.
Marine omega‐3 supplementation significantly lowered risk for most cardiovascular end points, even after excluding a trial testing very high‐dose supplementation.
Risk reductions were linearly associated with dose of marine omega‐3 supplementation.
What Are the Clinical Implications?
Daily marine omega‐3 supplementation is effective in lowering risk for coronary and most other cardiovascular end points, including myocardial infarction, coronary heart disease death, total coronary heart disease, cardiovascular disease death, and total cardiovascular disease; no benefits, however, were found for stroke.
Greater cardiovascular benefits may be achieved at higher doses of marine omega‐ 3 supplementation.
Introduction
Whether marine or long‐chain omega‐3 fatty acid supplementation has significant benefits in reducing risk of cardiovascular disease (CVD) is the subject of intense debate. Despite consistent findings from observational studies showing inverse associations between higher fish consumption and lower risk of heart disease,1, 2 recent evidence from randomized controlled trials (RCTs) testing marine omega‐3 supplementation, usually a moderate‐dose combination of eicosapentaenoic acid (EPA) and docosahexaenoic acid compared with placebo, have had largely null results.3, 4 Although the American Heart Association continues to recommend marine omega‐3 supplementation for patients with prevalent coronary heart disease to reduce mortality, it found insufficient evidence for use in prevention among patients at high CVD risk but without CVD.5 A recent meta‐analysis synthesizing study‐level data from 10 midsize‐to‐large RCTs with at least 1 year of follow‐up reported no significant favorable effects of marine omega‐3 fatty acid supplementation on fatal or nonfatal coronary heart disease (CHD) or any major vascular events.6 Another expanded meta‐analysis including smaller trials and dietary intervention trials reached the same conclusions.7 Inconsistent findings between observational studies and RCTs cast doubt on a causal relationship between fish oil supplements and CVD prevention.8
Against this backdrop, results from 3 recently published large RCTs have further fueled the debate. In ASCEND (A Study of Cardiovascular Events in Diabetes), which included 15 480 diabetes mellitus patients without existing CVD at baseline, marine omega‐3 supplementation did not reduce the primary end point of serious vascular events.9 The VITAL (Vitamin D and Omega‐3 Trial), which included 25 871 participants at “usual” risk of CVD from the general population, also did not find a statistically significant reduction in the primary end point of major CVD events.10 However, both ASCEND and VITAL found reductions in at least 1 prespecified secondary end point (vascular deaths in ASCEND and myocardial infarction [MI] in VITAL). The REDUCE‐IT (Reduction of Cardiovascular Events with Icosapent Ethyl‐Intervention Trial), in contrast, observed significant protective effects of icosapent ethyl, a highly purified and stable EPA ethyl ester, against occurrence of all fatal or nonfatal cardiovascular events among patients with established CVD or risk factors.11 Incorporating new data from these 3 recent large RCTs, with and without inclusion of REDUCE‐IT, is important to provide the most up‐to‐date evidence. Also, we explored dose–response relationships between marine omega‐3 supplementation and CVD risks, an important subject that has not been addressed by previous meta‐analyses.
Methods
The authors declare that all supporting data are available within the article and its online supplementary files.
Search Strategy
We performed an updated meta‐analysis of RCTs based on the published data of a previous study‐level meta‐analysis6 by incorporating data from the ASCEND, VITAL, and REDUCE‐IT. All 3 additional studies met inclusion criteria of RCTs using marine omega‐3 fatty acids supplementation versus placebo or open label control, with a sample size of at least 500 participants and a follow‐up duration ≥1 year. Study‐level data from these 3 studies were extracted from published results.
The end points of interest included MI (fatal and/or nonfatal MI), death from CHD, total CHD (MI, death from CHD, or coronary revascularization), total stroke (fatal and/or nonfatal stroke), death from CVD, total CVD (nonfatal MI, nonfatal stroke, death from CVD, or hospitalization because of a cardiovascular cause), and major vascular events (nonfatal MI, nonfatal stroke, any revascularization, or death from CVD). All the participants included in the current analysis provided written informed consent.
Statistical Analyses
For calculating the pooled rate ratio (RR) and 95% CIs, we constructed 2×2 contingency tables for each trial. The pooled RR, 95% CI, and P value for heterogeneity were calculated using a fixed‐effect model using the Mantel‐Haenszel method. Because REDUCE‐IT used a significantly higher dose (4000 mg/d) of marine omega‐3 supplements than all other trials, which might introduce substantial heterogeneity, we performed separate analyses with and without this study. To assess trials having an adequate dose use, treatment duration, and sample size, we conducted a sensitivity analysis by restricting to studies using at least 840 mg/d total marine omega‐3 supplementation, that had at least 1000 participants, and that lasted at least 2 years. Four studies (DOIT,12 SU.FOL.OM3,13 Alpha.Omega,14 and OMEGA15) were excluded according to these stricter criteria in this subset analysis. We also conducted a sensitivity analysis that excluded 2 open‐label trials (GISSI‐P16 and JELIS17) to eliminate potential bias introduced in the unblinding design. Finally, to assess the joint impact of both open‐label and smaller trials, we excluded the aforementioned 6 studies.
