Abstract
Background
Troponin release in ST‐segment–elevation myocardial infarction (STEMI) has predictable kinetics with early levels reflective of ischemia duration. Little research has examined the value of admission troponin levels in STEMI patients undergoing primary percutaneous coronary intervention. We investigated the relationship between troponin on presentation and mortality in a large, real‐world cohort of STEMI patients undergoing primary percutaneous coronary intervention.
Methods and Results
We used multivariable adaptive regression modeling to examine the association between admission troponin levels and in‐hospital mortality for patients who underwent primary percutaneous coronary intervention for STEMI. We adjusted for known clinical risk factors using a validated mortality risk model derived from the NCDR (National Cardiovascular Data Registry) CathPCI database, and this same model was used to calculate patients’ predicted mortality based on clinical and demographic factors. Patients were then stratified by troponin groups to compare predicted versus observed mortality. Of the 14 061 patients included in the cohort, 47.2% had initial troponin levels that were undetectable or within the reference range. Admission troponin was an independent predictor of in‐hospital mortality, and any value above the reference range was associated with increased mortality (1.8% versus 5.1%, [standardized difference, 18.2%]). Patients with the highest predicted risk for mortality (13% predicted) in the highest admission troponin grouping experienced an observed mortality of 19.5%. Patients in low troponin groupings consistently demonstrated lower than predicted mortality based on their clinical and demographic risk profile.
Conclusions
Nearly half of patients undergoing primary percutaneous coronary intervention had normal troponin on presentation and had a relatively good outcome. Mortality increases with elevated admission troponin levels, regardless of baseline clinical risk. The substantial number of patients who present with markedly elevated troponin and their relatively worse outcomes highlights the need for continued improvement in prehospital STEMI detection and care.
Keywords: myocardial infarction, percutaneous transluminal coronary angioplasty, troponin
Subject Categories: Mortality/Survival
Clinical Perspective
What Is New?
Nearly half of ST‐segment‐elevation myocardial infarction patients have a low or undetectable standard troponin level at the time of hospital presentation.
Admission troponin levels in the early phase of ST‐segment‐elevation myocardial infarction provide prognostic information in patients undergoing primary percutaneous coronary intervention.
Patients with lower troponin levels on admission have lower than predicted in‐hospital mortality at all levels of clinical risk.
What Are the Clinical Implications?
Troponin on presentation may have value in objectively quantifying the degree and duration of prehospital ischemia.
Admission biomarker data could be used to evaluate the effectiveness of quality improvement interventions aimed at improving prehospital ST‐segment‐elevation myocardial infarction detection and care.
Introduction
Cardiac troponin levels are routinely measured in the setting of known or suspected myocardial infarction. Though not part of formal diagnostic criteria, baseline troponin levels are frequently collected in the emergency department evaluation of patients with ST‐segment–elevation myocardial infarction (STEMI). Troponin release has predictable kinetics in the setting of acute infarction, and peak levels correlate with both ischemic time and quantity of myocardium at risk.1, 2, 3, 4 A previous investigation has shown that troponin kinetics can be used to estimate the onset of ischemia in STEMI patients, and this “biochemical ischemic time” correlates well with infarct size and long‐term mortality.5 Despite the potential value of admission troponin as an indicator of prehospital ischemia, there is a paucity of research evaluating the relationship between the magnitude of admission troponin and mortality for STEMI patients undergoing percutaneous coronary intervention (PCI). In this context, we examined the association between admission troponin level and in‐hospital mortality for a large, contemporary cohort of patients who underwent primary PCI for the treatment of STEMI.
Methods
The database used for this analysis is not available for wider sharing given the limitations of the data‐use agreements with participating institutions. Study code is available upon reasonable request.
Cohort
The Blue Cross Blue Shield of Michigan Cardiovascular Consortium is an ongoing, prospective, multicenter quality‐improvement registry of all patients undergoing PCI at every nonfederal hospital in the state of Michigan. The consortium includes a total of 47 hospitals, and details of the registry's formation and data collection procedures have been described previously.6, 7 Briefly, participating centers collect demographic, clinical, procedural, and in‐hospital outcomes data for consecutive patients undergoing PCI for all indications. Data collection procedures and diagnostic definitions are based on those used in the NCDR (National Cardiovascular Data Registry) CathPCI database.8 STEMI patients are identified based on clinical symptoms and standard ECG criteria.9 All participating sites undergo a periodic audit (including clinical and ECG data) by a central auditor to ensure the reliability of patient diagnoses and clinical data entry.
Our study cohort for this analysis included patients who presented to the emergency department and underwent primary PCI for STEMI over a 6‐year period from January 1, 2010 to December 31, 2015. Patients who were transferred to another facility for PCI or those who had PCI while admitted for another reason were excluded. We also excluded patients without baseline troponin levels obtained at hospital presentation and those who suffered prehospital cardiac arrest, were on chronic dialysis therapy, or had missing demographic or clinical data for estimation of predicted mortality risk. The primary outcome of interest was in‐hospital mortality following PCI.
