Abstract
Background
Disrupted circadian rhythm of blood pressure is commonly observed in patients in the intensive care unit (ICU). This study assessed the association of nocturnal mean arterial pressure rising (NMAPR) with short‐ and long‐term mortality in critically ill adult patients.
Methods and Results
Adult patients with a complete record of mean arterial pressure monitoring during the first 24 hours of ICU stay in the Multiparameter Intelligent Monitoring in Intensive Care II (MIMIC‐II) database were included in this retrospective cohort study. All patients were divided into the non‐NMAPR group (≤1) or the NMAPR group (>1), according to the value of mean nighttime divided by daytime mean arterial pressure. The associations of NMAPR with ICU, hospital, 28‐day, and 1‐year mortality were assessed using multivariable logistic regression or a Cox proportional hazards model. Interaction and subgroup analyses were performed for those patients who had a first Sequential Organ Failure Assessment (SOFA) score of ≥8 or <8. The overall cohort comprised 5185 patients. The patients with NMAPR (n=1865) had higher ICU, hospital, 28‐day, and 1‐year mortality than the non‐NMAPR group (n=3320). After adjusting for covariates, the analysis showed that NMAPR was significantly associated with mortality in the ICU (odds ratio: 1.34; 95% CI, 1.10–1.65), in the hospital (odds ratio: 1.35; 95% CI, 1.12–1.63), at 28 days (hazard ratio: 1.27; 95% CI, 1.10–1.48), and at 1 year (hazard ratio: 1.24; 95% CI, 1.10–1.40). All results of the interaction analysis had no statistical significance, and similar results persisted in the patients with different SOFA scores.
Conclusions
NMAPR may aid in the early identification of critically ill patients at high risk of ICU, hospital, 28‐day, or 1‐year mortality.
Keywords: circadian rhythm, intensive care unit, mean arterial pressure, mortality, Multiparameter Intelligent Monitoring in Intensive Care II
Subject Categories: Mortality/Survival, Risk Factors, Clinical Studies
Clinical Perspective
What Is New?
Nocturnal mean arterial pressure rising within the first 24 hours of admission might serve as an independent risk factor for intensive care unit, hospital, 28‐day, and 1‐year mortality.
The impact of nocturnal mean arterial pressure rising on mortality was consistent in patients with different Sequential Organ Failure Assessment scores.
What Are the Clinical Implications?
These results highlight the importance of circadian rhythms for early risk stratification and personalized mean arterial pressure management in the intensive care unit.
Patients in the intensive care unit (ICU) are exposed to many pathophysiological, psychological, environmental, and iatrogenic factors including artificial light, noise, ventilation, parenteral nutrition, and medications.1, 2 These factors may affect physiological homeostasis, including the circadian rhythms of sleep architecture, core body temperature, and blood pressure (BP).3 Previous studies have reported that these disturbances may contribute to a series of adverse outcomes. Sleep disruption has been hypothesized to contribute to emotional distress, ICU delirium,4, 5 prolonged duration of mechanical ventilation,6 deranged immune functions,7 and neurocognitive dysfunction.8 The degree of abnormal circadian rhythm of core body temperature is associated with greater Acute Physiology and Chronic Health Evaluation III scores, suggesting that changes in circadian rhythms may directly or indirectly contribute to the severity of illness in critically ill patients.3
BP, which is constantly monitored in the ICU, is a physiological parameter with diagnostic value in hypotension and hypertension. Circadian BP rhythm is recognized as an independent risk factor for cardiovascular disease and mortality.9 Our previous studies have shown that abnormal circadian variation of BP might elevate the risk of cardiovascular disease, diabetes mellitus, formation of carotid plaque, and metabolic syndrome.10, 11, 12, 13, 14 Mean arterial pressure (MAP), which is calculated as 1/3 (systolic BP, SBP) +2/3 (diastolic BP, DBP), is a routinely monitored parameter used to assess tissue perfusion in the ICU setting.15 Our group found that lower MAP fluctuation in patients during their ICU stay was associated with both ICU and hospital mortality.16 In this study, we extracted the MAP records of the first 24 hours of ICU admission from the Multiparameter Intelligent Monitoring in Intensive Care II (MIMIC‐II) database. We aimed to explore the associations of circadian MAP characteristics with mortality to facilitate early risk stratification in the ICU.
Methods
Study Design
The data that support the findings of this study are available from the corresponding author on reasonable request. We performed a retrospective cohort study using the patients’ data from the MIMIC‐II database maintained by Beth Israel Deaconess Medical Center (Boston, MA) and the Massachusetts Institute of Technology (Cambridge, MA). The database contains the medical information of >32 000 patients who were hospitalized in the ICU at Beth Israel Deaconess Medical Center from 2001 to 2008, including demographic characteristics, vital signs, laboratory and radiology results, medications, comorbidities, nursing notes, physician discharge summaries, and survival outcomes.17 Any researcher who agrees to the terms of the database must complete the “protecting human subjects” training and then can obtain access to the data.
