Abstract
Background
There has been an increase in the prevalence of drug abuse (DA) in the national opioid epidemic. With increasing DA, there is an increased risk of infective endocarditis (IE). There are limited recent data evaluating national trends on the incidence and geographical distribution of DA‐IE. We aim to investigate those numbers as well as the determinants of outcome in this patient population.
Methods and Results
Hospitalized patients with a primary or secondary diagnosis of IE based on the International Classification of Diseases, Ninth and Tenth Revisions (ICD‐9, ICD‐10) were included. We described the national and geographical trends in DA‐IE. We also compared DA‐IE patients’ characteristics and outcomes to those with IE, but without associated drug abuse (non‐DA‐IE) using Poisson regression models. Incidence of DA‐IE has nearly doubled between 2002 and 2016 All US regions were affected, and the Midwest had the highest increase in DA‐IE hospitalizations (annual percent change=4.9%). Patients with DA‐IE were younger, more commonly white males, poorer, had fewer comorbidities, and were more likely to have human immunodeficiency virus, hepatitis C, concomitant alcohol abuse, and liver disease. Their length of stay was longer (9 versus 7 days; P<0.001) and were more likely to undergo cardiac surgery (7.8% versus 6.2%; P<0.001), but their inpatient mortality was lower (6.4% versus 9.1%; P<0.001).
Conclusions
DA‐IE is rising at an alarming rate in the United States. All regions of the United States are affected, with the Midwest having the highest increase in rate. Young‐adult, poor, white males were the most affected.
Keywords: drug abuse, epidemiology, infective endarteritis, morbidity/mortality
Subject Categories: Valvular Heart Disease, Infectious Endocarditis, Epidemiology, Quality and Outcomes
Clinical Perspective
What Is New?
We found that patients with drug‐abuse–associated infective endocarditis were younger, more commonly male and white, more likely to have Medicaid insurance, and in the lowest quartile of median household income.
These patients underwent cardiac or valve surgery more often, had a higher median length of stay, and a higher hospitalization cost, but a lower inpatient mortality.
Geographically, we found that drug abuse infective endocarditis is increasing across all regions of the United States, with the Midwest having the highest annual percent increase.
What Are the Clinical Implications?
We believe these findings are alarming from a public health standpoint and outline the need for an immediate tailored action plan.
Introduction
Drug abuse (DA) continues to rise in the United States, and otherwise healthy populations have been particularly affected.1, 2 The number of deaths secondary to an opioid‐related drug overdose in 2016 was 5 times higher than in 1999, and drug‐related deaths from heroin nearly tripled during this time period.3, 4 In 2017, the US Department of Health and Human Services declared the US opioid epidemic a public health emergency.5 The opioid epidemic has led to increased infectious‐related morbidity and mortality, including infective endocarditis (IE).6
Drug abuse is a major risk factor for IE, and patients who have IE from intravenous DA have significant morbidity and mortality.7, 8, 9 Regardless of DA, IE is a life‐threatening condition with an associated mortality of 10% to 30%10, 11, 12 and has been steadily increasing over the past 20 years.6, 13, 14, 15 Admissions for IE in patients aged <30 years with intravenous DA increased from 11% in 2008 to 27% in 2014 in the Centers for Disease Control and Prevention Multiple Cause‐of‐Death database.16 Additionally, it was demonstrated that there was an alarming 2‐fold increase in IE deaths among young people (aged <35 years), white, and with intravenous DA from 1999 to 2016 compared with all‐cause IE mortality.17
There are limited data evaluating national trends on the incidence and geographical distribution of IE since 2014. A recent study looked at North Carolina state data between 2007 and 2017 and showed that the rate of DA‐related IE (DA‐IE) hospitalizations increased by 12‐fold, and a single‐center retrospective study in Virginia between 2000 and 2006 showed a 10% annual increase in intravenous DA‐IE.18, 19
In this analysis, we describe national and geographical trends in DA‐IE using the National Inpatient Sample (NIS) registry from 2002 to 2016. We also compare patient characteristics and outcomes in DA‐IE to those with non‐DA‐IE.
Methods
We performed a retrospective cohort study of patients admitted with IE using the NIS from years 2002 to 2016. The NIS is part of a family of database and software tools developed for the Healthcare Cost and Utilization Project. It is the largest publically available all‐payer inpatient healthcare database in the United States. Unweighted, it contains data from more than 7 million hospital stays each year; and weighted, it estimates more than 35 million hospitalizations nationally.20 These data are exempted from institutional review board approval because it is de‐identified and publically available. Thus, informed consent was waived.
We first included all hospitalizations from 2002 to 2016. We excluded patients with missing information (discharge weighting variable, age, or year of hospitalization), age <18 years, and hospital transfers. Out of this cohort, we excluded patients without a primary or secondary diagnosis of IE based on the International Classification of Diseases, Ninth and Tenth Revisions, Clinical Modification (ICD‐9‐CM, ICD‐10‐CM) diagnoses (Table S1). The authors declare that supporting ICD‐9 and ICD‐10 data are available within the article and its online supplementary file and are available to other researchers for purposes of replicating the procedure. Patient demographics, payer status, income, length of stay, charges, hospital region, and comorbidities were obtained from the database. Comorbidities and surgeries were identified according to their ICD‐9 and ICD‐10 codes (Table S1) and/or Elixhauser comorbidity variables, when appropriate.21, 22 Death was determined by inpatient mortality as provided in the NIS database. The data were then stratified based on DA status, which was defined based on 97 ICD‐9 codes and 253 ICD‐10 codes (Table S1).