Troponin Levels
Baseline troponin levels were obtained for all patients on presentation before PCI using each hospital's standard clinical troponin I (TnI) or troponin T (TnT) assay. Values from point‐of‐care assays were not included in the study. In most cases, the troponin draw occurred in the emergency department during the initial patient assessment. Each center's clinical laboratory was queried to determine the institution‐specific fourth‐generation assay and reference ranges in use during the study period. To adjust for interhospital heterogeneity in troponin assay use and differences in assay calibration between TnI and TnT,10 values were transformed into “troponin folds,” calculated using the ratio of the absolute value of the patient's presenting troponin level to the site‐specific assay's upper limit of normal, an approach that has been used previously in the analysis of multicenter cardiac biomarker data.11 A troponin fold of 1 represents a value matching the upper limit of the reference range. Patients with an undetectable troponin level were assigned a troponin fold of 0.
Statistical Analysis
Comparisons of continuous variables were performed using Student t tests, and comparisons of categorical variables were performed using Pearson chi‐squared tests. For both continuous and categorical variables, imbalance in patient clinical and demographic characteristics between groups defined by preprocedural troponin values within or exceeding site‐specific cutoffs (ie, troponin folds ≤1 versus >1) was assessed using the standardized difference measure.12 Standardized differences are reported in lieu of P values, given the tendency of the latter to indicate statistically significant, yet clinically trivial, differences in the setting of large sample sizes.13 A threshold of standardized difference ≥10% was used to identify cases of substantial imbalance.
Baseline predicted risk of in‐hospital mortality for each patient was calculated using coefficients from the NCDR CathPCI pre‐PCI risk model derived by Peterson et al.14 To evaluate the relationship between troponin and mortality, multivariable adaptive regression spline models15 were fitted using the R statistical program package “earth,”16 with troponin fold value and baseline predicted mortality from the CathPCI model as continuous predictors, allowing for second‐degree interactions. The troponin values identified by the model as points at which the underlying relationship with mortality changed functionally (spline knots) were subsequently used to group cases into troponin ranges reflecting mortality impact. Local weighted polynomial regression17 models were utilized to explore and visualize the relationship between baseline troponin fold values and mortality across the range of presenting troponin values.
The Spearman coefficient was used to assess correlation between symptom‐to‐door time and baseline troponin fold values among the subset of cases where symptom‐to‐door time was available. The correlation between troponin fold values and door‐to‐device times was also examined for all patients. Median and interquartile ranges for these time intervals were calculated for patients in troponin fold groups 0 to 0.5, 0.5 to 1.0, 1 to 10, and >10.
The University of Michigan Institutional Review Board has waived the need for approval of studies based on the data collected by the BMC2 (Blue Cross Blue Shield of Michigan Cardiovascular Consortium) registry.
Results
A total of 25 694 patients underwent primary PCI for STEMI at participating institutions from 2010 through 2015. Of these, 6301 patients were not admitted to the emergency department, leaving 19 393 patients eligible for inclusion. Figure 1 shows the patient inclusion flow diagram for the cohort. A total of 5332 patients met one of the exclusion criteria, leaving 14 061 patients in the final cohort. Of this cohort, 6645 (47.2%) had initial troponin levels that were undetectable or within the institution‐specific reference range (troponin folds 0–1).
Figure 1.
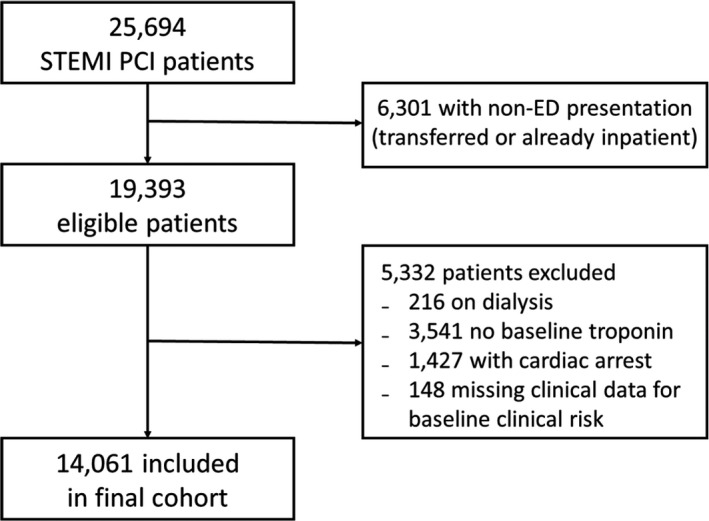
Patient inclusion diagram for study cohort. ED indicates emergency department; PCI, primary percutaneous coronary intervention; STEMI, ST‐segment‐elevation myocardial infarction.
Table 1 depicts the baseline demographic and clinical characteristics of STEMI patients with troponin folds 0 to 1 and troponin fold >1 on presentation. Mean (±SD) absolute TnI and TnT values for the negative fold group were 0.04±0.07 and 0.02±0.01 ng/mL, respectively. Mean TnI and TnT values for patients with an elevated troponin fold were 5.88±17.63 and 0.70±1.32 ng/mL, respectively. A total of 492 patients died during the index hospitalization, with an overall in‐hospital mortality of 3.5% for the cohort. Mortality was lower for those with normal or negative troponin values compared with patients who had troponin folds >1 (1.8% versus 5.1%).
Table 1.