Study Population
All patient information was obtained from the MIMIC‐II database (v2.6). The inclusion criteria were (1) age ≥15 years at ICU admission and (2) day and night MAP records during the first 24 hours in the ICU. The exclusion criteria were (1) multiple ICU admissions, (2) length of ICU stay <1 day, (3) vasoactive and sedative medication usage on day 1 of admission, (4) MAP measured <12 times on the day 1 of admission, and (5) missing covariate data for multivariate adjustments. All diseases in our study were classified using the International Classification of Diseases, Ninth Revision (ICD‐9).
Given that all patient records in the MIMIC‐II database were anonymized, the requirement for individual patient consent was waived by the institutional review board of Beth Israel Deaconess Medical Center.17
Data Extraction
The following patient data were extracted from the MIMIC‐II database: age, sex, ethnicity, length of ICU stay, the first Sequential Organ Failure Assessment (SOFA) score, mechanical ventilation (noninvasive and invasive), medication usage on day 1 of admission to the ICU (including vasopressor medications and sedatives), diseases (diabetes mellitus, hypertension, hypotension, heart failure, coronary heart disease, cerebral hemorrhage, shock, respiration failure, renal failure, self‐poisoning, infection, coma, and delirium), complete MAP records, number of daily MAP records, and survival outcomes (ICU, hospital, 28 days, 1 year). Vasopressor medications included norepinephrine, phenylephrine, dopamine, isoproterenol, epinephrine, and vasopressin, and vasodepressor medications included nitroprusside, nicardipine, labetalol, esmolol, and diltiazem. The sedatives indexed in the database were pentobarbital, propofol, midazolam, dexmedetomidine, and diazepam. Data were extracted using SAS v9.4 (SAS Institute).
Circadian Rhythm of MAP
MAP was measured via invasive monitoring from an arterial line using a bedside monitor (Component Monitoring System IntelliVue MP‐70; Philips Healthcare) during the first 24 hours in the ICU. In this study, we focused on differences between average nighttime and daytime MAP (mm Hg). Consequently, the circadian rhythm of MAP was calculated as mean nighttime MAP divided by mean daytime MAP. Mean nighttime MAP was calculated as all MAP values during the night divided by the number of MAP examinations, and the mean daytime MAP was calculated as all MAP values during the day divided by the number of MAP examinations. Patients were divided into 2 groups according to the circadian rhythm of MAP: the nocturnal MAP rising (NMAPR) group (>1) and the non‐NMAPR group (≤1). The mean daytime MAP value was computed as the average of all MAP values from 7 am to 11 pm, and mean nighttime MAP was from 11 pm to 7 am the next day.
Outcomes
The primary outcome measures in our study were ICU and hospital mortality. The secondary outcome measures were 28‐day and 1‐year mortality. In addition, ICU, hospital, and 28‐day mortality were defined as short‐term mortality, and 1‐year mortality was defined as long‐term mortality.
Statistical Analysis
Data for continuous and categorical variables were presented as median with interquartile range and frequency with percentage, respectively. Continuous and categorical variables were compared using the Mann–Whitney test and the χ2 or Fisher exact test, respectively. To assess the association of NMAPR with the study outcomes, we used univariate and multivariate logistic regression models for ICU and hospital mortality and Cox proportional hazards models for 28‐day and 1‐year mortality. In multivariate regression analyses, 5 models were built to show the modeling process, which could prove the stability of the association of NMAPR with mortality. Model 1 was adjusted for age, sex, and ethnicity. Model 2 was adjusted for the covariates included in model 1 plus average of MAP. Model 3 was adjusted for the covariates included in models 1 and 2 and for SOFA score. Model 4 was adjusted for the covariates included in model 3 plus ventilation. Model 5 was the best model, which incorporated model 4 along with some diagnoses, for example, heart failure, diabetes mellitus, hypertension, hypotension, coronary heart disease, cerebral hemorrhage, shock, respiration failure, renal failure, self‐poisoning, infection, coma, and delirium. The odds ratios (ORs) were generated for logistic regression and hazard ratios (HRs) were generated for Cox proportional hazards, with their 95% CIs. Kaplan–Meier survival analysis was performed for 28‐day and 1‐year mortality.
Sensitivity Analyses
The SOFA score, a measure of organ dysfunction, is associated with patient outcomes. We conducted interaction and subgroup analyses to determine whether the results persisted even when the severity of the clinical status changed.18 Based on the median first SOFA score, all patients were divided into the low (<8) and high (≥8) SOFA score groups. Logistic regression and Cox proportional hazards models were used to evaluate the circadian rhythm of MAP and outcomes in the subgroups.
All statistical analyses were performed using SAS v9.4. A 2‐sided P<0.05 was considered significant.