In the exploratory dose–response analysis, we used the total marine omega‐3 dose from EPA and docosahexaenoic acid combined. A meta‐regression was used to assess linear dose–response relationships between marine omega‐3 supplements dose measured as mg/d and risk for each outcome of interest. The nonlinear relationship was not explored because of the limited number of included trials. We also conducted separate analyses with and without REDUCE‐IT to assess whether any significant dose–response relationship was driven by its extremely high dose. In a sensitivity analysis, we additionally adjusted for the median follow‐up duration. Statistical analyses were performed using STATA version 15.0 (StataCorp LP, College Station, TX) and R (version 3.3.2, R Foundation) package metareg was used for the dose–response analysis.18
Results
The 13 included trials9, 10, 11, 12, 13, 14, 15, 16, 17, 19, 20, 21, 22 had a total number of 127 477 participants, of whom 59.7% were male (Table). On average, the participants were 64.3 years of age at baseline, had a body mass index of 28 kg/m2, and were treated for 5 years. The addition of ASCEND, VITAL, and REDUCE‐IT increased the sample size by 63.6% and extended the mean follow‐up duration by 0.6 year compared with the previous meta‐analysis.6 Overall, 39.7% of participants had prevalent diabetes mellitus and 72.6% used cholesterol‐lowering medication at enrollment. The range of marine omega‐3 supplementation dose was 376 to 4000 mg/d, although the relative proportion of EPA and docosahexaenoic acid varied among different trials. The JELIS and REDUCE‐IT trials tested EPA alone.
Table 1.
Baseline Characteristics of RCTs Investigating Effects of Marine Omega‐3 Supplementation and CVDs
| Study | Year | Sample Size | Mean Age, y | Marine Omega‐3 Dose, mg/d | Mean Follow‐up Duration, y | Male, No. (%) | BMI, kg/m2 | Diabetes Mellitus, No. (%) | Cholesterol‐Lowering Drug Use, No. (%) |
|---|---|---|---|---|---|---|---|---|---|
| GISSI‐P16 | 1999 | 11 334 | 59.4 | 866 | 3.5 | 9658 (85.2) | 26.5 | 2139 (18.9) | NA |
| JELIS17 | 2007 | 18 645 | 61.0 | 1800 | 4.6 | 5859 (31.4) | 24.0 | 3040 (16.3) | 18 645 (100.0) |
| GISSI‐HF22 | 2008 | 6975 | 67.0 | 866 | 3.9a | 5459 (78.3) | 27.0 | 1974 (28.3) | NA |
| DOIT12 | 2010 | 563 | 70.0 | 1320 | 3.0 | 563 (100) | NA | 46 (8.2) | NA |
| SU.FOL.OM313 | 2010 | 2501 | 61.0a | 600 | 4.2 | 1987 (79.4) | 27.2 | 440 (17.9) | 2079 (83.1) |
| Alpha Omega14 | 2010 | 4837 | 69.0 | 376 | 3.4a | 3783 (78.2) | 27.8 | 1014 (21.0) | 4122 (85.2) |
| OMEGA15 | 2010 | 3818 | 64.0a | 850 | 1.0 | 2841 (74.4) | 27.5 | 948 (27.0) | 3566 (94.2) |
| ORIGIN19 | 2012 | 12 536 | 63.5 | 840 | 6.2a | 8150 (65.0) | 29.8 | 11 081 (88.4) | 6739 (53.8) |
| R&P20 | 2013 | 12 505 | 64.0 | 866 | 5.0 | 7687 (61.5) | 29.4 | 7494 (59.9) | 12 505 (100.0) |
| AREDS‐221 | 2014 | 4203 | 74.0 | 1000 | 4.8a | 1816 (43.2) | NA | 546 (13.0) | 1866 (44.4) |
| VITAL10 | 2018 | 25 871 | 67.1 | 840 | 5.3a | 12 786 (49.4) | 28.1 | 3549 (13.7) | 9524 (37.5) |
| ASCEND9 | 2018 | 15 480 | 63.3 | 840 | 7.4 | 9684 (62.6) | 30.8 | 14 569 (94.1) | 11 653 (75.3) |
| REDUCE‐IT11 | 2018 | 8179 | 64.0a | 4000 | 4.9a | 5822 (71.2) | 30.8 | 3389 (41.4) | 8145 (100)b |
| Total | NA | 127 477 | 64.3 | NA | 5.0 | 76 095 (59.7) | 28.0 | 50 229 (39.4) | 78,844 (72.6) |
BMI indicates body mass index; CVDs, cardiovascular diseases; NA, not applicable; RCTs, randomized controlled trials.
Data are median values.