Baseline Demographic and Clinical Characteristics of Patients With Troponin Fold 0 to 1 and >1 on Presentation
| Troponin Fold 0 to 1 N=6645 | Troponin Fold >1 N=7416 | Absolute Standardized Difference (%) | |
|---|---|---|---|
| Demographic characteristics | |||
| Age, y | 60.16±12.43 | 63.11±13.54 | 22.6 |
| Male | 4863 (73.2%) | 4869 (65.7%) | 16.4 |
| Female | 1782 (26.8%) | 2547 (34.3%) | 16.4 |
| Black or African American | 856 (12.9%) | 926 (12.5%) | 1.19 |
| White | 5654 (85.1%) | 6345 (85.6%) | 1.33 |
| Uninsured | 728 (11.0%) | 706 (9.5%) | 4.74 |
| History and clinical characteristics | |||
| Hypertension | 4547 (68.4%) | 5190 (70.0%) | 3.47 |
| Dyslipidemia | 4218 (63.5%) | 4480 (60.5%) | 6.32 |
| Diabetes mellitus | 1567 (23.6%) | 2003 (27.0%) | 7.88 |
| Tobacco use | 3081 (46.4%) | 3135 (42.3%) | 8.2 |
| Previous MI | 1721 (25.9%) | 1482 (20.0%) | 14.1 |
| Previous PCI | 1845 (27.8%) | 1474 (19.9%) | 18.6 |
| Previous HF | 343 (5.2%) | 487 (6.6%) | 5.98 |
| Peripheral artery disease | 418 (6.3%) | 596 (8.0%) | 6.78 |
| Chronic lung disease | 823 (12.4%) | 925 (12.5%) | 0.27 |
| Cardiogenic shock at time of PCI | 305 (4.6%) | 471 (6.4%) | 7.75 |
| Creatinine, mg/dL | 1.04±0.38 | 1.10±0.51 | 11.8 |
| Pre‐PCI | |||
| Troponin I, ng/mL | 0.04±0.07 | 5.88±17.63 | 46.8 |
| Troponin T, ng/mL | 0.02±0.01 | 0.70±1.32 | 73.0 |
| Pre‐PCI TIMI | |||
| 0 flow | 4276 (64.3%) | 4637 (62.5%) | 3.78 |
| 1 flow | 596 (9.0%) | 649 (8.8%) | 0.77 |
| 2 flow | 757 (11.4%) | 959 (12.9%) | 4.71 |
| 3 flow | 739 (11.2%) | 824 (11.1%) | 0.03 |
| In‐hospital mortality | 117 (1.8%) | 375 (5.1%) | 18.2 |
Variables expressed as mean±SD or n (%). HF indicates heart failure; MI, myocardial infarction; PCI, percutaneous coronary intervention; TIMI, thrombolysis in myocardial infarction.
The multivariable adaptive regression spline model identified 2 troponin fold values where the relationship between troponin and mortality significantly changed function. These cut points occurred at troponin folds 4.75 and 124.33. The local weighted polynomial regression curve modeling the association between troponin and mortality is shown in Figure 2, along with the delineation of regression knots including the point fold=1, reflecting the cutoff for a site‐defined abnormal troponin value. Patients were divided into 4 troponin groupings using fold cut points from the upper reference range and the previously identified knots (troponin categories: folds 0–1, 1–4.75, 4.75–124.33, and >124.33). To examine the relationship between troponin and mortality among different clinical risk categories, these groups were further stratified into quartiles of predicted mortality risk based on the CathPCI model for comparison of predicted and observed mortality, thus creating 16 patient groups. Figure 3 shows the mean predicted mortality risk and actual observed mortality for these patient groups.
Figure 2.
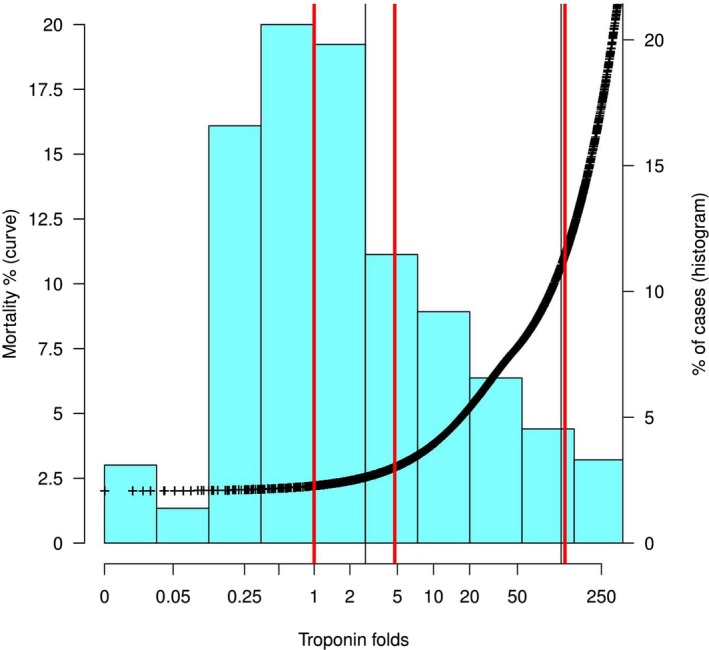
The association between admission troponin and mortality across the study cohort. Left y‐axis: estimated mortality (black curve) derived using LOESS regression. Right y‐axis: percent of cases in each troponin fold range (blue histogram bars). Vertical red lines identify nodes in the multivariable adaptive regression analysis. LOESS indicates Locally Estimated Scatterplot Smoothing.
