Abstract
Background
A higher circulating plasma ceramide ratio (C16:0/C24:0) is associated with an increased risk of heart failure, even after accounting for standard risk factors including lipid markers. However, the pathobiological mechanisms that underlie this association are incompletely understood. We tested the hypothesis that plasma ceramide ratio (C16:0/C24:0) is associated with adverse cardiac remodeling in the community.
Methods and Results
We evaluated 2652 Framingham Offspring Study participants (mean age, 66±9 years; 55% women) who attended their eighth examination cycle and underwent routine echocardiography and liquid chromatography–tandem mass spectrometry–based assays for circulating ceramide concentrations. We used multivariable linear regression models to relate C16:0/C24:0 (independent variable) to the following echocardiographic measures (dependent variables; separate models for each): left ventricular mass, left ventricular ejection fraction, left atrial emptying fraction, left atrial end‐systolic volume, E/e′ (a measure of left ventricular diastolic function), and left ventricular global circumferential and longitudinal strain by speckle‐tracking echocardiography. In multivariable‐adjusted analyses, higher C16:0/C24:0 per standard deviation increment was associated with lower left ventricular ejection fraction (0.991‐fold change in left ventricular ejection fraction; P=0.0004), worse global circumferential strain (β=0.34, P=0.004), higher left atrial end‐systolic volume (β=2.48, p<0.0001), and lower left atrial emptying fraction (0.99‐fold change; P<0.0001). The C16:0/C24:0 ratio was not associated with either E/e′ or global longitudinal strain, and the association with higher left ventricular mass was rendered statistically nonsignificant upon correction for multiple comparisons.
Conclusions
Our cross‐sectional observations in a large community‐based sample are consistent with a potential detrimental impact of higher ceramide ratio (C16:0/24:0) on cardiac remodeling traits, which may partly explain the associations of these molecular species with clinical heart failure.
Keywords: ceramides, lipids and lipoproteins, cardiac remodeling, left ventricle, left atrium, cardiac function
Subject Categories: Epidemiology, Cardiovascular Disease, Risk Factors
Clinical Perspective
What Is New?
We used a high‐throughput liquid chromatography–mass spectrometry assay to quantify ratios of ceramide molecular species in the plasma and subsequently assessed their relations to echocardiographic measures of cardiac remodeling.
Data from the Framingham Offspring Study demonstrate that higher plasma ceramide ratio (C16:0/24:0) is associated with potentially detrimental changes in echocardiographic measures of cardiac structure and function.
What Are the Clinical Implications?
Our study observations support the notion that the association between higher ceramide ratio (C16:0/24:0) and the subsequently increased risk of clinical heart failure may be partly explained by relations with unfavorable subclinical cardiac structural and functional alterations.
Introduction
Heart failure (HF) remains a leading cause of morbidity and mortality, affecting 6.5 million Americans, and its prevalence is expected to rise with the aging of the US population.1 Given the substantial and rising burden of HF, ongoing efforts are needed to uncover novel mechanistic pathways that may underlie disease pathogenesis and progression. One area of recent interest is the role of plasma ceramide biomarkers in the pathogenesis of cardiovascular disease (CVD), including HF. Ceramides are bioactive lipids with a sphingoid base and a fatty acyl chain that are present in cell membranes and plasma, and have major influences on cellular signaling, differentiation, senescence, and programmed cell death.2 Ceramide synthases facilitate variable acetylation of the sphingoid base, producing a spectrum of ceramide molecular species.2 Expression and biomolecular effects of ceramides can vary on the basis of detectable relative proportions of distinct circulating ceramide species and the CVD risk profile.3 Recent studies have focused, therefore, on analyzing the ratio of select ceramides, such as the ratio of long‐chain to very‐long‐chain ceramide species.4, 5 In the FHS (Framingham Heart Study), higher circulating levels of ceramide 16:0 relative to ceramide 24:0 were associated with an increased HF risk even after adjusting for standard CVD risk factors, including blood lipid levels.5 These findings have been supported recently by a report from the CHS (Cardiovascular Health Study), which noted that lower circulating concentrations of fatty acids with 24 carbons and no unsaturated bonds, was associated with a higher risk of HF.6 However, the mechanisms underlying these relations are incompletely understood. In this context, it is widely accepted that antecedent alterations in cardiac structure and function (cardiac remodeling) antedate the onset of overt HF.7, 8Accordingly, we investigated the association between the plasma ceramide ratio (C16:0/C24:0) and echocardiographic measures of cardiac structure and function in a large community‐based sample where both sets of measures were contemporaneously assessed.
Methods
Study Design and Participant Selection
The objectives and study design of the FOS (Framingham Offspring Study) have been published previously.9 Briefly, offspring of the original FHS cohort and their spouses were enrolled in the FOS in 1971, and participants have been evaluated approximately every 4 years. Of the 3021 attendees at the eighth examination cycle (2005–2008), we excluded participants who did not have plasma samples for ceramide quantification (n=177) and those with missing covariates (n=192), yielding an analytic sample size of 2652 study participants who underwent routine transthoracic echocardiography and phlebotomy to obtain plasma samples for targeted measurement of circulating ceramide species. The institutional review boards at the Boston University Medical Center and the Washington University School of Medicine, St. Louis, Missouri, approved the study protocols, and all participants provided written informed consent. FOS data and materials in the current study will be made publicly available through the Biologic Specimen and Data Repositories Information Coordinating Center (BioLINCC) of the National Heart Lung and Blood Institute, Bethesda.