Statistical Analysis
Continuous variables are described as medians and interquartile ranges. Categorical variables are described as percentages. The Mann–Whitney U test was used to compare 2 continuous variables, and the chi‐square test and Fisher's exact test were used to compare 2 categorical variables. P<0.05 was considered statistically significant. Statistical analyses were performed with SPSS software (version 25.0; IBM Corp, Armonk, NY). We used the Cochran–Armitage test for trend to compare incidence rate ratios of IE and DA‐IE across all calendar years. Multivariable Poisson regression models were used to assess the trends of IE, DA, and DA‐IE nationwide and by region, which was adjusted for potential confounders such as age, sex, race, and drug use.
Results
Population/Patient Selection
A total of 568 648 355 hospitalizations were recorded between 2002 and 2016 (Figure 1). Patients with missing discharge weighting factor (n=856 807), missing age, or year of admission (n=478 894), age <18 (92 676 715), and hospital transfers (n=23 188 164) were excluded. A total of 455 404 161 hospitalizations were included. Of those, 954 709 had a primary or secondary diagnosis of IE; 94 350 (9.9%) participants had DA and 860 359 did not (Figure 1).
Figure 1.
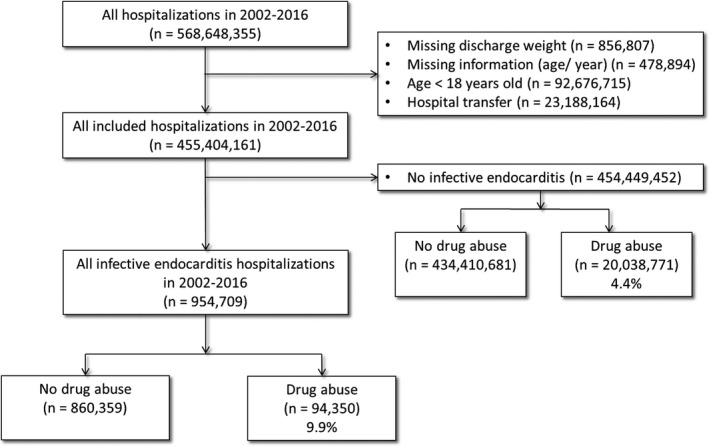
Flow diagram of the screened and enrolled population.
Baseline Characteristics, Costs, and Outcomes
The median age of patients with IE was 68 years (interquartile range, 52–80), 51% were males, and 72% were white (Table 1). When comparing patients with DA‐IE to those with non‐DA‐IE, they were younger (median age, 38 versus 70 years; P<0.001), more commonly male (55.5% versus 50.8%; P<0.001), on Medicaid (45.1%), and were in the lowest quartile of median household income (42.4%). They were less likely to have hypertension (24.7% versus 53.8%; P<0.001), diabetes mellitus (10.3% versus 28.7%; P<0.001), congestive heart failure (9.1% versus 21.9%; P<0.001), renal disease (10.3% versus 27%; P<0.00), or chronic lung disease (16.4% versus 22%; P<0.001), but were more likely to have hepatitis C (8.2% versus 1.2%; P<0.001), liver disease (18.6% versus 4.9%; P<0.001), human immunodeficiency virus (6% versus 1.2%; P<0.001), and concomitant alcohol abuse (13.6% versus 3.0%; P<0.001). In the DA‐IE group, Staphylococcus sp. was the most common organism identified (47.2%; Table 2). In terms of costs and outcomes (Table 3), DA‐IE underwent cardiac or valve surgery more often (7.8% versus 6.2% and 7.1% versus 5.1%, respectively; both P<0.001), had a higher median length of stay (9 versus 7 days; P<0.001), and higher hospitalization costs ($52 744 versus $37 373; P<0.001), but lower inpatient mortality (6.4% versus 9.1%; P<0.001). Regarding the microorganisms involved (regardless of DA status), the highest mortality was for Staphylococcus (13.4%) and fungal infections (13.1%), with the remaining microorganisms having lower comparable mortalities (Streptococus, Gram‐negative bacteria, and others having mortality rates of 7.3%, 7.4%, and 7.2%, respectively).
Table 1.