Results
Patient Selection, Characteristics, and Outcomes
Data from a total of 32 536 patients were screened from the MIMIC‐II database. Among these, 12 680 patients had day and night MAP records during the first 24 hours in the ICU. We excluded 3298 patients with multiple ICU admissions, 867 patients with ICU length of stay <1 day, 3087 patients with vasoactive medications and sedatives, 131 patients with MAP measured <12 times on the day 1 of admission, and 112 patients with missing covariates. Finally, 5185 patients were included in the analysis (Figure 1). The NMAPR group had 1865 patients. The median age of all patients was 65 years, and 60.0% were men. The average daily measurement frequency of MAP was 30.76±10.28. On the first day of admission, 1401 patients (27.0%) were ventilated. Table 1 shows the details of all values across the classifications of the circadian rhythm of MAP.
Figure 1.
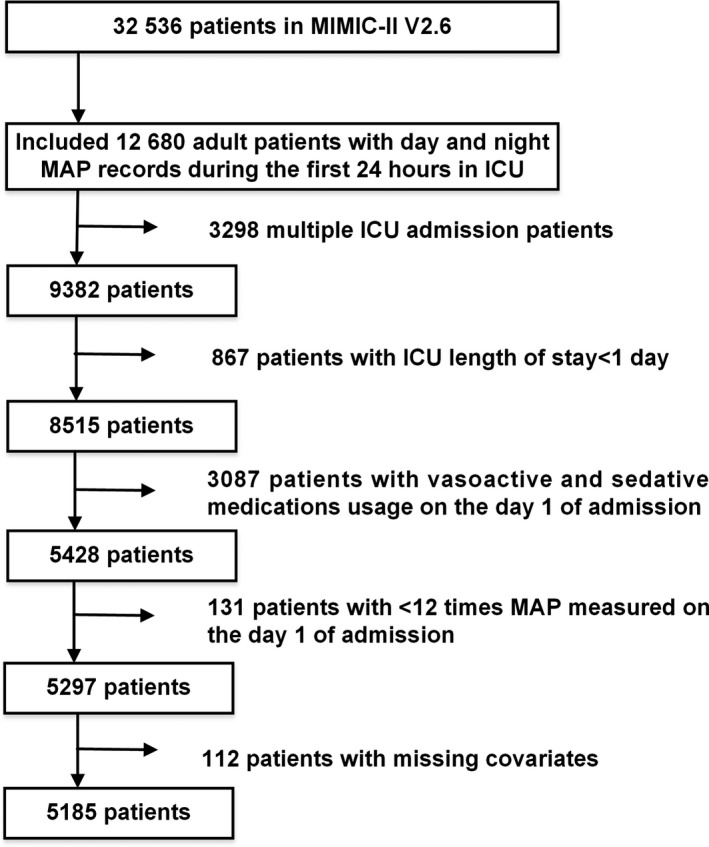
Flowchart of participant selection. A total of 5158 patients were included in the analysis. ICU indicates intensive care unit; MAP, mean arterial pressure; MIMIC‐II, Multiparameter Intelligent Monitoring in Intensive Care II.
Table 1.
Characteristics of Study Participants
| Characteristic | Non‐NMAPR Group (n=3320) | NMAPR Group (n=1865) |
|---|---|---|
| Age, y, n (%) | ||
| 15–44 | 420 (12.7) | 279 (15.0) |
| 45–59 | 730 (22.0) | 408 (21.9) |
| ≥60 | 2170 (65.4) | 1178 (63.2) |
| Male, n (%) | 2016 (60.7) | 1096 (58.8) |
| Ethnicity, n (%) | ||
| White | 2418 (72.8) | 1382 (74.1) |
| Black | 141 (4.2) | 108 (5.8) |
| Asian | 97 (2.9) | 49 (2.6) |
| Hispanic/Latino | 97 (2.9) | 47 (2.5) |
| Unknown/other | 567 (17.1) | 279 (15.0) |
| Day 1 SOFA, median (IQR) | 8 (5–10) | 8 (5–10) |
| MAP, mm Hg, median (IQR) | 77.8 (72.17–85.59) | 78.9 (72.54–86.71) |
| Day 1 ventilation, n (%) | 841 (25.3) | 560 (30.0) |
| Diseases by ICD‐9, n (%) | ||
| Diabetes mellitus | 849 (25.6) | 484 (26.0) |
| Hypertension | 1256 (37.8) | 681 (36.5) |
| Hypotension | 235 (7.1) | 124 (6.6) |
| Heart failure | 596 (18.0) | 384 (20.6) |
| Coronary heart disease | 585 (17.6) | 274 (14.7) |
| Cerebral hemorrhage | 170 (5.1) | 118 (6.3) |
| Shock | 285 (8.6) | 217 (11.6) |
| Respiration failure | 487 (14.7) | 343 (18.4) |
| Renal failure | 602 (18.1) | 403 (21.6) |
| Self‐poisoning | 37 (1.1) | 26 (1.4) |
| Infection | 71 (2.1) | 39 (2.1) |
| Coma | 133 (4.0) | 97 (5.2) |
| Delirium | 20 (0.6) | 5 (0.3) |
| Mortality, n (%) | ||
| ICU | 307 (9.2) | 250 (13.4) |
| Hospital | 373 (11.2) | 298 (16.0) |
| 28 d | 408 (12.3) | 313 (16.8) |
| 1 y | 612 (18.4) | 443 (23.8) |
ICD‐9 indicates International Classification of Diseases, Ninth Revision; ICU, intensive care unit; IQR, interquartile range; MAP, mean arterial pressure; NMAPR, nocturnal mean arterial pressure rising; SOFA, Sequential Organ Failure Assessment.