Measurement of Plasma Ceramides
The targeted plasma ceramide assay and quantification of C24:0 and C16:0 in the FHS Offspring cohort have been previously reported and demonstrated an excellent reproducibility profile.5 Briefly, plasma ceramides were obtained using a sensitive, accurate, and high‐throughput liquid chromatography–mass spectrometry assay to quantify the long‐chain (C16:0) and the very‐long‐chain (C24:0) ceramides in fasting plasma samples. The coefficients of variation for C16:0 and C24:0 assays were 7.8% and 6.9%, respectively, whereas the interassay and intra‐assay accuracy values were within ±3.2% and ±4.9% deviation for C16:0 and C24:0 ceramides, respectively. Ten percent of samples were tested in duplicate for quality assurance purposes.
Covariates
FHS Offspring study participants underwent a detailed medical history, physical examination, and laboratory assessment for CVD risk factors using methods described previously.10, 11 Briefly, systolic and diastolic blood pressure were assessed by an FHS physician, and weight and height by technicians using standardized protocols. Participants were considered to be current smokers if they reported smoking ≥1 cigarettes on a daily basis during the year preceding their FHS examination. Assays were performed on fasting biosamples using standard enzymatic methods for blood glucose, serum creatinine, serum total cholesterol, and high‐density lipoprotein cholesterol. Estimated glomerular filtration rate was calculated using the chronic kidney disease epidemiology collaboration equation.12
Echocardiographic Measures
Echocardiographic assessment in the FHS Offspring Study at the eighth examination cycle has been described previously.11, 13, 14 For our investigation, we evaluated the following echocardiographic measures: left ventricular (LV) mass (LVM), LV ejection fraction (LVEF), left atrial (LA) emptying fraction (LAEF), LA end‐systolic volume (LAVes), ratio of mitral inflow velocity to early diastolic mitral annular velocity (E/e′), LV global longitudinal strain (GLS), and global circumferential strain (GCS). Using Digisonics DigiView System Software (version 3.7.9.3; Digisonics Inc, Houston, TX) and digitized images, LV volumes were measured by Simpson's method. LA maximum and minimum volumes were obtained by averaging the respective volumes in apical 2‐ and 4‐chamber views that were measured using the area‐length method.15 Early peak systolic mitral annulus velocity was measured at the lateral mitral annulus using Doppler tissue imaging. Transmitral Doppler flow velocities were recorded using a standardized protocol. Repeated analysis of LV diastolic function measures yielded interobserver correlation with coefficients of >0.97. We used an offline speckle‐tracking software package (2D Cardiac Performance Analysis v1.1; TomTec Imaging Systems, Unterschleißheim, Germany) to analyze LV myocardial deformation, including GLS and GCS, according to a standardized protocol with excellent reproducibility.16 GCS and GLS are markers of myocardial deformation, and more positive values indicate worse systolic function. Lower values of LAEF indicate worse LA function.
Statistical Analyses
Descriptive characteristics of study participants were summarized as mean±SD or as median (25th, 75th percentile) for continuous variables, while categorical variables were summarized using proportions. Skewed echocardiographic measures were transformed by using natural logarithms to normalize their distributions.
We used multivariable linear regression to relate the plasma ceramide ratio C16:0/24:0 (independent variable) to the following echocardiographic measures (dependent variables, separate model for each): LVM, LVEF, GLS, GCS, E/e′, LAVes, and LAEF. Multivariable regression models were adjusted for the following covariates: age, sex, resting heart rate, height, weight, diabetes mellitus, systolic blood pressure, antihypertensive medication use, current smoking, and estimated glomerular filtration rate the ratio of total to high‐density lipoprotein cholesterol. We plotted cubic splines with 4 knots at the 5th, 25th, 75th, and 95th percentiles with a reference equal to the median C16:0/C24:0 value (0.07) to visualize the associations between C16:0/24:0 and echocardiography measures. All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC). A Bonferroni corrected 2‐sided P<0.007 (0.05/7) was used to indicate statistical significance to account for multiple testing. Authors (V.X. and M.D.) had access to all the data and take responsibility for the integrity of the analyses.
Results
Baseline Characteristics
The baseline clinical, biochemical, and echocardiographic characteristics of our study sample are shown in Table 1. Study participants in our sample had a mean age of 66±9 years, and 48.1% were men. The mean plasma concentration of C16:0 was roughly one tenth as abundant as that of plasma C24:0.
Table 1.