Baseline Characteristics of Patients With Infective Endocarditis According to Drug Abuse Status
| Total N=954 709 | Drug Abuse N=94 350 | No Drug Abuse N=860 359 | P Value | |
|---|---|---|---|---|
| Age, median (IQR) | 68 (52–80) | 38 (30–49) | 70 (57–81) | <0.001 |
| Male sex, % | 51.3 | 55.5 | 50.8 | <0.001 |
| Race, % | ||||
| White | 72.1 | 67.8 | 72.6 | <0.001 |
| Black | 15.3 | 18.3 | 15 | |
| Hispanic | 7.9 | 10.2 | 7.6 | |
| Asian/Pacific Islander/Native American/other | 4.7 | 3.8 | 4.8 | |
| Hypertension, % | 50.9 | 24.7 | 53.8 | <0.001 |
| Diabetes mellitus, % | 26.9 | 10.3 | 28.7 | <0.001 |
| Congestive heart failure, % | 20.6 | 9.1 | 21.9 | <0.001 |
| Liver disease, % | 6.2 | 18.6 | 4.9 | <0.001 |
| Chronic lung disease, % | 21.5 | 16.4 | 22 | <0.001 |
| Renal disease, % | 25.3 | 10.3 | 27 | <0.001 |
| Peripheral vascular disease, % | 10.4 | 8.2 | 10.7 | <0.001 |
| Hepatitis C, % | 1.9 | 8.2 | 1.2 | <0.001 |
| Human immunodeficiency virus, % | 1.7 | 6 | 1.2 | <0.001 |
| Alcohol abuse, % | 4.1 | 13.6 | 3 | <0.001 |
| Median household income national quartile for patient ZIP code, % | ||||
| 0 to 25th percentile | 31.8 | 42.4 | 30.7 | <0.001 |
| 26th to 50th percentile | 26.2 | 25.1 | 26.3 | |
| 51st to 75th percentile | 22.3 | 19.8 | 22.6 | |
| 76th to 100th percentile | 19.6 | 12.7 | 20.4 | |
| Primary expected payer, % | ||||
| Medicare | 62.2 | 14.8 | 67.3 | <0.001 |
| Medicaid | 12.4 | 45.1 | 8.9 | |
| Private | 18 | 12.8 | 18.6 | |
| Self‐pay/no charge/other | 7.4 | 27.3 | 5.3 | |
| Organisms, % | ||||
| Staphylococcus bacteremia | 24.6 | 47.2 | 22.1 | <0.001 |
| Streptococcus bacteremia | 15.5 | 13 | 15.8 | |
| Gram‐negative bacteremia | 1.2 | 1.3 | 1.1 | |
| Fungemia | 1.5 | 1.9 | 1.4 | |
| Unknown/other | 58.6 | 39 | 60.7 | |
Table 2.
Baseline Characteristics of Patients With Infective Endocarditis According to US Geographical Region
| All Regions N=94 350 | Northeast N=20 732 (22%) | Midwest N=14 988 (15.9%) | South N=37 695 (40%) | West N=20 935 (22.2%) | P Value | |
|---|---|---|---|---|---|---|
| Age, median (IQR) | 38 (30–49) | 39 (29–48) | 40 (30–50) | 37 (30–47) | 41 (30–51) | <0.001 |
| Male sex, % | 55.5 | 58.8 | 54.2 | 53.3 | 57.2 | <0.001 |
| Race, % | <0.001 | |||||
| White | 67.8 | 59.6 | 69.5 | 72.9 | 66.2 | |
| Black | 18.3 | 19.6 | 25.6 | 18.7 | 11.5 | |
| Hispanic | 10.2 | 14.6 | 2.3 | 6.4 | 17.1 | |
| Asian/Pacific Islander/Native American/other | 3.8 | 6.2 | 2.6 | 2 | 5.2 | |
| Hypertension, % | 24.7 | 20.8 | 29 | 25.3 | 24.1 | <0.001 |
| Diabetes mellitus, % | 10.3 | 8.9 | 11.3 | 9.3 | 12.7 | <0.001 |
| Congestive heart failure, % | 9.1 | 7.1 | 9.8 | 9 | 10.6 | <0.001 |
| Liver disease, % | 18.6 | 20.9 | 16.7 | 16.4 | 21.4 | <0.001 |
| Chronic lung disease, % | 16.4 | 17.4 | 19.7 | 15 | 15.7 | <0.001 |
| Renal disease, % | 10.3 | 8.5 | 12.7 | 10 | 10.8 | <0.001 |
| Peripheral vascular disease, % | 8.2 | 7 | 8.9 | 8.5 | 8.6 | <0.001 |
| Hepatitis C, % | 8.2 | 9.4 | 8.4 | 8.3 | 6.8 | <0.001 |
| Human immunodeficiency virus, % | 5.5 | 6.9 | 4.6 | 5.7 | 4.5 | <0.001 |
| Alcohol abuse, % | 13.6 | 14.9 | 14.7 | 12.2 | 13.9 | <0.001 |
| Median household income national quartile for patient ZIP code, % | ||||||
| 0 to 25th percentile | 42.4 | 36.6 | 45.7 | 51.3 | 28.9 | <0.001 |
| 26th to 50th percentile | 25.1 | 21.6 | 28.6 | 25.3 | 25.5 | |
| 51st to 75th percentile | 19.8 | 21 | 17.8 | 16.2 | 26.9 | |
| 76th to 100th percentile | 12.7 | 20.7 | 7.9 | 7.2 | 18.7 | |
| Primary expected payer, % | ||||||
| Medicare | 14.8 | 13.2 | 16.7 | 14.9 | 15 | <0.001 |
| Medicaid | 45.1 | 56 | 47.6 | 36.1 | 48.7 | |
| Private | 12.8 | 12.8 | 15.1 | 11.4 | 13.5 | |
| Self‐pay/no charge/other | 27.3 | 18 | 20.6 | 37.6 | 22.9 | |
| Organisms, % | ||||||
| Staphylococcus bacteremia | 47.2 | 43 | 46.8 | 47.4 | 51.1 | <0.001 |
| Streptococcus bacteremia | 13 | 11.9 | 12.3 | 11 | 11.6 | |
| Gram‐negative bacteremia | 1.3 | 0.4 | 0.5 | 0.8 | 0.6 | |
| Fungemia | 1.9 | 1.5 | 1.3 | 1 | 0.8 | |
| Unknown/other | 39 | 43.2 | 39.1 | 39.7 | 35.8 | |
IQR indicates interquartile range.