Figure 3.
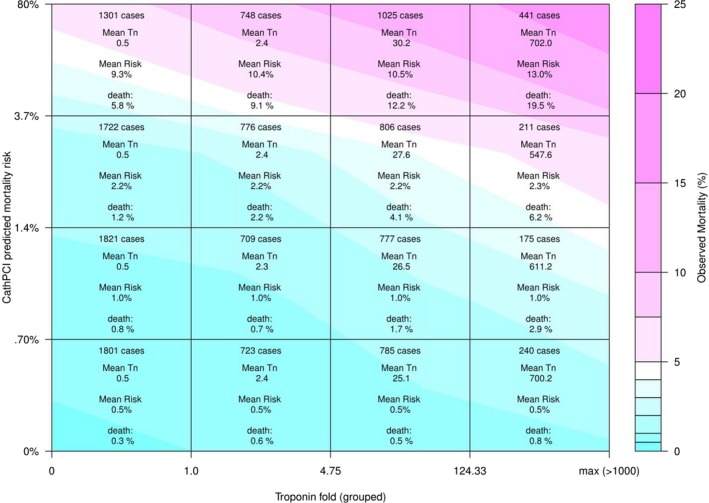
Troponin fold groupings and NCDR CathPCI risk quartile mortality heatmap. The x‐axis divides patients by troponin fold on presentation, with groupings determined by multivariable adaptive regression. The y‐axis divisions separate patients of each troponin grouping into quartiles of predicted risk based on the NCDR CathPCI prediction model. Observed mortality (%) is expressed with gradations of color found on the right heatmap calibration bar. NCDR indicates National Cardiovascular Data Registry; Tn, troponin fold.
The mean predicted mortality based on the CathPCI model ranged from 0.5% in the lowest risk quartile of patients with troponin folds 0 to 1, increasing to 13% for the highest‐risk quartile with troponin folds >124.33 on presentation. The actual observed mortality ranged from 0.3% to 19.5% in these same cohort groupings. The observed mortality for all patient groups with a troponin fold of 0 to 1 was consistently lower than expected, based on predicted clinical risk. This difference between predicted and observed mortality was particularly evident for the highest‐risk quartile in this troponin group (5.8% observed versus 9.3% predicted), but also persisted in patients with the lowest predicted risk (0.3% observed versus 0.5% predicted).
Conversely, for those patients presenting to the hospital with an initial troponin fold >4.75, the observed mortality generally exceeded what was predicted based on the CathPCI model. Differences between predicted and observed mortality were even more pronounced in patients with troponin folds >124.33, where observed mortality exceeded predicted mortality for all patient groups. As might be expected, patients in the highest predicted CathPCI risk quartile (>3.7% predicted mortality) who also fell into the highest troponin fold grouping (>124.33) had the worst outcomes with an observed mortality of 19.5%, substantially higher than the mean predicted risk of 13.0% based on the CathPCI model alone.
Symptom‐to‐door time was available for 47% of patients included in the cohort. Symptom‐to‐door time ranged from a median of 68 minutes (interquartile range, 47–120) for troponin folds 0 to 0.5, to 150 minutes (66–387.5) for patients with troponin folds >10 (Table 2). There was a statistically significant, albeit modest, correlation between troponin fold and symptom‐to‐door time (ρ=0.2858; P<0.001).
Table 2.
Symptom‐to‐Door and Door‐to‐Device Times by Troponin Fold on Presentation
| Troponin Folds | Median Symptom‐to‐Door in Minutes (IQR) | Median Door‐to‐Device in Minutes (IQR) |
|---|---|---|
| 0 to 0.5 | 68 (47–120) | 63 (49–77) |
| 0.5 to 1 | 72 (47–130) | 67 (53–81) |
| 1 to 10 | 99 (59–183) | 69 (54–84) |
| >10 | 150 (66–387.5) | 73 (58–91) |
Spearman correlation between troponin (folds) and symptom‐to‐door time (minutes) ρ=0.2858, P<0.0001. Spearman correlation between troponin (folds) and door‐to‐device time (minutes) ρ=0.1465, P<0.0001. IQR indicates interquartile range.
Discussion
The primary findings of this study are that nearly half of patients with STEMI had troponin levels that were undetectable or within the reference range on hospital presentation, and that the degree of troponin elevation on admission is a strong and independent predictor of in‐hospital mortality for STEMI patients undergoing primary PCI, even after adjustment for other baseline demographic and clinical factors. Patients with low or undetectable troponins experienced lower‐than‐expected mortality, regardless of their predicted risk based on demographic and clinical risk factors. Conversely, those with markedly elevated troponins on presentation had mortality rates that were higher than anticipated by the clinical risk model.