Characteristics of Study Sample
| Characteristics | N=2652 |
|---|---|
| Age, y | 66±9 |
| Men, % | 44.8 |
| Height, cm | 166.9±10 |
| Weight, kg | 78.8±18 |
| Systolic blood pressure, mm Hg | 128±17 |
| Diastolic blood pressure, mm Hg | 74±10 |
| Use of antihypertensive medication, % | 47.5 |
| Heart rate, bpm | 59.6±10 |
| Diabetes mellitus, % | 3.1 |
| Current smoking, % | 9.1 |
| LDL‐C, mg/dL | 105.8±31 |
| HDL‐C, mg/dL | 57.7±18 |
| Triglycerides, mg/dL | 117.5±69 |
| Total cholesterol, mg/dL | 186.6±37 |
| Lipid lowering medication use, % | 42.1 |
| eGFR, mL/min/1.73 m2 | 77.8±16 |
| Prior heart failure, % | 2.38 |
| Plasma ceramide concentrations | |
| Plasma C16:0 ceramide, μg/mL | 0.2±0.04 |
| Plasma C24:0 ceramide, μg/mL | 2.3±0.7 |
| Plasma C16/24 ceramide, μg/mL | 0.08±0.02 |
| Echocardiographic measures | |
| LV mass index, g | 162.2 (134.5, 162.6) |
| LV ejection fraction, % | 67.3 (62.9, 71.7) |
| Left atrial emptying fraction, % | 48 (46, 50) |
| Left atrial end‐systolic volume, mL | 56.8±19.3 |
| LV global longitudinal strain, % | −20.6±3 |
| LV global circumferential strain, % | −31.9±6 |
| E/e′ | 6.6 (5.5, 8.1) |
Values are mean±standard deviation, median (Q1, Q3), or percentage. E/e′ indicates mitral inflow velocity to early diastolic mitral annular velocity; eGFR, estimated glomerular filtration rate; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; LV, left ventricular.
Circulating Ceramide Ratio Cardiac Structure and Function
Results from multivariable linear regression models relating ceramide ratio to echocardiographic traits are shown in Table 2. In multivariable‐adjusted models, higher ceramide ratio C16:0/C24:0 was associated with lower LVEF, worse (more positive) LV GCS, lower LAEF, higher LAVes, and a higher LVM, although the association with LVM was no longer statistically significant after adjustment for multiple comparisons. C16:0/24:0 ratio was not associated with either E/e′ or GLS. Figure S1 shows the least squared mean plots per tertile of C16:0/24:0 ratio.
Table 2.
Ceramide Ratio (C16:0/24:0) and Cardiac Structure and Function
| Echocardiography Indices | Beta Estimate (Standard Error) |
|
P Value | |
|---|---|---|---|---|
| LV ejection fractiona | −0.009 (0.002) | 0.991 | 0.0004b | |
| LV global circumferential strain | 0.34 (0.12) | NA | 0.004b | |
| LV global longitudinal strain | 0.07 (0.06) | NA | 0.26 | |
| E/e′a | 0.006 (0.005) | 1.006 | 0.26 | |
| Left atrial emptying fractiona | −0.008 (0.002) | 0.992 | <0.0001b | |
| Left atrial end‐systolic volume | 2.48 (0.4) | NA | <0.0001b | |
| LV massa | 0.009 (0.004) | 1.009 | 0.02 |
Multivariable linear regression showing the relation between C16:0/C24:0 and echocardiographic measures (dependent variable). Beta estimates represent change in echocardiography variable per 1 SD increment in ceramide ratio. Models were adjusted for age, sex, heart rate, height, weight, diabetes mellitus, systolic blood pressure, antihypertensive medication use, current smoking, estimated glomerular filtration rate, and the ratio of total to high‐density lipoprotein cholesterol. E/e′ indicates mitral inflow velocity to early diastolic mitral annular velocity; LV, left ventricular; NA, not applicable.
These dependent variables were natural logarithmically transformed to satisfy the normality assumption of linear regression models. In these models represents the fold change in Y per standard deviation increase in C16:0/C24:0. More positive global strain values signify worse LV systolic function.
Denotes associations that retain statistical significance following a Bonferroni correction for multiple testing.
In the Figure, restricted cubic splines illustrating the association between C16:0/24:0 ratio and echocardiography measures (LAEF, GCS, LVM, and LVEF) are shown. Higher C16:0/24:0 ratio above the set reference level showed an approximately linear relation with LAEF, LVM, and LVEF, whereas the association with GCS was monotonic.
Figure 1.