Table 3.
Outcomes in Patients With Infective Endocarditis According to Drug Abuse
| Total N=954 709 | Drug Abuse N=94 350 | No Drug Abuse N=860 359 | P Value | |
|---|---|---|---|---|
| Any open cardiac surgery, % | 6.4 | 7.8 | 6.2 | <0.001 |
| Any valve surgery, %a | 5.2 | 7.1 | 5.1 | <0.001 |
| Tricuspid valve surgery, % | 0.6 | 2.3 | 0.5 | |
| Pulmonary valve surgery, % | 0.1 | 0.1 | 0.1 | |
| Mitral valve surgery, % | 2.7 | 2.7 | 2.7 | |
| Aortic valve surgery, % | 3 | 3.4 | 2.9 | |
| Length of stay | ||||
| Median, days (IQR) | 7 (4–13) | 9 (4–19) | 7 (3–12) | <0.001 |
| Mean, days (±SD) | 10.57 (12.76) | 14.12 (15.03) | 10.19 (12.42) | <0.001 |
| Total charges, median (IQR) | $38 545 ($17 305–$89 287) | $52 744 ($22 625–$125 644) | $37 372 ($16 852–$85 647) | <0.001 |
| Inpatient mortality, % | 8.8 | 6.4 | 9.1 | <0.001 |
IQR indicates interquartile range.
We assessed valves independently, because patients may have had more than 1 valve operated on.
Characteristics and Surgical Outcomes of Patients With DA‐IE According to Region
The median age by region varied from 37 years in the South to 41 years in the West. The majority of patients were whites and males across all regions. Concomitant human immunodeficiency virus and hepatitis C were both most common in the Northeast (9.4% and 6.9%, respectively) and least common in the West (6.8% and 4.5%, respectively). In all regions, patients diagnosed with DA‐IE were more likely to have a median household income of 0 to 25th percentile and predominantly had Medicaid insurance. Staphylococcus bacteria were the most commonly identified organism (Table 2). The Midwest had the highest incidence rate of cardiac surgery (Table 4), and the aortic valve was the most common valve operated on across all regions, regardless of DA status.
Table 4.
Regional Distribution of Surgical Outcomes in Patients With Drug‐Abuse–Related Infective Endocarditis
| Northeast | Midwest | South | West | P Value | |
|---|---|---|---|---|---|
| Any open cardiac surgery, % | 7 | 9.1 | 8.4 | 6.8 | <0.001 |
| Any valve surgery, %a | 6.2 | 8.1 | 7.7 | 6.1 | <0.001 |
| Tricuspid valve surgery, % | 2 | 2.4 | 2.5 | 2.2 | 0.001 |
| Pulmonary valve surgery, % | 0.1 | 0.2 | 0.1 | 0.1 | 0.002 |
| Mitral valve surgery, % | 2.4 | 3.3 | 2.9 | 2.3 | <0.001 |
| Aortic valve surgery, % | 3.1 | 3.8 | 3.7 | 2.9 | <0.001 |
We assessed valves independently, because patients may have had more than 1 valve operated on.
Trends in IE, DA‐IE, and DA From 2002 to 2016
Overall incidence rate of IE increased from 18 per 10 000 in 2002 to 29 per 10 000 in 2016 (Figure 2A). In those with DA‐IE, incidence rate increased from 48 per 10 000 in 2002 to 79 per 10 000 in 2016. When looking at specific incidence rate ratio, there was a positive trend of IE incidence rate between 2002 and 2016 (P<0.001), with annual percent change (APC) of 1.8% (95% CI, 1.7–1.9) across the United States; however, the trend was nonlinear with a decrease of incidence rate ratio between 2002 and 2008, followed by increasing incidence rate ratio between 2009 and 2016. The trend was increasing in each of 4 regions (P<0.001). The APC was 2.5% (95% CI, 2.4–2.7) in Southern states, 1.9% (95% CI, 1.7–2) in Western states, 1.5% (95% CI, 1.4–1.7) in the Midwest, and 0.6% (95% CI, 0.5–0.8) in the Northeast.
Figure 2.