It is thought that the onset of myocardial infarction precedes a clinically abnormal troponin elevation by 3 to 4 hours when standard (ie, non‐high‐sensitivity) TnT and TnI assays are used, with a peak in enzyme levels at 12 to 24 hours depending on successful reperfusion.4 Though the rapid rise in biomarkers following reperfusion of the infarct‐related artery by thrombolysis and angioplasty in STEMI patients has been well described18 and can be used to predict the success of thrombolytic therapy,19 an elevated presenting troponin in an unrevascularized STEMI patient should never be confused with the “washout phenomenon” that accompanies successful reperfusion. Whereas peak troponin levels are driven by the success of revascularization and the territory of myocardium at risk from the infarct‐related artery,1, 2, 3 troponin release kinetics dictate that the levels observed in the early acute phase are highly dependent on the time of ischemia. Our findings suggest that admission levels may provide an important objective insight into the degree and duration of prehospital ischemia, and that increased ischemic time is a significant driver of increased mortality for those in high‐troponin groups.
As in earlier studies,20, 21, 22, 23 we found that symptom‐to‐door time correlated with troponin elevation, though only modestly. The determination of ischemic time based on history can be problematic for groups more likely to present with atypical symptoms such as older individuals, women, and diabetic patients.24, 25 In our real‐world cohort, symptom onset could not be reliably estimated for over half of patients. Interestingly, Mahmoud et al5 have shown that “biochemical ischemic time” based on troponin data often disagrees with, and is substantially longer than, conventional ischemic time derived from retrospective patient symptom report. In our cohort, there was overlap in symptom‐to‐door time, with both “early presenters” and “late presenters” (based on symptom onset) in high‐ and low‐troponin groupings. The poor reliability of symptom duration as an indicator of ischemic time may, in part, explain why some studies have failed to show a convincing relationship between symptom‐to‐door time and mortality in STEMI.26, 27, 28 In many patients, progression to STEMI may be preceded by a more prolonged and dynamic process of plaque instability, thrombus formation, distal embolization, and restoration of flow, which is better appreciated with objective admission biomarker data.29, 30
The 47.2% of STEMI patients with normal or negative admission troponin necessarily presented in the initial window between ischemia onset and the appearance of an elevated troponin. These data serve as a reminder that biomarker levels should never be used to delay a decision to proceed to coronary angiography in a patient with suspected STEMI. This figure is similar to previous single‐center data reported by Giannitsis et al (42–54%),20, 21 Kurowski et al (47.7%),22 and Matetzky et al (49.0%)31 over 15 years before our study. Though direct comparisons are not valid given advances in troponin assay development, these numbers suggest that there is continued need to prioritize progress in prehospital STEMI recognition and expedited care, especially in an era when door‐to‐device time has generally been optimized.32 Admission troponin levels reflect prehospital delays at both the patient (symptom recognition and medical contact) and system (contact‐to‐door time) levels. Although earlier efforts have generally failed to produce sustainable improvements in clinical outcomes, this needs to be revisited in the era of ubiquitous use of mobile technology and emerging advances in biosensor technology. We believe that admission biomarker data could be used to evaluate the effectiveness of quality improvement interventions aimed at reducing prehospital delay and overall duration of ischemia in STEMI, eventually leading to improvement in clinical outcomes.
Study Limitations
Our study cohort was based on a subgroup of patients from a large registry comprised of geographically diverse institutions with intrinsic differences in STEMI systems of care. We hope that our study will spur further investigation of these regional and institutional differences using admission biomarkers as an objective tool for comparison. A number of other limitations may affect the generalizability of our findings. There was significant heterogeneity in clinical TnI and TnT assay use among participating institutions. Although we attempted to adjust for this by normalizing values to each institution's upper limit of normal, this may not have fully accounted for subtle differences in test sensitivity and performance characteristics between institutions. Admission troponins were not obtained for 18% of the eligible patients in the registry. It is unclear whether initial laboratory testing may have been bypassed in the most critically ill or unstable STEMI patients, though no significant difference in mortality was noted in patients without admission troponin data compared to the study cohort as a whole (data not shown). We additionally chose to exclude patients with prehospital cardiac arrest because of its uncertain effect on biomarker levels. Cardiac arrest is associated with varying degrees of troponin elevation on hospital presentation even in nonischemic settings, perhaps reflecting injury during resuscitation efforts and global myocardial hypoperfusion.33 Mortality in such patients is also driven, to a large degree, by the neurological insult sustained before PCI.34
Our study predates the recent approval and adoption of high‐sensitivity troponin assays in the United States, and the role of these assays in risk stratification for STEMI patients is still being explored.35 In theory, their higher temporal resolution may be helpful in refining estimates of prehospital ischemic time, and this concept warrants further investigation.
Conclusions
Troponin levels on presentation provide prognostic value for STEMI patients treated with primary PCI, and mortality increases with increasing admission troponin levels, regardless of baseline clinical risk. A low or undetectable troponin level on presentation, an objective measure of a shorter prehospital ischemic time, was present in nearly half of our patients and portends a good prognosis. The substantial number of patients who present with markedly elevated troponins in the setting of an acute infarction, and their relatively worse outcomes, highlights the continued need for improvement in prehospital STEMI detection and care.
Author Contributions
Gurm and Seth had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Seth, Gurm; Acquisition, analysis, or interpretation of data: Wanamaker, Seth, Sukul, and Gurm; Drafting of the manuscript: Wanamaker, Gurm; Critical revision of the manuscript for important intellectual content: Wanamaker, Seth, Sukul, Dixon, Bhatt, Madder, Rumsfeld, and Gurm; Statistical analysis: MMS; Obtained funding: Gurm; Study supervision: Gurm.