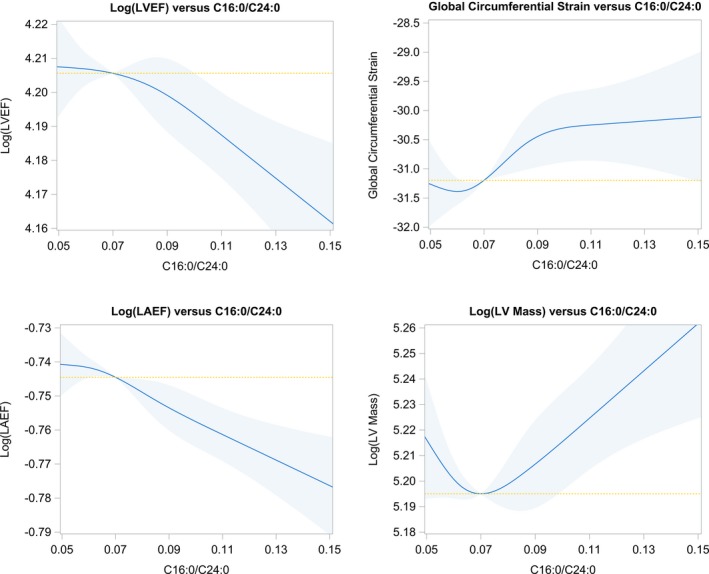
Restricted cubic splines plots showing the relations between ceramide ratio and LVEF, LAEF, global circumferential strain, and LV mass. Shaded areas represent the 95% CIs. The reference value in each panel is set to the median C16:0/C24:0 ratio of 0.07. The yellow hashed line serves as a reference line of “no association.” LAEF indicates left atrial emptying fraction; LV, left ventricular; LVEF, left ventricular ejection fraction.
Discussion
Principal Findings
In a large community‐based sample, we observed that a higher circulating plasma C16:0/C24:0 ceramide ratio was cross‐sectionally associated with alterations in cardiac structure and function, including higher LAVes, lower LV systolic function (determined by LVEF and LV GCS), lower LA function (assessed by LAEF), and a propensity toward an association with LVM upon correction for multiple comparisons. However, a higher C16:0/C24:0 ceramide ratio was not significantly associated with either E/e′ or GLS. Our findings raise the possibility that the association of higher circulating ceramide ratio (C16:0/C24:0) with an increased HF risk may in part be mediated by the association between specific ceramide species and measures of LV as well as LA remodeling.
Comparison With Published Literature
To our knowledge, this is the first investigation to explore the relations between plasma ceramide concentration ratios and cardiac structure and function in a community‐based sample. Circulating ceramide species are less abundant than cholesterol in plasma and have been challenging to assay precisely in the past. In recent years, the advent of liquid chromatography–mass spectrometry techniques has facilitated the more accurate measurement of several plasma ceramide molecular species. It is now increasingly recognized that distinct ceramide acyl chain lengths play important roles in biophysical properties of membranes, cell signaling, and apoptosis, with long‐chain and very‐long‐chain ceramides (including C16:0 and C24:0) being the predominant ceramides in mammalian tissues.17, 18, 19 C16:0 promotes programmed cell death by a cascade of events involving ceramide‐mediated channels and a loss of mitochondrial outer membrane integrity that facilitate the release of cytochrome c and other proapoptotic proteins into the cytoplasm, ultimately resulting in mitochondria‐induced apoptosis.2, 18 In contrast, C24:0 interferes with C16:0 ceramide‐associated channel formation in the mitochondria and has predominantly antiapoptotic effects.20 These species are also linked by intermediary metabolism enzymatic pathways.20 For example, in cells with selective knockdown of ceramide synthetase‐2 (which catalyzes the generation of very‐long‐chain ceramides, including C24:0) there was near elimination of C24:0 ceramide and higher C16:0 ceramide levels.18 Furthermore, the concentration or expression of plasma ceramides and their resultant effects are modified by prevalent CVD risk burden such as diabetes mellitus and smoking.4, 5 To mitigate these influences, rather than utilizing total ceramide levels, a ceramide ratio consisting of the indexation of the long‐chain ceramide species by very‐long‐chain ceramide species such as C16:0/24:0 ratio has emerged as a marker that provides greater information than either species alone in ceramide‐related analysis.3, 4 Multiple lines of evidence support the association of alterations in concentrations of plasma ceramide species with adverse cardiovascular outcomes and mortality in patients with prior CVD6, 21, 22, 23 and in asymptomatic community‐dwelling individuals.5 These studies demonstrate the additional prognostic value of specific plasma ceramides for predicting CVD risk beyond standard CVD risk factors, including plasma lipid levels.5 In one study of individuals with coronary artery disease, there were more pronounced differences in circulating ceramide profiles than in the standard lipid markers when subjects who died during follow‐up were compared with those who survived.4 Elevated plasma total ceramide levels have also been reported to be associated with New York Heart Association functional classes in individuals with HF and reduced ejection fraction.24 Similarly, in patients with HF, LV assist device placement reduced the detectable level of total ceramides present in the myocardium.25 In the CHS, a lower plasma level of the 24:0 fatty acid, lignoceric acid, was also associated with a higher risk of HF, which is consistent with data from experimental studies.6 Similarly, in the FHS, we previously reported that a higher level of C16:0 in relation to C24:0 was associated with an increased risk of incident HF. The present investigation is consistent with these findings and importantly offers additional insight into underlying mechanisms by demonstrating an association of higher circulating ceramide ratio (C16:0/C24:0) with adverse cardiac structural and functional alterations. Antecedent subclinical impairment in LV strain by speckle‐tracking echocardiography, LVEF, LV mass, and LA function have been widely established as strong independent predictors of future HF, CVD, and all‐cause mortality.7, 13, 14 In the present study, there was no association between C16:0/C24:0 and E/e′ (a surrogate for LV diastolic dysfunction). The reason for this observed lack of association with E/e′ is not entirely clear but may be partly related to the narrow distribution of E/e′ ratio in our relatively healthy sample with a median E/e′ of 6.6 (which is well below thresholds for LV diastolic dysfunction).26 Although there was no association of the ceramide ratio with E/e′, we observed statistically strong associations between C16:0/C24:0 and higher LAVes and lower LAEF—a sensitive marker for subclinical LA remodeling that may identify individuals at high risk for HF independent of LVEF.27, 28 Furthermore, we observed associations between C16:0/C24:0 and GCS but not with GLS. It is conceivable that distinct aspects of myocardial deformation may have differential associations with various biomarkers of CVD risk.14 Our observation of an association with GCS but not with GLS may suggest potential involvement of mesocardial more than endocardial myocardial function, a speculative premise that warrants further investigation.