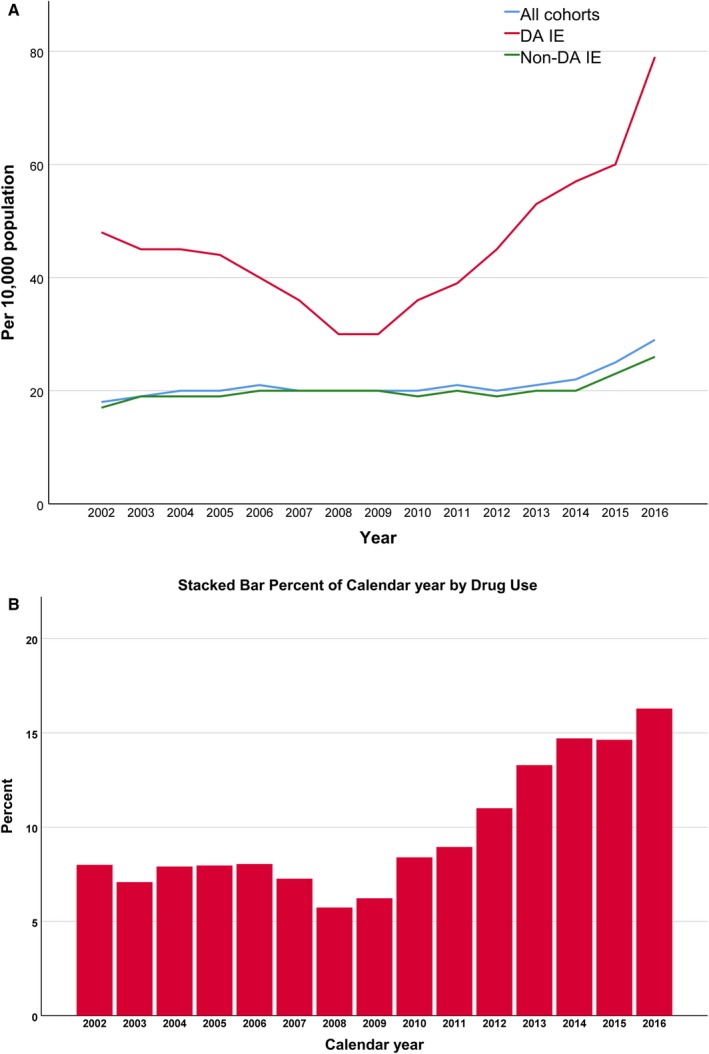
National trends of incidence rate of drug‐abuse–related infective endocarditis (DA IE) vs non‐drug‐abuse–related infective endocarditis (Non‐DA IE; A) and prevalence ratio of drug abuse in patients with infective endocarditis (B).
In patients with IE, there has been a near doubling in prevalence ratio of DA from 2002 (8.0%) to 2016 (16.3%; Figure 2B), with an APC of 3.5% (95% CI, 3.3–3.6) across the United States. The APC was highest in the Midwest (4.9%; 95% CI, 4.4–5.4), followed by the Western states (4.3%; 95% CI, 3.9–4.6), Southern states (3.3%; 95% CI 3.1–3.6), and the Northeast (2.4%; 95% CI 2.2–2.7).
When looking at DA across all hospitalizations (regardless of IE diagnosis), incidence rate has nearly doubled from 3% in 2002 to 5.9% in 2016 with a positive trend with an APC of 5% (95% CI, 4.9–5) across the United States. The highest trend was in the West (APC, 7.4%; 95% CI, 7.4–7.5), followed by Midwest (APC, 6.2%; 95% CI, 6.2–6.3), South (APC, 5.2%; 95% CI, 5.2–5.3), and Northeast (APC, 3.3%; 95% CI, 3.3–3.3). Figure 3 shows the various APCs per region in terms of DA‐IE, DA, and IE per region.
Figure 3.
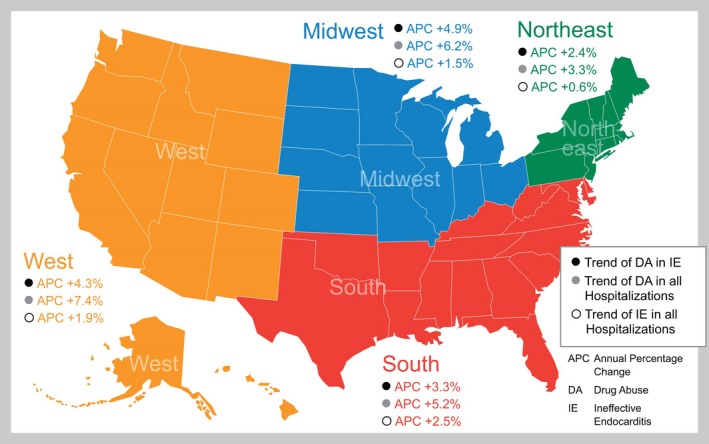
Geographical trends in annual percent change (APC) in drug abuse (DA) and infective endocarditis (IE) among hospitalizations from 2002 to 2016.
Discussion
To our knowledge, this study is the first to report population level trends of DA‐IE geographically across the entire United States. Overall, we found an increase in the incidence of all‐cause endocarditis across the entire United States during this period. DA‐IE also increased during this period. Patients with DA‐IE were younger, male, on Medicaid, and in the lowest quartile of median household income. The race most affected by DA‐IE was white. The DA‐IE cohort had fewer chronic comorbidities compared with non‐DA‐IE, including less hypertension, diabetes mellitus, congestive heart failure, and renal and lung disease; however, affected patients were more likely to have hepatitis C, human immunodeficiency virus, alcohol abuse, and liver disease. They had lower inpatient mortality, underwent cardiac surgery more often, and had an increased length of stay. Their hospitalization was also more expensive. Geographically, DA‐IE is increasing across all regions of the United States, with the Midwest having the highest prevalence ratio at 4.9%.