Sources of Funding
This work was supported by the Blue Cross Blue Shield of Michigan and Blue Care Network as part of the Blue Cross Blue Shield of Michigan Value Partnerships program. The funding source supported data collection at each site and funded the data‐coordinating center, but had no role in study concept, interpretation of findings, or in the preparation, final approval, or decision to submit the manuscript. Dr Sukul was supported by a National Institutes of Health T32 postdoctoral research training grant (T32‐HL007853).
Disclosures
Dr Bhatt discloses the following relationships: Advisory Board: Cardax, Elsevier Practice Update Cardiology, Medscape Cardiology, and Regado Biosciences; Board of Directors: Boston VA Research Institute, Society of Cardiovascular Patient Care; Chair: American Heart Association Quality Oversight Committee; Data Monitoring Committees: Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute), Cleveland Clinic, Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine, and Population Health Research Institute; Honoraria: American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org; Vice‐Chair, ACC Accreditation Committee), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; clinical trial steering committee), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Duke Clinical Research Institute (clinical trial steering committees), HMP Global (Editor in Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), Population Health Research Institute (clinical trial steering committee), Slack Publications (Chief Medical Editor, Cardiology Today's Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), and WebMD (CME steering committees); Other: Clinical Cardiology (Deputy Editor), NCDR‐ACTION Registry Steering Committee (Chair), VA CART Research and Publications Committee (Chair); Research Funding: Abbott, Amarin, Amgen, AstraZeneca, Bristol‐Myers Squibb, Chiesi, Eisai, Ethicon, Forest Laboratories, Idorsia, Ironwood, Ischemix, Lilly, Medtronic, PhaseBio, Pfizer, Regeneron, Roche, Sanofi Aventis, Synaptic, and The Medicines Company; Royalties: Elsevier (Editor, Cardiovascular Intervention: A Companion to Braunwald's Heart Disease); Site Co‐Investigator: Biotronik, Boston Scientific, St. Jude Medical (now Abbott), and Svelte; Trustee: American College of Cardiology; Unfunded Research: FlowCo, Merck, PLx Pharma, and Takeda. Dr Gurm discloses the following relationships: Consultant: Osprey Medical; Research Funding: National Institutes of Health, Blue Cross Blue Shield of Michigan Foundation. The remaining authors have no disclosures to report.
Acknowledgments
The authors are indebted to all the study coordinators, investigators, and patients who participated in the BMC2 registry. Disclaimer: Although Blue Cross Blue Shield of Michigan (BCBSM) and BMC2 work collaboratively, the opinions, beliefs, and viewpoints expressed by the authors do not necessarily reflect the opinions, beliefs, and viewpoints of BCBSM or any of its employees.
(J Am Heart Assoc. 2019;8:e013551 DOI: 10.1161/JAHA.119.013551.)
References
- 1. Selvanayagam JB, Porto I, Channon K, Petersen SE, Francis JM, Neubauer S, Banning AP. Troponin elevation after percutaneous coronary intervention directly represents the extent of irreversible myocardial injury: insights from cardiovascular magnetic resonance imaging. Circulation. 2005;111:1027–1032. [DOI] [PubMed] [Google Scholar]
- 2. Giannitsis E, Steen H, Kurz K, Ivandic B, Simon AC, Futterer S, Schild C, Isfort P, Jaffe AS, Katus HA. Cardiac magnetic resonance imaging study for quantification of infarct size comparing directly serial versus single time‐point measurements of cardiac troponin T. J Am Coll Cardiol. 2008;51:307–314. [DOI] [PubMed] [Google Scholar]
- 3. Chia S, Senatore F, Raffel OC, Lee H, Wackers FJ, Jang IK. Utility of cardiac biomarkers in predicting infarct size, left ventricular function, and clinical outcome after primary percutaneous coronary intervention for ST‐segment elevation myocardial infarction. JACC Cardiovasc Interv. 2008;1:415–423. [DOI] [PubMed] [Google Scholar]
- 4. Morrow DA, Cannon CP, Jesse RL, Newby LK, Ravkilde J, Storrow AB, Wu AH, Christenson RH; National Academy of Clinical Biochemistry. National Academy of Clinical Biochemistry Laboratory Medicine Practice Guidelines: clinical characteristics and utilization of biochemical markers in acute coronary syndromes. Circulation. 2007;115:e356–e375. [DOI] [PubMed] [Google Scholar]
- 5. Mahmoud KD, Hillege HL, Jaffe AS, Lennon RJ, Holmes DR. Biochemical validation of patient‐reported symptom onset time in patients with ST‐segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. JACC Cardiovasc Interv. 2015;8:778–787. [DOI] [PubMed] [Google Scholar]