Potential Mechanisms
There are several plausible mechanisms that support a relation between the circulating C16:0/C24:0 and subclinical cardiac remodeling and dysfunction. Sphingosine‐1‐phosphate, a signaling molecule formed by the phosphorylation of sphingosine, is interconvertible with ceramides.29 Sphingosine‐1‐phosphate can function to inhibit apoptosis and is also reported to suppress the proapoptotic effects of ceramides.29, 30 In porcine models, administration of sphingosine‐1‐phosphate receptor agonist reduced post–myocardial infarction LV remodeling and improved LV systolic function.31 Sphingosine‐1‐phosphate is also a constituent of high‐density lipoprotein and a contributor to many of the beneficial effects of high‐density lipoprotein on the heart and circulatory system.32
Animal and human studies have shown that total ceramides, and C16:0 in particular, are related to markers of inflammation (like interleukin‐6), insulin resistance, and diabetes mellitus through activation of inflammatory mediators such as tumor necrosis factor‐α as well as adiponectin receptor–associated ceramidase activity.33, 34 In a dietary study, consumption of a Western diet increased total ceramide content in myocardium with normal geometry and LV hypertrophy.35Aging, smoking, and prevalent CVD are also associated with higher ceramide ratio (C16:0/C24:0) in the community.5 Sphingosine, which is the main constituent of the ceramide backbone, has been reported to mediate the negative inotropic effects of tumor necrosis factor‐α in adult mammalian cardiomyocytes.36 In in vitro and murine studies, exogenous cell‐permeable ceramide can induce cardiomyocyte apoptosis and the inhibition of serine palmitoyl–coenzyme A transferase—which is vital for ceramide biosynthesis—mitigated atherosclerosis development.36, 37 Overall, these findings suggest that ceramides may play a contributory role in the cardiometabolic dysfunction that precedes cardiac remodeling and overt HF.
Strengths and Limitations
The large, well‐characterized community‐based sample; standardized measurement of echocardiographic measurements; and contemporaneous assessment of ceramides with liquid chromatography–mass spectrometry–based state‐of‐the art assays strengthen our investigation. Our study sample was mainly composed of middle‐aged to elderly white men and women of predominantly European ancestry; therefore, the generalizability of our findings to other age or racial groups is uncertain. Our cross‐sectional study design precludes any causal inferences.
Conclusions
In our cross‐sectional investigation of a moderate‐sized community‐based sample, we observed that a higher ceramide ratio (C16:0/C24:0) was associated with potentially unfavorable subclinical cardiac structural and functional alterations, which may represent an important underlying pathophysiological mechanism linking circulating ceramide species to HF risk in the community.
Sources of Funding
This work was supported by grants P20 HL113444 P30 DK020579, from the National Institutes of Health, and contracts N01‐HL25195 and HHSN268201500001I (Dr Vasan) from the National Heart, Lung, and Blood Institute and National Institutes of Health grants HL080124, HL071039, HL077447, HL107385, 1R01HL126136, 5R01HL107385, 1R01HL60040, 1RO1HL70100, R01HL131532, and R01HL134168. Dr McManus's time was supported by grants R01HL126911, R01HL137734, R01HL137794, R01HL13660, and R01HL141434, also from the National Heart, Lung, and Blood Institute. Dr Peterson's time was supported by grants from the NIH R34 HL138253‐01, 1R01AG060499‐01, and a grant from the Barnes Jewish Hospital Foundation.
Disclosures
Dr Vasan is supported in part by the Evans Medical Foundation and the Jay and Louis Coffman Endowment from the Department of Medicine, Boston University School of Medicine. Dr Cheng reports receiving consulting fees from Zogenix, unrelated to the submitted work. Dr McManus has received research support from Apple Computer, Bristol‐Myers Squibb, Boehringher‐Ingelheim, Pfizer, Samsung, Philips Healthcare, and Biotronik; has received consultancy fees from Bristol‐Myers Squibb, Pfizer, Flexcon, Boston Biomedical Associates, and Samsung; and has inventor equity in Mobile Sense Technologies, Inc (CT). Dr Mitchell is the president of Cardiovascular Engineering, Inc, a company that designs and manufactures devices that measure vascular stiffness. Dr Peterson serves as a consultant to and receives honoraria and grant support from Novartis and Servier. Dr Peterson consults for Radius Pharmaceuticals, unrelated to the submitted work. A patent application for use of the ceramide biomarkers is pending (Drs Peterson, Duncan, Vasan, and Xanthakis).