Thirty‐four participants with missing data.
The pooled associations between marine omega‐3 supplementation and risk of CHD subtypes are presented in Figure 1. In the analysis excluding REDUCE‐IT, the pooled RRs (95% CIs; P values) were 0.92 (0.86, 0.99; P=0.020) for MI, 0.92 (0.86, 0.98; P=0.010) for CHD death, and 0.95 (0.91, 0.99; P=0.008) for total CHD, where no significant heterogeneity was found. A linear dose–response relationship was not found between marine omega‐3 supplementation dose and these CHD outcomes (Figure S1). Including the REDUCE‐IT substantially strengthened the inverse associations for MI and total CHD while introducing statistically significant heterogeneity for the pooled estimates. The pooled RRs (95% CIs; I 2, P for heterogeneity) became 0.88 (0.83, 0.94; I 2=51.2%, P for heterogeneity 0.017) for MI (P<0.001) and 0.93 (0.89, 0.96; I 2=54.7%, P for heterogeneity 0.009) for total CHD (P<0.001). By including the REDUCE‐IT, a significant dose–response relationship was also observed but without introducing significant heterogeneity. Every 1000 mg/d marine omega‐3 supplementation corresponded to 9% (95% CI: 2%, 15%; P=0.012; P for heterogeneity 0.218) and 7% (95% CI: 0%, 13%; P=0.041; P for heterogeneity 0.068) lower risk of MI and total CHD, respectively (Figure S1A, and S1C).
Figure 1.
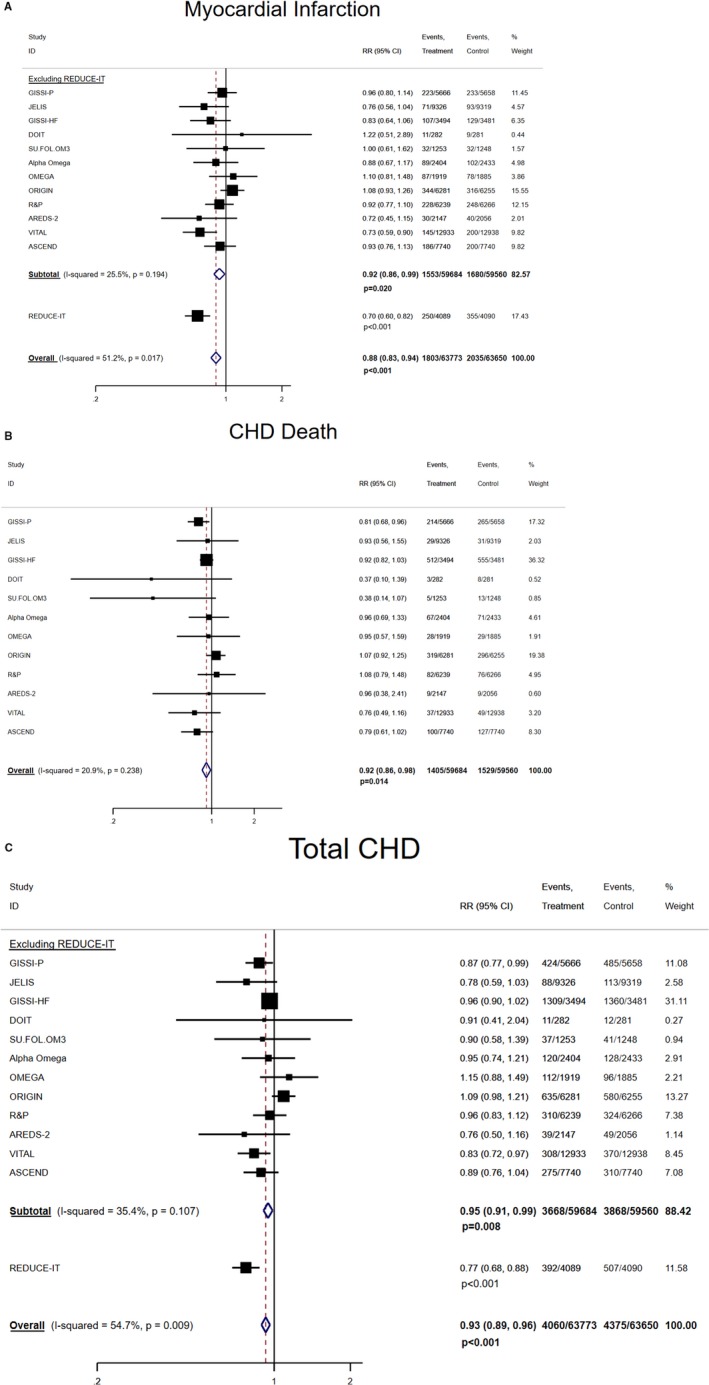
Pooled associations between marine omega‐3 supplementation and risk of subtypes of CHD. A, Marine omega‐3 supplementation and risk of MI, which includes fatal and/or nonfatal MI. B, Marine omega‐3 supplementation and risk of CHD death. C, Marine omega‐3 supplementation and risk of total CHD, which includes MI, death from CHD, or coronary revascularization. CHD indicates coronary heart disease; MI, myocardial infarction; RR, rate ratio.