Association Between NMAPR and Mortality
Patients in the non‐NMAPR group had lower ICU, hospital, 28‐day, and 1‐year mortality than those in the NMAPR group (9.2% versus 13.4%, 11.2% versus 16.0%, 12.3% versus 16.8%, and 18.4% versus 23.8%, respectively). To verify these findings, multivariate logistic regression analyses and multivariate Cox proportional hazards models were used by adjusting all covariates, including age, sex, ethnicity, average of MAP, first SOFA score, ventilation in the first day, heart failure, diabetes mellitus, hypertension, hypotension, coronary heart disease, cerebral hemorrhage, shock, respiration failure, renal failure, self‐poisoning, infection, coma, and delirium. The results were similar to those obtained in the univariate regression analyses (ICU mortality: OR: 1.34 [95% CI, 1.10–1.65]; hospital mortality: OR: 1.35 [95% CI, 1.12–1.63]; 28‐day mortality: HR: 1.27 [95% CI, 1.10–1.48]; 1‐year mortality: HR: 1.24 [95% CI, 1.10–1.40]; Table 2). Kaplan–Meier survival curves revealed that the 28‐day and 1‐year probability of survival was higher in the non‐NMAPR group than that in the NMAPR group (Figure 2).
Table 2.
ORs and HRs With 95% CIs for Mortality Associated With NMAPR in the ICU
| Regression Models | Mortality | |||
|---|---|---|---|---|
| ICU, OR (95% CI) | Hospital, OR (95% CI) | 28 d, HR (95% CI) | 1 y, HR (95% CI) | |
| Univariate | 1.52 (1.27–1.81) | 1.50 (1.28–1.77) | 1.40 (1.21–1.63) | 1.34 (1.18–1.51) |
| Multivariate | ||||
| Model 1 | 1.56 (1.30–1.86) | 1.54 (1.31–1.82) | 1.44 (1.24–1.67) | 1.38 (1.22–1.55) |
| Model 2 | 1.56 (1.30–1.87) | 1.54 (1.31–1.82) | 1.44 (1.24–1.67) | 1.37 (1.22–1.55) |
| Model 3 | 1.44 (1.20–1.73) | 1.44 (1.21–1.71) | 1.34 (1.16–1.55) | 1.31 (1.16–1.48) |
| Model 4 | 1.40 (1.15–1.70) | 1.40 (1.17–1.68) | 1.30 (1.12–1.50) | 1.28 (1.13–1.45) |
| Model 5 | 1.34 (1.10–1.65) | 1.35 (1.12–1.63) | 1.27 (1.10–1.48) | 1.24 (1.10–1.40) |
Model 1 was adjusted for age, sex, and ethnicity. Model 2 was adjusted for model 1 plus an average of mean arterial pressure. Model 3 was adjusted for model 2 plus Sequential Organ Failure Assessment score. Model 4 was adjusted for model 3 plus ventilation. Model 5 was adjusted for model 4 plus primary diagnoses such as heart failure, diabetes mellitus, hypertension, hypotension, coronary heart disease, cerebral hemorrhage, shock, respiration failure, renal failure, self‐poisoning, infection, coma, and delirium. HR indicates hazard ratio; ICU, intensive care unit; NMAPR, nocturnal mean arterial pressure rising; OR, odds ratio.
Figure 2.