Our findings of the rising incidence of IE‐DA are consistent with findings of earlier studies. Kim et al7 showed that intravenous DA is increasing among IE patients requiring surgery whereas another study showed rising rates of intravenous DA in IE in Virginia.19 Wurcel et al14 used the NIS with ICD‐9 codes and showed that the proportion of IE hospitalization from injection DA increased from 7% to 12.1% between 2000 and 2013. A previous study using the same database showed that the IE admission rate rose between 1998 and 2009.15 A state‐wide study in North Carolina between 2007 and 2017, using the North Carolina Hospital Discharge Database, showed a 12‐fold increase in overall hospitalizations in DA‐IE and a 13‐fold increase in DA‐IE hospitalizations with valve surgeries.18 A recent study that looked at the Centers for Disease Control and Prevention Multiple Cause‐of‐Death database showed a 1.5‐fold increase in the number of deaths among DA‐IE compared with non‐DA‐IE, as well as an increase in IE deaths in people who inject drugs in patients aged <35 years and white between 1996 and 2016.17 A study using the National Readmissions Database found that IE associated with intravenous DA has continued to rise between 2010 and 2015 and patients are more likely to be readmitted for subsequent episodes of IE.23 Our findings expand on this and show that the disease of DA‐IE is widespread across all geographical regions of the United States.
Hospitalizations for IE have been increasing side by side with the opioid epidemic.24, 25 The opioid crisis has reached epidemic levels, and now drug overdoses have been the leading cause of injury‐related death in the United States.24 Heroin deaths had remained relatively low from 1999 until 2010 whereas it then increased 3‐fold from 2010 to 2015.26 Our study using the NIS, and a previous study using the Nationwide Readmissions Database, showed a rise in DA‐IE that corresponds to this general period.23
Geographically, it is very interesting that the geographical area with the highest annual percent increase, as well as the highest incidence rate of cardiac surgery, is the Midwest. A previous study looking at geographical variations of cardiovascular mortality among US counties during 1980–2014 shows that counties with mortality rates attributed to IE >90th percentile reached all but 16 states and had clusters in Alaska, the Mountain West, Eastern Nebraska, Northern Indiana, Eastern Pennsylvania, Upstate New York, Southern Louisiana, and Central Georgia.27 A retrospective chart review of IE cases at 1 center in North Carolina showed an increasing rate of injection DA‐IE and specifically showed that these patients were more likely to reside in rural communities.28 Recently, opiates have been linked with community outbreaks of human immunodeficiency virus and hepatitis C in rural communities in the Midwest and Southeast United States.29, 30 Also, the US health system started using ICD‐10 for coding instead of ICD‐9 in October 2015, increasing the codes of DA from 97 to 253 codes, with an opioid‐related diagnosis code increase from 20 ICD‐9 to 100 ICD‐10 codes.31 Heslin et al32 examined the number of opioid‐related stays quarterly before, during, and after the transition and found that it increased by 5% just before, 14% during, and 3.5% after the transition. Accordingly, the sharp increase in opioid‐related stays indicates that we probably have had underestimated opioid abuse while using ICD‐9, which is less sensitive in diagnosing opioid‐related hospitalizations compared with ICD‐10.
Limitations
Our study has several limitations, most of which were inherent to the administrative database and billing codes that were used. This included reliance on reports using ICD‐9 and ICD‐10 codes. Observation hospitalizations are not part of the NIS database, and it is possible that the outcomes could be altered. In addition, hospital transfers were excluded per NIS database management recommendations to avoid double counting (the same patient would have 2 admissions in 2 different hospitals—the transferred from and the transferred to—and would thus be counted twice). This does lead to a bias in surgical selection, given that patients transferred to tertiary centers for surgical management may have been excluded. In addition, there is no definite and reliable way to ascertain that the death was related to DA or IE. Rather, we used the “overall” inpatient mortality as provided by the NIS database. Furthermore, the data analyzed only provide a geographical breakdown by region and do not break down the data further to a state level or urban versus rural communities. Finally, we acknowledge the limitation that APC assumes linear increase or decrease over time, and it is an average; however, DA‐IE has no linear relationship over time, and, accordingly, APC could be misleading. However, these limitations are balanced by the large and nationally represented cohort and the comprehensive nature of the data recorded.
Conclusions
We showed that DA‐IE is increasing annually in the United States over the past 14 years. This disease is widespread, and all regions of the United States are affected. The patient population most at risk is young, lower socioeconomic class, has less cardiac comorbidities, and is white. Our findings have important clinical and public health implications. Care for patients with DA‐IE is very complex, and we agree with the American Association for Thoracic Surgery guidelines that it involves a “specialized endocarditis team”—including cardiology, cardiac surgery, infectious disease, neurologist, nephrologist, addiction specialists, case management, and nursing.33 Unfortunately, treatment for opioid addiction after hospitalization is low.34 The 2 most common reasons for this are that patients were not ready to stop using illicit drugs or they did not have healthcare coverage to afford appropriate treatment.35 Care of patients with DA‐IE after discharge should include follow‐up with drug rehabilitation.36 Unfortunately, DA‐IE patients have a ≈10‐fold higher hazard of death or reoperation compared with patients who do not inject drugs 3 to 6 months after an operation for IE, and it is suspected that this is attributed to continued drug use.37 We highlight the need to have access to standardized endocarditis teams across hospital systems. Further research is needed on a geographical level to identify clusters or “hot zones” of DA‐IE outbreaks, such as in rural areas where opioids are more likely to be abused. Last, we show the need for resource allocations and public health interventions to target the young, poor, white population, most at risk at a national level.
Disclosures
None.