The pooled RR (95% CI) between marine omega‐3 supplementation and risk of stroke and other CVD event subtypes are shown in Figures 2 and 3. In the analysis excluding REDUCE‐IT, no significant association was found for stroke (1.05 [0.98, 1.14]; P=0.183) but significant inverse associations (RRs [95% CIs]; P values) were found for CVD death (0.93 [0.88, 0.99]; P=0.013) and total CVD (0.97 [0.94, 0.99]; P=0.015). For major vascular events, the RR was 0.97 (0.94, 1.00; P=0.058). Each 1000 mg/d marine omega‐3 supplementation lowered risk of total CVD by 17% (95% CI: 4%, 29%) and risk of major vascular events by 17% (95% CI: 3%, 28%) without evidence of heterogeneity (Figure S2B, and S2C). Including REDUCE‐IT only slightly strengthened the pooled inverse associations (RR [95% CI]; P value) for CVD death (0.92 [0.88, 0.97]; P=0.003) and total CVD (0.95 [0.92, 0.98]; P<0.001), but lowered the risk of major vascular events (0.95 [0.93, 0.98]; P<0.001). The P values for heterogeneity became statistically significant for total CVD (P=0.002) and major vascular events (P=0.003) but not for total stroke (P=0.093) or CVD death (P=0.388). The linear dose–response relationships were statistically significant for total stroke (RR [95% CI] per 1000 mg/d increment: 0.89 [0.82, 0.98]) (Figure S3), total CVD (RR [95% CI] per 1000 mg/d increment: 0.91 [0.88, 0.95]) and major vascular events (RR [95% CI] per 1000 mg/d increment: 0.92 [0.89, 0.95]) without evidence of heterogeneity after including REDUCE‐IT (Figure S2B, and S2C). Additional adjustment for follow‐up duration did not materially change the regression slopes for the CVD end points.
Figure 2.
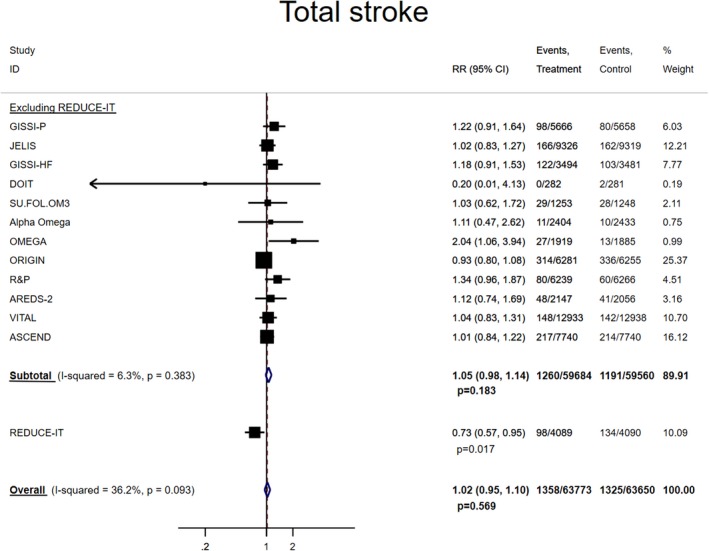
Pooled associations between marine omega‐3 supplementation and risk of total stroke. Total stroke includes fatal and/or nonfatal stroke.
Figure 3.