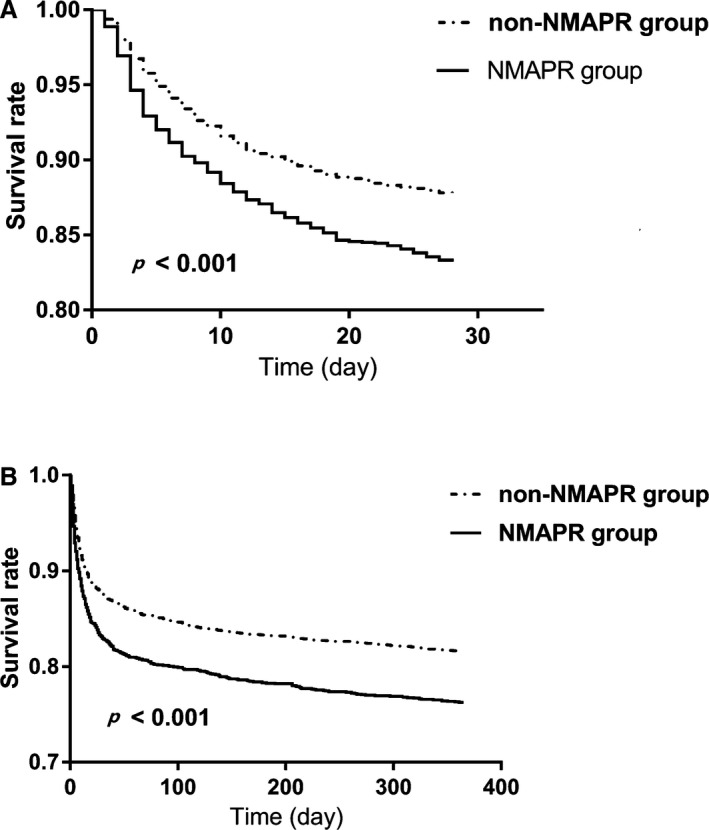
Kaplan–Meier survival analysis plot for 28‐day and 1‐year mortality with NMAPR. The curves show that patients with NMAPR in the ICU had lower rates of 28‐day survival (A) and 1‐year survival (B). ICU indicates intensive care unit; NMAPR, nocturnal mean arterial pressure rising.
Sensitivity Analysis
When stratified by SOFA score (≥8 [2522 patients] versus <8 [2663 patients]), no significant interaction was observed (ICU mortality, P interaction=0.866; hospital, P interaction=0.992; 28‐day, P interaction=0.980; 1‐year, P interaction=0.541). In the subgroup analyses, we found that our results persisted in the different subgroups after adjustment for all covariates, that is, the NMAPR group had a higher risk of mortality than the non‐NMAPR group. In the group with low SOFA score, ICU and hospital mortality had ORs of 1.43 (95% CI, 1.01–2.02) and 1.44 (95% CI, 1.06–1.95), respectively; 28‐day and 1‐year mortality had HRs of 1.34 (95% CI, 1.05–1.71) and 1.24 (95% CI, 1.02–1.50), respectively. In the group with high SOFA score, ICU and hospital mortality had ORs of 1.39 (95% CI, 1.08–1.79) and 1.37 (95% CI, 1.07–1.75), respectively; 28‐day and 1‐year mortality had HRs of 1.25 (95% CI, 1.04–1.51) and 1.27 (95% CI, 1.08–1.49), respectively (Table 3).
Table 3.
ORs and HRs With 95% CIs for Mortality Associated With NMAPR in Patients With Different SOFA Scores
| Regression Models | Mortality | |||
|---|---|---|---|---|
| ICU, OR (95% CI) | Hospital, OR (95% CI) | 28 d, HR (95% CI) | 1 y, HR (95% CI) | |
| SOFA <8 (n=2522) | ||||
| Univariate | 1.36 (1.00–1.86) | 1.38 (1.05–1.82) | 1.32 (1.03–1.68) | 1.22 (1.01–1.48) |
| Multivariate | ||||
| Model 1 | 1.44 (1.05–1.98) | 1.47 (1.11–1.93) | 1.39 (1.09–1.78) | 1.30 (1.07–1.57) |
| Model 2 | 1.43 (1.04–1.97) | 1.45 (1.10–1.92) | 1.39 (1.09–1.78) | 1.29 (1.07–1.57) |
| Model 3 | 1.41 (1.01–1.96) | 1.44 (1.08–1.93) | 1.37 (1.08–1.75) | 1.29 (1.07–1.56) |
| Model 4 | 1.43 (1.01–2.02) | 1.44 (1.06–1.95) | 1.34 (1.05–1.71) | 1.24 (1.02–1.50) |
| SOFA ≥8 (n=2663) | ||||
| Univariate | 1.60 (1.28–1.99) | 1.57 (1.28–1.93) | 1.45 (1.20–1.75) | 1.42 (1.21–1.67) |
| Multivariate | ||||
| Model 1 | 1.60 (1.28–1.99) | 1.57 (1.27–1.93) | 1.45 (1.20–1.75) | 1.43 (1.22–1.68) |
| Model 2 | 1.60 (1.29–2.00) | 1.57 (1.27–1.94) | 1.45 (1.20–1.75) | 1.43 (1.22–1.68) |
| Model 3 | 1.48 (1.17–1.87) | 1.45 (1.16–1.82) | 1.31 (1.09–1.58) | 1.32 (1.12–1.55) |
| Model 4 | 1.39 (1.08–1.79) | 1.37 (1.07–1.75) | 1.25 (1.04–1.51) | 1.27 (1.08–1.49) |
Model 1 was adjusted for age, sex, and ethnicity. Model 2 was adjusted for model 1 plus an average of mean arterial pressure. Model 3 was adjusted for model 2 plus ventilation. Model 4 was adjusted for model 3 plus primary diagnoses: heart failure, diabetes mellitus, hypertension, hypotension, coronary heart disease, cerebral hemorrhage, shock, respiration failure, renal failure, self‐poisoning, infection, coma, and delirium. HR indicates hazard ratio; ICU, intensive care unit; NMAPR, nocturnal mean arterial pressure rising; OR, odds ratio; SOFA, Sequential Organ Failure Assessment.