Supporting information
Figure S1. Least squared mean plots per tertile of C16/24 ratio for left ventricular GCS; LVM; LAEF; and LVEF. Models were adjusted for the following covariates: age, sex, heart rate, height, weight, diabetes mellitus, systolic blood pressure, antihypertensive medication use, current smoking, estimated glomerular filtration rate, and total/HDL cholesterol ratio. Per tertile of C16:0/24:0 ratio, there was a linear trend toward worse GCS and lower LAEF. In contrast, there was a nonlinear trend between LVM and C16:0/24:0. A propensity toward a trend was observed (albeit nonsignificant) of lower LVEF per tertile of C16:0/24:0 ratio. GCS indicates global circumferential strain; HDL, high‐density lipoprotein; LAEF, left atrial emptying fraction; LVEF, left ventricular ejection fraction; LVM, left ventricular mass.
(J Am Heart Assoc. 2019;8:e013050 DOI: 10.1161/JAHA.119.013050.)
References
- 1. Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R, de Ferranti SD, Ferguson JF, Fornage M, Gillespie C, Isasi CR, Jimenez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Lutsey PL, Mackey JS, Matchar DB, Matsushita K, Mussolino ME, Nasir K, O'Flaherty M, Palaniappan LP, Pandey A, Pandey DK, Reeves MJ, Ritchey MD, Rodriguez CJ, Roth GA, Rosamond WD, Sampson UKA, Satou GM, Shah SH, Spartano NL, Tirschwell DL, Tsao CW, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P. Heart disease and stroke statistics—2018 update: a report from the American Heart Association. Circulation. 2018;137:e67–e492. [DOI] [PubMed] [Google Scholar]
- 2. Park JW, Park WJ, Futerman AH. Ceramide synthases as potential targets for therapeutic intervention in human diseases. Biochim Biophys Acta. 2014;1841:671–681. [DOI] [PubMed] [Google Scholar]
- 3. Tippetts TS, Holland WL, Summers SA. The ceramide ratio: a predictor of cardiometabolic risk. J Lipid Res. 2018;59:1549–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tarasov K, Ekroos K, Suoniemi M, Kauhanen D, Sylvanne T, Hurme R, Gouni‐Berthold I, Berthold HK, Kleber ME, Laaksonen R, Marz W. Molecular lipids identify cardiovascular risk and are efficiently lowered by simvastatin and PCSK9 deficiency. J Clin Endocrinol Metab. 2014;99:E45–E52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Peterson LR, Xanthakis V, Duncan MS, Gross S, Friedrich N, Völzke H, Felix SB, Jiang H, Sidhu R, Nauck M, Jiang X, Ory DS, Dörr M, Vasan RS, Schaffer JE. Ceramide remodeling and risk of cardiovascular events and mortality. J Am Heart Assoc. 2018;7:e007931 DOI: 10.1161/JAHA.117.007931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lemaitre RN, McKnight B, Sotoodehnia N, Fretts AM, Qureshi WT, Song X, King IB, Sitlani CM, Siscovick DS, Psaty BM, Mozaffarian D. Circulating very long‐chain saturated fatty acids and heart failure: the cardiovascular health study. J Am Heart Assoc. 2018;7:e010019 DOI: 10.1161/JAHA.118.010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vasan RS, Xanthakis V, Lyass A, Andersson C, Tsao C, Cheng S, Aragam J, Benjamin EJ, Larson MG. Epidemiology of left ventricular systolic dysfunction and heart failure in the Framingham study: an echocardiographic study over 3 decades. JACC Cardiovasc Imaging. 2018;11:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nwabuo CC, Armstrong AAC, Ambale‐Venkatesh B, Vasconcellos HD, Mewton N, Lima JAC, Moreira HT, Opdahl A, Lloyd‐Jones D, Ogunyankin KO, Jacobs DR Jr, Schreiner PJ, Lewis CE, Gidding SS. Left ventricular global function index predicts incident heart failure and cardiovascular disease in young adults: the coronary artery risk development in young adults (CARDIA) study. Eur Heart J Cardiovasc Imaging. 2018;20:533–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. the Framingham Offspring Study. Am J Epidemiol. 1979;110:281–290. [DOI] [PubMed] [Google Scholar]
- 10. D'Agostino RB Sr, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, Kannel WB. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117:743–753. [DOI] [PubMed] [Google Scholar]