- 6. Moscucci M, Share D, Kline‐Rogers E, O'Donnell M, Maxwell‐Eward A, Meengs WL, Clark VL, Kraft P, De Franco AC, Chambers JL, Patel K, McGinnity JG, Eagle KA; the Blue Cross Blue Shield of Michigan Cardiovascular Consortium . The Blue Cross Blue Shield of Michigan Cardiovascular Consortium (BMC2) collaborative quality improvement initiative in percutaneous coronary interventions. J Interv Cardiol. 2002;15:381–386. [DOI] [PubMed] [Google Scholar]
- 7. Kline‐Rogers E, Share D, Bondie D, Rogers B, Karavite D, Kanten S, Wren P, Bodurka C, Fisk C, McGinnity J, Wright S, Fox S, Eagle KA, Moscucci M; the Blue Cross Blue Shield of Michigan Cardiovascular Consortium . Development of a multicenter interventional cardiology database: the Blue Cross Blue Shield of Michigan Cardiovascular Consortium (BMC2) experience. J Interv Cardiol. 2002;15:387–392. [DOI] [PubMed] [Google Scholar]
- 8. NCDR CathPCI Registry v4.4 Coder's Data Dictionary. American College of Cardiology Foundation; 2011. Available at: https://www.ncdr.com/WebNCDR/docs/public-data-collection-documents/cathpci_v4_codersdictionary_4-4.pdf. Accessed January 31, 2018. [Google Scholar]
- 9. O'Gara PT, Kushner FG, Ascheim DD, Casey DE, Chung MK, de Lemos JA, Ettinger SM, Fang JC, Fesmire FM, Franklin BA, Granger CB, Krumholz HM, Linderbaum JA, Morrow DA, Newby LK, Ornato JP, Ou N, Radford MJ, Tamis‐Holland JE, Tommaso CL, Tracy CM, Woo YJ, Zhao DX. 2013 ACCF/AHA guideline for the management of ST‐elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127:e362–e425. [DOI] [PubMed] [Google Scholar]
- 10. Apple FS, Sandoval Y, Jaffe AS, Ordonez‐Llanos J; IFCC Task force on Clinical Applications of Cardiac Bio‐Markers . Cardiac troponin assays: guide to understanding analytical characteristics and their impact on clinical care. Clin Chem. 2017;63:73–81. [DOI] [PubMed] [Google Scholar]
- 11. Roe MT, Peterson ED, Li Y, Pollack CV, Christenson RH, Peacock WF, Fesmire FM, Newby LK, Jesse RL, Hoekstra JW, Gibler WB, Ohman EM. Relationship between risk stratification by cardiac troponin level and adherence to guidelines for non‐ST‐segment elevation acute coronary syndromes. Arch Intern Med. 2005;165:1870–1876. [DOI] [PubMed] [Google Scholar]
- 12. Austin PC. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat Simul Comput. 2009;38:1228–1234. [Google Scholar]
- 13. Tijssen JG, Kolm P. Demystifying the new statistical recommendations: the use and reporting of p values. J Am Coll Cardiol. 2015;68:231–233. [DOI] [PubMed] [Google Scholar]
- 14. Peterson ED, Dai D, DeLong ER, Brennan JM, Singh M, Rao SV, Shaw RE, Roe MT, Ho KK, Klein LW, Krone RJ, Weintraub WS, Brindis RG, Rumsfeld JS, Spertus JA; NCDR Registry Participants . Contemporary mortality risk prediction for percutaneous coronary intervention: results from 588,398 procedures in the National Cardiovascular Data Registry. J Am Coll Cardiol. 2010;55:1923–1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Friedman J. Multivariate adaptive regression splines. Ann Stat. 1991;19:1–67. [Google Scholar]
- 16. Milborrow S. Earth: multivariate adaptive regression splines R statistical software package ed. 4.5.1. Available at: https://cran.r-project.org/web/packages/earth/index.html. Accessed October 9, 2017.
- 17. Cleveland WS. Robust locally weighted regression and smoothing scatterplots. J Am Stat Assoc. 1979;74:829–836. [Google Scholar]
- 18. Abe S, Arima S, Yamashita T, Miyata M, Okino H, Toda H, Nomoto K, Ueno M, Tahara M, Kiyonaga K. Early assessment of reperfusion therapy using cardiac troponin T. J Am Coll Cardiol. 1994;23:1382–1389. [DOI] [PubMed] [Google Scholar]
- 19. Stewart JT, French JK, Théroux P, Ramanathan K, Solymoss BC, Johnson R, White HD. Early noninvasive identification of failed reperfusion after intravenous thrombolytic therapy in acute myocardial infarction. J Am Coll Cardiol. 1998;31:1499–1505. [DOI] [PubMed] [Google Scholar]
- 20. Giannitsis E, Lehrke S, Wiegand UK, Kurowski V, Müller‐Bardorff M, Weidtmann B, Richardt G, Katus HA. Risk stratification in patients with inferior acute myocardial infarction treated by percutaneous coronary interventions: the role of admission troponin T. Circulation. 2000;102:2038–2044. [DOI] [PubMed] [Google Scholar]
- 21. Giannitsis E, Müller‐Bardorff M, Lehrke S, Wiegand U, Tölg R, Weidtmann B, Hartmann F, Richardt G, Katus HA. Admission troponin T level predicts clinical outcomes, TIMI flow, and myocardial tissue perfusion after primary percutaneous intervention for acute ST‐segment elevation myocardial infarction. Circulation. 2001;104:630–635. [DOI] [PubMed] [Google Scholar]