Discussion
Circadian rhythm is a universal intrinsic timekeeping system with a duration of close to 24 hours. This system is generated to adapt to changes in the external environment, specifically, to the cyclic changes in light and darkness caused by Earth's rotation around the sun.19 In the human body, circadian rhythms are controlled by a master clock in the suprachiasmatic nucleus of the anterior hypothalamus and are modulated by melatonin, the neurohormone of the pineal gland.1, 20 As a result of the alterations in various zeitgebers such as light/dark cycles, social interactions, eating and drinking patterns, and pharmacologic treatments, the circadian rhythms of sleep architecture, heart rate, core body temperature, and BP may be changed in the ICU, which may result in adverse outcomes.2, 21 Our group previously investigated the day/night variation of MAP during the full length of ICU stays and was the first to find that lower MAP fluctuations (between −5% and 5%) in critically ill patients were associated with both ICU and hospital mortality.16
Published studies have reported the relationship between circadian BP variation and cardiovascular or all‐cause mortality in community‐dwelling, hospitalized, and ICU patients. Fagard et al performed a meta‐analysis of 3468 hypertensive patients from 4 community‐based prospective studies in Europe and reported that the night/day BP ratio, including systolic and diastolic BP, was associated with all‐cause mortality.9 In another cohort with a mean follow‐up period of 6.3 years, nocturnal systolic BP decline of <10% was found to predict all‐cause mortality.22 MAP is one of the vital signs that can be used to evaluate organ perfusion and mortality in critically ill patients.23, 24 However, no studies have reported circadian BP variation and its prognostic value in the ICU. In this present study, we extracted complete MAP records of patients’ first 24 hours in the ICU and investigated the circadian characteristics of MAP to determine how to identify patients with a higher risk of mortality as early as possible. We found that NMAPR served as an important risk factor for higher mortality in the ICU (OR: 1.34; 95% CI, 1.10–1.65), in the hospital (OR: 1.35; 95% CI, 1.12–1.63), at 28 days (HR: 1.27; 95% CI, 1.10–1.48), and at 1 year (HR: 1.24; 95% CI 1.10–1.40) after adjusting for a series of covariates. To our knowledge, this study is the first in which the relationship between the circadian rhythm of MAP and short‐ and long‐term mortality has been evaluated.
The SOFA score is a powerful risk factor for ICU mortality.18, 25 To exclude the influence of the clinical status severity on the relationship between circadian rhythm of MAP and mortality, we divided the participants into 2 groups according to the SOFA score measured on day 1 of admission to the ICU and then conducted interaction and subgroup analyses. Findings suggested that the circadian rhythms of MAP have a similar effect on mortality in patients with different SOFA scores. The association between NMAPR and mortality remained significant in both subgroups. Consequently, NMAPR could be used as a reliable risk factor for ICU, hospital, 28‐day, and 1‐year mortality.
An abnormal circadian status of BP is mainly caused by endogenous neuroendocrine rhythm and day/night differences in physical activities and mental stress.26 Patients in the ICU usually experience tremendous anxiety and acute stress from infections, trauma, multiple organ dysfunctions, artificial light, noise, mechanical ventilation, enteral nutrition, and medications. These different factors could result in acute neuroendocrine responses, including metabolic, autonomic, and neurohumoral changes in, for example, growth hormone, adrenal steroids, thyrotropin, norepinephrine, gonadotropin, melatonin, and cortisol.2, 27, 28, 29 Previous studies have found that growth hormone is elevated in critically ill patients at night; this is associated with increased mortality.30, 31, 32 In addition, abnormal BP variation observed in patients aged about 70 years is associated with autonomic nervous system dysfunction and poor sleep quality, which is also common in ICU patients, unless they are sedated or unconscious.33 Nevertheless, to be clearly understood, the mechanism regulating circadian MAP variation should be explored in future studies.
This study has some limitations that should be considered. First, MIMIC‐II is a single‐center database, and thus selection bias (ie, Berkson bias) is inevitable. However, the recruited patients were from a variety of ICUs, including medical, surgical, and coronary care ICUs, and their data may reflect real‐world situations encountered by ICU physicians. Second, given the retrospective design, the data were previously collected. Therefore, some information such as the frequency of BP monitoring, noise level, and patient/nurse ratio, were missing. Although we have adjusted for as many covariates as possible and conducted a series of sensitivity analyses, a multicenter prospective study with adequate covariates is needed to further confirm the association between NMAPR and prognostic outcomes in critically ill patients. Third, we can provide only the association between NMAPR and mortality rather than causality. In the future, a well‐designed prospective study should be conducted to evaluate causality between NMAPR and mortality.