Supporting information
Table S1. ICD‐9 and ICD‐10 of Infective Endocarditis, Comorbidities, and Surgeries
(J Am Heart Assoc. 2019;8:e012969 DOI: 10.1161/JAHA.119.012969.)
References
- 1. Rudd RA, Seth P, David F, Scholl L. Increases in drug and opioid‐involved overdose deaths—United States, 2010–2015. MMWR Morb Mortal Wkly Rep. 2016;65:1445–1452. [DOI] [PubMed] [Google Scholar]
- 2. Substance Abuse and Mental Health Services Administration . Results From the 2013 National Survey on Drug Use and Health: Summary of National Findings, NSDUH Series H‐48, HHS Publication No. (SMA) 14‐4863. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2014. [Google Scholar]
- 3. Opioid overdose. Available at: https://www.cdc.gov/drugoverdose/epidemic/index.html. Accessed February 22, 2019.
- 4. Centers for Disease Control and Prevention . Trends in drug‐poisoning deaths involving opioid analgesics and heroin: United States, 1999–2012. Available at: http://www.cdc.gov/nchs/data/hestat/drug_poisoning/drug_poisoning.htm. Accessed November 28, 2018.
- 5. HHS acting secretary declares public health emergency to address national opioid crisis. Available at: https://www.hhs.gov/about/news/2017/10/26/hhs-acting-secretary-declares-public-health-emergency-address-national-opioid-crisis.html. Accessed December 9, 2018.
- 6. Ronan MV, Herzig SJ. Hospitalizations related to opioid abuse/dependence and associated serious infections increased sharply, 2002–12. Health Aff (Millwood). 2016;35:832–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kim JB, Ejiofor JI, Yammine M, Ando M, Camuso JM, Youngster I, Nelson SB, Kim AY, Melnitchouk SI, Rawn JD, MacGillivray TE. Surgical outcomes of infective endocarditis among intravenous drug users. J Thorac Cardiovasc Surg. 2016;152:832–841. [DOI] [PubMed] [Google Scholar]
- 8. Rabkin DG, Mokadam NA, Miller DW, Goetz RR, Verrier ED, Aldea GS. Long‐term outcome for the surgical treatment of infective endocarditis with a focus on intravenous drug users. Ann Thorac Surg. 2012;93:51–57. [DOI] [PubMed] [Google Scholar]
- 9. Murdoch DR, Corey GR, Hoen B, Miró JM, Fowler VG, Bayer AS, Karchmer AW, Olaison L, Pappas PA, Moreillon P, Chambers ST. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: the International Collaboration on Endocarditis‐Prospective Cohort Study. Arch Intern Med. 2009;169:463–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Thuny F, Grisoli D, Collart F, Habib G, Raoult D. Management of infective endocarditis: challenges and perspectives. Lancet. 2012;379:965–975. [DOI] [PubMed] [Google Scholar]
- 11. Ternhag A, Cederstrom A, Torner A, Westling K. A nationwide cohort study of mortality risk and long‐term prognosis in infective endocarditis in Sweden. PLoS One. 2013;8:e67519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Que YA, Moreillon P. Infective endocarditis. Nat Rev Cardiol. 2011;8:322–336. [DOI] [PubMed] [Google Scholar]
- 13. Toyoda N, Chikwe J, Itagaki S, Gelijns AC, Adams DH, Egorova NN. Trends in infective endocarditis in California and New York State, 1998–2013. JAMA. 2017;317:1652–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wurcel AG, Anderson JE, Chui KK, Skinner S, Knox TA, Snydman DR, Stopka TJ. Increasing infectious endocarditis admissions among young people who inject drugs. Open Forum Infect Dis. 2016;3:ofw157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bor DH, Woolhandler S, Nardin R, Brusch J, Himmelstein DU. Infective endocarditis in the U.S., 1998–2009: a nationwide study. PLoS One. 2013;8:e60033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Deo SV, Raza S, Kalra A, Deo VS, Altarabsheh SE, Zia A, Khan MS, Markowitz AH, Sabik JF, Park SJ. Admissions for infective endocarditis in intravenous drug users. J Am Coll Cardiol. 2018;71:1596–1597. [DOI] [PubMed] [Google Scholar]
- 17. Njoroge LW, Al‐Kindi SG, Koromia GA, ElAmm CA, Oliveira GH. Changes in the association of rising infective endocarditis with mortality in people who inject drugs. JAMA Cardiol. 2018;3:779–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schranz AJ, Fleischauer A, Chu VH, Wu LT, Rosen DL. Trends in drug use‐associated infective endocarditis and heart valve surgery, 2007 to 2017: a study of statewide discharge data. Ann Intern Med. 2019;170:31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gray ME, Rogawski McQuade ET, Scheld WM, Dillingham RA. Rising rates of injection drug use associated infective endocarditis in Virginia with missed opportunities for addiction treatment referral: a retrospective cohort study. BMC Infect Dis. 2018;18:532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. NIS Database Documentation . Healthcare Cost and Utilization Project (HCUP). Agency for Healthcare Research and Quality. Available at: https://www.hcup-us.ahrq.gov/db/nation/nis/nisdbdocumentation.jsp. Accessed February 26, 2018.