- 11. Sardana M, Nah G, Tsao CW, Ogunsua AA, Vittinghoff E, Thomas RC, Cheng S, Vaze A, Aragam JR, Mitchell GF, Benjamin EJ, Vasan RS, Aurigemma GP, Schiller NB, McManus DD, Parikh NI. Clinical and echocardiographic correlates of left atrial function index: the Framingham Offspring Study. J Am Soc Echocardiogr. 2017;30:904–912.e902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF III, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sardana M, Lessard D, Tsao CW, Parikh NI, Barton BA, Nah G, Thomas RC, Cheng S, Schiller NB, Aragam JR, Mitchell GF, Vaze A, Benjamin EJ, Vasan RS, McManus DD. Association of left atrial function index with atrial fibrillation and cardiovascular disease: the Framingham Offspring Study. J Am Heart Assoc. 2018;7:e008435 DOI: 10.1161/JAHA.117.008435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cheng S, McCabe EL, Larson MG, Merz AA, Osypiuk E, Lehman BT, Stantchev P, Aragam J, Solomon SD, Benjamin EJ, Vasan RS. Distinct aspects of left ventricular mechanical function are differentially associated with cardiovascular outcomes and all‐cause mortality in the community. J Am Heart Assoc. 2015;4:e002071 DOI: 10.1161/JAHA.115.002071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shah RV, Rong J, Larson MG, Yeri A, Ziegler O, Tanriverdi K, Murthy V, Liu X, Xiao C, Pico AR, Huan T, Levy D, Lewis GD, Rosenzweig A, Vasan RS, Das S, Freedman JE. Associations of circulating extracellular RNAS with myocardial remodeling and heart failure. JAMA Cardiol. 2018;3:871–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cheng S, Larson MG, McCabe EL, Osypiuk E, Lehman BT, Stanchev P, Aragam J, Benjamin EJ, Solomon SD, Vasan RS. Reproducibility of speckle‐tracking‐based strain measures of left ventricular function in a community‐based study. J Am Soc Echocardiogr. 2013;26:1258–1266.e1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Raichur S, Wang ST, Chan PW, Li Y, Ching J, Chaurasia B, Dogra S, Ohman MK, Takeda K, Sugii S, Pewzner‐Jung Y, Futerman AH, Summers SA. Cers2 haploinsufficiency inhibits beta‐oxidation and confers susceptibility to diet‐induced steatohepatitis and insulin resistance. Cell Metab. 2014;20:687–695. [DOI] [PubMed] [Google Scholar]
- 18. Sassa T, Suto S, Okayasu Y, Kihara A. A shift in sphingolipid composition from C24 to C16 increases susceptibility to apoptosis in HeLa cells. Biochim Biophys Acta. 2012;1821:1031–1037. [DOI] [PubMed] [Google Scholar]
- 19. Doroudgar M, Lafleur M. Ceramide‐C16 is a versatile modulator of phosphatidylethanolamine polymorphism. Biophys J. 2017;112:2357–2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stiban J, Perera M. Very long chain ceramides interfere with C16‐ceramide‐induced channel formation: a plausible mechanism for regulating the initiation of intrinsic apoptosis. Biochim Biophys Acta. 2015;1848:561–567. [DOI] [PubMed] [Google Scholar]
- 21. Anroedh S, Hilvo M, Akkerhuis KM, Kauhanen D, Koistinen K, Oemrawsingh R, Serruys P, van Geuns R‐J, Boersma E, Laaksonen R, Kardys I. Plasma concentrations of molecular lipid species predict long‐term clinical outcome in coronary artery disease patients. J Lipid Res. 2018;59:1729–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Laaksonen R, Ekroos K, Sysi‐Aho M, Hilvo M, Vihervaara T, Kauhanen D, Suoniemi M, Hurme R, März W, Scharnagl H, Stojakovic T, Vlachopoulou E, Lokki M‐L, Nieminen MS, Klingenberg R, Matter CM, Hornemann T, Jüni P, Rodondi N, Räber L, Windecker S, Gencer B, Pedersen ER, Tell GS, Nygård O, Mach F, Sinisalo J, Lüscher TF. Plasma ceramides predict cardiovascular death in patients with stable coronary artery disease and acute coronary syndromes beyond LDL‐cholesterol. Eur Heart J. 2016;37:1967–1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Havulinna AS, Sysi‐Aho M, Hilvo M, Kauhanen D, Hurme R, Ekroos K, Salomaa V, Laaksonen R. Circulating ceramides predict cardiovascular outcomes in the population‐based Finrisk 2002 cohort. Arterioscler Thromb Vasc Biol. 2016;36:2424–2430. [DOI] [PubMed] [Google Scholar]
- 24. Yu J, Pan W, Shi R, Yang T, Li Y, Yu G, Bai Y, Schuchman EH, He X, Zhang G. Ceramide is upregulated and associated with mortality in patients with chronic heart failure. Can J Cardiol. 2015;31:357–363. [DOI] [PubMed] [Google Scholar]