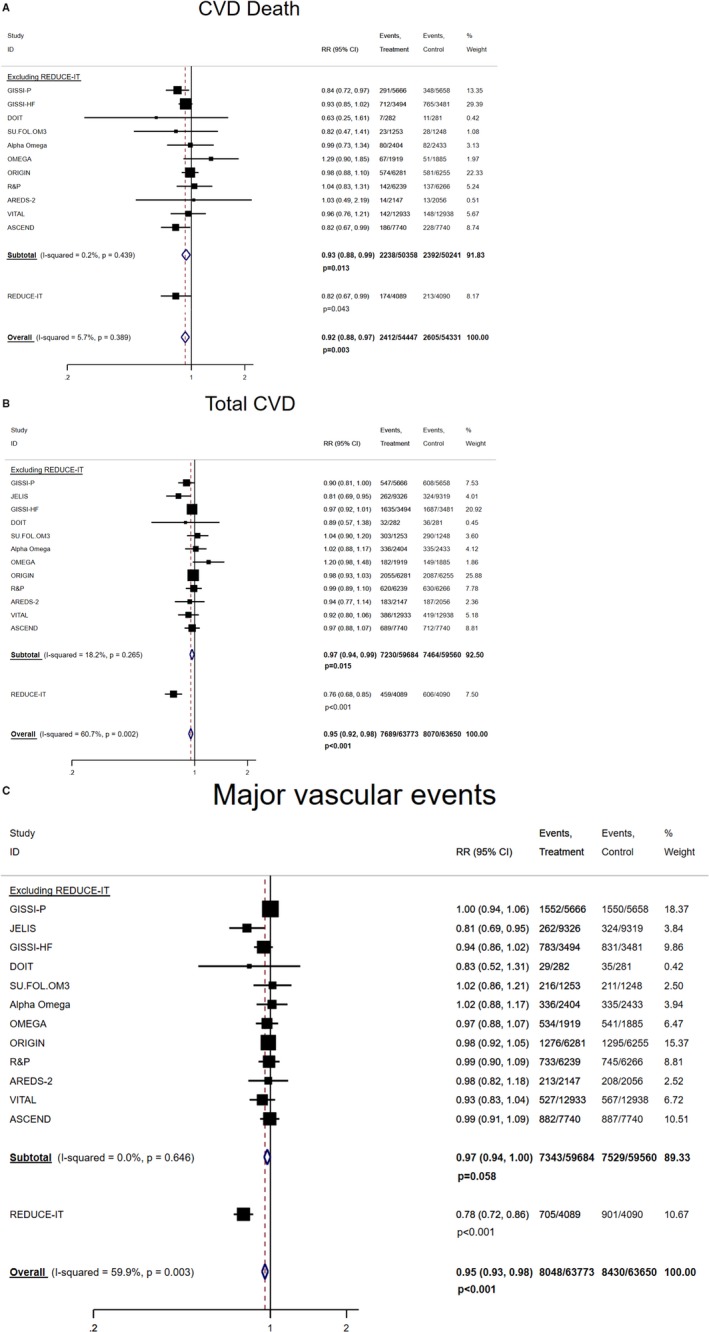
Pooled associations between marine omega‐3 supplementation and risks of other subtypes of CVD. A, Marine omega‐3 supplementation and risk of CVD death. B, Marine omega‐3 supplementation and risk of total CVD, which includes nonfatal MI, nonfatal stroke, death from CVD, or hospitalization because of a cardiovascular cause (except for JELIS and ALPHA OMEGA, which include revascularization). Removing JELIS and ALPHA OMEGA resulted in pooled RR 0.97 (0.94, 1.00), P=0.046 without including REDUCE‐IT, and 0.95 (0.93, 0.98), P=0.001 with REDUCE‐IT. C, Marine omega‐3 supplementation and risk of major vascular events, which include nonfatal MI, nonfatal stroke, death from CVD, or revascularization. CVD indicates cardiovascular disease; MI, myocardial infarction; RR, rate ratio.
In the sensitivity analysis that excluded DOIT, SU.FOL.OM3, Alpha.Omega, and OMEGA (because of considerably lower dose, duration, or size), inverse associations for most CVD end points were strengthened (Figure S4). In the analysis that excluded 2 open‐label trials, GISSI‐P and JELIS, the point estimates remained unchanged for most CVD end points except CHD death whose RR (95% CI) was attenuated to 0.94 (0.87, 1.01), and the 95% CIs for most end points became wider (Figure S5). Jointly excluding both open‐label and smaller trials also produced similar RRs with wider 95% CIs across CVD end points, with the largest attenuation for CHD death (Table S1).
Discussion
In this updated meta‐analysis, we found that marine omega‐3 supplementation significantly lowered the risk of MI, total CHD, total CVD, and because of CHD or CVD, even after excluding REDUCE‐IT. Including REDUCE‐IT resulted in stronger inverse associations for these outcomes while introducing significant heterogeneity. Linear dose–response relationships were persistent only for total CVD and major vascular events in the analyses with and without including REDUCE‐IT.
The current updated meta‐analysis builds upon a previous one including 10 large RCTs and provides an up‐to‐date assessment regarding the effects of marine omega‐3 supplementation and risks of multiple subtypes of CVD end points. The inclusion of 3 additional studies, increasing samples size by 64% and contributing 11% to 45% of the total weight of the CVD end points in the current analysis, has a substantial influence on the available evidence. In contrast with recent meta‐analysis, our study suggests that MI, total CHD, CHD death, total CVD, and CVD death are reduced by marine omega‐3 supplementation (even after excluding REDUCE‐IT) and that higher doses of marine omega‐3 supplementation are significantly associated with reduced risk of total CVD and major vascular events. Despite the modest effect sizes for some of the CVD outcomes, the use of marine omega‐3 supplementation may still help prevent large absolute numbers of CVD events, given the high incidence rates of CVD worldwide. Finally, our results were generally consistent with previous findings that indicated that marine omega‐3 supplementation was not associated with risk of stroke.