Conclusions
NMAPR may serve as an important risk factor for high ICU, hospital, 28‐day, and 1‐year mortality in critically ill patients.
Sources of Funding
Our study was only funded by the National Natural Science Foundation of China (81770057). This study was funded by the National Natural Science Foundation of China (81770057).
Disclosures
None.
(J Am Heart Assoc. 2019;8:e012388 DOI: 10.1161/JAHA.119.012388.)
References
- 1. Chan MC, Spieth PM, Quinn K, Parotto M, Zhang H, Slutsky AS. Circadian rhythms: from basic mechanisms to the intensive care unit. Crit Care Med. 2012;40:246–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Freedman NS, Gazendam J, Levan L, Pack AI, Schwab RJ. Abnormal sleep/wake cycles and the effect of environmental noise on sleep disruption in the intensive care unit. Am J Respir Crit Care Med. 2001;163:451–457. [DOI] [PubMed] [Google Scholar]
- 3. Gazendam JAC, Dongen HPAV, Grant DA, Freedman NS, Zwaveling JH, Schwab RJ. Altered circadian rhythmicity in patients in the ICU. Chest. 2013;144:483–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Helton MC, Gordon SH, Nunnery SL. The correlation between sleep deprivation and the intensive care unit syndrome. Heart Lung. 1980;9:464. [PubMed] [Google Scholar]
- 5. Trompeo AC, Vidi Y, Locane MD, Braghiroli A, Mascia L, Bosma K, Ranieri VM. Sleep disturbances in the critically ill patients: role of delirium and sedative agents. Minerva Anestesiol. 2011;77:604–612. [PubMed] [Google Scholar]
- 6. White DP, Douglas NJ, Pickett CK, Zwillich CW, Weil JV. Sleep deprivation and the control of ventilation. Am Rev Respir Dis. 1983;128:984–986. [DOI] [PubMed] [Google Scholar]
- 7. Bryant PA, Trinder J, Curtis N. Sick and tired: does sleep have a vital role in the immune system? Nat Rev Immunol. 2004;4:457–467. [DOI] [PubMed] [Google Scholar]
- 8. Devlin JW, Skrobik Y, Gelinas C, Needham DM, Slooter AJC, Pandharipande PP, Watson PL, Weinhouse GL, Nunnally ME, Rochwerg B, Balas MC, van den Boogaard M, Bosma KJ, Brummel NE, Chanques G, Denehy L, Drouot X, Fraser GL, Harris JE, Joffe AM, Kho ME, Kress JP, Lanphere JA, McKinley S, Neufeld KJ, Pisani MA, Payen JF, Pun BT, Puntillo KA, Riker RR, Robinson BRH, Shehabi Y, Szumita PM, Winkelman C, Centofanti JE, Price C, Nikayin S, Misak CJ, Flood PD, Kiedrowski K, Alhazzani W. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 2018;46:e825–e873. [DOI] [PubMed] [Google Scholar]
- 9. Fagard RH, Celis H, Thijs L, Staessen JA, Clement DL, De Buyzere ML, De Bacquer DA. Daytime and nighttime blood pressure as predictors of death and cause‐specific cardiovascular events in hypertension. Hypertension. 2008;51:55–61. [DOI] [PubMed] [Google Scholar]
- 10. Yan B, Sun L, Gao Y, Guo Q, Guo L, Wang X, Wang G. Blood pressure reverse dipping may associate with stable coronary artery disease in patients with essential hypertension: a cross‐sectional study. Sci Rep. 2016;6:25410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yan B, Yan H, Sun L, Yan X, Peng L, Wang Y, Wang G. Novel association between the reverse‐dipper pattern of ambulatory blood pressure monitoring and metabolic syndrome in men but not in women. Medicine (Baltimore). 2015;94:e2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sun L, Yan B, Gao Y, Su D, Peng L, Jiao Y, Wang Y, Han D, Wang G. Relationship between blood pressure reverse dipping and type 2 diabetes in hypertensive patients. Sci Rep. 2016;6:25053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yan B, Peng L, Han D, Sun L, Dong Q, Yang P, Zheng F, Ong H, Zeng L, Wang G. Blood pressure reverse‐dipping is associated with early formation of carotid plaque in senior hypertensive patients. Medicine (Baltimore). 2015;94:e604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yan B, Peng L, Dong Q, Zheng F, Yang P, Sun L, Gong S, Zeng L, Wang G. Reverse‐dipper pattern of blood pressure may predict lacunar infarction in patients with essential hypertension. Eur J Neurol. 2015;22:1022–1025. [DOI] [PubMed] [Google Scholar]