- 22. Kurowski V, Hartmann F, Killermann DP, Giannitsis E, Wiegand UK, Frey N, Müller‐Bardorff M, Richardt G, Katus HA. Prognostic significance of admission cardiac troponin T in patients treated successfully with direct percutaneous interventions for acute ST‐segment elevation myocardial infarction. Crit Care Med. 2002;30:2229–2235. [DOI] [PubMed] [Google Scholar]
- 23. Ohman EM, Armstrong PW, White HD, Granger CB, Wilcox RG, Weaver WD, Gibler WB, Stebbins AL, Cianciolo C, Califf RM, Topol EJ. Risk stratification with a point‐of‐care cardiac troponin T test in acute myocardial infarction. GUSTOIII Investigators. Global Use of Strategies To Open Occluded Coronary Arteries. Am J Cardiol. 1999;84:1281–1286. [DOI] [PubMed] [Google Scholar]
- 24. Canto JG, Rogers WJ, Goldberg RJ, Peterson ED, Wenger NK, Vaccarino V, Kiefe CI, Frederick PD, Sopko G, Zheng ZJ; NRMI Investigators . Association of age and sex with myocardial infarction symptom presentation and in‐hospital mortality. JAMA. 2012;307:813–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brieger D, Eagle KA, Goodman SG, Steg PG, Budaj A, White K, Montalescot G; GRACE Investigators . Acute coronary syndromes without chest pain, an underdiagnosed and undertreated high‐risk group: insights from the Global Registry of Acute Coronary Events. Chest. 2004;126:461–469. [DOI] [PubMed] [Google Scholar]
- 26. Cannon CP, Gibson CM, Lambrew CT, Shoultz DA, Levy D, French WJ, Gore JM, Weaver WD, Rogers WJ, Tiefenbrunn AJ. Relationship of symptom‐onset‐to‐balloon time and door‐to‐balloon time with mortality in patients undergoing angioplasty for acute myocardial infarction. JAMA. 2000;283:2941–2947. [DOI] [PubMed] [Google Scholar]
- 27. McNamara RL, Wang Y, Herrin J, Curtis JP, Bradley EH, Magid DJ, Peterson ED, Blaney M, Frederick PD, Krumholz HM; NRMI Investigators . Effect of door‐to‐balloon time on mortality in patients with ST‐segment elevation myocardial infarction. J Am Coll Cardiol. 2006;47:2180–2186. [DOI] [PubMed] [Google Scholar]
- 28. Berger PB, Ellis SG, Holmes DR, Granger CB, Criger DA, Betriu A, Topol EJ, Califf RM. Relationship between delay in performing direct coronary angioplasty and early clinical outcome in patients with acute myocardial infarction: results from the global use of strategies to open occluded arteries in Acute Coronary Syndromes (GUSTO‐IIb) trial. Circulation. 1999;100:14–20. [DOI] [PubMed] [Google Scholar]
- 29. Rittersma SZ, van der Wal AC, Koch KT, Piek JJ, Henriques JP, Mulder KJ, Ploegmakers JP, Meesterman M, de Winter RJ. Plaque instability frequently occurs days or weeks before occlusive coronary thrombosis: a pathological thrombectomy study in primary percutaneous coronary intervention. Circulation. 2005;111:1160–1165. [DOI] [PubMed] [Google Scholar]
- 30. Leibundgut G, Gick M, Morel O, Ferenc M, Werner KD, Comberg T, Kienzle RP, Buettner HJ, Neumann FJ. Discordant cardiac biomarker levels independently predict outcome in ST‐segment elevation myocardial infarction. Clin Res Cardiol. 2016;105:432–440. [DOI] [PubMed] [Google Scholar]
- 31. Matetzky S, Sharir T, Domingo M, Noc M, Chyu KY, Kaul S, Eigler N, Shah PK, Cercek B. Elevated troponin I level on admission is associated with adverse outcome of primary angioplasty in acute myocardial infarction. Circulation. 2000;102:1611–1616. [DOI] [PubMed] [Google Scholar]
- 32. Menees DS, Peterson ED, Wang Y, Curtis JP, Messenger JC, Rumsfeld JS, Gurm HS. Door‐to‐balloon time and mortality among patients undergoing primary PCI. N Engl J Med. 2013;369:901–909. [DOI] [PubMed] [Google Scholar]
- 33. Kontos MC, Ornato JP, Kurz MC, Roberts CS, Gossip M, Dhindsa HS, Reid RD, Peberdy MA. Prevalence of troponin elevations in patients with cardiac arrest and implications for assessing quality of care in hypothermia centers. Am J Cardiol. 2013;112:933–937. [DOI] [PubMed] [Google Scholar]
- 34. Valle JA, Smith DE, Booher AM, Menees DS, Gurm HS. Cause and circumstance of in‐hospital mortality among patients undergoing contemporary percutaneous coronary intervention: a root‐cause analysis. Circ Cardiovasc Qual Outcomes. 2012;5:229–235. [DOI] [PubMed] [Google Scholar]
- 35. Wang TK, Snow TA, Chen Y, Rostom H, White JM, Stewart JT, Webster MW, Ruygrok PN, Watson T, White HD. High‐sensitivity troponin level pre‐catheterization predicts adverse cardiovascular outcomes after primary angioplasty for ST‐elevation myocardial infarction. Eur Heart J Acute Cardiovasc Care. 2014;3:118–125. [DOI] [PubMed] [Google Scholar]