The differential associations frequently observed between composite CVD end points and individual components of composite outcomes imply that the potential beneficial effects of marine omega‐3 may not be uniform across all types of CVD. Findings from the current study are in line with previous meta‐analyses suggesting that marine omega‐3 supplementation may be particularly effective in reducing CHD events and mortality because of CVD causes, but not in reducing stroke.3, 23 Both ASCEND and REDUCE‐IT observed a lower incidence of vascular death with marine omega‐3 supplementation than with placebo, and VITAL also found a lower risk of MI and fatal MI in the treatment group. In contrast, the effects of marine omega‐3 supplementation on risk of stroke were mostly null, which was confirmed in the current meta‐analysis. However, given the substantial risk reduction of total stroke in REDUCE‐IT, it remains unclear whether higher doses of omega‐3 supplementation are required to attain these benefits. In addition, because most marine omega‐3 trials recruited participants with existing CVD or prevalent chronic conditions, the frequent use of statins, beta‐blockers, aspirin, anticoagulants, and hypoglycemic medications may impair the ability to detect additional CVD benefits from the marine omega‐3 supplementation. However, the generally similar results among those using and not using these medications in VITAL, the only trial conducted in a usual‐risk population, and in previous meta‐analyses argues against this explanation.
In the current study, the linear dose–response relationship observed between marine omega‐3 supplementation and several CVD end points is both clinically and biologically plausible. Because most included trials comprise patients at high risk of CVD and with advanced atherosclerosis, a high dose of marine omega‐3 supplementation may be needed to achieve potential benefits in this setting. A dose–response analysis based on 58 placebo‐controlled trials estimated that each 1 g/d increase of marine omega‐3 reduced triglyceride levels by 5.9 mg/dL and such linear association did not plateau even at 7 g/d.24 Nevertheless, our dose–response analysis was highly exploratory and should be interpreted cautiously. Because most included trials had a dose around 850 mg/d, the slope of the regression line was essentially determined by few distinctive doses within a narrow range, which may not be sufficient to delineate the underlying dose–response relationship. Although including REDUCE‐IT generated significant linear dose–response relationships between marine omega‐3 supplementation and most CVD outcomes, the substantially changed slopes suggested that the marine omega‐3 dose of 4000 mg/d was an influential outlier (most trials tested doses ≤1000 mg/d and the second largest dose was 1800 mg/d). Nevertheless, the general inverse trend in the dose–response analysis without including REDUCE‐IT suggested that the protective effects of marine omega‐3 may be evident even at moderate‐to‐high doses. Eventually, incorporating data from the ongoing trial STRENGTH (Statin Residual Risk Reduction With Epanova in High Cardiovascular Risk Patients with Hypertriglyceridemia), which is testing a high dose of marine omega‐3 supplementation, may help to further clarify the dose–response relationship between marine omega‐3 supplementation and CVD risk. Furthermore, results from our sensitivity analysis that excluded 4 smaller RCTs (Figure S4) suggested that adequate sample size, moderate‐to‐high marine omega‐3 dose, and longer treatment duration are required to ensure a rigorous and reliable assessment of the effect of marine omega‐3 supplementation on CVD end points. Finally, it is noteworthy that removing 2 open‐label trials attenuated the estimates for CHD death only (Table S1). This is largely because of the exclusion of GISSI‐P, the trial with the largest study weight (19.48%) among all included trials and showing a statistically significant risk reduction in this end point of nearly 20%. However, despite widening of CIs because of sample size reduction, the RR point estimates were virtually unchanged for MI, total CVD, and other vascular end points.
Our study has some limitations. First, we were unable to perform subgroup analysis by including 3 additional trials because the study‐level data were not available for these trials. However, because the associations did not differ across most subgroups such as age, sex, prior statin use, etc, in these 3 additional trials, it is unlikely that any significant effect modification would emerge, in view of the absence of interactions across these subgroups in previous meta‐analyses.6 Although VITAL suggested that marine omega‐3 supplementation may particularly benefit blacks and those with low fish consumption, we could not investigate such effect modifications in the current meta‐analysis because previous trials had predominantly white participants, and few studies assessed baseline fish intake. Second, because of the lack of published study‐level data, we were unable to include some end points such as subtypes of stroke and revascularization. Third, potential nonlinear relationships between marine omega‐3 supplementation and CVD end points could not be determined because of an insufficient number of trials. Finally, our study did not include some small trials or trials using dietary advice as the intervention. However, a previous study including these additional trials7 produced results identical to an earlier meta‐analysis involving 10 large trials only,6 suggesting that the results were unlikely to be influenced by inclusion of those studies.
Conclusions
The current updated meta‐analysis incorporating data from 13 RCTs, including 3 recent large trials, suggests that marine omega‐3 supplementation is associated with lower risk of MI, total CHD, total CVD, and death from CHD or CVD causes. Such inverse associations may be particularly evident at higher doses of marine omega‐3 supplementation. Additional large trials testing high doses of marine omega‐3 supplementation are warranted to confirm and extend these findings.