- 15. Lamia B, Chemla D, Richard C, Teboul JL. Clinical review: interpretation of arterial pressure wave in shock states. Crit Care. 2005;9:601–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gao Y, Wang Q, Li J, Zhang J, Li R, Sun L, Guo Q, Xia Y, Fang B, Wang G. Impact of mean arterial pressure fluctuation on mortality in critically ill patients. Crit Care Med. 2018;46:e1167–e1174. [DOI] [PubMed] [Google Scholar]
- 17. Saeed M, Villarroel M, Reisner AT, Clifford G, Lehman LW, Moody G, Heldt T, Kyaw TH, Moody B, Mark RG. Multiparameter Intelligent Monitoring in Intensive Care II: a public‐access intensive care unit database. Crit Care Med. 2011;39:952–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Raith EP, Udy AA, Bailey M, McGloughlin S, MacIsaac C, Bellomo R, Pilcher DV. Prognostic accuracy of the SOFA score, SIRS criteria, and qSOFA score for in‐hospital mortality among adults with suspected infection admitted to the intensive care unit. JAMA. 2017;317:290–300. [DOI] [PubMed] [Google Scholar]
- 19. Shibata S, Yu T, Hirao A. The adjustment and manipulation of biological rhythms by light, nutrition, and abused drugs. Adv Drug Deliv Rev. 2010;62:918–927. [DOI] [PubMed] [Google Scholar]
- 20. Telias I, Wilcox ME. Sleep and circadian rhythm in critical illness. Crit Care. 2019;23:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kiss K, Földesi I, Köves B, Csernus V, Molnár Z. Circadian rhythm disruption exists in ICU patients. Intensive Care Med Exp. 2015;3(Suppl 1):A428. [Google Scholar]
- 22. Brotman DJ, Davidson MB, Boumitri M, Vidt DG. Impaired diurnal blood pressure variation and all‐cause mortality. Am J Hypertens. 2008;21:92–97. [DOI] [PubMed] [Google Scholar]
- 23. De Backer D, Creteur J, Preiser JC, Dubois MJ, Vincent JL. Microvascular blood flow is altered in patients with sepsis. Am J Respir Crit Care Med. 2002;166:98–104. [DOI] [PubMed] [Google Scholar]
- 24. Hernandez G, Boerma EC, Dubin A, Bruhn A, Koopmans M, Edul VK, Ruiz C, Castro R, Pozo MO, Pedreros C, Veas E, Fuentealba A, Kattan E, Rovegno M, Ince C. Severe abnormalities in microvascular perfused vessel density are associated to organ dysfunctions and mortality and can be predicted by hyperlactatemia and norepinephrine requirements in septic shock patients. J Crit Care. 2013;28:538.e9–538.e14. [DOI] [PubMed] [Google Scholar]
- 25. Ferreira FL, Bota DP, Bross A, Melot C, Vincent JL. Serial evaluation of the sofa score to predict outcome in critically ill patients. JAMA. 2001;286:1754–1758. [DOI] [PubMed] [Google Scholar]
- 26. Fabbian F, Smolensky MH, Tiseo R, Pala M, Manfredini R, Portaluppi F. Dipper and non‐dipper blood pressure 24‐hour patterns: circadian rhythm‐dependent physiologic and pathophysiologic mechanisms. Chronobiol Int. 2013;30:17–30. [DOI] [PubMed] [Google Scholar]
- 27. Boldt J, Menges T, Kuhn D, Diridis C, Hempelmann G. Alterations in circulating vasoactive substances in the critically ill—a comparison between survivors and non‐survivors. Intensive Care Med. 1995;21:218–225. [DOI] [PubMed] [Google Scholar]
- 28. Muller B. Endocrine aspects of critical illness. Ann Endocrinol (Paris). 2007;68:290–298. [DOI] [PubMed] [Google Scholar]
- 29. Van den Berghe G. Dynamic neuroendocrine responses to critical illness. Front Neuroendocrinol. 2002;23:370–391. [DOI] [PubMed] [Google Scholar]
- 30. Van den Berghe G. Endocrine changes in critically ill patients. Growth Horm IGF Res. 1999;9(suppl A):77–81. [DOI] [PubMed] [Google Scholar]
- 31. Takala J, Ruokonen E, Webster NR, Nielsen MS, Zandstra DF, Vundelinckx G, Hinds CJ. Increased mortality associated with growth hormone treatment in critically ill adults. N Engl J Med. 1999;341:785–792. [DOI] [PubMed] [Google Scholar]
- 32. Schuetz P, Muller B, Nusbaumer C, Wieland M, Christ‐Crain M. Circulating levels of GH predict mortality and complement prognostic scores in critically ill medical patients. Eur J Endocrinol. 2009;160:157–163. [DOI] [PubMed] [Google Scholar]
- 33. Kario K, Pickering TG, Umeda Y, Hoshide S, Hoshide Y, Morinari M, Murata M, Kuroda T, Schwartz JE, Shimada K. Morning surge in blood pressure as a predictor of silent and clinical cerebrovascular disease in elderly hypertensives: a prospective study. Circulation. 2003;107:1401–1406. [DOI] [PubMed] [Google Scholar]