- 21. Creation of Format Library for Elixhauser Comorbidity Groups Elixhauser Comorbidity Software, Version 3.7. Available at: https://www.hcup-us.ahrq.gov/toolssoftware/comorbidity/comformat2012-2015.txt. Accessed February 26, 2018.
- 22. Creation of Format Library for Comorbidity Groups ICD‐10‐CM Elixhauser Comorbidity Software Version 2019.1. Available at: https://www.hcup-us.ahrq.gov/toolssoftware/comorbidityicd10/comformat_icd10cm_2019_1.txt. Accessed November 15, 2018.
- 23. Rudasill SE, Sanaiha Y, Mardock AL, Khoury H, Xing H, Antonios JW, McKinnell JA, Benharash P. Clinical outcomes of infective endocarditis in injection drug users. J Am Coll Cardiol. 2019;73:559–570. [DOI] [PubMed] [Google Scholar]
- 24. Theisen K, Jacobs B, Macleod L, Davies B. The United States opioid epidemic: a review of the surgeon's contribution to it and health policy initiatives. BJU Int. 2018;122:754–759. [DOI] [PubMed] [Google Scholar]
- 25. Kolodny A, Courtwright DT, Hwang CS, Kreiner P, Eadie JL, Clark TW, Alexander GC. The prescription opioid and heroin crisis: a public health approach to an epidemic of addiction. Annu Rev Public Health. 2015;36:559–574. [DOI] [PubMed] [Google Scholar]
- 26. Madras BK. The surge of opioid use, addiction, and overdoses: responsibility and response of the US health care system. JAMA Psychiatry. 2017;74:441–442. [DOI] [PubMed] [Google Scholar]
- 27. Roth GA, Dwyer‐Lindgren L, Bertozzi‐Villa A, Stubbs RW, Morozoff C, Naghavi M, Mokdad AH, Murray CJ. Trends and patterns of geographic variation in cardiovascular mortality among US counties, 1980–2014. JAMA. 2017;317:1976–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hartman L, Barnes E, Bachmann L, Schafer K, Lovato J, Files DC. Opiate injection‐associated infective endocarditis in the southeastern United States. Am J Med Sci. 2016;352:603–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zibbell JE, Iqbal K, Patel RC, Suryaprasad A, Sanders KJ, Moore‐Moravian L, Serrecchia J, Blankenship S, Ward JW, Holtzman D. Increases in hepatitis C virus infection related to injection drug use among persons aged </=30 years—Kentucky, Tennessee, Virginia, and West Virginia, 2006–2012. MMWR Morb Mortal Wkly Rep. 2015;64:453–458. [PMC free article] [PubMed] [Google Scholar]
- 30. Conrad C, Bradley HM, Broz D, Buddha S, Chapman EL, Galang RR, Hillman D, Hon J, Hoover KW, Patel MR, Perez A. Community outbreak of HIV infection linked to injection drug use of oxymorphone‐Indiana, 2015. MMWR Morb Mortal Wkly Rep. 2015;64:443–444. [PMC free article] [PubMed] [Google Scholar]
- 31. Moore BJ, Barrett ML. Case study: exploring how opioid‐related diagnosis codes translate from ICD‐9‐CM to ICD‐10‐CM. US Agency for Healthcare Research and Quality. 2017;29:2018.
- 32. Heslin KC, Owens PL, Karaca Z, Barrett ML, Moore BJ, Elixhauser A. Trends in opioid‐related inpatient stays shifted after the US transitioned to ICD‐10‐CM diagnosis coding in 2015. Med Care. 2017;55:918–923. [DOI] [PubMed] [Google Scholar]
- 33. Pettersson GB, Coselli JS, Hussain ST, Griffin B, Blackstone EH, Gordon SM, LeMaire SA, Woc‐Colburn LE. 2016 The American Association for Thoracic Surgery (AATS) consensus guidelines: surgical treatment of infective endocarditis: executive summary. J Thorac Cardiovasc Surg. 2017;6:1241–1258. [DOI] [PubMed] [Google Scholar]
- 34. Rosenthal ES, Karchmer AW, Theisen‐Toupal J, Castillo RA, Rowley CF. Suboptimal addiction interventions for patients hospitalized with injection drug use‐associated infective endocarditis. Am J Med. 2016;129:481–485. [DOI] [PubMed] [Google Scholar]
- 35. Lipari RN, Van Horn SL. Trends in Substance Use Disorders Among Adults Aged 18 or Older. The CBHSQ Report: Center for Behavioral Health Statistics and Quality Substance Abuse and Mental Health Services Administration (US) Available at: https://www.samhsa.gov/data/sites/default/files/report_2790/ShortReport-2790.html. Accessed December 11, 2018. [PubMed] [Google Scholar]
- 36. Hussain ST, Gordon SM, Streem DW, Blackstone EH, Pettersson GB. Contract with the patient with injection drug use and infective endocarditis: surgeons perspective. J Thorac Cardiovasc Surg. 2017;154:2002–2003. [DOI] [PubMed] [Google Scholar]
- 37. Shrestha NK, Jue J, Hussain ST, Jerry JM, Pettersson GB, Menon V, Navia JL, Nowacki AS, Gordon SM. Injection drug use and outcomes after surgical intervention for infective endocarditis. Ann Thorac Surg. 2015;100:875–882. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. ICD‐9 and ICD‐10 of Infective Endocarditis, Comorbidities, and Surgeries