Source of Funding
VITAL was supported by grants U01 CA138962 and R01 CA138962 from the National Institutes of Health. Pharmavite LLC of Northridge, California (vitamin D) and Pronova BioPharma of Norway and BASF (Omacor fish oil) donated the study agents and matching placebos.
Disclosures
Dr Frank B Hu reported being supported by grants HL60712, HL118264, and DK112940 from the National Institutes of Health and reported receiving research support from the California Walnut Commission and honoraria for lectures from Metagenics and Standard Process and honoraria from Diet Quality Photo Navigation, outside the submitted work. The remaining authors have no disclosures to report.
Supporting information
Figure S1. Dose–response relationship between marine omega‐3 supplementation and risk of coronary heart disease end points.
Figure S2. Dose–response relationships between marine omega‐3 supplementation and risk of other CVD subtypes
Figure S3. Dose–response relationships between marine omega‐3 supplementation and risk of stroke.
Figure S4. Pooled associations between marine omega‐3 supplementation and risk of cardiovascular disease end points excluding DOIT, SU.FOL.OM3, Alpha.Omega, and OMEGA because of lower dose, shorter follow‐up duration, or smaller sample size than the rest of included studies.
Figure S5. Pooled associations between marine omega‐3 supplementation and risk of cardiovascular disease end points excluding 2 open‐label trials, GISSI‐P and JELIS.
Table S1. Sensitivity Analysis That Excluded Open‐Label Trials and Trials With Smaller Sample Size, Lower Dose, and Shorter Follow‐Up Duration
(J Am Heart Assoc. 2019;8:e013543 DOI: 10.1161/JAHA.119.013543.)
References
- 1. Wang C, Harris WS, Chung M, Lichtenstein AH, Balk EM, Kupelnick B, Jordan HS, Lau J. n‐3 Fatty acids from fish or fish‐oil supplements, but not α‐linolenic acid, benefit cardiovascular disease outcomes in primary‐ and secondary‐prevention studies: a systematic review. Am J Clin Nutr. 2006;84:5–17. [DOI] [PubMed] [Google Scholar]
- 2. Zheng J, Huang T, Yu Y, Hu X, Yang B, Li D. Fish consumption and CHD mortality: an updated meta‐analysis of seventeen cohort studies. Public Health Nutr. 2012;15:725–737. [DOI] [PubMed] [Google Scholar]
- 3. Rizos EC, Ntzani EE, Bika E, Kostapanos MS, Elisaf MS. Association between omega‐3 fatty acid supplementation and risk of major cardiovascular disease events: a systematic review and meta‐analysis. JAMA. 2012;308:1024–1033. [DOI] [PubMed] [Google Scholar]
- 4. Kwak SM, Myung SK, Lee YJ, Seo HG. Efficacy of omega‐3 fatty acid supplements (eicosapentaenoic acid and docosahexaenoic acid) in the secondary prevention of cardiovascular disease: a meta‐analysis of randomized, double‐blind, placebo‐controlled trials. Arch Intern Med. 2012;172:686–694. [DOI] [PubMed] [Google Scholar]
- 5. Siscovick DS, Barringer TA, Fretts AM, Wu JHY, Lichtenstein AH, Costello RB, Kris‐Etherton PM, Jacobson TA, Engler MB, Alger HM, Appel LJ, Mozaffarian D. Omega‐3 polyunsaturated fatty acid (fish oil) supplementation and the prevention of clinical cardiovascular disease: a science advisory from the American Heart Association. Circulation. 2017;135:e867–e884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Aung T, Halsey J, Kromhout D, Gerstein HC, Marchioli R, Tavazzi L, Geleijnse JM, Rauch B, Ness A, Galan P, Chew EY, Bosch J, Collins R, Lewington S, Armitage J, Clarke R. Associations of omega‐3 fatty acid supplement use with cardiovascular disease risks: meta‐analysis of 10 trials involving 77 917 individuals. JAMA Cardiol. 2018;3:225–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Abdelhamid AS, Brown TJ, Brainard JS, Biswas P, Thorpe GC, Moore HJ, Deane KHO, Alabdulghafoor FK, Summerbell CD, Worthington HV, Song F, Hooper L. Omega‐3 fatty acids for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2018;11:CD003177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Abbasi J. Another nail in the coffin for fish oil supplements. JAMA. 2018;319:1851–1852. [DOI] [PubMed] [Google Scholar]
- 9. The ASCEND Study Collaborative Group . Effects of n−3 fatty acid supplements in diabetes mellitus. N Engl J Med. 2018;379:1540–1550. [DOI] [PubMed] [Google Scholar]