- 25. Chokshi A, Drosatos K, Cheema FH, Ji R, Khawaja T, Yu S, Kato T, Khan R, Takayama H, Knoll R, Milting H, Chung CS, Jorde U, Naka Y, Mancini DM, Goldberg IJ, Schulze PC. Ventricular assist device implantation corrects myocardial lipotoxicity, reverses insulin resistance, and normalizes cardiac metabolism in patients with advanced heart failure. Circulation. 2012;125:2844–2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nagueh SF, Smiseth OA, Appleton CP, Byrd BF III, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, Marino P, Oh JK, Alexandru Popescu B, Waggoner AD. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2016;17:1321–1360. [DOI] [PubMed] [Google Scholar]
- 27. Gupta S, Matulevicius SA, Ayers CR, Berry JD, Patel PC, Markham DW, Levine BD, Chin KM, de Lemos JA, Peshock RM, Drazner MH. Left atrial structure and function and clinical outcomes in the general population. Eur Heart J. 2013;34:278–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hedberg P, Selmeryd J, Leppert J, Henriksen E. Long‐term prognostic impact of left atrial volumes and emptying fraction in a community‐based cohort. Heart. 2017;103:687–693. [DOI] [PubMed] [Google Scholar]
- 29. Cuvillier O, Pirianov G, Kleuser B, Vanek PG, Coso OA, Gutkind S, Spiegel S. Suppression of ceramide‐mediated programmed cell death by sphingosine‐1‐phosphate. Nature. 1996;381:800–803. [DOI] [PubMed] [Google Scholar]
- 30. Reimann C‐M, Thuy AV, Weigel C, Gräler MH. Sphingosine‐1‐phosphate (S1P) in cancer immunity and development. Transl Cancer Res. 2015;4:460–468. [Google Scholar]
- 31. Santos‐Gallego CG, Vahl TP, Goliasch G, Picatoste B, Arias T, Ishikawa K, Njerve IU, Sanz J, Narula J, Sengupta PP, Hajjar RJ, Fuster V, Badimon JJ. Sphingosine‐1‐phosphate receptor agonist fingolimod increases myocardial salvage and decreases adverse postinfarction left ventricular remodeling in a porcine model of ischemia/reperfusion. Circulation. 2016;133:954–966. [DOI] [PubMed] [Google Scholar]
- 32. Sattler K, Graler M, Keul P, Weske S, Reimann CM, Jindrova H, Kleinbongard P, Sabbadini R, Brocker‐Preuss M, Erbel R, Heusch G, Levkau B. Defects of high‐density lipoproteins in coronary artery disease caused by low sphingosine‐1‐phosphate content: correction by sphingosine‐1‐phosphate‐loading. J Am Coll Cardiol. 2015;66:1470–1485. [DOI] [PubMed] [Google Scholar]
- 33. Turpin SM, Nicholls HT, Willmes DM, Mourier A, Brodesser S, Wunderlich CM, Mauer J, Xu E, Hammerschmidt P, Bronneke HS, Trifunovic A, LoSasso G, Wunderlich FT, Kornfeld JW, Bluher M, Kronke M, Bruning JC. Obesity‐induced CERS6‐dependent c16:0 ceramide production promotes weight gain and glucose intolerance. Cell Metab. 2014;20:678–686. [DOI] [PubMed] [Google Scholar]
- 34. Holland WL, Brozinick JT, Wang LP, Hawkins ED, Sargent KM, Liu Y, Narra K, Hoehn KL, Knotts TA, Siesky A, Nelson DH, Karathanasis SK, Fontenot GK, Birnbaum MJ, Summers SA. Inhibition of ceramide synthesis ameliorates glucocorticoid‐, saturated‐fat‐, and obesity‐induced insulin resistance. Cell Metab. 2007;5:167–179. [DOI] [PubMed] [Google Scholar]
- 35. Butler TJ, Ashford D, Seymour AM. Western diet increases cardiac ceramide content in healthy and hypertrophied hearts. Nutr Metab Cardiovasc Dis. 2017;27:991–998. [DOI] [PubMed] [Google Scholar]
- 36. Gulbins E, Li PL. Physiological and pathophysiological aspects of ceramide. Am J Physiol Regul Integr Comp Physiol. 2006;290:R11–R26. [DOI] [PubMed] [Google Scholar]
- 37. Hojjati MR, Li Z, Zhou H, Tang S, Huan C, Ooi E, Lu S, Jiang XC. Effect of myriocin on plasma sphingolipid metabolism and atherosclerosis in ApoE‐deficient mice. J Biol Chem. 2005;280:10284–10289. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Least squared mean plots per tertile of C16/24 ratio for left ventricular GCS; LVM; LAEF; and LVEF. Models were adjusted for the following covariates: age, sex, heart rate, height, weight, diabetes mellitus, systolic blood pressure, antihypertensive medication use, current smoking, estimated glomerular filtration rate, and total/HDL cholesterol ratio. Per tertile of C16:0/24:0 ratio, there was a linear trend toward worse GCS and lower LAEF. In contrast, there was a nonlinear trend between LVM and C16:0/24:0. A propensity toward a trend was observed (albeit nonsignificant) of lower LVEF per tertile of C16:0/24:0 ratio. GCS indicates global circumferential strain; HDL, high‐density lipoprotein; LAEF, left atrial emptying fraction; LVEF, left ventricular ejection fraction; LVM, left ventricular mass.