- 10. Manson JE, Cook NR, Lee I‐M, Christen W, Bassuk SS, Mora S, Gibson H, Albert CM, Gordon D, Copeland T, D'Agostino D, Friedenberg G, Ridge C, Bubes V, Giovannucci EL, Willett WC, Buring JE. Marine n−3 fatty acids and prevention of cardiovascular disease and cancer. N Engl J Med. 2018;380:23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, Doyle RT, Juliano RA, Jiao L, Granowitz C, Tardif J‐C, Ballantyne CM. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380:11–22. [DOI] [PubMed] [Google Scholar]
- 12. Einvik G, Ole Klemsdal T, Sandvik L, Hjerkinn EM. A randomized clinical trial on n‐3 polyunsaturated fatty acids supplementation and all‐cause mortality in elderly men at high cardiovascular risk. Eur J Prev Cardiol. 2010;17:588–592. [DOI] [PubMed] [Google Scholar]
- 13. Galan P, Kesse‐Guyot E, Czernichow S, Briancon S, Blacher J, Hercberg S. Effects of B vitamins and omega 3 fatty acids on cardiovascular diseases: a randomised placebo controlled trial. BMJ. 2011;341:C6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kromhout D, Giltay EJ, Geleijnse JM. n‐3 fatty acids and cardiovascular events after myocardial infarction. N Engl J Med. 2010;363:2015–2026. [DOI] [PubMed] [Google Scholar]
- 15. Rauch B, Schiele R, Schneider S, Diller F, Victor N, Gohlke H, Gottwik M, Steinbeck G, Del Castillo U, Sack R, Worth H, Katus H, Spitzer W, Sabin G, Senges J. OMEGA, a randomized, placebo‐controlled trial to test the effect of highly purified omega‐3 fatty acids on top of modern guideline‐adjusted therapy after myocardial infarction. Circulation. 2010;122:2152–2159. [DOI] [PubMed] [Google Scholar]
- 16. GISSI‐Prevenzione Investigators . Dietary supplementation with n‐3 polyunsaturated fatty acids and vitamin E after myocardial infarction; results of the GISSI‐Prevenzione trial. Lancet. 1999;354:447–455. [PubMed] [Google Scholar]
- 17. Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Saito Y, Ishikawa Y, Oikawa S, Sasaki J, Hishida H, Itakura H, Kita T, Kitabatake A, Nakaya N, Sakata T, Shimada K, Shirato K. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open‐label, blinded endpoint analysis. Lancet. 2007;369:1090–1098. [DOI] [PubMed] [Google Scholar]
- 18. Schwarzer G. meta: an R package for meta‐analysis. R News. 2007;7:40–45. [Google Scholar]
- 19. The ORIGIN Trial Investigators . N‐3 fatty acids and cardiovascular outcomes in patients with dysglycemia. N Engl J Med. 2012;367:309–318. [DOI] [PubMed] [Google Scholar]
- 20. The Risk and Prevention Study Collaborative Group . N‐3 fatty acids in patients with multiple cardiovascular risk factors. N Engl J Med. 2013;368:1800–1808. [DOI] [PubMed] [Google Scholar]
- 21. Bonds DE, Harrington M, Worrall BB, Bertoni AG, Eaton CB, Hsia J, Robinson J, Clemons TE, Fine LJ, Chew EY. Effect of long‐chain ω‐3 fatty acids and lutein+zeaxanthin supplements on cardiovascular outcomes: results of the age‐related eye disease study 2 (AREDS2) randomized clinical trial. JAMA Intern Med. 2014;174:763–771. [DOI] [PubMed] [Google Scholar]
- 22. GISSI‐HF investigators . Effect of n‐3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI‐HF trial): a randomised, double‐blind, placebo‐controlled trial. Lancet. 2008;372:1223–1230. [DOI] [PubMed] [Google Scholar]
- 23. Kotwal S, Jun M, Sullivan D, Perkovic V, Neal B. Omega 3 fatty acids and cardiovascular outcomes: systematic review and meta‐analysis. Circ Cardiovasc Qual Outcomes. 2012;5:808–818. [DOI] [PubMed] [Google Scholar]
- 24. Mozaffarian D, Wu JHY. Omega‐3 fatty acids and cardiovascular disease: effects on risk factors, molecular pathways, and clinical events. J Am Coll Cardiol. 2011;58:2047–2067. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Dose–response relationship between marine omega‐3 supplementation and risk of coronary heart disease end points.
Figure S2. Dose–response relationships between marine omega‐3 supplementation and risk of other CVD subtypes
Figure S3. Dose–response relationships between marine omega‐3 supplementation and risk of stroke.
Figure S4. Pooled associations between marine omega‐3 supplementation and risk of cardiovascular disease end points excluding DOIT, SU.FOL.OM3, Alpha.Omega, and OMEGA because of lower dose, shorter follow‐up duration, or smaller sample size than the rest of included studies.
Figure S5. Pooled associations between marine omega‐3 supplementation and risk of cardiovascular disease end points excluding 2 open‐label trials, GISSI‐P and JELIS.
Table S1. Sensitivity Analysis That Excluded Open‐Label Trials and Trials With Smaller Sample Size, Lower Dose, and Shorter Follow‐Up Duration


